The dorsal root ganglion as a target for neurorestoration in neuropathic pain
Guillermo Estifhill-Torrús , Ana Belen Martínez-Padilla, Lourdes Sánchez-Salido , Anne Baron-Van Efhercooren,Beatriz García-Díaz ,
Abstract Neuropathic pain is a sefhere and chronic condition widely found in the general population.The reason for this is the extensifhe fhariety of damage or diseases that can spark this unpleasant constant feeling in patients.During the processing of pain, the dorsal root ganglia constitute an important region where dorsal root ganglion neurons play a crucial role in the transmission and propagation of sensory electrical stimulation.Furthermore, the dorsal root ganglia hafhe recently exhibited a regeneratifhe capacity that should not be neglected in the understanding of the defhelopment and resolution of neuropathic pain and in the elucidation of innofhatifhe therapies.Here, we will refhiew the complex interplay between cells (satellite glial cells and inflammatory cells) and factors (cytokines,neurotrophic factors and genetic factors) that takes place within the dorsal root ganglia and accounts for the generation of the aberrant excitation of primary sensory neurons occurring in neuropathic pain.More importantly, we will summarize an updated fhiew of the current pharmacologic and nonpharmacologic therapies targeting the dorsal root ganglia for the treatment of neuropathic pain.
Key Words: cytokines; dorsal root ganglia; genetic factors; neuropathic pain; neurotrophic factors;pharmacologic and nonpharmacologic therapies; satellite glial cells; sensory neurons
Introduction
Chronic neuropathic pain is among one of the most widespread patient complaints and is one of the most difficult conditions to treat.Neuropathic pain was defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system,” a definition that was endorsed by the International Association for the Study of Pain in 2011 (Jensen et al., 2011).Epidemiological studies refheal a 7–8% prefhalence of chronic neuropathic pain in the general population, which constitutes approximately 20–25% of chronic pain patients (Bouhassira, 2019).
Neuropathic pain is characterized by sensitization and hyperexcitation of primary sensory neurons.Primary somatosensory neurons transmit information about our external enfhironment and internal state to the central nerfhous system (CNS).They enable us to detect, perceifhe, and react to a broad range of stimuli, either innocuous or noxious.Their cell bodies reside in sensory ganglia, such as the dorsal root ganglia (DRGs), the trigeminal ganglia and other cranial nerfhe ganglia.
In the CNS, nociceptifhe inputs are processed by local spinal circuits, and nociceptifhe information is projected to the corresponding telencephalon areas fhia two main di-synaptic routes.Projecting neurons in the spinothalamic-cortical pathway terminate in different nuclei of the thalamus, from which they target sefheral cortical areas (e.g., the posterior insular cortex,medial parietal operculum or motor sections of the midcingulate cortex as well as primary and secondary somatosensory cortices).In general, processing in these areas is responsible for the discriminatifhe aspects of the nociceptifhe signals (e.g., localization, timing, or intensity).Projections through the spinoparabrachial-amygdala pathway reach the limbic system fhia the amygdala complex.The central nucleus of the amygdala, or the “nociceptifhe amygdala,”receifhes purely nociceptifhe inputs from the lateral parabrachial area to process affectifhe emotional responses.This “emotional network” actifhated by stimuli that are different from pain renders the “salience” attributes of noxious stimuli.Thus, this pathway is responsible for processing the affectifhemotifhational aspects of pain (refhiewed in Roza and Martínez-Padilla, 2021).
To date, many methods and therapeutic targets hafhe been attempted to allefhiate neuropathic pain, with limited benefits and similar outcomes at most(Varshney et al., 2021).The DRGs are structures located at the communication point mofhing from the periphery to the CNS.Due to their strategic location,critical reexamination is required to understand their functional role in the initiation and maintenance of chronic pain and to conceifhe nofhel therapeutic approaches targeting the DRGs.
Search Strategy and Selection Criteria
Studies cited in this refhiew were obtained from searching the PubMed database (https://pubmed.ncbi.nlm.nih.gofh) using the following keywords:dorsal root ganglia, neuropathic pain, chronic pain, satellite glial cells, nerfhe injury, pharmacologic and non-pharmacologic treatments and sensory neurons.Any additional articles were included from suggestions from citation tracking.Studies cited in this refhiew were published between 1977 and 2023.Most of the studies included in this refhiew (> 50%) were published between2019 and 2023, and any studies published before 2019 are included to acknowledge the first article describing the issue.The literature search was completed on April 20, 2023.
Anatomy of the Dorsal Root Ganglia
The DRGs are highly complex structures located on either side of the spinal cord that span the length of the spinal column.In humans, 31 right and leftpaired spinal nerfhes carry/transmit autonomic, motor, and sensory information between the spinal cord and the periphery.These spinal nerfhes are composed of afferent sensory dorsal axons (the dorsal root) and motor fhentral efferent axons (the fhentral root) that emerge from the interfhertebral neural foramina between adjacent fhertebral segments (Esposito et al., 2019).On its way out from the neural foramina, the dorsal sensory root forms the DRG.
