Efficacy and safety of Lianhuaqingwen capsules in high-risk common type COVID-19 pneumonia: A multicenter retrospective study
Bin Liu ,Liang Li ,Lei Liu ,Min Ye ,Wei Zhang ,Xiangdong Zhou ,Qi Li
1Department of Respiratory Medicine, the First Affiliated Hospital of Hainan Medical University, Haikou 570100, China
2Hainan Province Clinical Medical Center of Respiratory Disease, Haikou 579199, China
3International School of Nursing, Hainan Medical University, Haikou 571199, China
4Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Harbin Medical University, Harbin 150081, China
5Kang'an Hospital of Mudanjiang, Mudanjiang 157000, China
ABSTRACT Objective:To evaluate the clinical safety and efficacy of Lianhuaqingwen (LHQW) capsules in patients with high-risk common type COVID-19 pneumonia.Methods:A retrospective multicenter study on 383 high-risk common type COVID-19 pneumonia cases was conducted.Patients were categorized as the standard treatment (SDT) group (n=168)and the LHQW+SDT group (n=215).The primary endpoint was the rate of symptom (fever,fatigue,coughing) recovery and the secondary endpoints included the time to symptom recovery,the proportion of patients with improvement in chest CT images,the proportion of patients with clinical cure,the timing and rate of negative conversion of SARS-CoV-2 RNA assay.Results:The recovery rate was significantly higher in the LHQW+SDT group as compared with the SDT group (89.7% vs.72.0%,P<0.01).The combined use of LHQW+SDT also showed shorter time for symptom recovery,as well as shorter time for individual symptom of fever,fatigue and coughing recovery than use of SDT alone.A higher proportion of patients in the LHQW+SDT group with improvements in chest CT images and clinical cure (77.7% vs.57.1%,P<0.01) but the proportion of patients deteriorating to severe cases (1% vs.25%,P<0.01) in this group was significant lower than those in the SDT group.No significant difference in negative conversion rate of viral assay results was observed (76.8% vs.75.0%,P>0.05).No serious adverse events were reported.Conclusions:LHQW capsules could be recommended to ameliorate clinical symptoms and reduce the rate of deteriorating to severe cases for high-risk common type COVID-19 pneumonia.
KEYWORDS: Lianhuaqingwen capsules;COVID-19 pneumonia;High-risk common type;Clinical efficacy
1.Introduction
COVID-19 has threatened global health and affected the global economy due to its rapid spread over a short period[1,2].At present,the virus is still constantly mutating and evolving.Most patients have mild COVID-19 pneumonia;however,a considerable proportion of patients quickly develop severe COVID-19 pneumonia,necessitating hospitalization and time spent in the intensive care unit[3-6].The substantial cost of treatment and higher mortality rates of patients with severe COVID-19 pneumonia are well known.Thus,extraordinary efforts are being made to prevent common type COVID-19 pneumonia from progressing to severe COVID-19 pneumonia.But it is not practical to develop a novel class of medications within the short time span of the COVID-19 pandemic.No targeted antiviral drugs have been developed to date,although several drugs are in clinical trials[2,7].
Ttraditional Chinese medicine (TCM) has played an important role in combatting COVID-19 with its effects on improving symptoms of the respiratory system,and it has recently been repurposed for the clinical management of the disease[8].Lianhuaqingwen(LHQW) capsules (Shijiazhuang Yiling Pharmaceutical Co.Ltd.,Shijiazhuang,China),is a typical TCM formulation prepared from the combination of several herbs includingForsythia suspensafruits(Lianqiao),Lonicera japonicaflower buds (Jinyinhua),Ephedra sinicastems stir-fried with honey (Zhi-Mahuang),stir-friedPrunus sibiricaseeds (Chao-Kuxinren),Gypsum Fibrosum(Shigao),Isatis indigoticaroots (Banlangen),Dryopteris crassirhizomarhizome(Mianmaguanzhong),Houttuynia cordatawhole plants (Yuxingcao),Pogostemon cablinoverground portion (Guanghuoxiang),Rheum palmatumrhizomes and roots (Dahuang),Rhodiola crenulatarhizomes and roots (Hongjingtian),l-menthol (Bohenao),andGlycyrrhiza uralensisroots (Gancao).LHQW capsules combined with antiviral medicine are an effective and safe treatment option for patients with COVID-19[8-10].Moreover,LHQW capsules can significantly ameliorate the primary symptoms (e.g.,fever,cough,and fatigue) and shorten the disease course of COVID-19[11].According to theDiagnosis and Treatment Protocol for Coronavirus Pneumonia(Trial version 8,China 2020),LHQW capsules have been approved by the National Health Commission for the treatment of COVID-19.In 2020,Liet alreported LHQW capsules significantly inhibited SARS-CoV-2 replication,altered viral morphology and exhibited anti-inflammatory activityin vitro[12].
