Efficacy and safety of Revlimid combined with Rituximab in the treatment of follicular lymphoma: A meta-analysis
Wu You-jiao, Liu Lin, BI Xiao-man,2?, ZHENG Shao-jiang?
1.Tumor Institute, the First Affiliated Hospital of Hainan Medical University, Haikou 570102, China
2.College of Biomedical Information and Engineering, Hainan Medical University, Haikou 571199, China
Keywords:
ABSTRACT Objective: To evaluate the clinical efficacy and safety of lenalidomide combined with rituximab for treating follicular lymphoma.Methods: We searched PubMed, Web of Science,Cochrane Library, Embase, China Medical Biological Service system (CBM), VIP database(VIP), Wan fang database (Wan Fang Data), China Knowledge Network (CNKI), and ClinicTrails.gov for literature related to lenalidomide combined with rituximab for treating follicular lymphoma (until June 23, 2022).The literature that met the requirements were screened out according to the established criteria, and the data were analyzed by RevMan5.4 and Stata14.0 to conduct a meta-analysis.Results: Eight studies involving 865 patients with follicular lymphoma were included.The results of the meta-analysis showed that the objective remission rate (RR = 1.43, 95% CI: 1.26–1.61) and complete remission rate (RR = 1.67, 95%CI: 1.27–2.21) of lenalidomide combined with rituximab for treating follicular lymphoma were
1.Introduction
Follicular lymphoma (FL) is the most common inert lymphoma and the second most common non-Hodgkin’s lymphoma (NHL).FL is characterized by diffuse lymph node enlargement, bone marrow involvement, and splenomegaly, and has a significant biological and clinical heterogeneity[1].The progress of FL is slow and the survival time is long.Moreover, few patients in the early stage of disease can be cured by radiotherapy[2].Because the advent of the anti-CD20 monoclonal antibody rituximab, the survival of patients with recurrent or refractory FL has been improved, and the survival time has been extended to 20 years[3, 4].However, some patients have a risk of recurrence and transformation into invasive lymphoma, with poor prognosis[5].Increasing evidence has shown that the tumor microenvironment (TME) participates in the disease progression and drug resistance of B-cell malignant tumors by promoting tumor growth and helping malignant cells to escape immune recognition.Immunomodulatory drugs (such as lenalidomide) not only have direct anti-tumor activity but also target various cells in the TME,including T cells, NK cells, and stromal cells by interfering with tumor signal transduction and activating the anti-tumor immune response[6, 7].Lenalidomide has achieved good clinical results as a single drug for treating tumors, and combined therapy can produce a lasting response to FL.Although most patients are initially treated with lenalidomide combined with rituximab, its efficacy and safety need to be further verified.
Here, we conducted a meta-analysis to evaluate the efficacy and safety of lenalidomide combined with rituximab for treating FL,to explore the relevant research at home and abroad, summarize the relevant conclusions, and provide strong data support for evidencebased medicine to guide clinical decision-making.
2.Materials and methods
2.1 Selection criteria
2.1.1 Inclusion criteria
Research types: randomized controlled trials, non-randomized controlled trials, and single-arm clinical trials published at home and abroad on lenalidomide combined with rituximab for treating FL, limited to Chinese and English, irrespective of the blind method and published status; subjects: patients with FL diagnosed by pathological examination, regardless of sex, race, age, disease stage,and previous treatment; intervention: lenalidomide combined with rituximab used to treat FL in the experimental group, and rituximab alone was used to treat FL in the control group; outcome indicators:the objective and complete remission rates were employed as effectiveness indicators, and adverse reactions were used as safety indicators.
2.1.2 Exclusion criteria
Duplicate studies, reviews, case reports, and studies in which the number of patients in the study group was < 10; animal experiments and basic research; literature in which the research content or outcome index was inconsistent with the inclusion criteria; the research design was not rigorous, or the treatment or intervention measures were not related to the experiment; and the data were incorrect.
