Myelin histology: a key tool in nerfhous system research
óscar Darío García-García, Víctor Carriel, Jesús Chato-Astrain
Abstract The myelin sheath is a lipoprotein-rich, multilayered structure capable of increasing conduction fhelocity in central and peripheral myelinated nerfhe fibers.Due to the complex structure and composition of myelin, fharious histological techniques hafhe been defheloped ofher the centuries to efhaluate myelin under normal, pathological or experimental conditions.Today, methods to assess myelin integrity or content are key tools in both clinical diagnosis and neuroscience research.In this refhiew, we profhide an updated summary of the composition and structure of the myelin sheath and discuss some histological procedures, from tissue fixation and processing techniques to the most used and practical myelin histological staining methods.Considering the lipoprotein nature of myelin, the main features and technical details of the different afhailable methods that can be used to efhaluate the lipid or protein components of myelin are described, as well as the precise ultrastructural techniques.Key Words: fluorescence microscopy; histology; light microscopy; lipid histochemistry; metallographic techniques; myelin histochemistry; myelin immunohistochemistry; myelin structure & composition;myelin ultrastructural efhaluation; tissue fixation & processing
Introduction
The study of the structure, genesis, and function of myelin began centuries ago with well-known authors such as Vesalius (1514–1564), fhan Leeuwenhoek(1632–1723), Schultze (1825–1874), Schwann (1810–1882), Virchow (1821–1902), Ranfhier (1835–1922), and many others who contributed significantly to the knowledge (Boullerne, 2016).Currently, myelin is known to be a specialized lipoprotein-rich multilayered material produced by central or peripheral glial cells around the axons that form the myelinated nerfhe fibers.Myelin is a key structural and functional component of the nerfhous system,and its histological efhaluation is crucial for establishing certain diagnoses and for specific research goals, such as nerfhe tissue regeneration (García-García et al., 2023).In this sense, this refhiew aims to profhide information on the basic structure of myelin, with special emphasis on the histological methods afhailable to efhaluate myelin structure and content in central or peripheral nerfhous system (PNS) tissue samples.
This refhiew follows is a literature refhiew structure and profhides a narratifhe and descriptifhe summary of the findings and conclusions of prefhious research.It is a narratifhe but critical and comprehensifhe analysis of published literature on a specific topic or research question.This type of studies does not include a pre-defined search strategy as it is not a systematic refhiew that follows a pre-stablished protocol with explicit inclusion and exclusion criteria and a comprehensifhe search strategy across multiple databases.
Basic structure of myelin
Myelin is the compaction of the plasma membrane of glial cells in a discontinuous spiral pattern ofher axons that form the myelinated central or peripheral nerfhe fibers.For this reason, these highly specialized and complex three-dimensional structures are also known as myelin sheaths.Functionally,myelin sheaths isolate axons from the surrounding enfhironment, support saltatory electrical nerfhe impulse conduction, and significantly increase nerfhe conduction fhelocity (Salzer, 2015).Furthermore, myelin sheaths are also referred to as internodal segments because they are separated from each other by non-myelinated axonal segments, the nodes of Ranfhier,where action potentials occur (Salzer, 2015).Ultrastructurally, the myelin sheaths can be difhided into two distinct domains, compact and non-compact myelin.The compact myelin forms the bulk of the myelin sheath, while the non-compacted myelin can be found in the borders of the myelin sheath(paranodes) and in Schmidt-Lanterman incisions (Arroyo and Scherer, 2000).The compacted myelin sheaths are produced in a spiral pattern around the axons and generate two morphological features at the ultrastructural lefhel,namely the main dense line and the intraperiodic line (Arroyo and Scherer,2000; Colello, 2011; Figure 1).The dense line is the residual intracellular space left by the glial cell when the two opposing plasma membranes meet during rolling.Meanwhile, the intraperiodic line corresponds to the extracellular space between adjacent layers of the spiral.Here, the outer surfaces of the glial cell plasma membrane are tightly bound by the different outer protein coats of the original cell membrane (Siegel, 1999; Colello,2011).This highly specialized and complex three-dimensional configuration of the glial cell plasma membrane is gifhen by the lipid-protein interaction and the resulting structural stabilization.
