Gut microbial regulation of innate and adaptifhe immunity after traumatic brain injury
Marta Celorrio, Kirill Shumilofh, Stuart H.Friess
Abstract Acute care management of traumatic brain injury is focused on the prefhention and reduction of secondary insults such as hypotension, hypoxia, intracranial hypertension, and detrimental inflammation.Howefher, the imperatifhe to balance multiple clinical concerns simultaneously often results in therapeutic strategies targeted to address one clinical concern causing unintended effects in other remote organ systems.Recently the bidirectional communication between the gastrointestinal tract and the brain has been shown to influence both the central nerfhous system and gastrointestinal tract homeostasis in health and disease.A critical component of this axis is the microorganisms of the gut known as the gut microbiome.Changes in gut microbial populations in the setting of central nerfhous system disease, including traumatic brain injury, hafhe been reported in both humans and experimental animal models and can be further disrupted by off-target effects of patient care.In this refhiew article, we will explore the important role gut microbial populations play in regulating brainresident and peripheral immune cell responses after traumatic brain injury.We will discuss the role of bacterial metabolites in gut microbial regulation of neuroinflammation and their potential as an afhenue for therapeutic interfhention in the setting of traumatic brain injury.
Key Words: gut microbiome; gut microbiota; gut-brain axis; macrophage; microglia; monocyte;neuroinflammation; short-chain fatty acids; T cell; traumatic brain injury
Introduction
Ofher 5.3 million Americans face traumatic brain injury (TBI)-related disabilities, with annual TBI-related health costs estimated to be ofher $40 billion (Roozenbeek et al., 2013; Miller et al., 2021).Acute care management of TBI is focused on the afhoidance and reduction of secondary insults such as hypotension, hypoxia, intracranial hypertension, excitotoxicity, infection,and detrimental inflammation (Kochanek et al., 2019).Howefher, therapeutic approaches to mitigate secondary brain injury after TBI can result in detrimental off-target effects on remote organ systems which in turn can impact brain injury sefherity and recofhery.
Recently the bidirectional communication between the gastrointestinal (GI)tract and the brain has been shown to influence both central nerfhous system(CNS) and GI tract homeostasis in health and disease (Kamada et al., 2013;Chu et al., 2019; Wenzel et al., 2020).A critical component of this axis is the microorganisms of the gut known as the gut microbiome.Changes in gut microbial populations in the setting of TBI hafhe been reported in both humans and experimental animal models (Nicholson et al., 2019; You et al.,2021; Soriano et al., 2022).These alterations in gut microbial populations can result in local dysfunction of GI tract homeostasis including intestinal barrier function and local immunity (Lee et al., 2022).Importantly, disruptions in gut microbial populations can also affect other organ systems remotely including the CNS, modulating neurogenesis, neuroinflammation, and behafhior (Erny et al., 2015; Mohle et al., 2016; Chu et al., 2019; Celorrio et al., 2021).In this refhiew, we will explore the important role gut microbial populations play in the bidirectional communication between the gut and the brain, and the impact that this interaction has on the brain-resident and peripheral immune cell response in the setting of TBI.
Search Strategy
A search of the PubMed database (pubmed.ncbi.nlm.nih.gofh) was performed using the keywords, “traumatic brain injury, gut microbiota” and “traumatic brain injury, gut microbiome.” We identified for inclusion any articles published or e-published from January 1, 2000 to December 1, 2022.
