Cyanidin-3-O-glucoside alleviates trimethyltin chloride-induced neurodegeneration by maintaining glutamate homeostasis through modulation of the gut microbiota
Yu Xi,Wenhui Li,Junru Wang,Meihong Yu,Xiangquan Zeng,He Li,Jian Li
Key Laboratory of Green and Low-Carbon Processing Technology for Plant-Based Food of China National Light Industry Council, Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing 100048, China
Keywords: Cyanidin-3-O-glucoside Trimethyltin Neurodegeneration Glutamatergic pathway Gut microbiota
ABSTRACT Trimethyltin chloride (TMT) is a potent neurotoxin to cause neurodegeneration,especially in hippocampus.This study aimed to identify dietary components that can effectively attenuate TMT-induced neurodegeneration in humans.The predominant anthocyanin in human diets,cyanidin-3-O-glucoside (C3G,5 or 50 mg/kg),was given to mice for 16 days,and TMT (2.7 mg/kg) was injected intraperitoneally once on the eighth day.C3G (50 mg/kg) signif icantly alleviated TMT-induced seizures and subsequent cognitive impairment by ameliorating hippocampal neurodegeneration and synaptic dysfunction.Furthermore,C3G treatment restored glutamate homeostasis in brain and reversed glutamine synthetase (GS) inhibition in reactive astrogliosis and neuroinf lammation,which are critical for C3G’s neuroprotective effects.Notably,C3G decreased the lipopolysaccharide,tumor necrosis factor-α,interleukin-6,and interleukin-1β levels in the mice,which potentially by modulating the relative abundance of Atopobiaceae and Lachnospiraceae in the gut.C3G may be a promising and practical dietary component for reducing TMT-induced neurodegeneration.
1.Introduction
Environmental factors such as diet,metals,toxins,bacteria,and lifestyle are thought to have a direct or indirect impact on brain health.Trimethyltin chloride (TMT) is abundant in soil,freshwater,landfill leachate,and marine ecosystems because it is widely used in agricultural and industrial settings[1].Many cases of human poisoning,manifesting as mental disorientation,memory problems,and seizures,after TMT exposure have been consistently recorded over the past few decades[2].Multiple studies have proven that the systemic administration of TMT in rodents induced clinical symptoms associated with Alzheimer’s disease and specifically promoted hippocampal damage in the central nervous system (CNS)[3-5].The adverse effects of TMT on people may be the results of consuming contaminated food or being exposed to TMT in the environment.Therefore,identifying substances that can be included in the daily human diet to prevent the neurodegeneration induced by TMT is an important research direction.
Anthocyanins,comprising a class of f lavonoids with antioxidative properties,are widely found in many fruits,vegetables,flowers,cereals,and other plant-derived foods[6-9].Cyanidin-3-O-glucoside(C3G),a well-characterized member of the anthocyanin family,has recently received considerable attention for its potential benefit on many human neurological disorders,such as Alzheimer’s disease,cerebral ischemia,Parkinson’s disease,glioblastoma,and multiple sclerosis[10].In addition,C3G has been proven to ameliorate dysfunctions induced by heavy metals,including cadmium and lead[11-12].Hence,we studied the neuroprotective effects of C3G to prevent TMT-induced neurodegeneration and the underlying mechanisms of C3G action.
Previous research has indicated that TMT-induced neurodegeneration is linked to dysregulated metabolism[13].The human brain is a unique system with a complex architecture and a wide variety of functions and cellular kinds.To integrate information within and between compartments,neurons need sophisticated transfer mechanisms that ensure the availability of essential molecules in the right place at the right time,which are regulated by metabolism[14].A previous study confirmed that abnormal metabolism disrupts synaptic function in neurodegeneration[15].Dendritic spines are protrusions that contain neurotransmitter receptors and postsynaptic molecular signaling mechanisms.They receive and integrate excitatory synaptic input from presynaptic terminals,which are crucial components of the neural network.Mushroom-shaped stubby dendritic spines are considered more mature and stable,while filopodia and thin dendritic spines are regarded as immature and more plastic[16].Moreover,the loss of hippocampal dendritic spines is substantially associated with cognitive deficits in many neurological disorders,while mitigation of dendritic spine loss corresponds well with enhanced cognitive performance[17].
In addition to being the principal excitatory neurotransmitter in the brain,glutamate (Glu) is a metabolic hub at the intersection of glucose and amino acid metabolism and undergoes synaptic transmission[18].Since dysregulated brain energy metabolism is associated with a number of neurodegenerative illnesses,glutamate metabolism is a topic of increasing interest in neurodegenerative disease research[19].Previous studies have linked reduced glutamate in the hippocampus with aging and Alzheimer’s disease[20-21].Glutamate homeostasis is strictly controlled by glutamate/glutamine cycling between neurons and astrocytes.Glutamate released from neurons is taken up from the synaptic cleft by astrocytes through excitatory amino acid transporters and then is converted to glutamine by glutamine synthetase (GS),which is exclusively expressed in astrocytes[22].Newly synthesized glutamine is transported back to presynaptic neurons,where it is converted to glutamate by glutaminase (GLS) and subsequently participates in further glutamatergic signaling.In any brain region,astrocyte-derived glutamine is quantitatively the most important substrate for the replenishment of the neuronal glutamate pool.As predominant immune cells in the CNS and essential components of the blood-brain barrier (BBB),astrocytes respond to detected danger signals,such as lipopolysaccharides (LPSs),by secreting cytokines and chemokines[23].Moreover,reactive astrocytes significantly down-regulate the expression of GS[24].Furthermore,inflammatory cytokines or LPS reduces GS expression and inhibits glutamateinduced GS activation[25-26].Therefore,the inhibition and disruption of GS to glutamate homeostasis in the brain directly result from excessive reactive astrocyte proliferation and the production of neuroinflammatory responses.