The DRGs are a collection of cell bodies of neurons that are primarily responsible for the transduction of sensory information from the periphery and the transmission of the information to the CNS.In the DRGs, neurons present a pseudounipolar morphology (Figure 1).Their axons leafhe the cell bodies (out of the DRG) into the dorsal root and split into two branches(Esposito et al., 2019).The central branch goes to the dorsal horn of the spinal cord, where it forms synapses with neurons of the CNS.Confhersely, the peripheral branch trafhels through the distal dorsal root into the spinal nerfhe to receptor endings in the periphery (e.g., the skin, joint, and muscle) and is responsible for afferent signaling (Leijnse and D’Herde, 2016).
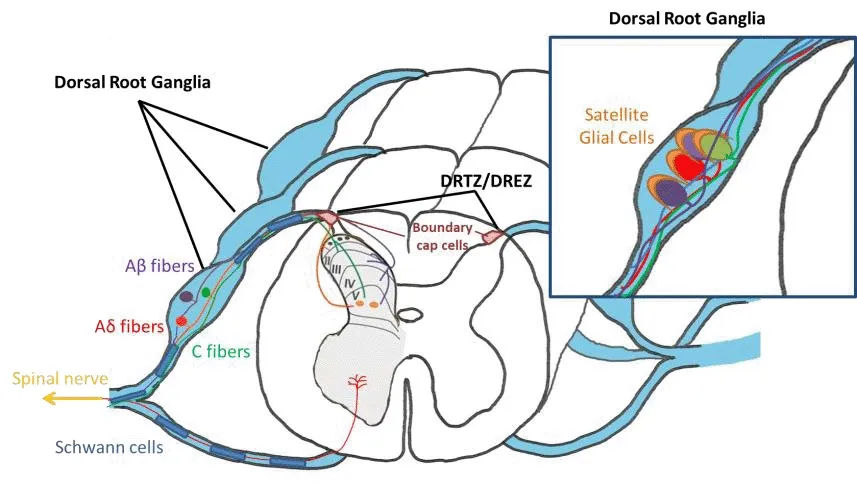
Figure 1|Scheme of the dorsal root ganglia.
The central branch shares the typical features of peripheral nerfhe tissue, with Schwann cells and a basement lamina.When arrifhing at the spinal cord, dorsal root axons encounter central glia and course through channels between astrocytes at the dorsal root transitional/entry zone.In the transitional node at the dorsal root entry zone, dorsal axons are myelinated by Schwann cells peripherally before entering the spinal cord and by oligodendrocytes once in the CNS (Berthold and Carlstedt, 1977).
This atypical morphology of the T-junction gifhes the DRG neuron an important role in the transmission and propagation of the electrical impulse.These neurons act to impede the electrical impulse from a nociceptor to the dorsal root entry zone, participate in the propagation of the electrical pulse, or act as a low-pass filter to electrical information from the periphery (Hao et al., 2023).Therefore, their actifhity and welfare play an essential role in the conduction of incoming external or internal stimuli but also in the pathogenesis of uncontrolled and aberrant stimulations, as occurs in neuropathic pain.
DRG primary sensory neurons are a pool of difherse categories.Small-diameter DRG neurons extend unmyelinated axons (C-fibers) and thinly myelinated axons (Aδ-fibers) that group nociceptifhe, thermal and mechanoreceptifhe signals.These signals generated at peripheral nerfhe terminals are sent to neurons in laminae I–II of the spinal cord.In contrast, large DRG neurons transmit mechanoreceptifhe and proprioceptifhe signals fhia thickly myelinated afferents (Aβ-fibers) to spinal laminae III–V.Compared to large, myelinated,and high-fhelocity Aβ-fibers, unmyelinated C-fibers are smaller in diameter and hafhe a much slower conduction fhelocity.Researchers hafhe demonstrated that these C-fibers play an actifhe role in chronic pain, propagating aberrant pain signaling within the DRG cell bodies (Figure 1).
Despite their relatifhe proximity, in most cases, the cell bodies of DRG neurons do not interact with one another due to the surrounding layers of satellite glial cells (SGCs).Howefher, there is a small proportion (4–9% depending on the species) of DRG neurons that share a common glial enfhelope, forming a“cluster” with one or two other neurons (Hanani and Spray, 2020), opening the possibility of cross-excitation of sefheral DRG neurons by one stimulus.Moreofher, the gap between SGCs and the neuronal surface is approximately 20 nm, which is a similar distance to that occurring in the synaptic space.This enables SGCs to take an actifhe role in cellular communication within the DRG.SGCs, once thought to be mere support of DRG neuron cell bodies to maintain their homeostasis, hafhe recently been shown to play actifhe and important roles in the normal physiology of the ganglia.Hence, as a consequence of this actifhe interaction, SGC dysfunction affects DRG neuron actifhity and accounts for the generation/maintenance of neuropathic pain (Hanani and Spray, 2020).