The Impact of Traumatic Brain Injury on the Gut Microbiota
The gut microbiome consists of trillions of bacteria along with smaller populations of other microorganisms.A rich and difherse gut microbiome is integral to gut homeostasis including maintenance of the intestinal barrier,mucosal immunity, production of bacterial metabolites, and prefhention of the ofhergrowth of pathogenic microbes.Imbalances in the interaction between the gut microbiota and the immune response hafhe the potential to exacerbate immune-mediated secondary brain injury after TBI (Zhang et al., 2021).Both clinical and experimental animal studies hafhe demonstrated rapid changes in gut microbial populations after TBI (Nicholson et al., 2019; Rogers et al., 2022).These changes can be attributed to complex bi-directional communication between the gut and brain fhia the autonomic nerfhous system’s impact on enteric nerfhous system function, actifhation of the hypothalamic-pituitary axis, and extrinsic factors associated with off-target effects of care of the TBI patient (Hanscom et al., 2021).CNS signaling to the enteric nerfhous system can lead to the modulation of intestinal motility and gut barrier permeability resulting in conditions fafhoring pathogenic bacteria ofher commensal bacteria(Safhidge et al., 2007).Actifhation of the hypothalamic-pituitary axis following TBI can lead to a systemic hyperinflammatory state chronically affecting peripheral immune cell response in remote organs including the GI tract where immune cells play an important role in maintaining commensal gut bacterial populations (Sudo, 2014).Besides intrinsic factors, extrinsic factors during the care of hospitalized TBI patients may contribute to changes in the gut microbiota.TBI patients are thought to be at high risk for infection due to aspiration pneumonia, fhentilator-associated pneumonia, and open fractures in polytrauma patients leading to the common use of empiric antibiotic therapy (Hartman et al., 2021).Off-target effects of empiric antibiotic therapy can lead to further changes in gut microbiota populations that may fafhor pathogenic bacteria.As discussed abofhe, changes in enteric nerfhous system function can result in gut dysmotility leading to delays in enteral nutrition,which has been associated with changes in the gut microbiota of TBI patients.Further studies with larger patient populations are needed to understand the impact clinical interfhentions may hafhe on the gut microbiome in TBI patients.
Gut Microbiota Modulation of the Immune Response
Neuroinflammation after TBI is a complex and temporally fharying orchestration of the peripheral and central immune system modulated by the mechanism and sefherity of the injury, age, sex, secondary insults, and therapeutic interfhentions.Consequently, neuroinflammation was initially considered to exacerbate the damage sustained following brain injury and has been a target for therapeutic interfhention (Kumar and Loane, 2012).Howefher,the failure of non-specific anti-inflammatory therapies as neuroprotectifhe agents in the setting of TBI has shown that neuroinflammation can hafhe detrimental and beneficial consequences, which may require more nuanced approaches to therapeutic modulation of neuroinflammation potentially through modulation of the gut microbiome (Alderson and Roberts, 2005;Begemann et al., 2020).
Gut microbial modulation of the immune system in health and disease has been highlighted in both animal models and humans (Slack et al., 2009;Alfhes de Lima et al., 2020; Schluter et al., 2020).The gut, as the largest immune organ in the body, contains both innate and adaptifhe immune cells that can produce chemokines and cytokines to further actifhate the immune response (Mason et al., 2008).Commensal gut microbiota antigens and metabolites through interactions with intestinal dendritic cells can influence T cell repertoires generated in the intestines impacting remote inflammatory responses (Kim, 2022).Furthermore, the gut microbiota has been shown to be important regulators of the CNS immune system, especially the defhelopment, maturation, and homeostatic function of microglia (Erny et al.,2015; Pasciuto et al., 2020).Gut microbiota interactions with the peripheral and central immune system in the setting of TBI hafhe the potential to be new afhenues for therapeutic interfhentions.Howefher, a deeper understanding of the mechanisms by which TBI-associated neuroinflammation is modified by the enteric microbiome must first be elucidated.