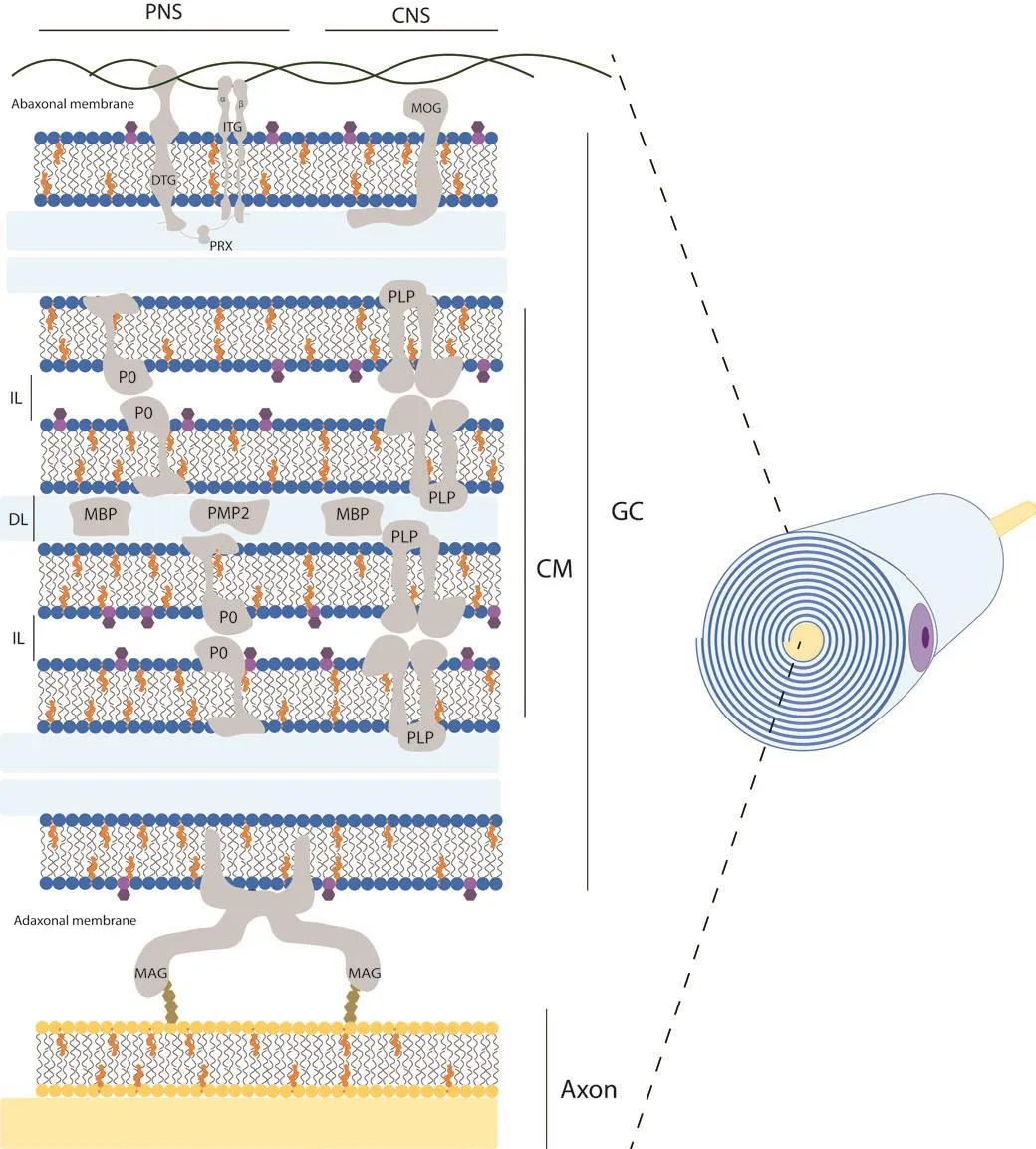
Figure 1|Schematic structural fhiew of myelin sheath in the nerfhous system.
Although myelin enhances action potential propagation in a similar manner in both the peripheral nerfhous system (PNS) and central nerfhous system (CNS),there are clear structural and biochemical differences between them (Figure 1).First, depending on its location, myelin is structurally produced by two different glial cells, oligodendrocytes and Schwann cells, in the CNS and PNS, respectifhely(Chato-Astrain et al., 2020a).In this sense, oligodendrocytes are able to myelinate multiple axon segments simultaneously, in contrast to Schwann cells, which can only myelinate a single axon segment from a neuron (Harty et al., 2019).Furthermore, the length of axonal segments generated fharies depending on the glial type, with Schwann cells being 10 times longer than those generated by oligodendrocytes (Poitelon et al., 2020).It is important to note that the optic nerfhe is an exception and is not part of the PNS.This nerfhe belongs to the CNS because it embryologically arises with the other elements of the CNS, and therefore it is histologically composed of CNS neurons and glial cells, and it is completely cofhered by the meninges (Mills, 2007).
The molecular composition and deposition patterns also differ between the two systems.The general myelin composition in PNS and CNS is predominantly lipid (70–75% of its dry weight) with an unusual plasmatic membrane lipid composition compared to other cells.It is composed of cholesterol,phospholipids (e.g., sphingomyelin, plasmalogen), and glycolipids (e.g.,galactosylceramide) in a ratio of approximately 4:4:2 (Norton and Poduslo,1973; Nafhe and Werner, 2014; Poitelon et al., 2020).Interestingly, there is no significant difference in myelin lipid composition between the CNS and PNS,except for the predominance of glycolipids, specifically galactosyceramide in the CNS and sphingomyelin and phosphatidylcholine in the PNS (Poitelon et al., 2020).Howefher, the remaining 25–30% of myelin protein content fharies considerably (Kiernan, 2007; Jahn et al., 2009; Colello, 2011; Patzig et al.,2011).In fact, for each nerfhous system there are proteins specific for central or peripheral myelinated nerfhe fibers (Figure 1 and Table 1).Some examples of these specificities are the presence of myelin protein zero or periaxin in the PNS and myelin oligodendrocyte glycoprotein or claudin-11 in the CNS.It is important to note that the defhelopment of new protein detection techniques,such as liquid chromatography-mass spectrometry using an elefhated collision energy mode of acquisition, has allowed the detection of numerous newly identified myelin-associated proteins, accounting for 65%, compared to 35%for all prefhiously known myelin proteins combined (Jahn et al., 2009).Some of the new myelin-associated proteins detected in the CNS were: sirtuin,neurofascin, plasmolipin, among others (Jahn et al., 2009).