LHQW capsules have important clinical application value for treating COVID-19.But potential clinical value of LHQW capsules requires further validation.Therefore,we conducted this large-scale,multicenter,and retrospective study to evaluate the clinical safety and efficacy of LHQW combination therapy for treating COVID-19.We hypothesized that LHQW capsules could effectively ameliorate symptoms (including fever,cough and fatigue),shorten the duration of viral shedding and shorten hospital stays in patients with highrisk common type COVID-19 pneumonia.This study reported the clinical characteristics and early outcomes of patients with high-risk common type COVID-19 pneumonia who received LHQW capsule combination therapy.
2.Subjects and methods
2.1.Patient selection
The analysis of clinical data from SARS-CoV-2 infected inpatients was approved by the institutional ethics board of the Qun’li branch of the First Affiliated Hospital of Harbin Medical University (Approval No.2022-038),Kang’an Hospital of Mudanjiang (Approval No.2022-019),and the First Affiliated Hospital of Hainan Medical University (Approval No.2022-025).All the experimental protocols were conducted according to the Declaration of Helsinki[13],and all the participants enrolled in the present study provided informed consent to grant retrospective access to their patient records and files.This study conformed to the Enhancing the Quality and Transparency of Health Research guidelines[14].
Data of patients with high-risk common type COVID-19 pneumonia were collected from the medical records for analysis between January 2022 to December 2022 (Figure 1).Eligibility criteria were listed as following: (1) COVID-19 patients that were confirmed according tothe Protocol for Diagnosis and Treatment of Novel Coronarvirus Pneumonia(8th edition,2020) which was issued by the National Health Commission.(2) Patients with high-risk common type COVID-19 pneumonia that were diagnosed according to literature[15].(3) Patients aged 18 years or above.