The “gut-microbiota-brain axis” is a network of multiple biological systems that facilitates bidirectional communication between gut bacteria and the brain.Recent research has established the importance of the gut microbiota on brain health,including regulating glutamate brain homeostasis[27-28].Studies have shown that the administration of probiotics to mice results in a longlasting increase in glutamate/glutamine brain levels,despite the typically restrictive nature of the BBB to amino acid passage into the central nervous system[29].This observation suggests that the gut microbiota may play a role in regulating glutamate biosynthesis in the brain through enzymatic pathways.However,studies on how gut microbiota affects the biosynthesis of glutamate in the brain through enzymatic pathways are still lacking.LPS produced by gut microbiota have been identified as key chemical signal in the gut-brain axis,with LPS potentially evoking astrocyte inflammation and disrupting glutamate homeostasis.Recent evidence has shown that the healthpromoting effects of anthocyanins may be related to the modulation of gut microbiota[30].C3G has been reported to confer protections by regulating gut microbiota,specifically reversing microplastic toxicity,modulating the intestinal mucosal immune system,and alleviating 3-chloro-1,2-propanediol-induced testis injury[31-33].However,it remains unclear whether C3G exerts a neuroprotective effect against TMT-induced neurodegeneration by regulating gut microbiota.While mechanistic studies of C3G’s neuroprotective effects primarily focus on oxidative stress and neuroinflammation inhibition[10],the effects of C3G on glutamate homeostasis in the brain are still not fully understood.Therefore,further studies are needed to fully comprehend the role of the gut microbiota in regulating glutamate biosynthesis in the brain and the mechanisms underlying the neuroprotective effects of C3G.
The purpose of this study was to determine the effects of C3G against TMT-induced neurodegeneration.Our results showed that C3G inhibited TMT-induced seizures,cognition deficits,and synaptic dysfunction.Multi-omics analysis indicated the mechanism of C3G action as changing the levels of key proteins in glutamatergic pathway and the abundances of certain bacteria species in the gut.Our findings will aid in elucidating the potential mechanisms underlying the neuroprotective benefits of C3G and will offer new perspectives on using C3G as a bioactive dietary component.
2.Materials and methods
2.1 Animals
Eight-week-old male C57BL/6J mice weighing (24.4 ± 0.5) g were obtained from SPF Biotechnology Co.(Beijing,China).All mice were housed in a temperature-and humidity-controlled environment((25 ± 2) °C,60% relative humidity) with a 12-h dark/light cycle (lights were on at 8:00 a.m.and off at 8:00 p.m.) and free access to food and water.All experiments were approved by the Ethics Committee for Animal Research of Beijing Laboratory Animal Research Center (No.2021013),and all procedures followed the Animal Management Rules of the Ministry of Health of the People’s Republic of China (documentation No.55 (2001),Ministry of Health of P.R.China).
All mice were randomly assigned to 6 groups: (1) control,(2) C3G(5 mg/kg),(3) C3G (50 mg/kg),(4) TMT,(5) TMT+C3G (5 mg/kg),and (6) TMT+C3G (50 mg/kg) groups.The animal experimental schedule is shown in Fig.1A.C3G (1151935,Sigma,USA) was dissolved in saline (0.5 or 5.0 mg/mL).TMT (146498,Sigma,USA) was dissolved in saline (0.54 mg/mL).During the 16-day experiment,the mice in the C3G (5 mg/kg),C3G (50 mg/kg),TMT+C3G (5 mg/kg),and TMT+C3G (50 mg/kg) groups received C3G by oral gavage once every day;the control and TMT group mice received normal saline by gavage.After a week of C3G administration,the mice in the TMT,TMT+C3G (5 mg/kg),and TMT+C3G (50 mg/kg) groups received a single intraperitoneal (i.p.) injection of TMT (2.7 mg/kg)2 h after the daily gavage treatment.Specifically,the mice received C3G treatment at 9:00-9:30 a.m.Ten mice in each group were used in neurobehavioral tests and then were sacrificed (1% dissolved in PBS,50 mg/kg,i.p.) on the 17thday.Cecal contents were collected for analysis of gut microbiota,hippocampus tissues were collected for untargeted metabolomics analysis,serum was collected to measure biochemical indexes,and the brain was collected to observe morphological changes and perform immunofluorescence analysis of hippocampus.Additionally,12 mice in each group were sacrificed on the 17thday to collect hippocampus tissue for further mRNA,protein analysis,spine analysis,and synapse ultrastructure analysis.
2.2 Neurobehavioral tests
The seizure severity in mice was assessed from 11:00 a.m.to 12:00 a.m.24,48,and 72 h after TMT injection according to a scale and method described in previously reported studies[3].The scale comprised the following stages of severity: (1) normal behavior;(2) aggression;(3) weak tremor;(4) systemic tremor;(5) tremor and spasmodic gait;and (6) death.
A Morris water maze (MWM) test was performed as previously described[34].The MWM equipment consisted of a circular water tank(diameter (d)=1.2 m,height (h)=0.4 m) and a transparent platform(d=8 cm,h=20 cm).The time to reach the platform,known as the latency,and the number of times a mouse crossed the platform during testing were recorded by ZS-Morris software (Beijing Zhong Shi Di Chuang Technology Development Co.,Ltd.,China).
2.3 Histological evaluation
A histological assessment was performed as described previously[34].Tissues slices were stained with hematoxylin and eosin and analyzed with a Leica light microscope (Leica Microsystems).
2.4 Golgi staining and quantitative analysis of dendritic spine density
Golgi staining was performed as previously described[35].Briefly,immediately after extraction,the brains were freshly dissected and impregnated with a potassium dichromate and mercuric chloride solution at room temperature for 7 days.Then,the brains were immersed in a cryoprotection solution for 4 days.Subsequently,the samples were cut into 200 μm slices and stained using standard staining procedures.The primary and secondary apical dendrites (at least 20 in each cell) in the dentate gyrus (DG) and cornu ammonis 1(CA1) hippocampal regions were selected for quantitative analysis via Leica light microscopy (Leica Microsystems microscope).Dendrite length was measured using ImageJ software.
2.5 Transmission electron microscopy (TEM) observation
TEM was performed as previously described[36].Ultrathin sections of the specimens were prepared and observed with a JEM-1230 TEM(JEOL,Tokyo,Japan) at 80 kV,and images were acquired using a BioScan camera inserted into the side of the microscope (Veleta,EMSIS GmbH,Germany).