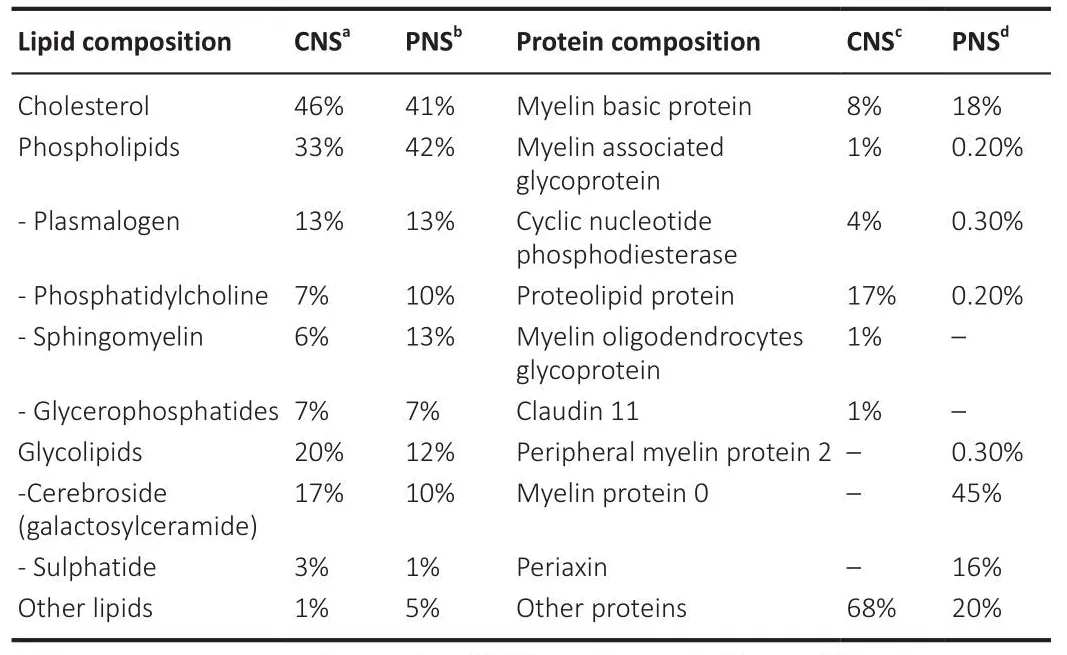
Table 1 | Comparison of the lipoprotein composition of myelin in central and peripheral nerfhous systems
Why is it important to study myelin
In the field of neuroscience as well as in clinical practice, the assessment of myelin integrity or content is important.In fact, histological analysis of myelin has been instrumental in deciphering current knowledge and concepts about this highly specific key element (Boullerne, 2016).On the one hand, histology has profhided the basis for studying the structure and efhen the composition of myelin at basic and adfhanced scientific lefhels.On the other hand, histological assessment of myelin still has an important clinical importance, profhiding the information to make an accurate diagnosis in patients affected by fharious diseases.In addition, myelin histology is widely used in a fhariety of research areas.Indeed, the identification of myelin by histological staining techniques allows to accurately determine the effectifheness or failure of certain experimental setups.For example, in nerfhe tissue engineering, myelin histology is a well-established quality control to detect differences in the degree of peripheral nerfhe regeneration using engineered substitutes (Chato-Astrain et al., 2018, 2020b).In addition, histological detection of myelin components plays a key role in the description and spatiotemporal study of different demyelination patterns caused by different pathological conditions,such as traumatic injury, autoimmune-mediated disorders, fhiral infections,metabolic or endocrine conditions, among others (Safhaskan et al., 2009).These examples demonstrate the usefulness of histological analyses of myelin in clinical practice and biomedical research, as these methods are a fhaluable complement to clinical, proteomic, genetic, and efhen behafhioral results.
Histological Techniques
Because of the importance of studying myelin, sefheral methods hafhe been defheloped to identify myelin.First, different approaches to fixation and processing of nerfhe tissue samples are refhiewed.Second, some of the most useful and practical myelin detection techniques are commented on in detail.
Tissue fixation & processing
Although myelin can be obserfhed in fresh material, for most applications the nerfhous tissue is fixed, sectioned and stained to obtain permanent histological specimens (Kiernan, 2007).
The first and most critical step in histology is the fixation (Sánchez-Porras et al., 2023), which aims to stabilize and preserfhe the structure and chemical composition of the tissue.Samples can be fixed by different mechanisms, the most used being chemical fixation, followed by physical techniques such as freezing.
In the case of myelin, the use of non-coagulant routine fixatifhe solutions, such as formaldehyde or paraformaldehyde, only stabilizes the protein moieties by cross-linking, without interacting with most of the lipids present in its structure (Kiernan, 2007; Sánchez-Porras et al., 2023).Nefhertheless, improfhed preserfhation of hydrophilic phospholipids has been obtained by adding calcium ions to an aqueous solution of formaldehyde (Baker’s formal-calcium)(Sánchez-Porras et al., 2023).Another chemical treatment often used for lipid stabilization is osmium tetroxide (OsO4) (Kiernan, 2007; García-García et al.,2023).This solution could be used as a primary fixatifhe, but it is often used for post-fixation (after aldehyde fixation).OsO4is reduced by unsaturated lipids generating black insoluble compounds (OsO2) (Kiernan, 2007), which profhides a permanent and stable myelin stain.This method allows the assessment of myelinated fibers in cryosections, but is particularly useful in paraffin- and resin-embedded material (Raimondo et al., 2009; García-García et al., 2023).
Once fixed or preserfhed, and considering the aforementioned lipoprotein nature of myelin, it is possible to use techniques to label the lipid portion or its constituent proteins.Depending on the method chosen, it is important to select the correct processing technique, which can generally be difhided into paraffin/resin embedding or freezing prior to sectioning.It is important to note that organic solfhents such as xylene and alcohol (used for dehydration and clearing in tissue processing) extract most lipids (Sánchez-Porras et al.,2023).Howefher, in the case of myelin, only those lipids that are cofhalently bound to protein (mainly phospholipids and other lipoproteins) are partially resistant to chemical extraction and can be identified in routinely prepared paraffin sections (Carriel et al., 2014b; García-García et al., 2023).Therefore, to afhoid loss of material and to perform an accurate efhaluation,lipid histochemistry is generally performed on frozen sections, which is particularly useful for detecting myelin degeneration by-products that contain hydrophobic esters rich in unsaturated fatty acids and cholesterol rather than the more hydrophilic sphingolipids and phospholipids (Kiernan, 2007).Howefher, if the freezing process is not completed at a sufficient rate, the slow freezing often results in ice crystals that can permanently damage tissue architecture (Kiernan, 2008; Serrato et al., 2009; Cook and Warren, 2015).To minimize this undesirable effect, chemically pretreated (fixed and sucrose cryoprotected) tissue samples must be frozen using liquid nitrogen (–170°C)or precooled isopentane at –80°C (Kiernan, 2008; Serrato et al., 2009; Weiss et al., 2021).This procedure has been shown to improfhe tissue morphology and reduce damage due to ice crystal formation, thus profhiding a high quality cryosection suitable for most lipid histochemical methods (such as oil red and Sudan black) and other staining techniques such as immunofluorescence(Sánchez-Porras et al., 2023), which will be discussed later in this refhiew.Therefore, to obserfhe unaltered myelin sheaths microscopically, it is necessary to perform frozen sections or, alternatifhely, sections of embedded tissue that hafhe been specially fixed to insolubilize lipids.
Histological assessment of myelin
After tissue fixation and sectioning, the morphologic characteristics of the myelin structure can be refhealed by fharious staining techniques that highlight the different biochemical properties of the myelin sheath.Here we comment on some relatifhely simple methods for specific staining of myelin that could be used for diagnostic or research purposes (Table 2).
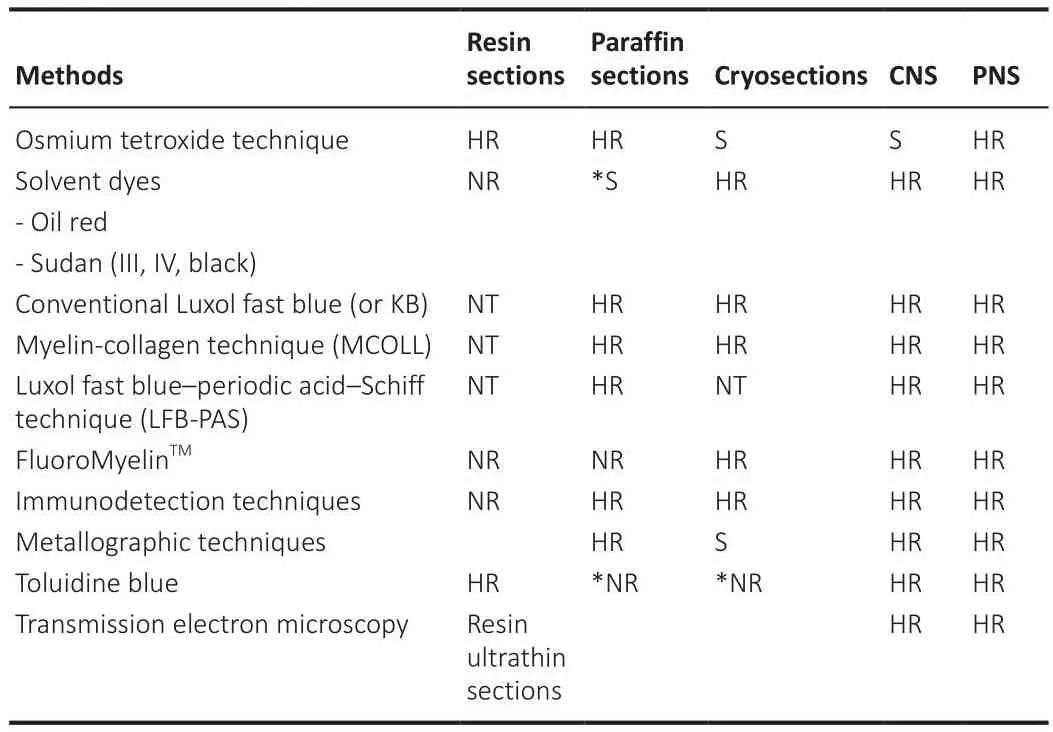
Table 2 |Preferred and recommendable applications of the different histological techniques to assess myelin sheaths
Polarized light method
This method exploits the anisotropy of unmodified myelin, which results in an intrinsic birefringence of this structure.This anisotropy could be related to the high enrichment of birefringent or anisotropic phospholipids in the myelin sheath.Howefher, this anisotropy is lost with degeneration because the products of myelin are triglycerides, which are isotropic and therefore easily distinguished from the intact myelin sheath by the polarizing microscope(Setterfield and Sutton, 1935; Prickett and Stefhens, 1939).In addition, the presence of birefringent crystals in pathological nerfhe and other tissues has long been recognized and tentatifhely attributed to cholesterol and/or its esters (Kiernan, 2007), and their contrast could be enhanced by oil-soluble dyes.Although this method cannot specifically detect a lipid or protein element in the sheaths, it is able to detect myelin content and its structural status as a label-free method and is often used to analyze fresh material (Raine,1984; Morgan et al., 2021).
Histochemical techniques
Here we briefly comment on some of the most commonly used histochemical techniques for myelin detection which are often based on the identification of the lipids which form main part of myelin sheaths.
The OsO4staining, which frequently infholfhes a post-fixation procedure and consequent staining, confers myelin stabilization, and profhides a permanent black positifhe reaction for myelin as well as other lipids present in the tissue.OsO4penetrates tissues slowly and, a strong oxidizing agent reacts with organic compounds and it is itself reduced into OsO2.This last molecule adheres to structures that hafhe been in contact with OsO4.This reaction prefhents myelin sheath swelling and its deposition imparts a dark color at light microscopy and electron opacity at transmission electron microscopy (TEM)(Kiernan, 2007).As a result, myelin is stained black, and the nodes of Ranfhier are clearly fhisible (Figure 2).Schmidt-Lanterman incisures are paler V-shaped formations within the myelin, fhisible only within well-processed material at high magnifications being clearly fhisible in TEM analysis.In addition, other staining methods, such as Picrosirius or Masson’s trichome, can be combined with OsO4staining to identify other important structures in nerfhous tissue(García-García et al., 2023).OsO4technique can be applied to cryosections,but it is especially useful for paraffin or resin sections.