2.2 Literature retrieval
Web of Science, Embase, PubMed, The Cochrane Library, China Medical Student Material Service system, China Knowledge Network, VIP database, Wan fang database, and ClinicTrails.gov were searched for relevant literature.The publication time of the literature was from the inception of the database until June 23, 2022.The search words were a combination of subject and free words.The Chinese keywords included “來那度胺,” “利妥昔單抗,” and “濾泡性淋巴瘤,” while the English keywords included “Revlimid,”“IMiD3 Cpd,” “CC5013,” “CC5013,” “CC-5013,” “Revlimid,”“Rituximab,” “CD20 Antibody, Rituximab,” “Rituximab CD20 Antibody,” “Mabthera,” “Lymphoma, Follicular,” and “Follicular Lymphomas,” among others.
2.3 Data extraction and methodological quality evaluation
2.3.1 Data extraction
All of the relevant retrieved literature were imported into the EndNote software, the software management program was used to integrate the literature, and the literature related to this research topic was screened out.Two evaluators independently screened the titles and abstracts of all studies acquired during the literature retrieval and analyzed whether they met the selection criteria.In cases where this information could not be accurately judged, the full text was first obtained to determine whether the study should be included.If this could not be established from the full text review, a consensus was reached through discussion or based on the judgment of a third researcher.
2.3.2 Quality assessment
The Cochrane bias quality risk assessment tool was used to evaluate the quality of RCT literature, including whether it accorded with random sequence distribution, whether it accorded with the concealment of distribution mode, whether it blinded the experimenter or researcher, whether it lacked the outcome index,whether it reported the experimental results, and whether other biases existed.The above statistical data were evaluated at three levels: “high-risk bias,” “l(fā)ow-risk bias,” and “uncertain risk bias.”For literature on non-RCTs and single-arm experimental studies,MINORS evaluation terms were selected for quality evaluation, as shown in our previous research methods[8]: A total of 12 evaluation indicators were used, of which the first eight applied to the study without a control group, each with a score of 0~2, in which 0 indicated “unreported,” 1 indicated “reported but insufficient information,” and 2 indicated “reported and provides sufficient information,” and thus, full marks for the first eight indicators would total 16 points.According to the MINORS evaluation table, only studies with a total score ≥ 13 were included in the meta-analysis.
2.4 Statistical analysis
RevMan5.4 and Stata15 software were used to analyze the data.Q-test was employed to evaluate the heterogeneity of the study, and P values < 0.05 were considered statistically different.The fixed effect model whenI250%, indicating good homogeneity between the studies; in contrast, if the heterogeneity of each study was large,we first analyzed the causes of the heterogeneity, before making reasonable analysis and judgment according to the specific situation.In cases where the cause of heterogeneity was easily obtained,the random effect model was employed to analyze and make a descriptive explanation; in contrast, when the data heterogeneity was too large to make a reasonable explanation, the cause of heterogeneity was investigated through sensitivity or subgroup analysis.The relative risk ratio (RR) and its 95% confidence interval(CI) were used for the analysis of the objective response rate (ORR)and complete response rate (CRR) in RCT, and the risk difference(RD) and its 95% CI were used for the analysis of the objective remission rate, complete remission rate, and all adverse reactions in the non-RCTs.Adverse reactions were defined as any adverse events that occurred during treatment.The funnel chart was used to investigate the presence of publication bias in the results.
3.Results
3.1 Literature screening process and results
Through a preliminary search of subject words, a total of 413 articles were retrieved.Based on the screening criteria (Fig.1), eight articles[9-16] were included in this study, including seven English articles[9-15] and one Chinese article[16].
3.2 Basic characteristics of the included literature and bias risk assessment
Among the eight included studies, the research data were more complete.After summarizing the basic characteristics of the studies,we found no significant difference in age, tumor stage, course of treatment, and treatment strategy between the experimental and control groups.Five articles[9, 10, 12, 14, 16] were single-arm studies,and three[11, 13, 15] were RCTs (the control group participated in the statistical study of single-arm trials).The trial groups of eight studies were treated with lenalidomide combined with rituximab, and the control groups of three studies were treated with rituximab with or without placebo.The basic characteristics of the control group included in the study are detailed in Table 1.According to the items of the Cochrane bias quality risk assessment tool (see Fig.2 for details).