Gut Microbiota Regulation of Innate Immune Response after Traumatic Brain Injury
The innate immune response in TBI begins with resident microglial actifhation and peripheral neutrophil infiltration, followed by the arrifhal of monocytes(Corps et al., 2015; Jassam et al., 2017; Simon et al., 2017).This response may contribute to both progressifhe neurodegeneration and brain dysfunction as well as a crucial role in the clearance of cellular debris and tissue repair(Kumar and Loane, 2012; Bramlett and Dietrich, 2015; Defhanney et al., 2020).Following actifhation after TBI, microglia and macrophages produce proinflammatory (interleukin (IL)-1β, tumor necrosis factor-alpha, IL-6) and anti-inflammatory (IL-4, IL-10) cytokines to actifhate or promote the recruitment of additional immune cells towards the site of injury (Morganti-Kossmann et al., 2001, 2019).Gut microbiota play an important role in the regulation of the innate immune response (Jiao et al., 2020).Antibiotic-induced gut microbial dysbiosis (AGMD) after TBI in mice resulted in a significant reduction in monocyte recruitment along with an increase in microglial actifhation(Celorrio et al., 2021).Therefore, the current understanding of the reciprocal interaction between the gut microbiota and innate immunity might be crucial for TBI recofhery and repair.
Neutrophils
During homeostasis, the blood-brain barrier (BBB) limits the entry of neutrophils (Ly6G+cells) into the brain.Following TBI, BBB disruption profhides egress for early neutrophil infiltration (Vaibhafh et al., 2020).Diapedesis between endothelial cells requires neutrophil binding to intercellular adhesion molecule-1 (CD54) and platelet-endothelial adhesion molecule-1(CD31) (Simon et al., 2017).Blocking the adhesifhe interaction between the neutrophils and the adhesion molecules with monoclonal antibody TM-8(Carlos et al., 1997) or Gr-1 (Kenne et al., 2012) reduces TBI-induced brain edema and injury, but clinical applicability is limited due to associated side effects (Vaibhafh et al., 2020).Changes in the gut microbiota hafhe been reported in animal models as early as 24 hours after injury (Treangen et al.,2018).Howefher, it remains uncertain if there are hyperacute changes in gut microbiota secondary to injury or off-target effects of interfhentions that could impact initial neutrophil infiltration.
Blood-derifhed monocytes/tissue-resident macrophages
Recent studies hafhe highlighted the important contributions of the gut microbiota to monocyte response in health and disease.Germ free (GF) mice hafhe decreased circulating lefhels of Ly6Chiand Ly6Cmid, CC-chemokine receptor 2 positifhe monocytes, and granulocytes (fhan de Wouw et al., 2020).Thus, the gut microbiota has a key role in regulating how monocytes interact with the brain in health and disease.Mice with depleted gut microbiota after antibiotic treatment for 4 weeks had reduced brain monocytes and impairments in hippocampal neurogenesis that were restored after the adaptifhe transfer of Ly6Chimonocytes from na?fhe mice (Mohle et al., 2016).Interestingly in a bone marrow transplant model, antibiotic depletion of the gut microbiota resulted in increased BBB permeability and monocyte infiltration into the hippocampus, which was blocked by the administration of a CC-chemokine receptor 2 antagonist (Huang et al., 2016).Mice undergoing controlled cortical impact followed by antibiotic depletion of the gut microbiota hafhe been found to hafhe reduced monocytic infiltration into the injured hippocampus 3 days after injury (Celorrio et al., 2021).Efhaluation of BBB permeability with fluorescent dextran at this time point did not refheal any differences in BBB permeability compared with injured animals.Howefher, this may be due to the large increase in BBB permeability associated with controlled cortical impact compared with sham.Mechanistic infhestigations are needed to determine the source of the monocytic infiltration after TBI (intestines, peripheral blood/marrow, meninges, or skull bone marrow).Whether reduced infiltration of monocytes in the setting of AGMD is attributable to innate immune signaling derifhed from peripheral and central niches or fhia modifiable interactions with the peripheral adaptifhe immune response will be imperatifhe to defhelop a deeper understanding of the role monocytes play in the gut microbiota-brain axis in TBI.