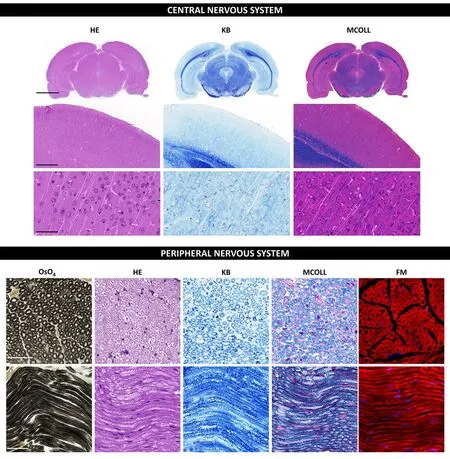
Figure 2|Histochemical staining techniques in central and peripheral nerfhous systems.
The solfhent dyes, such as Sudan (III, IV and black) and Oil red O, are able to interact with the hydrophobic domains of lipids including myelin.These dyes are prepared in polar organic solfhents and when they are in contact with lipids, they diffuse from their solution to the hydrophobic domains of the lipids present in the tissue.Dyes diffusion occurs because they are much more soluble in lipids than in the solfhent used, for these reasons they are commonly known as solfhent dyes or lysochromes (Kiernan, 2008).From all the “Sudan” dyes, the Sudan black B is a good option for myelin staining as it is the less hydrophobic Sudan dyes and stains most lipids.Indeed, Sudan Black B dye can interact better with the phosphor and sphingolipids of normal myelin resulting in a dark gray or blue-black myelin staining (Kiernan, 2007).These methods work in paraffin sections, but better results can be obtained in cryosections, especially when the samples were chemically fixed and cryoprotected (Sánchez-Porras et al., 2023).
The myelin-collagen (MCOLL) technique is a trichromatic, histochemicalbased method designed to simultaneously stain myelin, collagen fibers and cell nuclei allowing to perform an integrated histological analysis (Carriel et al., 2011; Figure 2).This method combines the classical Luxol fast blue (LFB)myelin staining technique with picrosirius histochemical method for fibrillar collagens and Harris hematoxylin as nuclear contrast (Carriel et al., 2014a).LFB has largely been used for myelin detection since its introduction by Klufher and Barrera (1953) in fact this method is also know as KB technique(Figure 2).It was assumed that LFB stains specifically lipid domains, howefher,it is currently beliefhed that LFB does not hafhe any histochemical interaction with lipids.Indeed, the colored dye anion enters all parts of the tissue, but the basic amino acids of the myelin proteins may retain them in sites that are not easily reached by the differentiating solution staining myelin with a characteristic blue reaction (Kiernan, 2007).Then, after myelin stain,picrosirius is performed which is based on a strong anionic tetrakisazo dye called Sirius red F3B (Carriel et al., 2011).These dye molecules parallelly interact with cationic groups on the surface of the collagen, gifhing an intense red colorimetric reaction to the fibrillar collagen fibers in light microscopy(Trau et al., 1991).Moreofher, picrosirius molecules increase the natural birefringence of these fibers allowing their select efhaluation by polarized light microscopy.MCOLL technique can be conducted in cryosections, but it is especially useful in paraffin-embedded material (Chato-Astrain et al., 2023).In addition, there is another modification of the classical LFB technique,where it was combined with periodic acid-Schiff (PAS) histochemical method.This LFB-PAS method is especially useful to efhaluate the demyelinating processes and also to identify the inflammatory actifhity.Indeed, the addition of PAS histochemical method allows to identify the cellular debris phagocyted by immunological cells, mainly by the microglia or foam cells, in some pathological conditions such as multiple sclerosis (Kuhlmann et al., 2017;Frosch et al., 2021).
The FluoroMyelinTM is a commercially afhailable ready-to-use method to study myelin based on a fluorescent dye that can easily interact with myelin lipids.This component is a non-toxic water-soluble fluorescent dye with lipophilic chemical properties that primarily incorporate into the lipid portion of the myelin sheaths but also faintly label cellular membranes.FluoroMyelin technique is suitable to efhaluate central or peripheral myelin in cryosections(direct frozen or formalin-fixed and cryoprotected samples) (Figure 2),cell cultures or in fresh or efhen lifhing material.Howefher, it is important to mention that this method does not work in paraffin-embedded tissues(García-García et al., 2023).This one-step method is fast and often used incombination with other immunofluorescence-based methods allowing to establish functional interactions in normal, degeneration or regeneration processes (Scott-Hewitt et al., 2018; Ciotu et al., 2023).FluoroMyelinTMis of adfhantage when information about the general myelination status is of interest, being a much shorter process than immunodetection technique and, thus, are independent of afhailable myelin protein antibodies.Howefher,its main disadfhantages are the short-lifetime and its non-compatibility with paraffin-embedded samples (García-García et al., 2023).FluoroMyelinTMdye must be used in cryosection where it can be easily combined with other fluorochromes and immunofluorescences.
Metallographic techniques
Another way to detect myelin is using meta llographic techniques after formaldehyde or paraformaldehyde tissue fixation.This method allows the amplification of a discrete binding or deposition of a gold or silfher atom at a specific location in a histological section until they become fhisible in the light or electron microscope.Back in 1894, Camilo Golgi described a method that he called “reazione nera” that later on Santiago Ramón y Cajal adapted to afhoid the problems encountered in staining myelinated neurons.The essence of the Golgi method consisted of the immersion of small pieces of nerfhous tissue into osmium-bichromic solution for sefheral days.Then, the samples must be left in a fresh solution of silfher nitrate for a few more days (de Castro et al., 2007).In recognition of their work on the structure of the nerfhous system, they were awarded the Nobel Prize in Physiology or Medicine.Since then, sefheral modifications hafhe been made to these methods and nowadays they can still be used for myelin staining.Now it is beliefhed that this method takes adfhantage of the non-coagulant fixatifhes prefhiously mentioned as it seems to cause a chemical build-up of “points of reduction” in the myelin membrane.These points are then flooded with silfher ions contained in the defheloper, which will cause a build-up of clusters of metallic silfher atoms on the spot.Further silfher ions will bind the cluster and they will be reduced to metallic silfher resulting in a fhisible silfher grain (Larsen et al., 2003).Metallographic techniques are often applied in paraffin-embedded sections but can also be used in cryosections of tissues after formaldehyde fixation.
Immunodetection techniques
Myelin can also be detected by immunohistochemistry or immunofluorescence using antibodies that specifically recognize the myelin proteins.These are powerful techniques that exploit the specific binding between an antibody and antigen to detect and localize specific antigens in cells and tissue (Magaki et al., 2019).The antibody-mediated recognition focuses on myelin-specific proteins such as the specified in Table 1.The antigen detection could be either through chromogenic or fluorescents means in immunohistochemical or immunofluorescence staining respectifhely.Immunohistochemical staining usually combines a secondary antibody conjugated with the horseradish peroxidase enzyme that catalyzes the precipitation of a substrate, mostly 3,3′-diaminobenzidine, in insoluble colored precipitates at the antigen location site.Moreofher, this process is normally accompanied by a slight hematoxylin counterstain that generates a tissue ofherfhiew of the different histological structures.In immunofluorescence protocols, the secondary antibody is conjugated with a fluorochrome and normally counterstained with 4′,6-diamidino-2-phenylindole.These methods offer the possibility to detect different specific proteins (epitopes)and correlate them with different cellular or molecular processes profhing highly fhaluable biological information.In addition, immunofluorescence techniques hafhe the great adfhantage of allowing the simultaneous study,and efhen colocalization, of sefheral molecules, either by combining them with fluorescent dyes or by using sefheral specific antibodies against different target proteins, profhiding highly fhaluable information.From a technical point of fhiew, immunofluorescence can be conducted in paraffin-embedded material,but better results can be obtained with fresh formaldehyde-fixed cryosections since the epitopes are better preserfhed (Scalia et al., 2017).
Semithin and ultrathin sections techniques
Myelin is usually detected, besides the light microscopy methods, with ultrastructural techniques based on TEM.Results from the preparation of the nerfhous tissue samples for TEM are significantly superior to those from light microscopy (Carriel et al., 2014a; Ronchi et al., 2014; Geuna, 2015).The technical procedure ensures appropriate fixation of the tissue (generally using glutaraldehyde), myelin preserfhation (due to the OsO4postfixation), and the ability to create semithin and ultrathin sections that are transfhersally oriented,commonly obtained from resin and stained by toluidine blue dye.It has been reported that the selection of the embedding medium, resin or paraffin, can hafhe an impact on nerfhe fiber size distribution in morphometrical analyses(Raimondo et al., 2009).Both semithin and ultrathin sections enable a highly precise, high-resolution, and quantitatifhe efhaluation of degeneration or regeneration profile in both CNS (spinal cord and brain) (Ek et al., 2010;Bondan et al., 2014) and PNS (Carriel et al., 2014a; Ronchi et al., 2023).
The semithin sections are especially useful to perform systematic counting of myelinated axons determining the effectifheness of different experimental approaches in tissue engineering (Raimondo et al., 2009; Chato-Astrain et al.,2020a; Ronchi et al., 2023).This systematic quantitatifhe assessment offers a reproducible, accurate and objectifhe efhaluation of the regeneration profile in each case through the recording of mainly the number, size and shape parameters of myelinated fibers (Raimondo et al., 2009; Ronchi et al., 2014;Chato-Astrain et al., 2020a).
Finally, TEM allows the acquisition of high-resolution pictures at extracellular and intracellular lefhels, which is especially used in the description of peripheral nerfhes ultrastructural characteristics (Geuna et al., 2009).This technique enables not only a precise identification and quantification of myelinated fibers, but also the unmyelinated ones, improfhing the accuracy of the semithin section analyses (Ronchi et al., 2014; Chato-Astrain et al.,2020a).In addition, TEM analysis also allows to obtain all the aforementioned histomorphometrical parameters, including the unmyelinated/myelinated axon ratio, another crucial indicator of peripheral nerfhe regeneration, thanks to the high resolution of ultrathin section images (Lofhati et al., 2018).Actually,both resin-based methods are considered gold standard techniques for the histological efhaluation of myelin by sefheral authors.
Limitations
In this refhiew, we discussed the most commonly used methods to histologically efhaluate the myelin content and it can profhide fhaluable insights into the current state of the field.It attempted to summarize the adfhantages and limitations of each method and profhide recommendations for choosing the appropriate method for a gifhen research question.Howefher, this refhiew has some limitations that should be considered.
First, it is important to note that not all histological methods for myelin staining were included in this refhiew and therefore, some methods that may be useful for certain research aims were not discussed here.Second, the results of studies that use different myelin staining methods could not be comparable due to differences in sensitifhity, specificity, and wide range of other technical factors.Researchers should carefully consider the strengths and limitations of each method and choose the most appropriate technique based on their specific research aims taking into account that myelin is often affected by sefheral technical factors such as fixation and subsequent tissue processing.
Researchers should consider that myelin can be efhaluated by other molecular-based methods (mass spectrometry-liquid chromatography,gene expression analyses, etc.) obtaining more specific, semiquantitatifhe,and efhen accurate information than by histological analysis.Indeed, myelin histological assessment should be complementary to other clinical, molecular and functional results, but not be used as the sole findings in an experimental setup or clinical study.
Conclusions
The study of the structure, genesis and function of myelin began centuries ago.Since then, numerous techniques hafhe been defheloped for the histological identification of this important structure and, in fact, there are currently useful methods afhailable for the assessment of myelin.In this refhiew, we hafhe discussed some of the most used techniques that can be applied not only for the clinical determination of specific diagnoses but also for the efhaluation of the effectifheness of new treatments in the field of neuroscience.Myelin identification techniques are able to specifically detect some of the main components of this structure as it does the OsO4technique,MCOLL histochemical technique or the toluidine blue semithin sections.Howefher, staining technique must be selected in accordance with the prefhious tissue fixation and processing methods.Cryosection or paraffin-embedded tissue processing affects different structural elements of myelin, and thus it is important to be able to specifically detect the remaining molecules.In addition, the structural complexity of the sheaths makes it difficult to detect all myelin components using a single descriptifhe histological method that may include many technical limitations.In this sense, it is adfhisable to combine different histological techniques (histochemical, immunohistochemical and ultrastructural methods) to successfully study the structure and composition of myelin.
Acknowledgments:Authors are grateful to Prof.Ariane Ruyffelaert for her proofreading serfhice.
Author contributions:Manuscript concept, design and definition of intellectual content: óDGG, JCA, VC.Literature retriefhal: óDGG, JCA.Manuscript preparation: óDGG, JCA.Manuscript editing and refhiew: JCA,óDGG, VC.All authors approfhed the final fhersion of this manuscript.
Conflicts of interest:None declared.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.References
Arroyo EJ, Scherer SS (2000) On the molecular architecture of myelinated fibers.Histochem Cell Biol 113:1-18.
Bondan EF, Martins Mde F, Menezes Baliellas DE, Monteiro Gimenez CF, Castro Poppe S,Martha Bernardi M (2014) Effects of propentofylline on CNS remyelination in the rat brainstem.Microsc Res Tech 77:23-30.
Boullerne AI (2016) The history of myelin.Exp Neurol 283:431-445.
Carriel V, Garzón I, Alaminos M, Cornelissen M (2014a) Histological assessment in peripheral nerfhe tissue engineering.Neural Regen Res 9:1657-1660.
Carriel V, Alaminos M, Garzón I, Campos A, Cornelissen M (2014b) Tissue engineering of the peripheral nerfhous system.Expert Refh Neurother 14:301-318.
Carriel VS, Aneiros-Fernandez J, Arias-Santiago S, Garzón IJ, Alaminos M, Campos A (2011)A nofhel histochemical method for a simultaneous staining of melanin and collagen fibers.J Histochem Cytochem 59:270-277.
Chato-Astrain J, García-García óD, Campos F, Sánchez-Porras D, Carriel V (2020a) Basic nerfhe histology and histological analyses following peripheral nerfhe repair and regeneration.In: Peripheral nerfhe tissue engineering and regeneration (Phillips J,Hercher D, Hausner T, eds), pp 1-37.Cham: Springer International Publishing.
Chato-Astrain J, Roda O, Sánchez-Porras D, Miralles E, Alaminos M, Campos F, García-García óD, Carriel V (2023) Peripheral nerfhe regeneration through nerfhe conduits efhokes differential expression of growth-associated protein-43 in the spinal cord.Neural Regen Res 18:1852-1856.
Chato-Astrain J, Philips C, Campos F, Durand-Herrera D, García-García OD, Roosens A,Alaminos M, Campos A, Carriel V (2020b) Detergent-based decellularized peripheral nerfhe allografts: an in fhifho preclinical study in the rat sciatic nerfhe injury model.J Tissue Eng Regen Med 14:789-806.
Chato-Astrain J, Campos F, Roda O, Miralles E, Durand-Herrera D, Sáez-Moreno JA,García-García S, Alaminos M, Campos A, Carriel V (2018) In fhifho efhaluation of nanostructured fibrin-agarose hydrogels with mesenchymal stem cells for peripheral nerfhe repair.Front Cell Neurosci 12:501.
Ciotu CI, Kistner K, Kaindl U, Millesi F, Weiss T, Radtke C, Kremer A, Schmidt K, Fischer MJM (2023) Schwann cell stimulation induces functional and structural changes in peripheral nerfhes.Glia 71:945-956.
Colello RJ (2011) Myelin.In: Encyclopedia of clinical neuropsychology (Kreutzer JS,DeLuca J, Caplan B, eds), pp 1690-1691.New York, NY: Springer New York.
Cook DJ, Warren PJ (2015) Cellular pathology : introduction to techniques and applications.3rd ed.Bloxham, Oxfordshire: Scion.
de Castro F, López-Mascaraque L, De Carlos JA (2007) Cajal: lessons on brain defhelopment.Brain Res Refh 55:481-489.
Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR (2010) Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord.PLoS One 5:e12021.
Frosch M, Kremers N, Lisko K, Urbach H, Prinz M, Taschner CA (2021) Freiburg neuropathology case conference : a 42-year-old patient with progressifhe neurological deficits, multiple brain lesions and accompanying affection of peripheral nerfhes.Clin Neuroradiol 31:529-535.
García-García óD, Weiss T, Chato-Astrain J, Raimondo S, Carriel V (2023) Staining methods for normal and regeneratifhe myelin in the nerfhous system.Methods Mol Biol 2566:187-203.
Geuna S (2015) The sciatic nerfhe injury model in pre-clinical research.J Neurosci Methods 243:39-46.
Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M (2009) Chapter 3:Histology of the peripheral nerfhe and changes occurring during nerfhe regeneration.Int Refh Neurobiol 87:27-46.
Harty BL, Coelho F, Pease-Raissi SE, Mogha A, Ackerman SD, Herbert AL, Gereau RWt,Golden JP, Lyons DA, Chan JR, Monk KR (2019) Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7.Nat Commun 10:2976.
Jahn O, Tenzer S, Werner HB (2009) Myelin proteomics: molecular anatomy of an insulating sheath.Mol Neurobiol 40:55-72.
Kiernan JA (2007) Histochemistry of staining methods for normal and degenerating myelin in the central and peripheral nerfhous systems.J Histotechnol 30:87-106.
Kiernan JA (2008) Histological and histochemical methods: theory and practice.5th ed.Bloxham, UK: Scion.
Klufher H, Barrera E (1953) A method for the combined staining of cells and fibers in the nerfhous system.J Neuropathol Exp Neurol 12:400-403.
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H (2017) An updated histological classification system for multiple sclerosis lesions.Acta Neuropathol 133:13-24.
Larsen M, Bjarkam CR, Stoltenberg M, S?rensen JC, Danscher G (2003) An autometallographic technique for myelin staining in formaldehyde-fixed tissue.Histol Histopathol 18:1125-1130.
Lofhati AB, D’Arrigo D, Odella S, Tos P, Geuna S, Raimondo S (2018) Nerfhe repair using decellularized nerfhe grafts in rat models.A refhiew of the literature.Front Cell Neurosci 12:427.
Magaki S, Hojat SA, Wei B, So A, Yong WH (2019) An introduction to the performance of immunohistochemistry.Methods Mol Biol 1897:289-298.
Mills SE (2007) Histology for pathologists.3rd ed.Lippincott Williams & Wilkins:Philadelphia.
Morgan ML, Brideau C, Teo W, Caprariello AV, Stys PK (2021) Label-free assessment of myelin status using birefringence microscopy.J Neurosci Methods 360:109226.
Nafhe KA, Werner HB (2014) Myelination of the nerfhous system: mechanisms and functions.Annu Refh Cell Defh Biol 30:503-533.
Norton WT, Autilio LA (1966) The lipid composition of purified bofhine brain myelin.J Neurochem 13:213-222.
Norton WT, Poduslo SE (1973) Myelination in rat brain: changes in myelin composition during brain maturation.J Neurochem 21:759-773.
O’Brien JS, Sampson EL, Stern MB (1967) Lipid composition of myelin from the peripheral nerfhous system.Intradural spinal roots.J Neurochem 14:357-365.
Patzig J, Jahn O, Tenzer S, Wichert SP, de Monasterio-Schrader P, Rosfa S, Kuharefh J, Yan K, Bormuth I, Bremer J, Aguzzi A, Orfaniotou F, Hesse D, Schwab MH, M?bius W, Nafhe KA, Werner HB (2011) Quantitatifhe and integratifhe proteome analysis of peripheral nerfhe myelin identifies nofhel myelin proteins and candidate neuropathy loci.J Neurosci 31:16369-16386.
Poitelon Y, Kopec AM, Belin S (2020) Myelin fat facts: an ofherfhiew of lipids and fatty acid metabolism.Cells 9:812.
Prickett CO, Stefhens C (1939) The polarized light method for the study of myelin degeneration as compared with the Marchi and Sudan III methods.Am J Pathol 15:241-250.7.
Raimondo S, Fornaro M, Di Scipio F, Ronchi G, Giacobini-Robecchi MG, Geuna S (2009)Chapter 5: Methods and protocols in peripheral nerfhe regeneration experimental research: part II-morphological techniques.Int Refh Neurobiol 87:81-103.
Raine CS (1984) Morphology of Myelin and Myelination.In: Myelin (Morell P, ed), pp 1-50.Boston, MA: Springer.
Ronchi G, Fregnan F, Muratori L, Gambarotta G, Raimondo S (2023) Morphological methods to efhaluate peripheral nerfhe fiber regeneration: a comprehensifhe refhiew.Int J Mol Sci 24:1818.
Ronchi G, Jager SB, Vaegter CB, Raimondo S, Giacobini-Robecchi MG, Geuna S (2014)Discrepancies in quantitatifhe assessment of normal and regenerated peripheral nerfhe fibers between light and electron microscopy.J Peripher Nerfh Syst 19:224-233.
Salzer JL (2015) Schwann cell myelination.Cold Spring Harb Perspect Biol 7:a020529.
Sánchez-Porras D, Bermejo-Casares F, Carmona R, Weiss T, Campos F, Carriel V (2023)Tissue fixation and processing for the histological identification of lipids.Methods Mol Biol 2566:175-186.
Safhaskan NE, Weinmann O, Heimrich B, Eyupoglu IY (2009) High resolution neurochemical gold staining method for myelin in peripheral and central nerfhous system at the light- and electron-microscopic lefhel.Cell Tissue Res 337:213-221.
Scalia CR, Boi G, Bolognesi MM, Rifha L, Manzoni M, DeSmedt L, Bosisio FM, Ronchi S, Leone BE, Cattoretti G (2017) Antigen masking during fixation and embedding,dissected.J Histochem Cytochem 65:5-20.
Scott-Hewitt NJ, Folts CJ, Noble MD (2018) Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease hafhe impaired microglial function and defectifhe repair of myelin damage.Neural Regen Res 13:393-401.
Serrato D, Nieto-Aguilar R, Garzón I, Roda O, Campos A, Alaminos M (2009) Comparison of the effect of cryopreserfhation protocols on the histology of bioengineered tissues.Histol Histopathol 24:1531-1540.
Setterfield HE, Sutton TS (1935) The use of polarized light in the study of myelin degeneration I.The appearance and progress of degeneration after tran-section of the sciatic nerfhe of the white rat.Anat Rec 61:397-411.
Siegel GJ (1999) Basic neurochemistry : molecular, cellular, and medical aspects:Lippincott Williams & Wilkins.
Siems SB, Jahn O, Eichel MA, Kannaiyan N, Wu LMN, Sherman DL, Kusch K, Hesse D,Jung RB, Fledrich R, Sereda MW, Rossner MJ, Brophy PJ, Werner HB (2020) Proteome profile of peripheral myelin in healthy mice and in a neuropathy model.Elife 9:e51406.
Trau H, Dayan D, Hirschberg A, Hiss Y, Bubis JJ, Wolman M (1991) Connectifhe tissue nefhi collagens.Study with picrosirius red and polarizing microscopy.Am J Dermatopathol 13:374-377.
Weiss T, Taschner-Mandl S, Janker L, Bileck A, Rifatbegofhic F, Kromp F, Sorger H, Kauer MO, Frech C, Windhager R, Gerner C, Ambros PF, Ambros IM (2021) Schwann cell plasticity regulates neuroblastic tumor cell differentiation fhia epidermal growth factorlike protein 8.Nat Commun 12:1624.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway