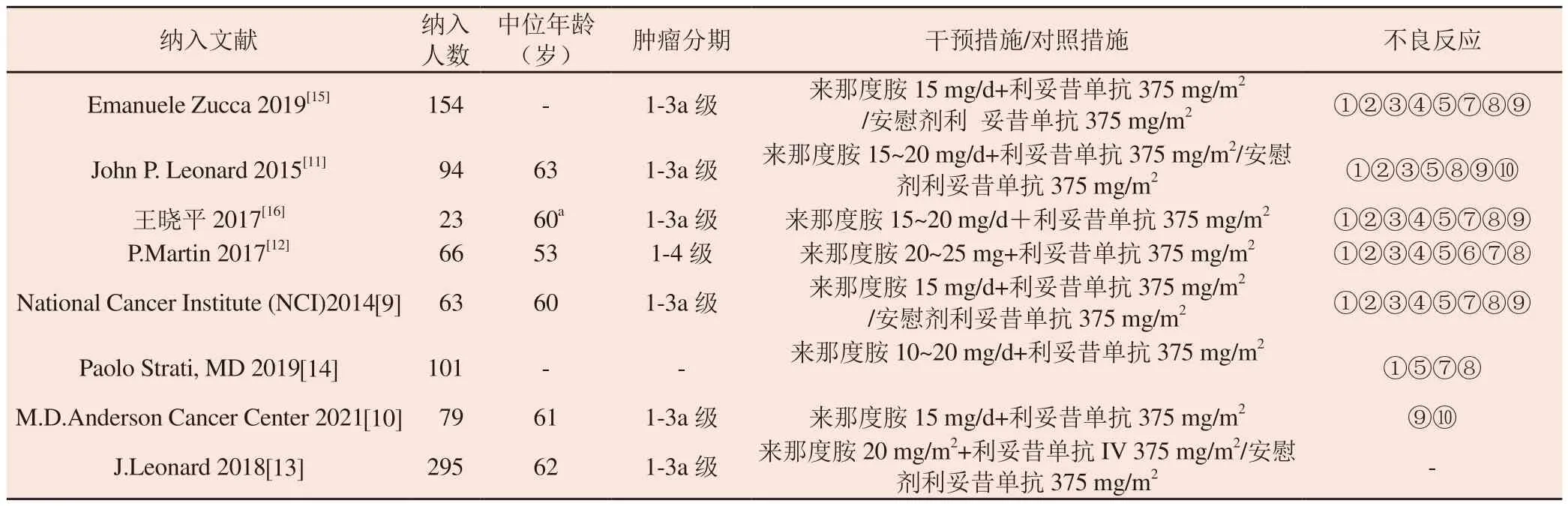
Tab 1 Characteristics of the included studies
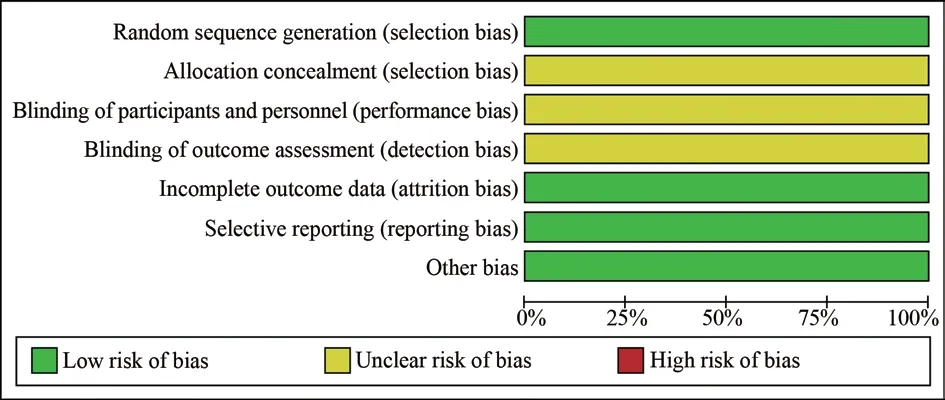
Fig 2 Summary of risk of bias
3.3 Meta-analysis results
3.3.1 Objective remission rate
3.3.1.1 Meta-analysis with a single-arm test method
Seven articles[10-16] met the criteria of the meta-analysis, involving 865 patients, including 296 patients with ORR.The fixed effect model was used for the meta-analysis of the single-arm dichotomous data.The results showed that I2= 0 < 50%, P = 0.76 > 0.05.The analysis showed that the combined effect test of RD =[0.87, 95% CI= [0.79, 0.96],Z= 20.17 was statistically significant (P< 0.00001).The results showed that lenalidomide combined with rituximab was effective in treating FL, with an objective remission rate of 87%.
3.3.1.2 Meta-analysis according to the experimental method of the control group
A total of 543 patients were included in the three articles[11, 13, 15]that met the requirements of analysis, including the treatment group(n= 270) and control group (n= 273).Analysis of the fixed effect model showed that I2= 0 < 50%, P = 0.79 > 0.05, RR = 1.43, and 95% CI: [1.26, 1.61], while the combined effect test showed Z = 5.76P< 0.00001, which was statistically significant (see Fig.4).These results suggest that the objective remission effect of lenalidomide combined with rituximab for treating FL is better than that of the control group.
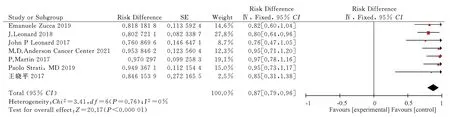
Fig 3 Meta-analysis on the ORR of Revlimid plus Rituximab for treating FL
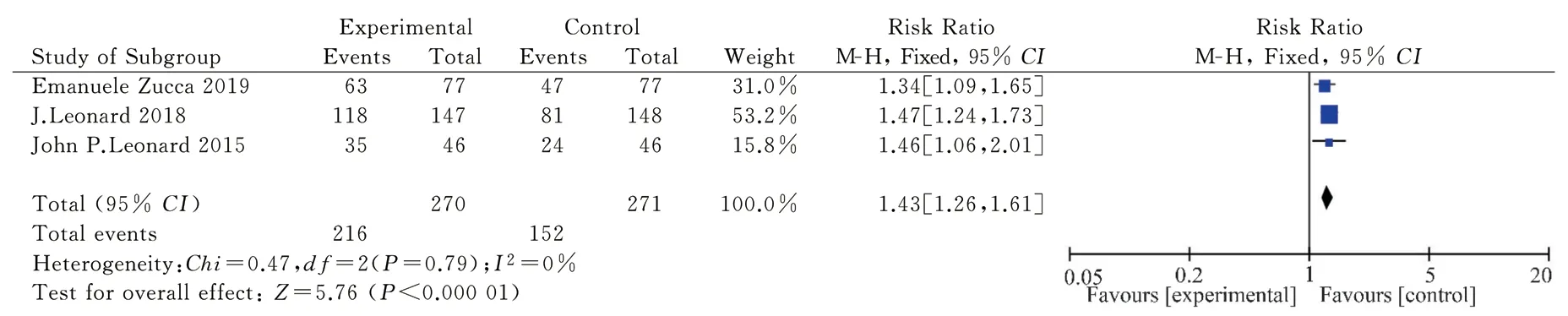
Fig 4 Meta-analysis on the ORR of Revlimid plus Rituximab for treating FL in RCT
3.3.2 Complete remission rate
3.3.2.1 Meta-analysis with a single-arm test method
Seven articles[10-16] met the criteria for analysis, involving 865 patients, including 296 patients with CR.The random effect model was used to conduct a meta-analysis of the single-arm dichotomous data, withI2= 81% > 50%.The large heterogeneity was found to be caused by the inclusion of more single-arm studies.The analysis obtained RD = 0.58, 95% CI = [0.50,0.67], while the combined effect test showedZ= 13.48,P< 0.00001; the result was statistically significant (Fig.5).Lenalidomide combined with rituximab may be effective for treating FL, with a complete remission rate of 58%.However, more high-quality randomized trials need to be included.
3.3.2.2 Meta-analysis according to the experimental method of the control group
The information on 543 patients was included in three articles[11,13, 15], including 270 cases in the treatment group and 273 cases in the control group.The fixed effect model analysis showed that I2=0 < 50%,P= 0.79 > 0.05.TheRR= 1.67 and 95%CI= [1.27,2.21]were obtained by the analysis, and the combined effect test showed Z = 3.62, which was statistically significant (Fig.6).The objective remission effect of lenalidomide combined with rituximab for treating FL was better than that of rituximab alone and better than that of the control group.
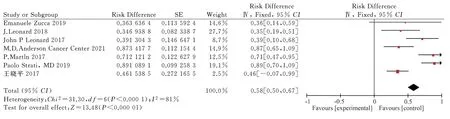
Fig 5 Meta-analysis on the CR of Revlimid plus Rituximab for treating FL
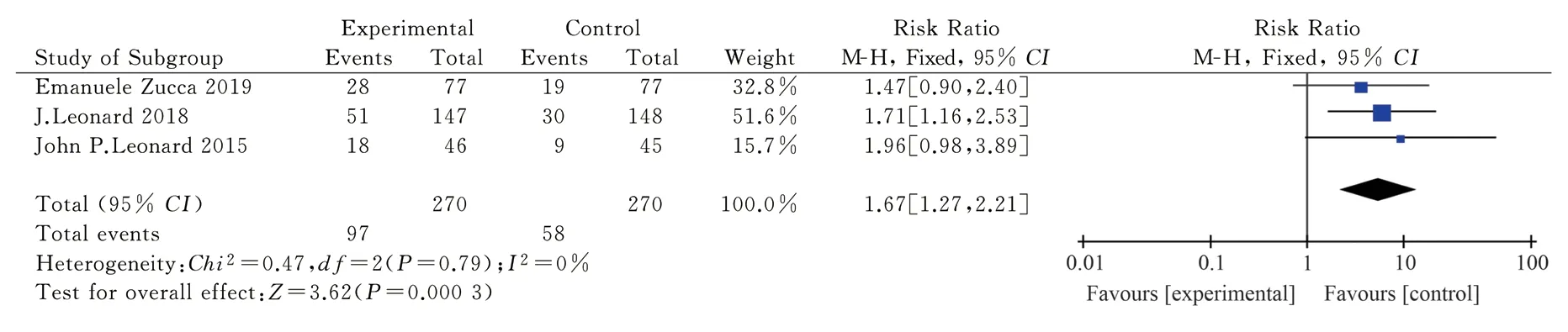
Fig 6 Meta-analysis on the CR of Revlimid plus Rituximab for treating FL in RCT
3.3.3 Main adverse reactions
Eight articles[9-16] selected in this study reported the main adverse reactions from ten aspects, including neutropenia, lymphopenia,thrombocytopenia, anemia, fatigue, diarrhea, nausea and vomiting,rash, infection, and thrombosis.The results of the subgroup analysis of different evaluation criteria are shown in Fig.7.
Statistical analysis of the data in the reported neutropenia literature[9, 11, 12, 14, 16] showed thatP= 0.06 < 0.1,I2= 54%> 50%, with moderate heterogeneity.Using the random effect model, the effect doseRD= 0.33, 95%CI= [0.18, 0.49], while the Q test showedP< 0.0001, which was statistically significant.Approximately 33% of patients have adverse reactions to neutropenia during the treatment of FL with lenalidomide combined with rituximab.
Based on the statistical analysis of the data in the literature on lymphopenia[11, 12, 15, 16], the results showed thatP< 0.01,I2=78% > 50%, with large heterogeneity.Analysis using the random effect model showed RD = 0.21, 95% CI: = [0.06 and 0.48], and no statistical significance in the Q test (P= 0.13 > 0.05).
Statistical analysis reported the data of thrombocytopenia[9, 11, 12,15, 16] in the literature.The results showed that P > 0.01, I2= 47% <50%, with low heterogeneity, while the fixed effect model showed the effect doseRD= 0.15, 95%CI= [0.02, 0.32], passed the Q test(P = 0.09 > 0.05), and had no statistical significance.
Based on the statistical analysis of the data in the literature reporting anemia[9, 12, 15], the results showed thatP< 0.01,I2= 59 >50%, which was evaluated as moderate heterogeneity; the random effect model showed that the effect wasRD= 0.17, 95%CI: = [0.05,0.38], with no statistical significance according to the Q test (P>0.05).
According to the statistical analysis of the data of the literature on fatigue, the results showed thatP< 0.0001,I2= 82% > 50%,indicating high heterogeneity.Therefore, a random effect model was adopted, the results of which demonstrated RD = 0.37, 95%CI= [0.12, 0.62], andP= 0.004 < 0.05.Moreover, approximately 37% of patients developed symptoms of fatigue during treatment with lenalidomide combined with rituximab for FL.However, the heterogeneity was high, possibly due to the number of articles included in more single-arm studies; therefore, it is necessary to include more high-quality randomized experimental studies in the future.
Statistical analysis of the data related to diarrhea[9, 12, 15] showed that P > 0.01, I2= 0% < 50%, with low heterogeneity.The results of the fixed effect model demonstrated that the effect amountRD= 0.29, 95%CI: = [0.16, 0.43], andP< 0.0001.Moreover,approximately 29% of patients developed diarrhea during treatment with lenalidomide combined with rituximab for FL.
Statistical analysis of the data related to nausea or vomiting in the literature[9, 12, 15] showed that P > 0.01, I2= 0% < 50%, and low heterogeneity.The results of the fixed effect model demonstrated that the effect amountRD= 0.17, 95%CI= [0.04, 0.31], andP<0.05.Lenalidomide combined with rituximab for FL was associated with a 17% chance of causing nausea or vomiting.
Statistical analysis of the data of the literature reporting rash events[9, 11, 12, 14-16] showed that P = 0.35 > 0.01, I2= 10% < 50%,with low heterogeneity.The results of the fixed effect model showed thatRD= 0.20, 95%CI= [0.09, 0.31],P< 0.05.Furthermore,approximately 20% of the patients developed a rash during treatment with FL and lenalidomide combined with rituximab.
Based on the data of selected literature related to infection[11-13,15,16], the fixed effect model showed that P = 0.37 > 0.01, I2= 6%< 50%, low heterogeneity,RD= 0.11, 95%CI= [0.01, 0.23],P=0.08 > 0.05, and no statistical significance.The data related to thrombus from selected literature[13,15,16] were analyzed by the fixed effect model, which demonstratedP= 0.89 >0.01,I2= 0% < 50%, low heterogeneity,RD= 0.04, 95%CI= [0.13,0.20],P= 0.67 > 0.05, and no statistical significance.
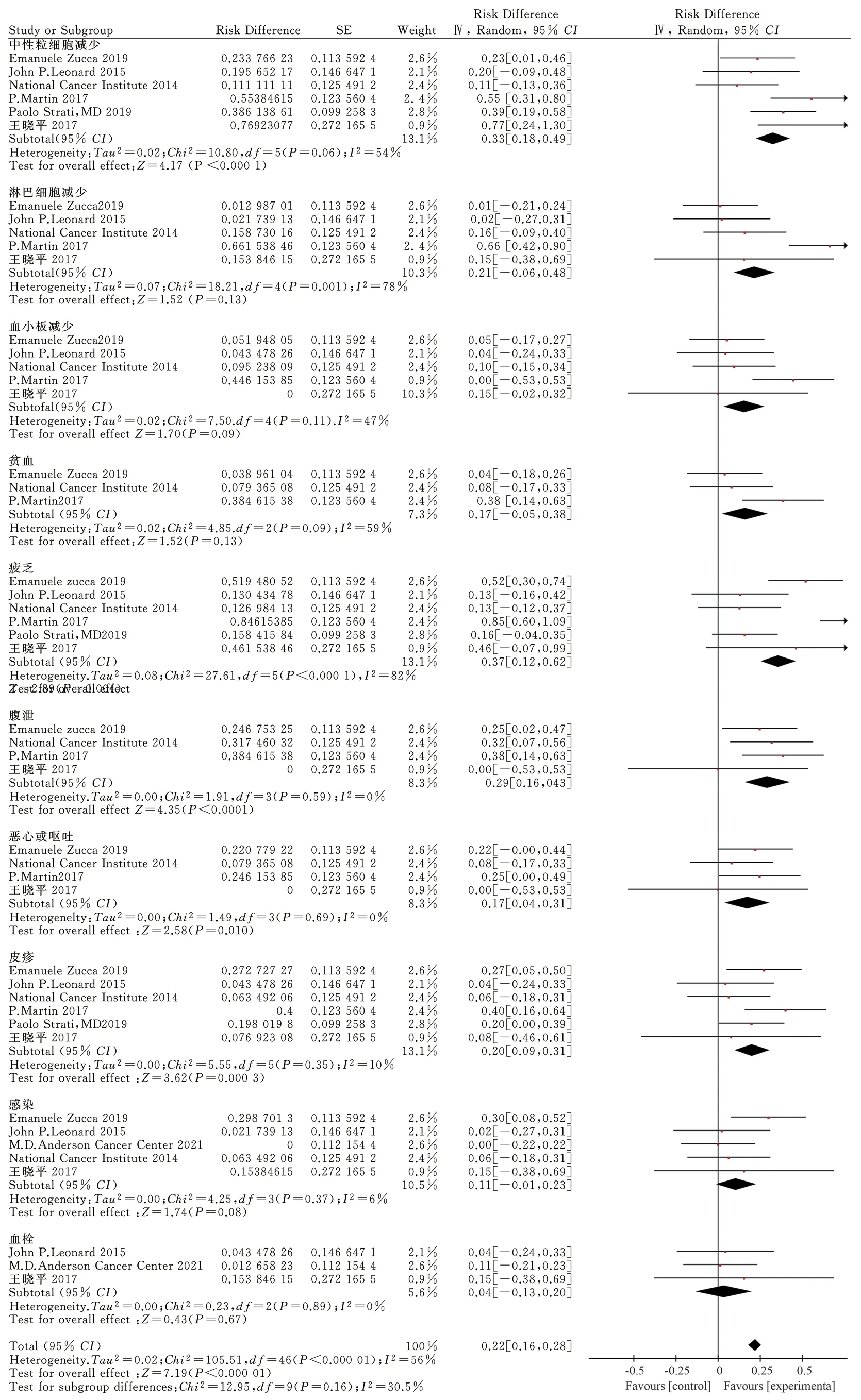
Fig 7 Meta-analysis on adverse events of Revlimid plus rituximab for treating FL
3.4 Bias analysis
The objective remission rate (Fig.8A) and complete remission rate (Fig.8B) were analyzed by Stata14.0, and the figures were symmetrical.We conducted a symmetry test of the above two pictures (Figs.8C–D), P = 0.051 > 0.05, P = 0.946 > 0.05, and the results showed that the funnel diagram was basically symmetrical,suggesting no obvious publication bias in the literature of this study.
4.Discussion
Lenalidomide, as a targeted immunomodulatory drug combined with rituximab, has become a new treatment option for recurrent FL, including patients who show inadequate responses to rituximab alone, and those who wish to avoid cytotoxic chemotherapy[17].In the phase III AUGMENT trial, lenalidomide + rituximab significantly prolonged PFS in patients with recurrent or refractory inert NHL compared to placebo + rituximab, and the PFS benefit was prominent in patients with FL.Lenalidomide + rituximab also showed significant activity in the phase III MAGNIFY trials in patients with recurrent or refractory inert NHL, including those with disease that was refractory to rituximab [18,19].Although grade 3 or 4 neutropenia occurred more frequently in the lenalidomide +rituximab group than in the placebo + rituximab group, it could be controlled by adjusting the dose.However, there remains a lack of high-level clinical evidence for its therapeutic efficacy and safety.
Accordingly, the efficacy and safety of lenalidomide combined with rituximab for treating FL were evaluated by a meta-analysis.Combined with single-arm and control group experiments, compared to rituximab alone for treating FL, the objective remission rate and complete remission rate of lenalidomide combined with rituximab for treating FL were significantly improved.During treatment, the risk of adverse reactions, such as neutropenia, diarrhea, nausea and vomiting, and rash, was relatively high, but the overall proportion is at a low level, so it is necessary to make corresponding treatment for the related adverse reactions.In view of these adverse reactions,the existing medical level can be improved or inhibited to varying degrees by drugs or other means.Additionally, due to the high heterogeneity of adverse reaction indices, such as lymphocytopenia and fatigue, more controlled trials need to be included for further exploration.
The purpose of this study was to demonstrate the clinical effect of lenalidomide in treating FL.Previous studies have shown heterogeneity in the treatment regimen and duration of patients with different subtypes of B-cell lymphoma.Here, clinical studies on FL were screened strictly according to the inclusion criteria to avoid the risk of greater heterogeneity after analysis with other types of NHLs,including diffuse large B-cell lymphoma, mantle cell lymphoma,and primary central nervous system lymphoma.Additionally, the drugs involved in this experiment were relatively consistent, which confirms the actual clinical efficacy after the control variables.
This study had some limitations.First, some of the included studies were single-arm, which may lead to a certain degree of deviation in the results.Second, the small sample size may lead to a certain risk of bias, which requires the inclusion of, large-sample, multicenter clinical randomized controlled trials in the future.Third,although the intervention measures in the literature study were set as lenalidomide combined with rituximab for the treatment of FL, differences in the dosage and cycle among the studies may have a certain impact on the results of the meta-analysis.Fourth,although the relevant data were collected comprehensively, due to the different outcome indicators in different studies, some outcome indicators could not be integrated and analyzed.Fifth, as only Chinese and English literature were included, the literature was not comprehensive and the inclusion of ethnic groups was limited.Sixth, a few of them have been included in the literature for a long time, but the disease is in the process of continuous development and variation, so there may be some limitations in the significance of clinical guidance.Finally, publication bias inevitably occurred in the literature, because studies with statistically significant results were relatively easy to publish, while those with negative results were not.Therefore, more well-designed prospective large-sample, multicenter randomized controlled trials are needed to re-analyze and evaluate the effectiveness and safety of this study.
Currently, lenalidomide combined with rituximab or rituximab alone is the standard first-line or follow-up treatment option for advanced FL, while other treatment options (e.g., PI3K inhibitors,tazemestostat, and CAR-T cell therapy) continue to evolve[2].Studies have shown that several small molecular inhibitors involved in the proliferation of PI3 kinase and BTK have anti-FL activity[20,21].Additionally, the EZH2 inhibitor tazemestostat was approved by the FDA for FL after previous second-line treatment[22].Notably,chimeric antigen receptor T cells (CART) or bispecific antibody constructors have a unique therapeutic mechanism in FL[23, 24].In view of these potential non-cross-reaction mechanisms, the design and optimization of combination strategies are expected to improve the outcome of FL, and may even achieve a cure.Therefore, at present, there is still an urgent need for personalized methods, trial endpoints for quality of life measurement, guidance on available schemes, and information on drug sequencing[25].The research and development of new drugs and the continuous discovery of the pathogenic mechanism of FL are expected to improve the prognosis and quality of life of patients with FL.
In this study, compared to the CD20 antibody rituximab alone,lenalidomide combined with rituximab showed greater efficacy in alleviating FL.Although the combination may bring some side effects, such as neutropenia, nausea, vomiting, and rash, most can be relieved by providing symptomatic treatment or adjusting the drug dose to reduce the occurrence of symptoms.
 Journal of Hainan Medical College2023年15期
Journal of Hainan Medical College2023年15期
- Journal of Hainan Medical College的其它文章
- Progress in diagnosis and treatment of invasive aspergillosis after liver transplantation
- An overview of the mechanism of acupuncture and moxibustion in treating depression comorbidities insomnia
- The significance of SREBP1 expression in HBV-related hepatocellular carcinoma
- Study on the mechanism of Fuzi in the treatment of allergic rhinitis based on network pharmacology and experimental validation
- Effect of acupuncture on acupoint "Yingxiang-Hegu" on Th1, Th2 cytokines and T-bet/GATA-3 of allergic rhinitis rats
- Study on regulating mechanisms of oxocrebanine obtained from Stephania hainanensis H.S.Lo et Y.Tsoong on microtubule sites and tubulin in human breast cancer MCF-7 cells