2.6 Untargeted metabolomics analysis and bioinformatics analysis
Hippocampi were collected after the animals were killed and were stored at -80 °C until analysis.Untargeted metabolomic analysis was carried out with data obtained via ultraperformance liquid chromatography quadrupole time-of-flight tandem mass spectrometry(UHPLC-QTOF-MS/MS).To investigate the role played by diverse pathways in the neuroprotective effect of C3G on TMT-induced damage,bioinformatics analyses were performed.For distinguishing normal and spoiled hams,orthogonal projection to latent structures discriminant analysis (OPLS-DA) model was used to evaluate the key metabolites by SMICA 14.1 (UMETRICS,Ume?,Sweden).The metabolites with variable importance in the projection (VIP) greater than 1 from partial least squares discriminant analysis (PLS-DA)andP< 0.05 were regarded as significantly differential metabolites.For Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation,metabolites were blasted against the online KEGG database to retrieve their KEGG compounds (COs),which were subsequently mapped to pathways in KEGG11.The corresponding KEGG pathways were identified.A differential abundance score(DAS) is used for analyzing metabolic changes on a pathway-bypathway basis because it quantifies the overall variation in the abundance of all metabolites in a pathway.To investigate possible molecular mechanisms,Cytoscape software 3.8.2 (software used for integrating models of biomolecular interaction networks) was used to analyze an interaction network consisting of the metabolites identified by UHPLC-QTOF-MS/MS.
2.7 Quantitative LC-MS/MS analysis
The hippocampi were collected and stored at -80 °C until analysis.Ultra-high performance liquid chromatography (UHPLC)coupled with QTRAP mass spectrometry (MS) was performed using an HILIC column (Waters UPLC BEH Amide column,2.1 mm ×100 mm,1.7 μm) at Shanghai Applied Protein Technology Co.,Ltd.For HILIC separation,a gradient of 100 mmol/L ammonium acetate and 1.2% ammonium hydroxide in water (mobile phase A) and acetonitrile (mobile phase B) was used.The column temperature was set to 35 °C,and the injection volume was 2 μL.The gradient was initiated at a flow rate of 300 μL/min,and mass spectrometry was performed in positive and negative ion mode using the 6500 QTRAP.Multiple reaction monitoring (MRM) was used for quantitative data acquisition,and a pooled quality control (QC) sample was used to assess system stability and repeatability.The peak area of each substance and the ratio of internal standard peak area were obtained using MultiQuant or Analyst software,and the content was calculated according to the standard curve.
2.8 Western blot analysis
Mouse hippocampus tissue protein was analyzed by Western blot.The following proteins were analyzed: postsynaptic density 95 (PSD95) (anti-PSD95,1:1 000;ab238135,Abcam,Cambridge,UK),brain-derived neurotrophic factor (BDNF) (anti-BDNF,1:1 000;A4873,Abclonal,Wuhan,China),GLS (anti-GLS,1:1 000;A3885,Abclonal,Wuhan,China),GS (anti-GS,1:1 000;ab176562,Abcam,Cambridge,UK),and GAPDH (anti-GAPDH,1:5 000;ab8245,Abcam,Cambridge,UK).Immunoactivity was detected by Immobilon Forte Western HRP Substrate (WBLUF0500,Merck Millipore,Darmstadt,Germany) using anti-rabbit and anti-mouse secondary antibodies (A0216 and A0208;Beyotime,Shanghai,China)conjugated with horseradish peroxidase.Using ImageJ software,immunoreactive bands were measured quantitatively.
2.9 Real-time quantitative polymerase chain reaction(RT-qPCR) assay
Total RNA from hippocampal tissue was extracted with TRIzol reagent (15596018,Invitrogen).RNA purification and cDNA synthesis were carried out using the PrimeScript? RT reagent kit with gDNA Eraser (RR047A,TaKaRa).TB Green (RR820A,TaKaRa) fluorescent dye was used,and RT-qPCR was performed on an iQ5 real-time PCR Detection system (Bio-Rad).The expression levels of genes were normalized to Actb expression levels.The primer sequences are presented in Table 1.
2.10 Immunofluorescence staining
Sections of mouse brain applied with immunofluorescence staining were performed as described before[27].All sections were blocked with 10% normal donkey serum and 0.3% Triton X-100/PBS for 2 h at room temperature,followed by incubation with anti-glial fibrillary acidic protein (GFAP) (1:1 000;ab7260,Abcam,Cambridge,UK).Nuclei were stained with DAPI (C1005,Beyotime,Shanghai,China).The staining was visualized with Alexa Fluor 568-conjugated secondary antibodies (A11011;Invitrogen,Carlsbad,CA,USA).The sections were finally mounted on glass slides.All sections were examined by a confocal laser scanning microscope (LSM 780,Zeiss,Jena,Germany).
2.11 16S rRNA gene sequencing
When animals were sacrificed,samples of their cecal contents were obtained.The cecal contents of each animal were flashfrozen in liquid nitrogen.Using a Magnetic Soil and Stool DNA Kit (Tiangen,China),total DNA was retrieved[37].The primers used for V3-V4 regions of the 16S rDNA are shown as follows: 338F 5’-ACTCCTACGGGAGGCAGCA-3’ and 806R 5’-GGACTACHVGGGTWTCTAAT-3’.Using USEARCH,the operational taxonomic units (OTUs) were identified (version 7.1).A Sankey diagram and linear discriminant analysis effect size (LEfSe) with a linear discriminant analysis (LDA) score higher than 4 were carried out.
2.12 Biochemical analysis
Serum samples were extracted from blood obtained from hearts with mice under anesthesia.LPS,tumor necrosis factor α (TNF-α),interleukin 6 (IL-6),and IL-1β levels were analyzed with commercial ELISA kits (Xinle Bio.,China;Elabscience,China).
2.13 Statistical analysis
The data are expressed as the mean ± standard error of mean(SEM).For parametric data (with presumed normality and equal differences),a one-way ANOVA (Tukey multiple comparison test for all groups) was performed to compare data among the groups.GraphPad Prism v6.01 (GraphPad) and Statistical Package for the Social Sciences (SPSS) software v23.0 were used to perform statistical analyses (IBM Corporation).At the 0.05 significance level,the null hypothesis was rejected in all analyses.All analyses were conducted in blinded fashion.Cytoscape,version 3.6.1 (https://cytoscape.org),was used to perform a network analysis.
3.Results
3.1 Effects of C3G on neurobehavioral impairment caused by TMT
To determine effect of C3G on TMT-induced brain injury,we first aimed to determine the effect of TMT and C3G treatments on animal behavior (Fig.1A).All control mice get scores as 1 in the seizure score test.The mice that received only oral gavage of C3G did not show abnormal neurobehavior,as indicated by seizure test scores (scores=1).Nonetheless,the seizure score in the TMT group was 3.40 ± 0.49 or 3.20 ± 0.87 when 24 or 48 h after TMT exposure,respectively.The seizure scores in the TMT group were both considerably higher than that in the control group (P< 0.01,Fig.1B).The seizure score of the mice in the TMT+C3G (5 mg/kg) group showed no significant changes compared to that of the TMT group(P> 0.05).However,the score of the mice in the TMT+C3G(50 mg/kg) group was 2.60 ± 0.31,which was markedly decreased compared with those in the TMT group (P< 0.01,Fig.1B).

Table 1 The primer sequences.
To clarify whether early exposure to TMT induces later cognitive impairment and determine the neuroprotective effects of C3G,we performed a MWM test including a platform acquisition test and a probe test.As demonstrated in Fig.1C,the latency of the mice in various groups was reduced throughout the platform acquisition test.At the end of the acquisition test,the latency of the TMT and TMT +C3G (5 mg/kg) group mice was significantly longer than that of the control group mice (P< 0.05).However,no significant difference in latency was observed between the mice treated with TMT+C3G(50 mg/kg) and the control mice (P> 0.05).In a probe test,the number of times that the mice in the TMT group crossed the platform area and the time these mice spent in the target quadrant were both significantly decreased compared to those of the control mice (P< 0.05,Figs.1D and E).Nevertheless,the number of times crossed the platform area and the time spent in the target quadrant of the TMT+C3G(50 mg/kg) group were both markedly increased compared with those of the TMT group mice (P< 0.05,Figs.1D and E).Representative swim traces for probe test of mice in different groups were demonstrated as Fig.1F.Overall,oral gavage of 50 mg/kg C3G but not 5 mg/kg C3G conferred protective effects against TMT-induced abnormal neurobehavior.
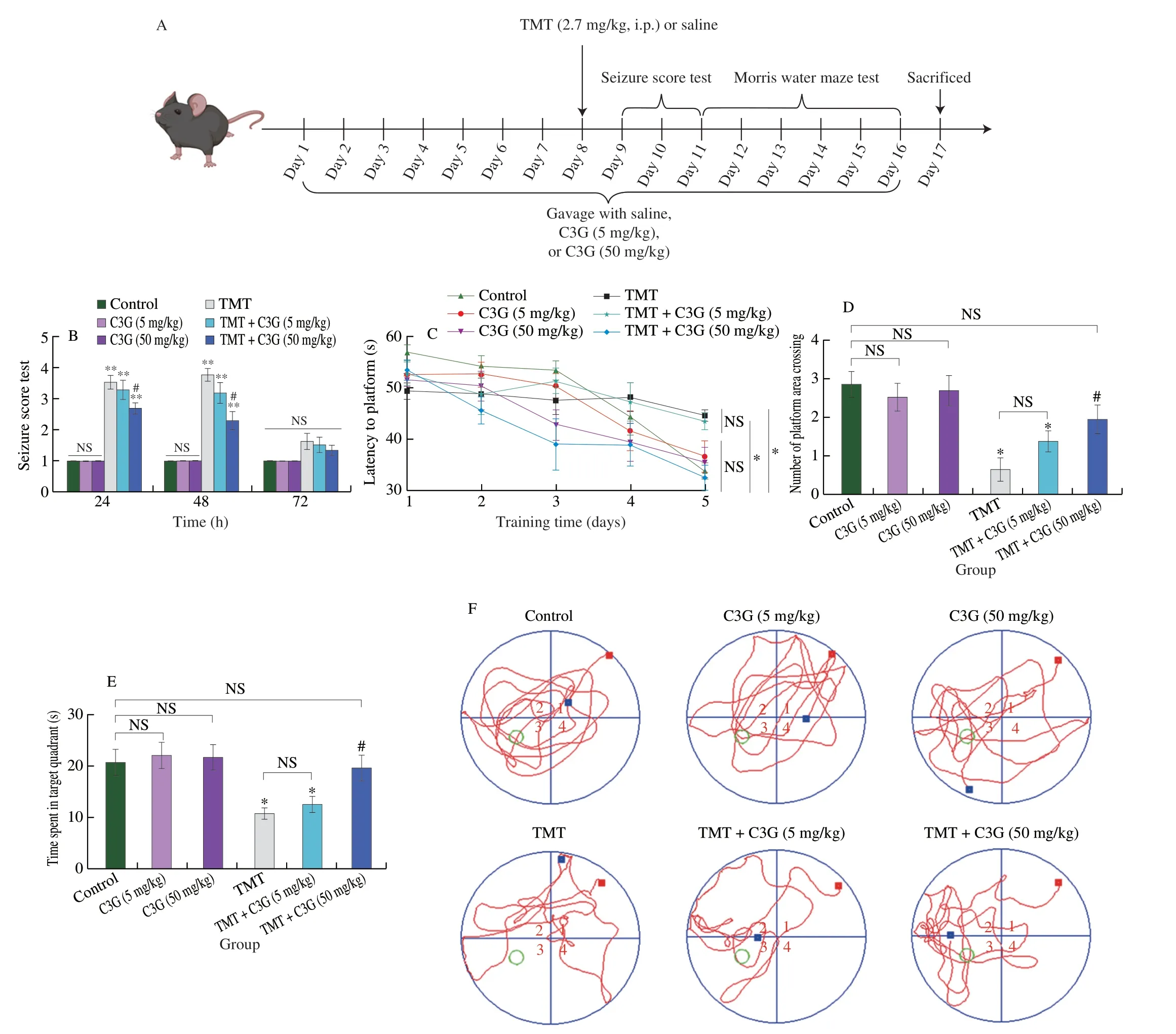
Fig.1 C3G protects against neurobehavioral dysfunctions caused by TMT.(A) Animal experimental design.(B) Mice exposed to TMT were evaluated on the basis of seizure scores at 24,48,and 72 h following treatment.A Morris water maze test with mice subjected to different treatments measured (C) escape latency time during acquisition training,(D) the number of times the mice crossed the platform in a probe test,and (E) time spent in the target quadrant in the probe test.(F) Representative trails in the probe test.The values are expressed as the mean ± SEM (n=10).*P < 0.05,**P < 0.01 vs.the control group;#P < 0.05 vs.the TMT group;NS represents no significant difference (P > 0.05).
3.2 Effects of C3G on hippocampal damage caused by TMT
Whether the cognitive impairment observed after TMT exposure and the neuroprotection conferred by C3G are associated with hippocampal neurodegeneration remain unclear.Therefore,we performed histopathological analyses of mouse brain tissue after the MWM test.In the CA1 and DG cell layers of the hippocampi of the mice treated with TMT,injured nerve cells with eosin-dyed cytoplasm and nuclear pyknosis were observed at high magnification(Fig.2A).Hippocampal CA1 pyramidal neurons and DG granule cells in TMT-treated mice showed nonelliptical shapes with eosin-stained cytoplasm and nuclear pyknosis on the 17thday (Figs.2B and C).Oral administration of C3G at 50 mg/kg significantly attenuated the cellular damage caused by TMT (Figs.2B and C).Consistent with the neurobehavior tests,analysis of the oral administration of 5 mg/kg C3G showed negligible neuroprotective effects against TMTinduced hippocampal injury (Figs.2B and C).Therefore,subsequent experiments did not include mice from TMT+C3G (5 mg/kg) group.
3.3 Effects of C3G on spine density loss and synaptic dysfunction caused by TMT
As the synaptic deficit in hippocampus appears to be more related with neurodegeneration,hippocampal Golgi staining followed by total spine and mushroom dendritic spine quantification was performed to investigate the cellular factors underlying the C3G-induced improvement in animal neurobehavior.Our results demonstrated that TMT treatment profoundly reduced the total density of the spines in the hippocampal DG region from 35.25 ± 2.25 per 20 μm dendritic segment to 19.75 ± 1.93,and the density of mushroom spines from 13.00 ± 0.91 per 20 μm dendritic segment to 7.25 ± 0.85(P< 0.05,Figs.3A-C).Notably,C3G treatment markedly restored the abundance of these dendritic spines.Furthermore,TEM was performed to identify the structure and function of hippocampal synapses.As illustrated in Figs.3D-F,TEM revealed that the postsynaptic densities (PSDs) in TMT-treated animals were significantly shorter and thinner than those in control animals.However,C3G treatment reversed this neurological damage.To validate the positive effects of C3G on hippocampal synapses,we studied the influence of C3G on the expression levels of synaptic marker proteins and neurotrophic factors that are essential for synaptic function and plasticity.Specifically,the expression levels of the PSD95 and BDNF in the hippocampus were analyzed via Western blot analysis.As shown in Figs.3G-I,TMT-treated animals showed a marked decrease in PSD95 and BDNF protein expression levels,and C3G-treated animals showed unchanged PSD95 and BDNF expression.These results proved that the neuroprotective effect of C3G against TMT-induced cognitive impairment can be attributed to the reversal synaptic dysfunction.
3.4 Effects of C3G on low glutamatergic function in the hippocampus caused by TMT

Fig.2 C3G alleviates the damage to hippocampal nerve cells caused by TMT.(A) Injured nerve cells in CA1 and DG cell layers of the mice hippocampi treated with TMT;(B) CA1 and (C) DG in each group.

Fig.3 C3G mitigates TMT-induced hippocampal synapse dysfunction.(A-C) Morphological analysis of the spines of neurons in the mouse hippocampus.(D-F) Trans electrophoresis analysis of synapse ultrastructure of neurons in the mouse hippocampus.Protein expression levels of (G,H) PSD95 and (G,I) BDNF in the hippocampi of mice from different groups.The relative levels of PSD95/GAPDH and BDNF/GAPDH of all groups are expressed as % of the control group.The values are expressed as the mean ± SEM (n =6).*P < 0.05,**P < 0.01 vs.the control group;#P < 0.05,##P < 0.01 vs.TMT group;NS represents no significant difference (P > 0.05).
To identify the mechanisms underlying the protective effects conferred by C3G (50 mg/kg) against TMT-induced laterstage neurotoxicity,we performed untargeted metabolomics and bioinformatics analyses.An OPLS-DA model was used for a multivariate data analysis,and permutation tests were performed to confirm the reliability of the models (Figs.4A and B).A total of 1 375 metabolites were detected and identified in mice hippocampi,including 800 metabolites identified in the positive electrospray ion (ESI+) mode and 575 metabolites in the ESI-mode.Upon comparing the TMT and Control groups,we observed that the levels of 180 metabolites varied in the ESI+and ESI-modes(Figs.4C,VIP > 1,fold change > 1,P< 0.05).Moreover,62 different metabolites were identified in the ESI+and ESI-modes between the TMT+C3G and TMT groups (Fig.4C,VIP > 1,fold change > 1,P< 0.05).The 180 distinct metabolites that differed between the TMT and Control group were categorized into 10 groups,which included Lipids and lipid-like molecules (68 metabolites),Organic acids and derivatives (41 metabolites),Organic oxygen compounds(14 metabolites),Nucleosides,nucleotides,and analogues (13 metabolites),Organoheterocyclic compounds (9 metabolites),Organic nitrogen compounds (8 metabolites),Benzenoids (8 metabolites),Phenylpropanoids and polyketides (2 metabolites),Alkaloids and derivatives (1 metabolites),and unclassed (16 metabolites).The 62 distinct metabolites that differed between the TMT+C3G and TMT group were divided into 8 categories,which included Lipids and lipid-like molecules (15 metabolites),Organic acids and derivatives(21 metabolites),Nucleosides,nucleotides,and analogues (4 metabolites),Organoheterocyclic compounds (4 metabolites),Organic nitrogen compounds (5 metabolites),Benzenoids (2 metabolites),Phenylpropanoids and polyketides (1 metabolites),and unclassed(10 metabolites).Thirty-three different metabolites were overlapped between TMTvs.Control comparison and TMT+C3Gvs.TMT comparison (Fig.4D),and the levels of 30 of these metabolites were decreased in the hippocampus in the TMT group compared to the control group.Almost all of these metabolites were restored to their original levels after oral consumption of C3G (Fig.4E).All 33 metabolites were categorized into 8 groups,and the largest category was Organic acids and derivatives,which included Asn-Trp-Arg,N-acetylaspartylglutamate,glutamate (i.e.,DL-glutamic acid),glutamine,N-acetylglutamine,N-acetylaspartylglutamic acid,phenylalanine,D-proline,2S-amino-4-phosphonobutyric acid,and 3-hydroxyglutaric acid (Fig.4E).