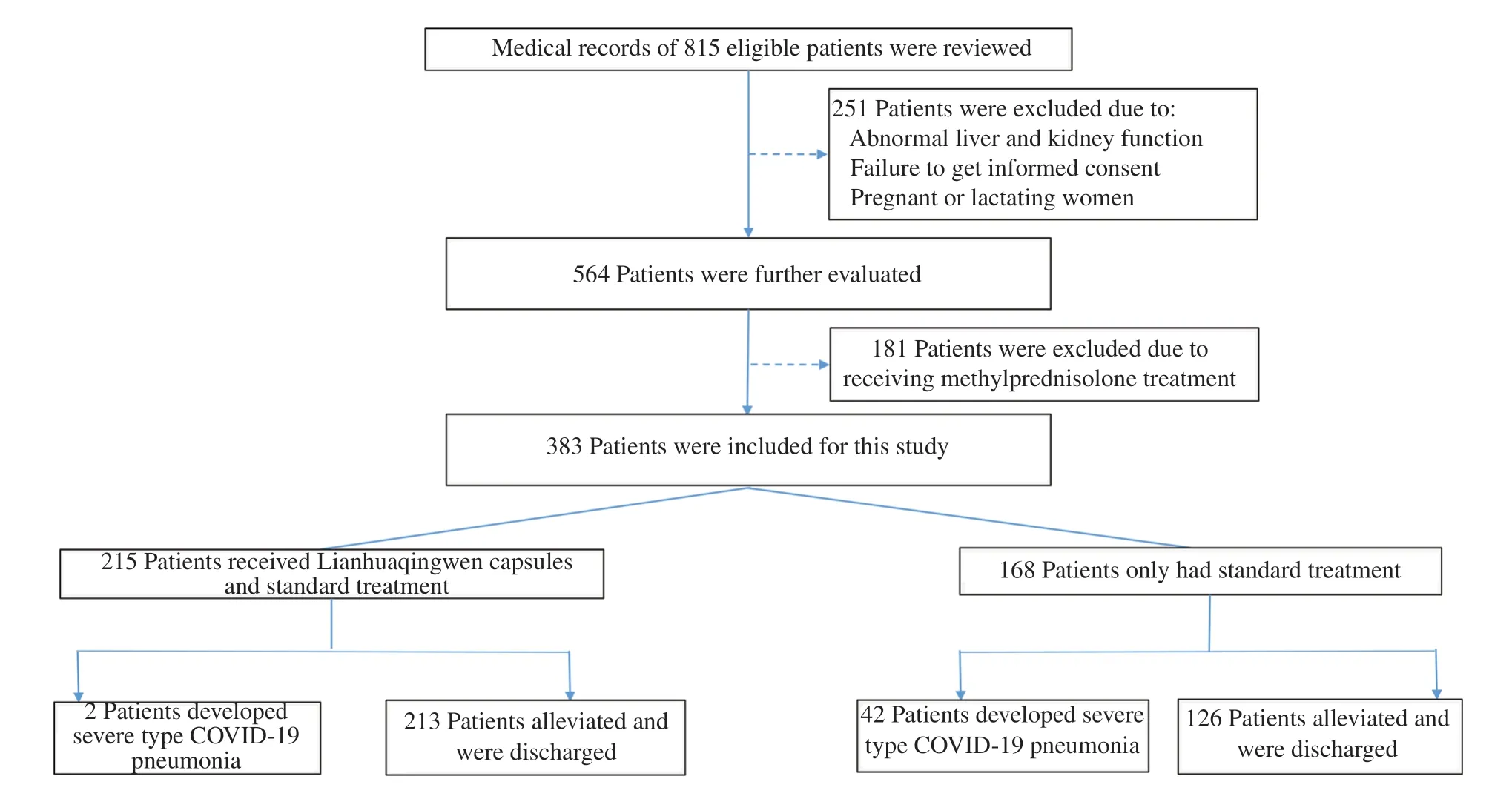
Figure 1.Study flow chart.
Key exclusion criteria included: (1) patients with severe pneumonia that needed mechanical ventilation;(2) patients with severe systemic diseases (i.e.,malignancy,autoimmune diseases,liver or renal diseases) or those underwent surgeries (splenectomy,organ transplantation) that could affect the treatment efficacy;(3) patients with respiratory tract bacterial infections due to primary or secondary immunodeficiency,congenital respiratory malformation,congenital heart disease,gastroesophageal reflux,or lung malformation;(4)patients with asthma or other chronic respiratory diseases that need maintenance therapy,acute respiratory tract bacterial infection (i.e.,bronchiectasis,tonsillitis,bronchitis,rhinosinusitis,otitis media),or severe pulmonary interstitial diseases;(5) women during pregnancy or lactation;(6) those participated in other clinical trials within the past 3 months;(7) allergy to gradients of the LHQW capsule;(8)other conditions not appropriate for participate in this study.
2.2.LHQW capsules
LHQW capsules were manufactured based on The Pharmacopedia of People’s Republic of China (Shijiazhuang YiLing Pharmaceutical Co.,Ltd,China).LHQW capsules were provided by the manufacturer,supplied to the participating sites,and dispensed by the designated research nurses.The standard treatment (SDT) in this study refers to therapy determined based onthe Diagnosis and Treatment Protocol for Coronavirus Pneumonia(Trial version 8,2020),at the discretion of the attending clinicians (General Office of The National Health and Health Commission,2021).The SDT treatment generally consisted of the supportive treatment such as oxygen therapy,antiviral medications and symptomatic therapies[15].