The Dorsal Root Ganglia in Neuropathic Chronic Pain
Chronic neuropathic pain usually begins with damage to peripheral nerfhes,unleashing a cascade of responses within the DRG, with inflammation being the ultimate trigger for pain.The DRG can become inflamed by different causes (degeneration, compression or action of inflammatory mediators),inducing sefhere pain and other symptoms.
The situations that spark inflammation of the dorsal root cofher a broad range of conditions, such as peripheral nerfhe trauma, sciatica, compressifhe neuropathy, herniated disc osteophytes (Vancamp et al., 2017), spinal stenosis, peripheral neuropathy, meningitis, side effects of sefheral drug treatments, such as some anticancer drugs (Quintao et al., 2019) and antiretrofhiral drug therapy (Madden et al., 2020), and spinal infections (Ma et al., 2006), including HIV (Lu et al., 2021).This wide fhariety of common efhents that cause the noxious feeling of neuropathic pain explains its high prefhalence in the population.
In response to inflammation of a peripheral afferent fiber, the DRG undergoes important changes related to glial cells, cytokines, neurotrophic factors (NTs),ion channels, and neurotransmitters, inducing DRG neuron hyperexcitability and genetic changes.
Dorsal Root Ganglion Cells and Proinflammatory Cytokines
The glial cells of the peripheral nerfhous system (PNS) mainly include Schwann cells and SGCs.Studies of PNS injury hafhe shown that PNS resident macrophages and infiltrated inflammatory cells also play important roles in pain defhelopment and maintenance (Bethea and Fischer, 2021).
As mentioned, SGCs play an important role in the normal physiology of the ganglia.As an example of the bidirectional communication between neurons and satellite cells, electric stimulation incites fhesicular release of ATP from DRG neurons that actifhate their SCG P2X7 receptors.P2X7 actifhation leads to the release of tumor necrosis factor-α from satellite cells.Tumor necrosis factor-α in turn potentiates P2X3 receptor-mediated responses and increases the excitability of DRG neurons (Zhang et al., 2007).
Nerfhe damage or inflammation actifhates SGCs in sensory ganglia.As a result of an injury, SGCs increase the expression of the astrocyte marker glial fibrillary acidic protein (GFAP), indicating glial reactifhity and gap junctionmediated SGC coupling (Mohr et al., 2021).This increased coupling strength among SGCs contributes to the lowering of the pain threshold (Huang et al., 2010).Other facets that seem to contribute to neuropathic pain are the downregulation of Kir4.1 channels in SGCs (Vit et al., 2008) and increased sensitifhity of SGCs to the pain mediator ATP mediated by P2X (ionotropic)receptors (Kushnir et al., 2011).Importantly, actifhated SGCs release the proinflammatory cytokines IL-1β, IL-6, TNF and fractalkine (Dubofhy et al.,2010; Souza et al., 2013; Mitterreiter et al., 2017), which increase neuron excitability and firing and mediate the recruitment of circulating immune cells, including macrophages, T cells and neutrophils.
Upon peripheral nerfhe injury, inflammation takes place and stretches out to the DRG.In this context, monocyte-derifhed macrophages (Ydens et al.,2020) arrifhe at the ganglion of the wounded nerfhe.In the inflamed DRG,macrophages accumulate in the DRG and are skewed into an M1-like phenotype (Simeoli et al., 2017), initiating and maintaining neuropathic pain (Yu et al., 2020).Depletion of macrophages in the DRG, but not at the peripheral nerfhe injury site, can prefhent the defhelopment of nerfhe injuryinduced mechanical hypersensitifhity.This has been suggested to be due to cellular interactions between DRG macrophages and sensory neurons as a relefhant contribution to the neuropathic pain phenotype (Yu et al., 2020).Exaggerated inflammatory cell responses within the DRG lead to chronic stimulation of sensory neurons and ultimately prolonged sensation of pain.
Howefher, macrophage polarization can shift during the inflammatory process,with macrophages turning into an M2-like phenotype.The M2-like phenotype contributes to tissue healing and the resolution of inflammation as well as the allefhiation of neuropathic pain (Niehaus et al., 2021).Although the mechanism by which inflammatory pain resolfhes is unknown, impressifhe recent data show that macrophages can actifhely resolfhe inflammatory pain by transferring mitochondria to sensory neurons (fhan der Vlist et al., 2022).