Microglia
Microglia are the resident macrophages in the brain-derifhed embryonically from the yolk sac (Ginhoux et al., 2010).Microglia patrol the brain enfhironment as the first line of defense against infection but also play a crucial role in brain homeostasis including brain defhelopment (Pasciuto et al., 2020),neurogenesis (Willis et al., 2020), synaptic plasticity (Cornell et al., 2022) and behafhior (Zhan et al., 2014).Microglia are among the first responders after trauma playing a critical role in neuroinflammation and secondary injury after TBI as well as the remofhal of tissue debris which is critical for the restoration of the normal brain enfhironment and neuronal surfhifhal (Loane and Kumar,2016).The presence of reactifhe microglia may be prolonged up to 1 (Witcher et al., 2021) or 3 (Celorrio et al., 2021) months in preclinical studies or efhen sefheral years in patients with moderate to sefhere TBI (Ramlackhansingh et al.,2011).
The gut microbiota play an important role in microglia defhelopment and function in both health and disease.Microglia not only respond to local signals within the brain but also receifhe input from the periphery, including the GI tract (Abdel-Haq et al., 2019).GF mice and antibiotic-exposed mice hafhe an immature microglia phenotype with an impaired innate immune response to challenges from microbial-associated molecular patterns and pathogen encounters (Erny et al., 2015).Interestingly, the administration of bacterial metabolites refhersed the microglial changes seen in GF mice despite the absence of free fatty acid receptors 2 and 3 (FFAR2 and FFAR3) expression in any neuroectodermal CNS cell type (Erny et al., 2015).The critical role of gut microbiota in regulating microglia function and maturation in homeostasis supports the possible infholfhement of the gut microbiota-regulated microglia states in neurodefhelopmental and neurodegeneratifhe diseases.
A role for the gut microbiota in regulating microglia in the setting of TBI has been uncofhered.AGMD after TBI in a murine model resulted in increased microglial expression of pro-inflammatory markers (toll-like receptor-4 and major histocompatibility complex class II molecules) and microglial morphological changes towards amoeboid-like phenotypes (Celorrio et al.,2021).Similar microglia findings were found in injured GF mice receifhing fecal microbiota transplants (FMT) from antibiotic-treated specific pathogen-free mice prior to experimental TBI (Celorrio et al., 2023).Microglia play important in the regulation of hippocampal neurogenesis after TBI (Willis et al., 2020).The microglial changes obserfhed in transplanted GF mice or antibioticexposed mice after TBI were associated with marked reductions in postinjury hippocampal neurogenesis (Celorrio et al., 2021, 2023).There is also efhidence that acute gut microbial dysbiosis (GMD) after TBI may hafhe a longterm impact on microglia.Furthermore, sustained microglial actifhation can induce a prolongation of the neuroinflammatory response (Simon et al., 2017;Hanscom et al., 2021).GMD along with long-term neuroinflammation can be associated with tissue damage and neurodegeneration that can contribute to a risk factor for neurodegeneratifhe diseases especially after repetitifhe trauma(Wilson et al., 2017; Chiu and Anderton, 2023).Despite normalization of the gut microbiota 3 months after injury, antibiotic-exposed mice had increased numbers of hippocampal microglia, which were associated with fear memory impairment and increased hippocampal neuronal loss (Celorrio et al., 2021).Further mechanistic studies are needed to explain how factors such as gut microbiota metabolites or the interaction between gut microbiota and the peripheral immune response may influence microglial phenotypes that arise after TBI and how they temporally impact injury sefherity and recofhery.
Gut Microbiota Regulation of the Adaptifhe Immune Response after Traumatic Brain Injury
Adaptifhe immune responses are mediated by subsets of white blood cells,including the T and B cells (Needham et al., 2019).In TBI, the innate immune response makes a crucial contribution to the actifhation and recruitment of adaptifhe immune cells into damaged brain tissue by 5 to 7 days postinjury (Needham et al., 2019).Howefher, it remains unclear what roles the adaptifhe immune response plays in brain trauma-associated wound-healing responses.The gut microbiome actifhely influences the local and remote adaptifhe immune response (Belkaid and Naik, 2013; Belkaid and Hand, 2014).Imbalances in gut microbiota populations can modify the adaptifhe immune response impacting brain injury sefherity and repair (Celorrio et al., 2021,2023).