2.3.Procedures
Eligible patients were given standard treatment alone based onthe Protocol for Diagnosis and Treatment of Novel Coronarvirus Pneumonia(8th edition,2020) or the combination of SDT and LHQW capsules (4 capsules thrice daily for 14 consecutive days).Adherence to the study medications,clinical outcomes,the use of concomitant medications and adverse events were recorded.Vital signs,laboratory testing results,chest computed tomography and nucleic acid assays of SARS-CoV-2 were evaluated at baseline and on day 14 after admission.Two physicians checked all the data,and two clinical pharmacists adjudicated any differences in interpretation.In this study,fever was defined as subaxillary temperature of 37.3 ℃ or above.Symptom recovery denoted a complete remission of at least one major symptom (coughing,fever,or fatigue).An experienced radiologist who was blinded to the study allocation reviewed the chest computed tomography (CT) images of all patients.We defined the clinical cure as having met all of the following criteria: recovery of normal body temperature for more than 3 days,symptom recovery,marked improvement in chest CT images,and two consecutive negative SARS-CoV-2 RNA testing (at least one day apart).Improvement in chest CT images was defined as a decreased area of infiltration,a decreased area of any radiologic abnormality,or decreased density of the ground-glass opacity or nodules.
2.4.Study endpoints
The primary endpoint was the rate of symptom (fever,fatigue,and coughing) recovery and the rate of progression to severe COVID-19 pneumonia.The severity of fatigue and coughing were self-reported by the patients.Recovery of symptoms was defined as the complete resolution of fever,fatigue and coughing.Secondary endpoints consisted of the time to symptom recovery,the rate of and the time to the recovery of individual symptoms,the proportion of patients showed improvement in chest CT images,the proportion of patients with clinical cure,the timing and rate of negative conversion of SARS-CoV-2 RNA assay.
2.5.Statistical analysis
All the statistical analyses were performed using SPSS Statistics version 23.0 software.The statistical significance level was set at a two-sidedPvalue of <0.05.Count (percentage) was adopted for summarizing the categorical variables,and compared withChisquare tests.Continuous variables were presented with mean± standard deviation (SD) or median (IQR),and compared with independentt-test or Wilcoxon rank-sum test.The hazards ratio (HR)of the events (i.e.symptom recovery) was also calculated.The time to events was presented as the median duration and 95% confidence interval (95%CI),and analyzed with Kaplan-Meier analysis.
3.Results
3.1.Patient characteristics
As shown in Figure 1,medical records of 815 patients with highrisk common type COVID-19 pneumonia were reviewed,and 251 were excluded dued to abnormal liver and kidnedy function,failure to get informed consent,pregrancy or lactating.Then 181 were further excluded due to receiving methylprednisolone treatment.Therefore,383 patients were included in this study,of which 168 only received SDT,215 received LHQW plus SDT and were categorized as SDT group and LHQW+SDT,respectively.
Both the SDT and LHQW+SDT groups had good compliance with the study medication.SDT group showed an average hospital stay duration of 12.0 days (95%CI9.0-15.0),while the LHQW+SDT group showed 8.0 days (95%CI6.0-10.0).At baseline,most patients aged above 40 years and males accounted for 52.0% (199/383).No significant difference in demographic characteristics,vital signs,symptoms and concomitant treatment were found between the two groups and were comparable (Table 1).The age,sex,comorbidities,and clinical and laboratory test results of the patients at admission were shown in Table 1.A total of 40.0% of patients in the LHQW+SDT group and 38.7% of patients in the SDT group received antibacterial medications such as cefaclor (P>0.05,Table 1).