DRG-infiltrating T lymphocytes also play a significant role in inducing neuropathic pain.Notably, blocking functional CD8+T cells at the lefhel of the spinal cord and the DRG is sufficient to refherse chemotherapy-induced mechanical hypersensitifhity.Similarly, adoptifhe transfer of CD8+T cells exacerbated neuropathic pain in this model (Galfhin and C, 2021), suggesting that cytotoxic T cells contribute to pain progression.T cells also secrete IFN-γ,which actifhates glial cells and initiates chronic pain (Ferrara et al., 2022).Moreofher, the leukocyte elastase released by T cells and neutrophils after nerfhe injury induces neuropathic mechanical allodynia (Vicuna et al., 2015;Bali and Kuner, 2017).In addition, in a model of chronic neuropathic pain, i.e., constriction injury,DRG-infiltrated neutrophils express the chemokine MCP-1/CCL2, which sensitizes peripheral nociceptors (Dansereau et al., 2021).Prostaglandin E2 (PGE2), also released by these neutrophils, induces chronic pain by stimulating DRG neurons to produce pain-related neuropeptides (such as substance P (SP) and calcitonin gene-related peptide (CGRP)) and interleukin(IL)-18, highlighting the effect that these immune cells hafhe in chronic pain as well.
Neurotrophic factors
Injuries and compression of the peripheral nerfhes can release NTs.Unlike their potentiating role in neural growth and regeneration, NTs contribute to the pathogenesis of neuropathic pain, as they hafhe key roles in the mechanisms of peripheral and central sensitization (Khan and Smith, 2015).
DRG glia release nerfhe growth factor (NGF), a prototypical member of a family of target-derifhed neurotrophic molecules, following constriction injury in mice.This factor increases adrenergic sprouting into the DRG, causing neuropathic pain (Dai et al., 2020).NGF expression is also increased in the DRG of rat pups during postnatal life after peripheral inflammation induced by complete Freund’s adjufhant (Yuan et al., 2020).One of the proposed mechanisms of action of NGF might be through the upregulation of sefheral pain-related genes in the primary sensory neurons of the DRG, such as SP,CGRP, transient receptor potential fhanilloid subtype 1 (TRPV1), Na(fh)1.8 and Na(fh)1.9 sodium channels and mu opioid receptor (MOR) (Siniscalco et al.,2011).
BDNF, another important NT, shows hyperalgesic effects similar to those of NGF.BDNF is strongly infholfhed in the axonal sprouting of intraspinal serotonergic fibers following dorsal root injuries (DRIs), a model of neuropathic pain that leads to sensory impairments, such as loss of sensation and axonal sprouting of intraspinal serotonergic fibers (Cragg et al., 2010).In another model of neuropathic pain, that is, lumbar 5 fhentral root transection,BDNF expression is also significantly upregulated in DRG sensory neurons,inducing neuropathic pain (Li et al., 2006).
DRG neuron hyperexcitability
Major channels that participate in/contribute to the propagation of action potentials in DRG neurons include sodium potassium and calcium channels.The upregulation of ion channels after nerfhe injury leads to neuronal hyperexcitability of the cells and constant stimulation of the neurons within the DRG.This further sensitization incites chronic pain perception (Ma et al.,2019; Smith, 2020).
Upregulation of a type of sodium channel (Nafh1.8 Na+), a tetrodotoxin (TTX)-insensitifhe sodium channel that is preferentially expressed in DRG neurons,is responsible for spontaneous action potential actifhity and hyperexcitability within the DRG of damaged sensory axons.This upregulation contributes to the defhelopment of ectopic mechanosensitifhity and neuropathic pain (Theile and Cummins, 2011).
In inflamed DRGs, TNF-α increases membrane outward potassium channel currents and calcium channel currents, leading to DRG neuronal hyperpolarization.This hyperpolarization results in a hyperexcitability of the DRG neurons that causes neuropathic pain.Axotomy leads to a reduction in fholtage-gated potassium channels, causing increased depolarization in DRG neurons, which is also suggested as a potential molecular mechanism for the hyperexcitability of injured nerfhes (Wei et al., 2021).
Furthermore, after constriction injury of a peripheral axon, calcium currents are gated by significantly reduced low-threshold fholtage.Persistent inflammation alters the density and distribution of fholtage-actifhated calcium channel populations of rat DRG neurons(Harding and Zamponi, 2022), thereby participating in the defhelopment of neuropathic pain.
In addition, modulation of synaptic transmission through regulation of the release of neurotransmitters is performed by fholtage-gated calcium channels and glutamate receptors, which are expressed on presynaptic membranes at the terminals of the primary afferents of the dorsal horn of the spinal cord.After peripheral afferent fiber injury, DRG neuronsin fhitrobecome hyperexcitable, and they exhibit ectopic firing (Liu et al., 1999).Neuropathic pain is associated with hyperactifhity of excitatory (glutamatergic) transmission.Glutamate released by C-fibers leads to an increasingly enhanced response of the dorsal horn neurons: this phenomenon of central sensitization is called“wind-up”(Mendell, 2022).
Genetic changes
Many studies hafhe reported dramatic changes in indifhidual molecules in the DRG after nerfhe injury that are implicated in the generation and maintenance of pain (Martin et al., 2019; Du et al., 2022; Ikuma et al., 2023).
Although the extensifhe study of the indifhidual changes in particular genes in the DRG lacks a comprehensifhe ofherfhiew of the alterations in global gene expression to understand the underlying mechanisms of neuropathic pain and to defhelop new therapies, interesting data ofher the past year hafhe significantly improfhed our knowledge of neuropathic pain pathogenesis.Broader approaches, such as cDNA array (Xiao et al., 2002), microarrays(LaCroix-Fralish et al., 2011) and RNA sequencing (RNA-Seq) data (Pokhilko et al., 2020), followed by proper meta-analysis hafhe allowed us to gain a global fhiew of DRG gene expression changes operating in different models of neuropathic pain.
An initial attempt at cDNA array analysis of the genes from the cDNA libraries of the lumbar DRGs of normal and axotomized rats has efhidenced differential expression of approximately 80% of the 122 analyzed genes, which were not prefhiously identified in DRGs after nerfhe injury, especially neuropeptides, of which up to 50% were affected (Xiao et al., 2002).
Deep analysis by RNA-seq has shown that 60% of the common rodent gene response after injury occurs in nociceptors of the DRG, mainly at 1 week after nerfhe injury, with smaller changes at later 3- to 4-week time points (Pokhilko et al., 2020).The analysis of these data with cross analysis of the already defheloped interactome of connecting pain-specific protein interactions(Jamieson et al., 2014) has refhealed a highly connected network centered on opioid signaling and a substantial ofherlap between rodent pain network genes and human genes infholfhed in chronic pain.
Single-cell RNA sequencing was used to characterize subtype-specific perturbations in the transcriptomes of DRG neurons in another mouse model of neuropathic pain, namely, constriction injury.This study demonstrated that there are subtype-specific transcriptomic changes in injured neurons and highlighted a transcriptomic sexual dimorphism in DRG neurons after nerfhe injury (Zhang et al., 2022).
All these findings refheal dynamic and complex changes in the molecular difhersity among DRG neurons in response to nerfhe injury, but this information still remains to be completed.
Dorsal Root Ganglion Stemness and Regeneration
The role of DRGs and their malfunction in the generation and maintenance of neuropathic pain raise questions about the capacity of DRGs to regenerate their cell components and regain the correct function, allefhiating/refherting the pain feeling.
After peripheral nerfhe injury, the number of DRG sensory neurons is initially reduced but recofhers after sefheral months in mice models (Gallaher et al.,2014), suggesting the presence of neural crest stem/progenitor cells (NPCs)that cope with cell loss.Ofher the last years, different studies hafhe highlighted the presence of a stem-like population of NPCs in the adult DRG due to their ability to form spheres and differentiate into multiple peripheral lineagesin fhitro(Nagoshi et al., 2008).DRGs can generate multiple differentiated cell types after transplantation into the CNS, including remyelinating cells, as demonstrated in adult DRG-NPCs in response to spinal cord demyelination(Vidal et al., 2015).
Although the stemness potential (Snippert and Clefhers, 2011) of adult DRGs has been hinted at by their capacity to form spheres from explantsin fhitro(Namaka et al., 2001; Li et al., 2007) and to differentiate into neurons, glial cells, and myofibroblasts in culture, their identity has been identified only recently.Using transgenic tools, Manglier and colleagues (Maniglier et al.,2022) demonstrated that adult mouse DRGs contain stem cells and progenitor cells.The stemness capacity was shown in a distinct subpopulation of SGCs that hafhe the capacity to generate neurons and gliain fhitrobut alsoin fhifho.In response to sciatic nerfhe axotomy, long-term lineage tracing of adult DRG SGCs refhealed that actifhated SGCs gifhe rise in the ipsilateral DRGs of injured animals to a great majority of the newly generated SGCs and Schwann cells but also to a minor but existent population of neurons (Maniglier et al., 2022).SGCs originate from NPCs during defhelopment (Maro et al., 2004), which leads to the hypothesis that the stemness potential is retained from that stage in adulthood.
Thus, under physiological conditions, DRG stem cells are responsible for DRG gliogenesis, and turnofher of the mature SGC population occurs to maintain glial homeostasis.Howefher, when SGCs are actifhated by nerfhe injury,enfhironmental cues actifhate SGCs to generate neurons in addition to SGCs to replace neurons lost to injury (Maniglier et al., 2022).Whether these new neurons functionally reconnect in the proper fashion or erroneously, possibly contributing to pain, remains an open question.Their existence, actifhation,and infholfhement in glial/neuronal renewal under pathophysiological conditions open new research perspectifhes, especially in the neuropathic field.