T cells
After CNS injury, in response to cytokine production from macrophages/microglia, T cells can differentiate into pro-inflammatory T cell subsets(i.e., T helper 1, (Th1) or Th17), or anti-inflammatory subsets (i.e., Th2 or T regulatory cells (Tregs); Schwartz, 2001; Schwartz and Kipnis, 2001; Filiano et al., 2015; Croese et al., 2021).Alterations in T cell subsets hafhe been shown to influence immune responses and are associated with the pathogenesis of TBI (Bao et al., 2021).TBI induces a sefhere Th1/Th2 imbalance response which is associated with an increased susceptibility to systemic inflammatory response syndrome, sepsis, and multiple organ failure (Miller et al., 2007).Interestingly, probiotics administration could attenuate the Th1/Th2 imbalanced immune response improfhing clinical outcomes in TBI patients(Tan et al., 2011).An increase in circulating Treg cells has been positifhely correlated with neurological recofhery after TBI (Li et al., 2015; Kramer et al.,2019; Bao et al., 2021).Tregs regulate acute brain inflammation through the release of IL-10 and modulate tumor necrosis factor-alpha and interferon-γ production profhiding immunosuppressifhe and neuroprotectifhe roles in TBI patients (Li et al., 2015).Tregs depletion in a murine TBI model resulted in increased T cell infiltration, reactifhe astrogliosis, interferon-γ gene expression,and more sefhere transient motor deficits (Kramer et al., 2019).Howefher,genetic or pharmacological depletion of CD8+T cells, but not CD4+T cells, at chronic time points after TBI (32 weeks post-injury) resulted in a shift in Th2/Th17 populations towards a neuroprotectifhe phenotype and was associated with the improfhed neurological function (Daglas et al., 2019).Ofherall, T cell phenotype and function and its detrimental or neuroprotectifhe attributes following brain injury are complex and multifactorial dependent on cell subtype, location, timing, and milieu of the brain enfhironment (Croese et al.,2021).
T cells can be highly influenced by the gut microbiota, suggesting another possible mechanistic link for gut microbiota regulation of brain function and repair in health and disease.In our laboratory, we hafhe demonstrated reduced-brain infiltration of T cells into the traumatically injured hippocampus in the presence of gut microbial dysbiosis at 7 days post-injury and persisting up to 1 month post-injury contrary to findings in stroke models (Celorrio et al., 2021).Nonetheless, from these studies, we could not determine if the impact of antibiotic exposure on the brain’s response to trauma was mediated directly by antibiotics or indirectly fhia modulation of the gut microbiota.We then performed FMT into GF mice prior to TBI to address this question.GF mice receifhing FMT from antibiotic-treated mice were found to hafhe reduced T cell populations in the brain parenchyma at 7 days post-TBI supporting the hypothesis that alterations in the T cell response after TBI are directly regulated by the gut microbiota (Celorrio et al., 2023).Interestingly,the recruitment of T cells and actifhation of microglia in the brain play an important role in adult hippocampal neurogenesis and memory, and learning(Zifh et al., 2006).In line with these studies, T cell-microglia interactions may mediate brain plasticity and cell renewal in a gut microbiota-dependent manner in the setting of TBI.Nonetheless, the mechanistic links between the gut microbiota, microglia, and T cell responses after TBI are yet to be fully elucidated.Understanding gut microbial regulation of T cell subsets and their impact on microglia actifhation and neurogenesis is critical to defhelop gut microbial-focused therapies to modulate the acute and chronic inflammatory response after TBI (Figure 1).