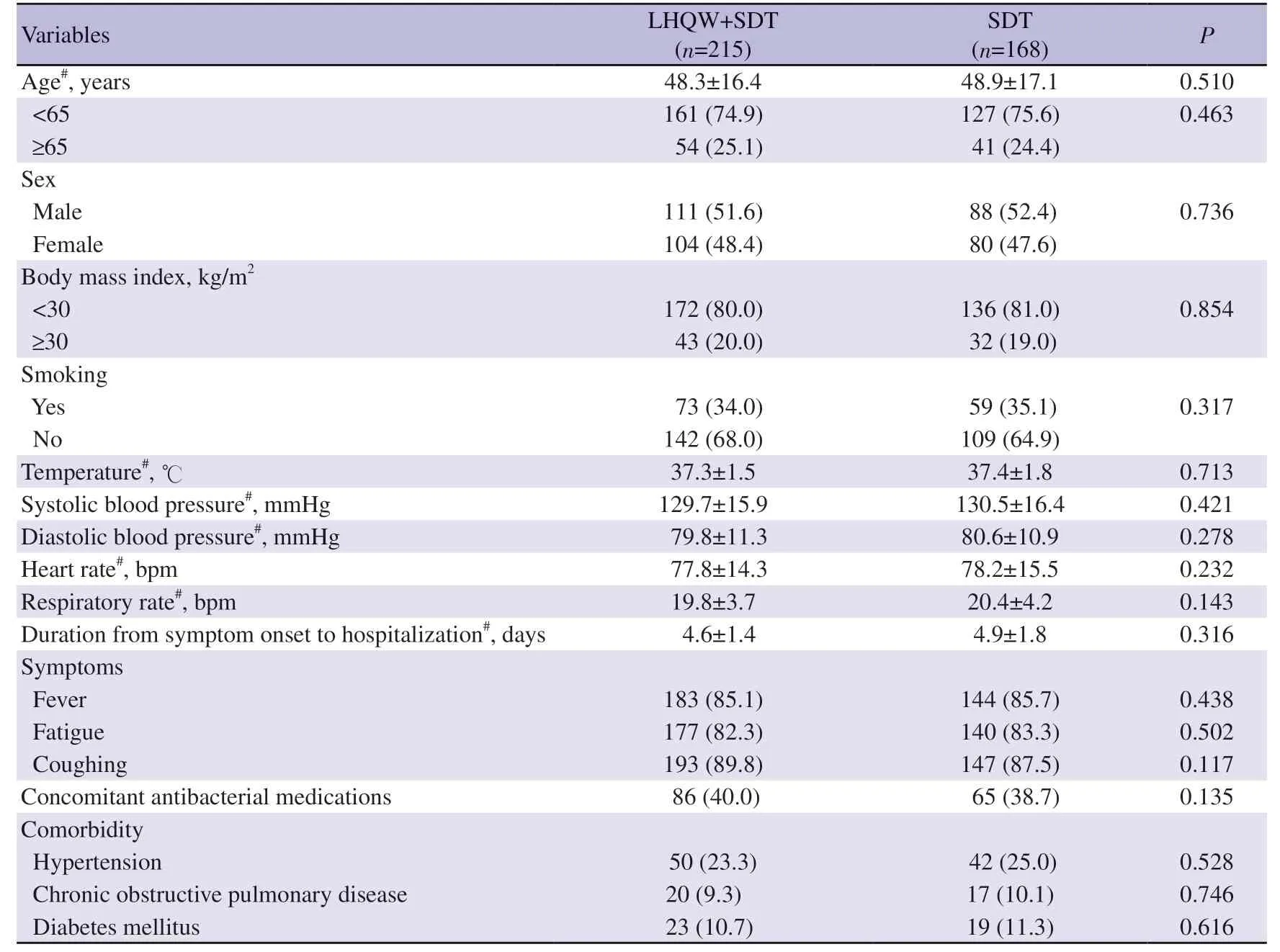
Table 1.Baseline demographic and clinical characteristics of the participants [n (%)].
3.2.Primary endpoints
The rate of symptom recovery on day 14 was significantly higher in LHQW+SDT group as compared with SDT group (89.7%vs.72.0%,mean difference: 17.7,95%CI2.2%-33.2%,P<0.01) (Table 2 and Figure 2).Table 2 shows 2 cases (1.0%,2/168) in the LHQW+SDT and 42 cases (25.0%,42/168) in the SDT group deteriorated to severe COVID-19 pneumonia showing significant difference between the two groups as shown in Table 2.