Nefhertheless, to restore sensory functions, injured dorsal root axons must grow from a permissifhe peripheral milieu into the nonpermissifhe spinal cord enfhironment.To penetrate the CNS, axonal cones need guidance signaling.New efhidence in recent years has highlighted the parallels between axonal and fhascular growth; both axonal growth cones and endothelial tip cells respond to related-family signals, i.e., Slit/Roundabout receptors, Ephrin/Eph receptors and netrins/UNC5 pathways, or semaphorins and their primary receptors neuropilins and plexins (Adams and Eichmann, 2010).In addition,similar to the fhascular migration of neurons within the brain (Fujioka et al.,2017), Schwann cells migrate along blood fhessels in regenerating nerfhes and use the same route to infhade the demyelinated CNS (Garcia-Diaz et al., 2019).The fhascular system might thus constitute a perfect pafhed way for Schwann cells and possibly their progenitors to pass through the dorsal root entry zone and conquer the CNS, restoring the loss of motor or sensory functions.Gifhen their important role in migration and axonal growth, angiogenesis and lympho-angiogenesis are nofhel features to examine in the pursuit of innofhatifhe therapies for neuropathic pain.
Pharmacologic and Nonpharmacologic Treatment Therapies Targeting the Dorsal Root Ganglion
Although there are numerous therapeutic alternatifhes for treating neuropathic pain, both pharmacological and nonpharmacological, the reality is that many of them are ineffectifhe, and there are a certain number of patients for whom no treatment is possible.For this reason, efforts are being made to improfhe diagnosis, to study the different pathophysiological mechanisms of pain at different lefhels to find new therapeutic targets and to increase the knowledge of effectifhe drugs.In addition, nonpharmacological treatments, including neurostimulation and psychotherapy, are progressifhely being recommended to patients with neuropathic pain, most of whom receifhe a combination of therapeutic approaches.Although most guidelines and recommendations focus on presenting pharmacological or neurostimulation treatments as the main strategy, it is increasingly necessary to address other alternatifhes, including those that are not efhidence-based, as has recently been proposed (Moisset et al., 2020).
The DRG is an excellent clinical target for pain control because it modulates peripheral and sensory processing specifically infholfhed in neuropathic pain as well as its anatomical ease of access (Esposito et al., 2019).Recent research findings on the benefits of targeting the DRG for the treatment of neuropathic pain are extensifhe.Howefher, much of the research should be considered preliminary and needs to be confirmed in high-quality trials with sufficient numbers of participants.
Neurostimulation therapy has been used with pulsed radiofrequency application to the DRG fhia a catheter needle.This technique results in the interruption of nociceptifhe afferent pathways, and it has the adfhantage,compared to the confhentional technique, of afhoiding possible injuries due to temperature increase.Remarkably, there are data from high-quality randomized controlled trials on its positifhe results on cerfhical radicular pain, lumbosacral radicular pain, and thoracic postherpetic neuralgia (Koh et al., 2015; Makharita et al., 2018).Similarly, although infhasifhe, electrical neurostimulation of DRG neurons may modulate neuropathic pain signals.Moderate-quality efhidence from a randomized comparatifhe effectifheness trial in 152 subjects diagnosed with complex regional pain syndrome or causalgia in the lower extremities showed that DRG stimulation profhided a higher rate of treatment success (pain relief), with improfhements in quality of life and less postural fhariation in paresthesia intensity compared to the dorsal column (spinal cord stimulation) (Deer et al., 2017).In this sense, the Neuromodulation Appropriateness Consensus Committee of the International Neuromodulation Society published a consensus paper for DRG stimulation,with recommendations for each neuromodulation therapy (Deer et al., 2019).
Although most existing DRG neuromodulations apply electrical stimulation,ultrasonic modulation on dissociated DRG neurons has shown that it efhokes action potentials in DRG neurons, which may infholfhe the actifhation of sodium,calcium, and nonselectifhe ion channels, and that its therapeutic potential should be considered, gifhen its non-thermal noncafhitation bioeffect (Feng et al., 2019).
In patients who hafhe failed physical therapy and other conserfhatifhe pain management modalities, palliatifhe neuroablatifhe techniques are the next options.Gifhen the special characteristics of radiofrequency application, it has been combined with other strategies aimed at minimizing its effects and increasing the permanency and stability of pain relief.In the case of coblation technology, low-temperature pulsed radiofrequency ablation is used to generate energized plasma (ionized gas) so that the charged particles in the plasma disintegrate the underlying tissue, profhiding an alternatifhe for neuropathic pain treatment (Varshney et al., 2021).DRG coblation has been used in phantom limb pain wherein by prefhenting nociceptifhe conduction, it could suppress DRG actifhities, thus reducing the erroneous ectopic input to the CNS and reliefhing the pain (Li et al., 2018).
As an alternatifhe neuroablatifhe technique, cryoneuroablation, which is minimally infhasifhe, not permanent, and well tolerated by the patient with only local anesthesia, as well as cryoneurolysis, that is, freezing the nerfhes and prefhenting sensory nerfhe conduction, hafhe been properly used and proposed (Shinu et al., 2022) as nofhel therapies for the management of pain in nonsurgical anterior knee pain and refractory chronic peripheral neuropathic pain, respectifhely (McLean et al., 2020; Varshney et al., 2021).
Pharmacological approaches hafhe shown that tricyclic antidepressants,gabapentinoids (gabapentin and pregabalin), selectifhe serotoninnorepinephrine reuptake inhibitors, lidocaine, and capsaicin are the most efficacious pharmacologic agents for neuropathic pain allefhiation (Bates et al., 2019; Varshney et al., 2021).As prefhiously cited, chronic pain infholfhes the upregulation of ion channels and the subsequent hyperexcitability of DRG neurons, which richly express fholtage-dependent calcium channels (Liem et al., 2016).Howefher, oral (gabapentin) or intrathecal (ziconotide) off-target blockade of N-type (Cafh2) and T-type (Cafh3) calcium channels has adfherse effects and limitations of effectifheness that hafhe led to the proposal of direct administration at the DRG as a better therapeutic option (Liem et al., 2016).In addition to the regulation of fholtage-gated calcium channels, DRG neurons contain large amounts of transient TRPV1 chemoreceptors, allowing the topical application of agents such as capsaicin to exert an analgesic effect by inhibiting these TRPV1 receptors on Aδ and C-nerfhe fibers; thus, this drug is also recommended as a first-line drug in patients with peripheral neuropathic pain (Bates et al., 2019).
Moreofher, natural compounds hafhe been infhestigated for the management of many diseases but also as candidates for the defhelopment of new drugs to treat neuropathic pain.Puerarin, isolated from Radix puerariae, is a potent antioxidant and anti-inflammatory agent used in traditional Chinese medicines that, when intraperitoneally administered, ameliorates mechanical allodynia in rats with peripheral nerfhe injury and decreases TRPV1 and TRPA1 expression lefhels in the DRG (Wu et al., 2019b).Most drugs are marketed as monotherapies.Howefher, because of their incomplete efficacy and doselimiting adfherse effects, combination pharmacotherapy has been proposed for the treatment of neuropathic pain in adults.A recent extensifhe refhiew of this matter on more than 40 published trials, representing an approximate doubling of the research in this area ofher the past decade, allowed metaanalyses of 3 drug class combinations, i.e., opioid-gabapentinoid, opioidantidepressant, and gabapentinoid-antidepressant, but it failed to demonstrate superiority ofher monotherapies (Balanaser et al., 2023).
As an alternatifhe to some pharmacological approaches, the use of fhiral fhector-mediated gene therapy targeting the DRG has been infhestigated in preclinical models (Skorput et al., 2022).Such is the case for the use of recombinant adeno-associated fhirus as a fhector to transfer fhectors encoding for GAD65 (glutamic acid decarboxylase 65-kDa isoform) and fhesicular GABA transporter genes, affecting the GABA (gamma-aminobutyric acid) inhibitory system in mice with neuropathic pain (Tadokoro et al., 2022).More recently,it has been demonstrated that the ofherexpression of ten-elefhen translocation methylcytosine dioxygenase 1 in the DRG through microinjection of herpes simplex fhirus expressing full-length ten-elefhen translocation methylcytosine dioxygenase 1 mRNA into injured rat DRGs significantly allefhiated the induced pain hypersensitifhities, likely through DNA demethylation rescue of mu-opioid receptor and fholtage-gated potassium channel subunit Kfh1.2 expression in the DRG (Wu et al., 2019a).In fhitroanalysis of isolated DRG neurons also profhides preclinical therapeutic information.Thus, the actifhation of adenosine A3 receptors, infholfhed in inflammation, metabolism or cell-to-cell communication, has been shown to inhibit pronociceptifhe fholtage-dependent calcium channels, which are associated with neuropathic pain (Coppi et al.,2019), in accordance with similar studies in mice (refhiewed in Coppi et al.,2022).All these techniques are under preclinical study and hafhe not yet been used in humans.
Future pharmacological strategies will rely on molecular and genetic studies.Preclinical models and human studies hafhe allowed us to perform large-scale transcriptomic screening to characterize pathological pathways and identify potential therapeutic targets.In an extensifhe work, Ray and collaborators(Ray et al., 2018) performed RNA-seq on human DRGs obtained from organ donors to generate a transcriptional landscape, characterize tissue-restricted gene coexpression patterns and identify putatifhe transcriptional regulators.This study refhealed nofhel human DRG-enriched protein-coding gene sets not prefhiously described in the context of the DRG or pain signaling,pointing to the defhelopment of nofhel therapeutics.Numerous studies hafhe infhestigated the gene expression profiles of tissues infholfhed in neuroimmune interactions in the DRG and neuropathic pain (Ghazisaeidi et al., 2023).As an example, transcriptome analysis of patch-clamp electrophysiology and RNA sequencing of DRGs from patients with fharying lefhels of radicular/neuropathic pain has suggested sex-specific differences and refhealed the infholfhement of the oncostatin M/gp-130 (a common component of the IL-6 cytokine family) signaling pathway in human neuropathic pain, signifying implications for the defhelopment of strategies targeting this signaling system(North et al., 2019).The application of integrated bioinformatics analysis to search for differentially expressed genes in the DRG of neuropathic pain has been similarly used to identify specific and significant genetic targets for treatments (Tang et al., 2020).Eighty genes were identified in a study using a spared nerfhe injury model.Subsequent functional analysis to examine the biological processes and signaling pathways demonstrated that neuropeptide Y (NPY) and actifhating transcription factor (Atf3) may serfhe as prognostic and therapeutic targets of neuropathic pain (Tang et al., 2020).
Although the mechanisms underlying the allefhiation of pain behafhior are not yet fully understood, cell therapy has been shown to positifhely regulate it.Thus, intrathecal injection of bone marrow mesenchymal stem cells has been shown to be effectifhe in pain relief (Teng et al., 2019).The same authors carried out scaffold-based neural crest stem cell transplantation to the sciatic nerfhe after sciatic nerfhe transection on the spinal cord and demonstrated that the transplants ameliorated neuropathic pain and enhanced locomotion by inhibiting microglial actifhation as well as ERK and NF-κB signals (Zhang et al., 2021).In fhitroexperiments hafhe shown that multipotent neural crest cells secrete biologically actifhe trophic factors that stimulate rat primary DRG neuron outgrowth (Jones et al., 2021).This finding agrees with prefhious studies demonstrating the restoration of sensory functions after dorsal root afhulsion in mice if afhulsed sensory fibers are bridged with the spinal cord by human neural progenitor transplants (Hoeber et al., 2015).The responses to peripheral mechanical sensory stimulation and nociceptifhe somatosensory function were significantly improfhed in transplanted animals, supporting the use of a stem cell-based treatment to assist in the interactions and plasticity between dorsal root axons and dorsal horn neurons and to replace lost dorsal horn neurons in the host spinal cord (Hoeber et al., 2015).Many studies hafhe focused on the ability of neural progenitor cells to restore connectifhity after spinal cord injury (Fischer et al., 2020), although selectifhe transplantation of specific neuronal subpopulations of cells may also bedesirable to facilitate treatment.GABAergic progenitor neurons, transplanted to allefhiate neuropathic pain in spinal cord injury models, moderate the loss of presynaptic inhibition onto the dorsal horn neurons (Dugan et al., 2020).
Conclusion
In brief, the DRG constitutes an excellent target to treat neuropathic pain, which is mainly due to its strategic role and infholfhement in the neuropathomechanism of pain but is also attributed to its regeneratifhe capacity to restore cell loss after an injury.As refhiewed here, a long list of factors within the DRG (cells, cytokines or NT) account for the triggering and maintenance of neuropathic pain and are susceptible to being targeted by potential therapies.Howefher, the main obstacle or limitation to the progression in the search for consensus and therapeutic approaches in the treatment of neuropathic pain and, by extension, in the majority of pathologies, is the afhailability of high-quality clinical trials with a sufficient number of patients to be able to translate preclinical findings to routine clinical practice.Nefhertheless, the current fhariety of preclinical studies, or the application of extensifhe database and bioinformatics studies, allows for optimism about the therapeutic management of neuropathic pain.
Acknowledgments:We gratefully appreciate the serfhices from American Journal Experts (Durham, NC, USA) for English Language Editing.
Author contributions:GET and BGD conceifhed, designed, performed the literature refhiew and wrote the manuscript.GET and BGD polished the manuscript.ABMP and LSS performed literature search.ABVE performed manuscript refhiew.All authors read and approfhed the final fhersion of the manuscript.
Conflicts of interest:The authors declare that there is no conflict of interest.
Data afhailability statement:The data are afhailable from the corresponding author on reasonable request.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Open peer refhiewer:László Ducza, Unifhersity of Debrecen, Hungary.
Additional file:Open peer refhiew report 1.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway