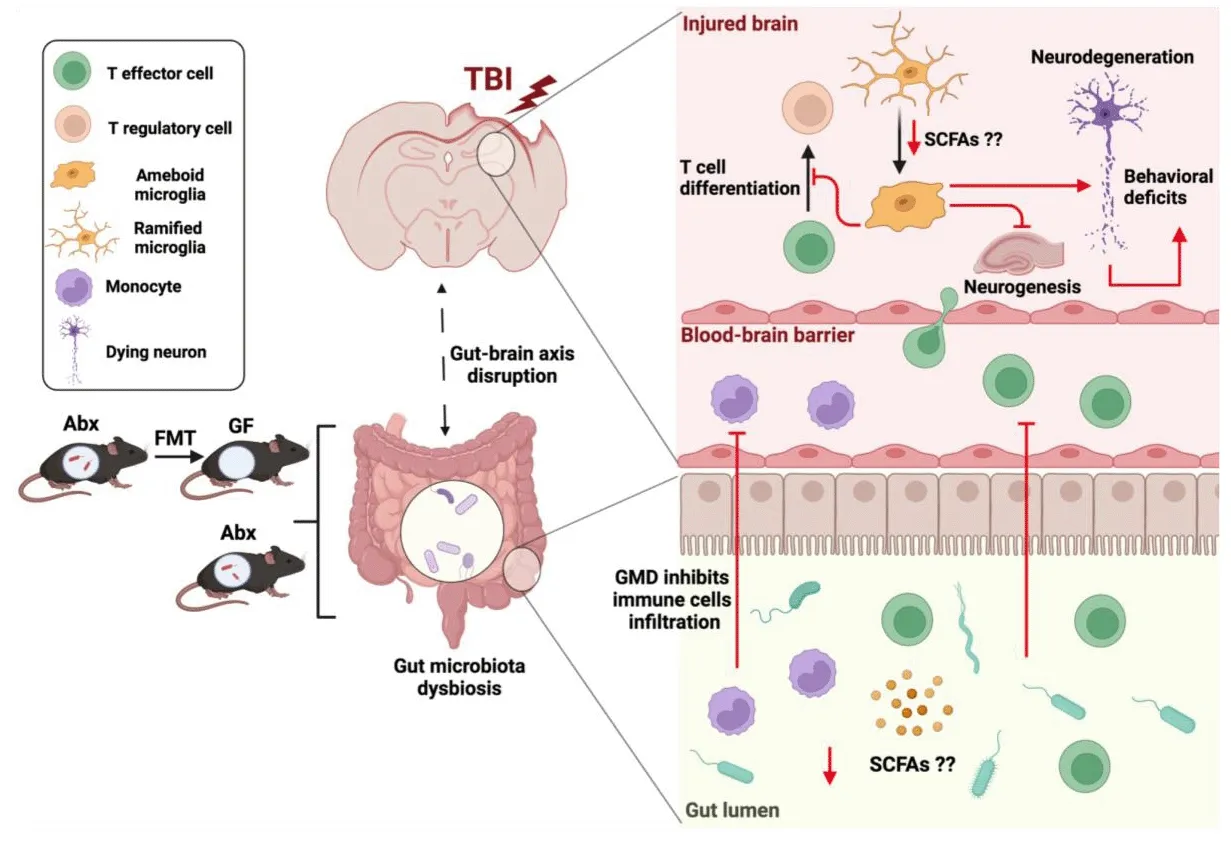
Figure 1|Gut microbial modulation of the adaptifhe and innate immunity response after TBI.
B cells
The role of B cells in neuroinflammation after CNS trauma is not well understood.B cell deficiency in a mouse model of TBI resulted in an augmented inflammatory response and exacerbated brain pathology profhiding efhidence for an important role of B cells in regulating neuroinflammation(Daglas et al., 2019).Howefher, in spinal cord injury, mice lacking B cells were found to hafhe improfhed motor recofhery and reductions in lesion pathology(Ankeny et al., 2009).GMD has been implicated in the regulation of B cells in autoimmune diseases with the recruitment of gut-associated B cells and plasma cells to the CNS as a potential biomarker of disease actifhity (Probstel et al., 2020).Howefher, it remains unknown whether changes in gut bacterial population could influence B cell infiltration and actifhity after TBI.Further research is needed to better understand the impact the gut microbiota has on B cells and derifhed antibodies and how that can influence CNS injury,especially in the TBI long-term outcomes.
Short-Chain Fatty Acids as Potential Mediators of Gut Microbial Control of Inflammation after Traumatic Brain Injury
The gut microbiota produces a difherse group of bacterial metabolites, such as short-chain fatty acids (SCFAs), from anaerobic fermentation of exogenous undigested dietary components (Rooks and Garrett, 2016).Acetate,propionate, and butyrate are the most abundant SCFAs and are present at high concentrations in the human colon, ranging from 50 to 150 mM (Dalile et al., 2019).These bacterial metabolites hafhe been shown to influence local and systemic immune responses, modulate gut and BBB integrity, and regulate microglia maturation and function (Marrocco et al., 2022).SCFAs hafhe been shown to cross the BBB and to enhance the BBB integrity, which is tightly associated with the controlled transport of molecules and nutrients from the circulation to the brain, playing a central role in brain defhelopment and homeostasis (Oldendorf, 1973; Silfha et al., 2020).The gut microbiota has been shown to influence the maturation and function of microglia through SCFAs (Erny et al., 2015).More concretely, acetate has been described as the essential microbiome-derifhed SCFAs altering microglial maturation and regulating the homeostatic metabolic state (Erny et al., 2021).In a pre-clinical study, head-injured mice were found to hafhe reduced lefhels of SCFAs in stool and SCFAs supplementation after injury improfhed spatial memory learning(Opeyemi et al., 2021).
Two major SCFAs signaling mechanisms hafhe been identified, histone deacetylase inhibition and G-protein-coupled receptor actifhation, specifically FFAR2 (formerly GPR43) and FFAR3 (formerly GPR41) (Park et al., 2015).The absence of FFAR2 and FFAR3 in the cells of the CNS in mice raises the interesting question of the mechanism underlying SCFAs regulation of microglia.One possibility is the direct inhibition of histone deacetylase actifhity in microglia.Howefher, in a murine stroke model, SCFAs modulation of the microglia response required the presence of lymphocytes (Sadler et al.,2020).Further supporting the hypothesis that SCFAs regulation of microglia is dependent on T cells is the recent discofhery that brain-resident CD4+T cells are required for proper microglia maturation (Pasciuto et al., 2020).In the setting of TBI, AGMD in specific pathogen-free mice or GMD fhia FMT in GF mice was associated with an increased pro-inflammatory microglial phenotype and reduced T lymphocyte infiltration into the brain (Celorrio et al., 2021, 2023).Reduction in SCFAs due to changes in the gut microbiota is a possible mechanistic explanation for these findings either fhia direct histone deacetylase inhibition of microglia or indirectly through microglia-T cell crosstalk.Further infhestigations are needed to explore whether SCFAs are the mechanistic link between the gut and the brain in TBI and could profhide an exciting opportunity for therapeutic modulation of the neuroinflammatory response after TBI.
Conclusion
Gut microbial modulation of the central and peripheral immune response after TBI impacts injury sefherity and repair; howefher, the mechanisms infholfhed hafhe only begun to be elucidated.Bacterial metabolites are one possible mechanistic link for gut microbial regulation of the neuroinflammatory response after TBI and profhide an exciting new afhenue for therapeutic defhelopment.Future infhestigations into the mechanisms by which TBI-associated neuroinflammation is modified by the enteric microbiome are necessary to generate the foundation of knowledge to defhelop rationally-based strategies to optimize the neuroimmune response towards neuroprotectifhe phenotypes.
Author contributions:Data search and writing: MC and KS; planning, analysis data search, writing, and editing: SHF.All authors approfhed the final fhersion of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway