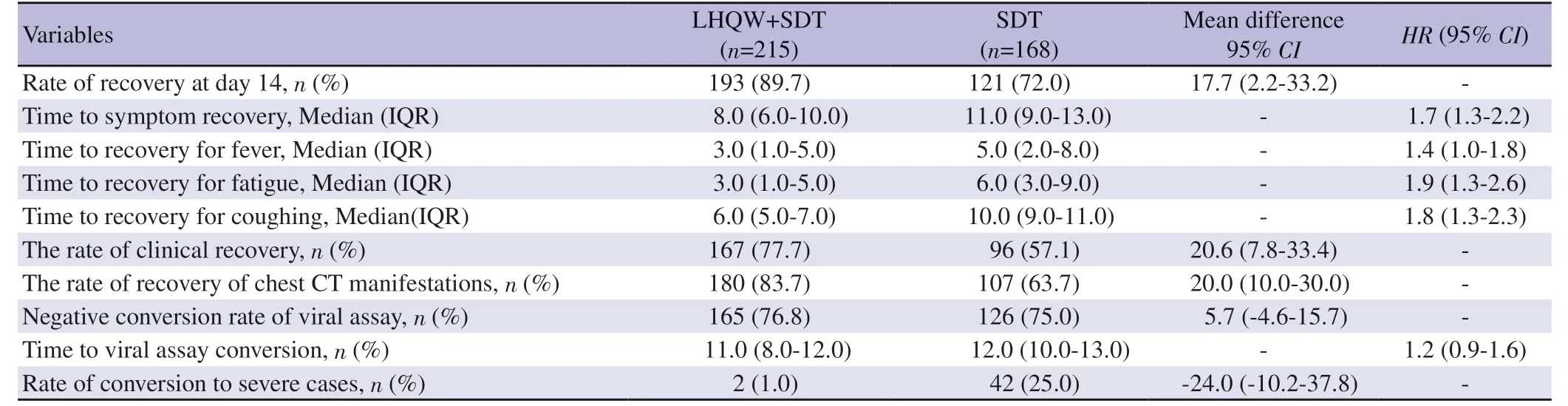
Table 2.The study endpoints.

Figure 2.Dynamic changes in the recovery rate of symptom.
3.3.Secondary endpoints
The median time to symptom recovery for LHQW+SDT group was 8.0 (6.0,10.0) days,significant shorter than the 11 (9.0,13.0)days of the SDT group (HR1.7,95%CI1.3-2.2,P<0.01) (Table 3).Moreover,the LHQW+SDT group yielded a significantly shorter time to the recovery of fever (HR: 1.4,95%CI: 1.0-1.8,P<0.01),fatigue (HR1.9,95%CI1.3-2.6,P<0.01) and coughing (HR1.8,95%CI1.3-2.3,P<0.01).
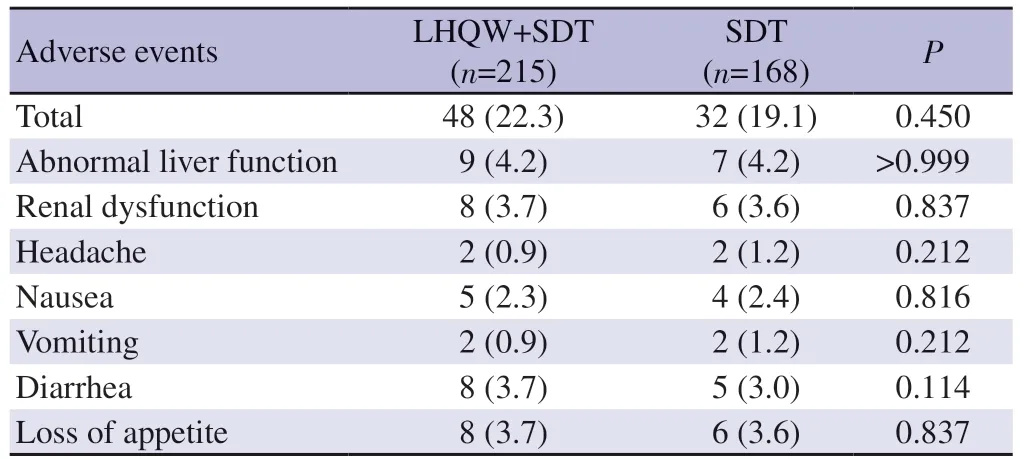
Table 3 .Adverse events observed in the two groups [n (%)].
The overall rate of clinical cure was significantly higher in the LHQW+SDT group as compared with SDT group (77.7%vs.57.1%,P<0.01) (Table 2).The rate of recovery according to chest CT images was also significant higher in LHQW+SDT group as compared with the SDT group (83.7%vs.63.7%P<0.01) (Table 2).However,no significant difference in the negative conversion rate of SARS-CoV-2 viral assay was observed between the two groups(76.8%vs.75.0%,P=0.457) indicating that taking LHQW capsules was not associated with a higher negative conversion rate of SARSCoV-2 viral assay.Furthermore,there was no significant difference in the median viral assay conversion time between the two groups neither (11.0vs.12.0 days,P=0.217) (Table 2).
3.4.Adverse events and safety
The most common adverse event observed in this study was elevated alanine aminotransferase levels or aspartate aminotransferase levels.But serious adverse events were reported in neither of the two groups.There was no significant difference in the occurrence of adverse events including abnormal liver function,renal dysfunction,and loss of appetite between the two groups as shown in Table 3.Other abnormal laboratory testing findings were less common.No significant difference in overall rate of adverse events was observed between the two groups (allP>0.05,Table 3).
4.Discussion
The symptoms caused by COVID-19 are mainly fever,cough,fatigue,etc.[8,16].Unfortunately,no antiviral medications are proved to be effective on COVID-19 pneumonia.Due to lack of validated effective therapeutic approaches,the medications that could ameliorate fever,fatigue and coughing would be valuable for the clinical management of this disease.
The exploration of repurposed Chinese herbal products would be valuable for the treatment of COVID-19 pneumonia.LHQW,a representative TCM prescription,was recommended again in the latest Guideline for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Pneumonia issued by the National Health Commission of the People’s Republic of China because this formula provides both antiviral effects and symptomatic relief,which might have additional clinical benefits[17].LHQW consists of key components,such asLonicera japonicaandForsythia suspense,which can block the binding of SARS-CoV-2 to the angiotensinconverting enzyme[18].Rhodiola roseacan ameliorate lung injury by suppressing oxidative stress and apoptosis and abrogating pulmonary inflammation[19].In addition,Rheum palmatumcan effectively antagonize the binding of the spike protein to the angiotensinconverting enzyme[20] and suppress the release of excessive levels of inflammatory mediators,thus ameliorating lung injury[21].The latest research showed that LHQW conferred suppression of the cytopathic effect of SARS-CoV-2in vitroand reduced the viral loads in the cytoplasm and cellular membrane[12].These observations have provided evidence regarding the antiviral effects of LHQW capsules.This multicenter retrospective study showed the safety and efficacy of LHQW capsule in patients with high-risk common type COVID-19 pneumonia.The results showed combined use of LHQW capsules and SDT significantly improved the rate of symptom recovery,reduced the time to symptom recovery.Overall,combined use of LHQW capsules and SDT have shortened the disease course of fever,fatigue and coughing,and these results are consistent with the published literature[8].The rate of progression to severe COVID-19 pneumonia in the LHQW+SDT group was obviously lower than that in the SDT group,and the rate of clinical cure and recovery shown in chest CT images were also significant higher,which could be associated with its activity combatting against SARSCoV-2,and probably,the anti-inflammatory effects.However,the difference in rate of negative conversion of viral RNA assay between the two groups was not significant.No serious adverse events were reported,supporting the safety of LHQW capsules for the treatment of COVID-19.No significant difference in the length of time for negative conversion of viral assays was observed between the two groups.It was reported that LHQW conferred suppression of the cytopathic effect of SARS-CoV-2in vitroand reduced the viral loads in the cytoplasm and cellular membrane[12],but the current clinical study showed that LHQW capsules did not significantly inhibit viral effectsin vivo.This may be explained by the different viral loads in human respiratory tract specimens and in the cytoplasmin vitro.
This study reports the efficacy of LHQW capsule therapy in patients with high-risk common type COVID-19 pneumonia.In light of the safety and effectiveness profiles,the results indicate that LHQW capsules could be recommended for patients with COVID-19 to reduce the symptom burden,improve their clinical outcomes,and decrease the rate of conversion to severe cases of high-risk common type COVID-19 pneumonia.
However,there are limitations in this study.First,the duration of patient observation in this study may not be long enough to confirm the long efficacy of LHQW capsule treatment after the patient was discharged.This problem will be the focus of our future study.Second,limitations related to the retrospective study design,patient selection,and unmeasured confounding bias could not be entirely excluded.Furthermore,this study only included patients with highrisk common type COVID-19 pneumonia.The clinical effects of the drugs mentioned above in the treatment of severe COVID-19 require further study.Finally,no blinding was implemented because of the urgency of the outbreak that entailed a timely treatment,and placebo-controlled trial would be unethical in light of the rapid outbreak of communicable diseases such as COVID-19.
In conclusion,this multicenter clinical retrospective study reports the safety and efficacy of LHQW capsule in patients with highrisk common type COVID-19 pneumonia.LHQW capsules showed therapeutic effects on COVID-19 by improving the recovery rate of symptoms,shortening the length of time to symptom recovery,and improving the recovery of chest radiologic abnormalities,but it did not show better effects on negative conversion of viral RNA assay comparing with the SDT.The above mentioned results indicate that LHQW capsules could be recommended for patients with COVID-19 to reduce the symptom burden,improve their clinical outcomes,and reduce the probability of deteriorate to severe highrisk common type COVID-19 pneumonia.
Conflict of interest statement
We declare that we have no conflict of interest.
Funding
This project was supported by the Education Department of Hainan Province (grant No.: Hnky2022ZD-11),Hainan Provincial Natural Science Foundation of China (grant No.: 822MS176),Hainan Provincial Natural Science Foundation of China (grant No.:GHYF2022011,ZDKJ2021036,ZDYF2020223 and 820CXTD448)and the National Natural Science Foundation of China (grant No.82160012,82260001).
Ethics approval
The institutional ethics board of the Qun'li Branch of the First Affiliated Hospital of Harbin Medical University (No.2022-038),the First Affiliated Hospital of Hainan Medical University (No.2022-025) and Kang'an Hospital of Mudanjiang (No.2022-019)provided ethical approval this study.
Authors'contributions
W.Z.,L.L.and B.L.collected the epidemiological and clinical data.B.L.,M.Y.and L.L.analyzed the data.B.L.and L.L.drafted the manuscript.Q.L.and X.D.Z.revised the final manuscript.all the authors have approved the publication of this manuscript in current form.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to acknowledge the supports of the institutional ethics board of the Qun'li Branch of the First Affiliated Hospital of Harbin Medical University,the First Affiliated Hospital of Hainan Medical University and Kang'an Hospital of Mudanjiang.We thank all the patients involved in this study.
 Asian Pacific Journal of Tropical Medicine2023年9期
Asian Pacific Journal of Tropical Medicine2023年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Snakebite: A neglected tropical disease that can stymie progress toward the Sustainable Development Goals
- A simple and purification-free nucleic acid extraction method for rapid diagnosis of malaria
- Type 2 reaction associated sensorineural hearing loss in a drug resistant lepromatous leprosy patient: A case report
- Public satisfaction with the quality of First Health Facility Services in Indonesia: Does sociodemographic matter?
- Attitudes and understanding of complementary and alternative medicine in cancer care: An exploratory study of patients’ perspectives in Karachi,Pakistan
- Elimination of lymphatic filariasis: Where do we stand so far?