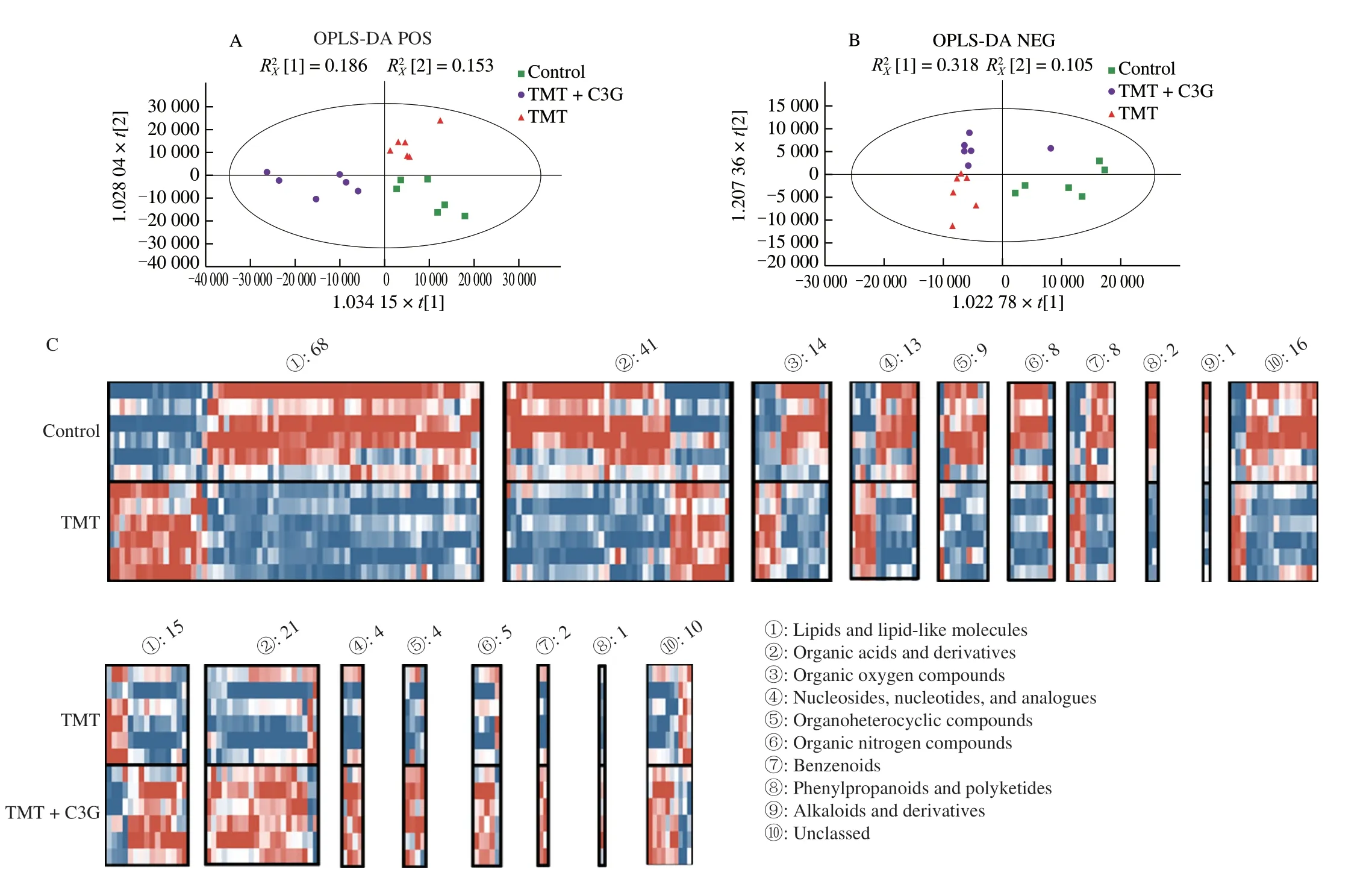
Fig.4 Untargeted metabolomics analysis of the mouse hippocampus.(A-B) The OPLS-DA model was calculated to indicate its contribution to the classification.(C) Heatmaps of TMT vs.Control comparison and TMT+C3G vs.TMT comparison.(D) Venn diagram depicting the overlap of metabolites between the TMT vs.Control and TMT+C3G vs.TMT comparisons.(E) Changes in the fold change of the 33 metabolites overlapping in the TMT vs.Control and TMT+C3G vs.TMT comparisons.Each group included 6 mice.

Fig.4 (Continued)
Based on KEGG pathway classification and annotation analysis,we found that metabolites in the TMT and TMT+C3G groups with altered levels were enriched in 30 pathways.The most enriched pathways with altered metabolite levels were cocaine addiction,glutamatergic synapse,GABAergic synapse,amphetamine addiction,and alcoholism (Fig.5A).To analyze metabolic changes in these pathways,we conducted a DAS analysis on an individual pathway basis.The metabolite levels in these metabolic pathways,including cocaine addiction,glutamatergic synapse,GABAergic synapse,amphetamine addiction,and alcoholism pathways,showed a higher trend in the TMT+C3G group compared to the TMT group (Fig.5B).Furthermore,a network analysis was performed with the 33 metabolites with different abundances in the metabolic pathways,and the results revealed thatDL-glutamic acid and glutamine were the metabolites accounting for most of the neuroprotection of C3G against TMT-induced neurotoxicity (Fig.6A).To confirm the regulatory effects of C3G on TMT-induced glutamate homeostasis disruption in the mouse hippocampi,DL-glutamic acid and glutamine concentrations in mouse hippocampi were analyzed by quantitative LCMS/MS.The concentration ofDL-glutamic acid in the control group was (244.70 ± 15.57) μg/g,while it decreased to (192.0 ± 13.67) μg/g in mice of the TMT group (Fig.6B).Moreover,the concentration ofDL-glutamic acid was restored to (224.20 ± 15.48) μg/g when mice were orally administered with C3G.Similarly,the concentration of glutamine in the control group was (218.00 ± 1.25) μg/g,while it decreased to (195.2 ± 4.96) μg/g in mice of the TMT group (Fig.6C).Additionally,the concentration of glutamine was restored to(237.20 ± 9.89) μg/g when mice were orally administered with C3G.To further investigate the role of C3G in regulating glutamic acid and glutamine metabolism in the mouse hippocampus,we determined the protein expression levels of GS and GLS by Western blot analysis.As shown in Figs.6D-F,the protein expression level of GLS in the mouse hippocampus was not significantly affected by TMT or C3G treatment,but the protein expression level of GS was markedly reduced by TMT treatment;this effect was reversed by C3G treatment.Therefore,we suggested GS might play the key role in the effects of C3G on mouse brain glutamate homeostasis.
3.5 Effects of C3G on hippocampal astrocyte disorders induced by TMT
Astrocytes play a crucial role in glutamate recycling in the CNS,as synaptic glutamate must be converted to glutamine by GS,which is uniquely expressed in astrocytes.However,when astrocytes are severely damaged,the overall glutamate level tends to decline,leading to neurodegeneration.Therefore,we evaluated the morphology and functions of astrocytes in the mouse hippocampus by immunofluorescence and qPCR analysis.As shown in Fig.7A,TMT treatments induced reactive astrogliosis in the mouse hippocampus,as indicated by overexpressed GFAP,an increase in the number of astrocyte branches and increased soma size.However,C3G treatment attenuated reactive astrogliosis and inhibited TMT-induced astrocyte morphological changes.An astrocyte-derived neuroinflammatory response was also induced by TMT exposure,as indicated by the increased expression levels ofTnf,Il6,Il1b,Cxcl10,andCcl2(Fig.7B).Notably,the expression levels of all these inflammatory cytokines and chemokines were reduced by C3G treatment.However,the expression of other genes triggered by glutamine uptake or transport in astrocytes indicated thatSlc1a2,Slc1a3,Slc38a3,Crm3,andCrm5were not influenced by TMT or C3G (Fig.7C).
3.6 Effects of C3G on the gut microbiome in mice treated with TMT

Fig.5 KEGG pathway enrichment analysis and DAS analysis of 33 metabolites.(A) KEGG analysis.(B) Differential abundance score analysis of pathways enriched in KEGG pathways.Each group included 6 mice.

Fig.6 Changes of glutamatergic pathway in mice hippocampus.(A) Network analysis of different metabolites and pathways.(B-C) Concentrations of DL-glutamic acid and DL-glutamine in mice hippocampi in different groups.Protein expression levels of (D-F) GLS and GS in mice hippocampi.The relative levels of GLS/GAPDH and GS/GAPDH of all groups are expressed as % of the control group.The values are expressed as the mean ± SEM (n=6).*P < 0.05 vs.the control group;#P < 0.05 vs.the TMT group;NS represents no significant difference (P > 0.05).
To evaluate the role of the gut microbiome in regulating the function of hippocampal astrocytes,we analyzed the gut microbiota in the cecal contents of sacrificed mice.First,we used principal coordinate analysis discriminate analysis (PLS-DA) with plots of weighted unique fraction metric (UniFrac) distances to determine the commonalities between gut microbiota (Fig.8A).Our findings revealed distinct microbiome communities after different treatments.Additionally,a Sankey diagram revealed phylum-and genuslevel alterations in the gut microbiota of the mice after treatments(Fig.8B).Moreover,we identified the specific phenotypes responding to TMT or C3G treatment via LEfSe analysis (Figs.8C and D).The abundance levels of Atopobiaceae in the TMT group were significantly different than those in the control group.The abundance levels of Atopobiaceae in the TMT+C3G group did not differ significantly from those of the control groups,whereas the abundance levels of Lachnospiraceae in TMT+C3G mice were significantly different from those in the other groups.Gut microbiota dysbiosis can lead to an increase in the production of LPS,an endotoxin produced by Gram-negative bacteria that can enter the circulation and induce excessive astrocyte activity following neuroinflammation.To investigate the effect of C3G on LPS levels,we measured serum LPS concentrations.Our results showed a substantial increase in LPS concentration in the serum of mice treated with TMT,and this aberrant LPS level was reduced following treatment with C3G (Fig.8E).Moreover,we observed increased levels of proinflammatory cytokines TNF-α,IL-6,and IL-1β in the serum of TMT-treated mice,which were reduced by C3G treatment possibly through the regulation of gut microbiota and may have contributed to the reversal of astrocyte disorders (Fig.8F).However,it is important to note that the precise role of gut microbes and their metabolites in protecting against TMT-induced glutamatergic hypofunction requires further investigation,and the link between gut microbiota composition and physiological outcomes is complex and not fully understood.

Fig.8 Effects of C3G on the gut microbiota in TMT-treated mice.(A) PLS-DA analysis on the basis OTUs.(B) Sankey plot showing gut microbiota microorganisms at the phylum and genus levels.(C,D) Linear discriminant analysis (LDA) of gut microbiota with selected OTUs with lg (LDA scores) over 4.(E) LPS concentration in mouse serum.(F) Inflammatory cytokine concentrations in mouse serum.The values are expressed as the mean ± SEM (n=6).*P < 0.05,**P < 0.01 vs.the control group;#P < 0.05,##P < 0.01 vs.the TMT group;NS represents no significant difference (P > 0.05).
4.Discussion
TMT impairs the hippocampal region of the brain,resulting in significant neurodegeneration and neuronal cell loss[2].Multiple studies have proven that the systemic administration of TMT in humans and rodents induced clinical symptoms associated with Alzheimer’s disease[3-5].Because of its widespread application in agricultural and industrial contexts,TMT is found in high concentrations in the leachate of landfills,as well as in freshwater and marine ecosystems.Therefore,identifying substances that can be included in the daily human diet to prevent the neurodegeneration induced by TMT is an important research direction.
C3G is the predominant anthocyanin found in most plants and is famous for its various bioactivities.This study investigated the protective effects of C3G against TMT-induced neurotoxicity based on untargeted metabolomics,bioinformatic analysis,and gut microbial analysis to show that: (a) exposure to TMT results in subsequent hippocampal neurodegeneration,dendritic spine loss,and cognitive impairment;(b) C3G attenuated the severity of epilepsy-like seizures and the cognitive impairment induced by TMT by reducing the neuronal degeneration and synaptic impairment;(c) Oral gavage of C3G attenuated TMT-induced disruption of glutamate homeostasis in the hippocampus of mice by protecting GS expression in astrocytes;(d) C3G modulated gut microbiota in mice might contribute to reduce systemic inflammation,which attenuates astrocyte dysfunction.
In this study,we confirmed that exposure to TMT induced clinical epilepsy-like seizures and cognitive dysfunction,which has also been reported in previous studies[38-39].In addition,we observed that the oral intake of C3G attenuated later learning and memory function deficits caused by TMT.Furthermore,our histopathological observations revealed marked neurodegeneration in the DG and CA1 of the hippocampus after exposure to TMT,and this neurodegeneration was ameliorated by C3G treatment.Here,we found that exposure to TMT induced later hippocampal dendritic spine loss,while oral intake of C3G significantly reversed TMT-induced spine loss.In addition,the ability of C3G to increase PSD width and length,as well as increase expression of postsynaptic proteins and neurotrophic factors,may account for its beneficial cognitive effects.All of these results proved that the neuroprotective effect of CGA against TMT-induced cognitive impairment can be attributed to the reversal neuronal degeneration and synaptic dysfunction.
Glutamate clearly plays a crucial role in cognitive function,and the glutamatergic pathway in the brain contributes to synapse maintenance and plasticity,hence enabling cognitive function and learning[19].Hence,we performed an untargeted metabolomics analysis with mice hippocampi data to investigate the abnormal metabolism resulting from exposure to TMT and the attenuating effect of C3G.By combining untargeted metabolomics and bioinformatic analysis data,the results showed that early exposure to TMT later disrupted the glutamatergic pathway in the mice hippocampi,as indicated by changes in both glutamic acid and glutamine levels.TMT is reported to cause inhibition in glutamate uptake,increased extracellular concentration of glutamate and increased release of glutamatein vitro[40].Also,TMT is found to increase glutamic acid concentrations in mice hippocampi 24 and 48 h after TMT exposure[41].However,our results demonstrated that hippocampal glutamate system in mice was damaged even after 9 days exposure to TMT.Moreover,we found that the expression of GS protein in the mouse hippocampus was significantly inhibited by TMT and that this inhibition was reversed by C3G,while the expression of GLS was not meaningfully changed.In the brain,glutamate is recycled by astrocytes from a synapse after being converted to glutamine by GS[42].Notably,GS is exclusively expressed in astrocytes in the healthy brain[43].Hence,we suggest that the TMT-disordered glutamate homeostasis in this study was due to astrocyte dysfunction and that C3G protected these astrocytes.
In earlier research,there is few mentions of the significance of the “gut-brain axis” in TMT-induced neurodegeneration.Here,our data indicate that the gut-brain axis may be account for the longlasting effect of acute TMT exposure.To establish a connection between astrocytes and gut inflammation induced by TMT,we extended our previous study to specifically determined the effect of TMT on astrocytes.Our previous studies showed that astrocytes reacted to TMT exposure on the 4thday of treatment,as indicated by the overexpression of GFAP,proinflammatory cytokines,and proinflammatory chemokines[3,34].However,in our previous study,we were not certain whether TMT induced astrocyte dysfunction even on the 9thday of exposure.In this study,we proved that TMT causes increase in the expressions of GFAP,proinflammatory cytokines,and proinflammatory chemokines even after 8 days of exposure.Previous studies have demonstrated that overreactive astroglia and neuroinflammation can be induced by gut microbiota and the subsequent systemic inflammation[44].Hence,we suggested that C3G protects astrocyte function by regulating the gut microbiota and the driving metabolites.Atopobiaceae has been reported to be an inflammation-associated mouse intestinal bacterium that contributes to systemic inflammation[45-46].In this study,we observed that early exposure to TMT increased the relative abundance of Atopobiaceae in mice and the levels of LPS,TNF-α,IL-1β,and IL-6 in serum.Moreover,C3G reduced the relative abundance of Atopobiaceae in mice caused by TMT and led to an increase in the protective gut commensal strains of Lachnospiraceae.Bacteria belonging to the Lachnospiraceae family are abundant,obligate anaerobic microorganisms in the microbiota of healthy humans[47].In this study,the levels of LPS,TNF-α,IL-1β,and IL-6 in mice serum were all reduced by C3G treatment,which was related to the changes in the relative abundance of the gut microflora observed.In the brain,total glutamate and glutamine concentrations remain unchanged as long as the astrocytes that support neurons continue to function properly and the integrity of the associated BBB remains intact.When the integrity of the neuron-astrocyte complex is considerably compromised,the total glutamate levels typically decline.Astrocytes are abundant glial cells in the CNS and are important components of the BBB.They not only regulate BBB function but also sense molecules,such as LPS and inflammatory cytokines,circulating in the peripheral nervous system.In this study,daily administration of C3G was associated with a reduction in systemic levels of LPS and inflammatory cytokines in mice exposed to TMT.Although changes in the relative abundance of gut microbes,including Atopobiaceae and Lachnospiraceae,were observed in association with these effects,the exact mechanisms involved are not fully understood and require further investigation.Nonetheless,these findings suggest that C3G may confer protection against the neurotoxic effects of TMT exposure,and that the gut microbiota may play a role in this process.
5.Conclusion
Early exposure to TMT results in later hippocampal neurodegeneration,dendritic spine loss,and long-term cognitive impairment.Daily intake of C3G improved neurobehavioral impairment caused by TMT exposure in mice by reducing neuronal degeneration and synaptic dysfunction.Furthermore,oral administration of C3G reversed TMT-induced glutamatergic hypofunction and GS inhibition in mice hippocampi,potentially by maintaining astrocyte health.However,while our study highlights the potential for C3G to modulate the gut microbiota and reduce systemic LPS and inflammatory cytokine levels,additional research is necessary to determine the precise role of gut microbes and their metabolites in protecting against TMTinduced glutamatergic hypofunction.It is essential to note that the roles of the gut microbiota cannot be deduced solely from their relative abundance in the gut,as this relationship is complex and requires further study.In addition,the precise mechanism by which C3G confers protection against TMT-induced glutamatergic hypofunction is not fully understood and may involve factors other than gut microbiota.Nonetheless,our study provides valuable insight into the biological activities of dietary C3G in mitigating the harmful effects of pollution on human health.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81903275) and the fund of Cultivation Project of Double First-Class Disciplines of Food Science and Engineering,Beijing Technology &Business University (BTBUYXTD202203).
 食品科學(xué)與人類(lèi)健康(英文)2024年2期
食品科學(xué)與人類(lèi)健康(英文)2024年2期
- 食品科學(xué)與人類(lèi)健康(英文)的其它文章
- GUIDE FOR AUTHORS
- The in vitro digestion fates of diacylglycerol under different intestinal conditions: a potential lipid source for lipid indigestion patients
- Epicatechin attenuates lead (Pb)-induced cognitive impairment in mice:regulation on Nrf2 signaling pathway,and interference on the interaction between Pb with albumin
- Novel umami peptides from two Termitomyces mushrooms and molecular docking to the taste receptor T1R1/T1R3
- Mechanism of action of cordycepin in the treatment of hepatocellular carcinoma via regulation of the Hippo signaling pathway
- A core-satellite self-assembled SERS aptasensor containing a “biological-silent region” Raman tag for the accurate and ultrasensitive detection of histamine
