Diversity and species composition of microbiota associated with dengue mosquito breeding habitats: A cross-sectional study from selected areas in Udapalatha MOH division, Sri Lanka
Yashoda Kumari, Deepika Amarasinghe, Koshila Ranasinghe
Department of Zoology and Environmental Management, Faculty of Science, University of Kelaniya, Dalugama, Kelaniya GQ 11600, Sri Lanka
ABSTRACT
Objective: To determine the diversity of microbiota associated with different breeding habitats of dengue vector mosquitoes Aedes (Ae.)aegypti and Ae.albopictus and to identify any parasitic, epibiont,pathogenic, competitive or predatory species.
Methods: Sampling was performed from a variety of breeding habitats using dipping, pipetting and siphoning techniques.Microbiota in water samples were preserved using Rose Bengal solution and Lugol’s iodine, and were identified.Live samples of microbiota were kept under laboratory conditions to observe any pathogenic or parasitic microbiota interacting with larvae.
Results: A total of eleven microbiota species (Canthocamptus staphylinus, Canthocamptus microstaphylinus, Parastenocaris brevipes, Lepadella ovalis, Lepadella patella, Rotatoria rotatoria,Rotatoria macrura, Asplanchna brightwelli, Trichocerca rattus,Euglena variabilis, and Flagilaria capucina) belonging to four (4)phyla (Arthropoda, Rotifera, Euglenozoa, and Ochrophyta) and 8 microbiota species belonged to four phyla (Arthropoda, Rotifera,Euglenozoa, and Ochrophyta) were identified from Ae.aegypti and Ae.albopictus breeding habitats respectively.There was a higher percentage (54.54%) of larval habitats positive for the secondary vector Ae.albopictus than through the primary vector Ae.aegypti in the Gampola urban area indicating higher possibility of transmitting the dengue virus through the secondary vector.However, no pathogenic or parasitic ciliates on mosquito larvae were encountered in the present study.Those findings may be due to sampling maingly from temporary container-type breeding habitats.
Conclusions: The relative distribution of microbiota associated with mosquito species differed significantly among Ae.aegypti and Ae.albopictus.The overall findings of this study could help in implementing novel eco-friendly vector-control strategies in the study area.
KEYWORDS: Aedes; Biological; Mosquito-control; Vectors
Significance
Identification of parasitic, epibiont, pathogenic, competitive or predatory microbiota in larval habitats and their interactions with associated mosquito larvae, in terms of controlling agents,would be beneficial for potential larval-controlling approaches.The degree of such parasitic, pathogenic, or predatory effects may vary with the geographical location.During the present study, a total of eleven and eight microbiota species were identified fromAedes aegyptiandAedes albopictusbreeding habitats respectively from Udapalatha MOH division.The relative distribution of microbiota associated with mosquito species differed significantly among theAedes aegyptiandAedes albopictusrevealing the relationship of microbiota abundance with different mosquito species which helps in implementing novel vector-control strategies in the study area in an ecofriendly manner.
1.Introduction
In both tropical and temperate climates, mosquitoes spread a variety of vector-borne diseases to people, making them a significant group of pathogen-transmitting vectors[1].Therefore, the study of mosquitoes' ecological and environmental factors that determine their abundance is a vital necessity[2,3].The selectivity of a suitable oviposition site is an important factor in determining the success of the life cycle.Adult mosquitoes use different cues like visual, tactile,etc.to select oviposition locations[4].The factors that influence oviposition site selectivity mainly include the water quality of the breeding habitat.It is one of the most critical parameters that determine the success of egg hatching and the development of progeny.Therefore, females choose breeding places based on biotic and abiotic constituents of water[5,6].Such abiotic parameters include pH, salinity, breeding site temperature, ionic concentrations and vegetation[7].Meanwhile, biotic parameters also include competitors,predators, and the presence of parasites which influence mosquito larval development[8-10].
Naturally occurring microbiota is another biotic factor associated with mosquito breeding habitats.Among this diverse microbiota,food sources for mosquitoes, competitors, parasites, epibionts and predators may present.Such parasitic or pathogenic microbiota species may cause lethal effects on mosquito larvae[11].Further, there are competitors such as bacteria, protists, and algae that consume the same food items as mosquito larvae.Predatory microbes such as Calanoida, Harpacticoida, and Cladocera could interfere with the development of mosquito larvae, thereby influencing their survival rates[12].Meanwhile, it may negatively affect the egg-laying of gravid female mosquitos[10].Therefore, some microbiota in the mosquito breeding habitats may operate as natural mosquito larvae biocontrol agents[11].
Mosquitoes harbor communities of symbiotic microbes exhibiting various functions in their digestive tract including promoting or assisting the gut infection of incoming pathogens and significantly contributing to disease transmission and host-parasite interactions.The naturally occurring microbiota acquired by larvae from breeding habitats may establish as symbiotic flora in mosquito larval gut.Therefore associated microbiota species can affect the ability of mosquitoes to transmit disease-causing pathogens too[13].Identification of naturally occurring microbiota and their interactions with mosquito larvae, in terms of epibiont, parasitic, pathogenic,competitive or predatory organisms against mosquito larvae as controlling agents would be beneficial for potential larval controlling approaches in an environmental-friendly manner.
2.Materials and methods
2.1.Study area
Gampola (7° 7' 36.3972'' N, 80° 33' 52.8372'' E) is a town located in Kandy District, Central Province, Sri Lanka, and consists of an extent of land of about 94.02 km2.From Udapalatha MOH division,Gampola urban area was selected as the study area for the present study.
2.2.Sampling of mosquito breeding habitats for microbiota and mosquito larvae
Sampling was performed bi-monthly from November 2021 to January 2022.A total of 50 breeding habitat with mosquito larvae was collected from the sampling site, including blocked drains,discarded pots and plastic cups accumulated with rain water,discarded coconut shells, bamboo tree holes, ornament ponds, leaf axils, discarded tires and discarded roof tiles.
Breeding sites were selected within the district randomly, and each sampling site was geo-referenced (GARMIN-etrex SUMMIT)(Figure 1).Breeding habitats were categorized as man-made and natural[14].Water samples were collected using a standard 250 mL dipper.When dipping is impossible, sampling was performed using pipetting or siphoning methods (maximum 250 mL) into a larvalrearing container (Height 12 cm, Diameter 6.5 cm).Dipping was performed for larger temporary habitats with a greater volume of water.For this, the metal scooper (250 mL volume scoop with a 30 cm long handle) was held vertically into the water body and a sample of water was taken maximum at the handle depth to comprise subsurface and bottom layers.When dipping is impossible, in small and flatwater sources, sampling was performed by pipetting out the water using a pasture pipette; siphoning was done in places such as tree holes, leaf axils, and tires where both dipping and pipetting is impossible.
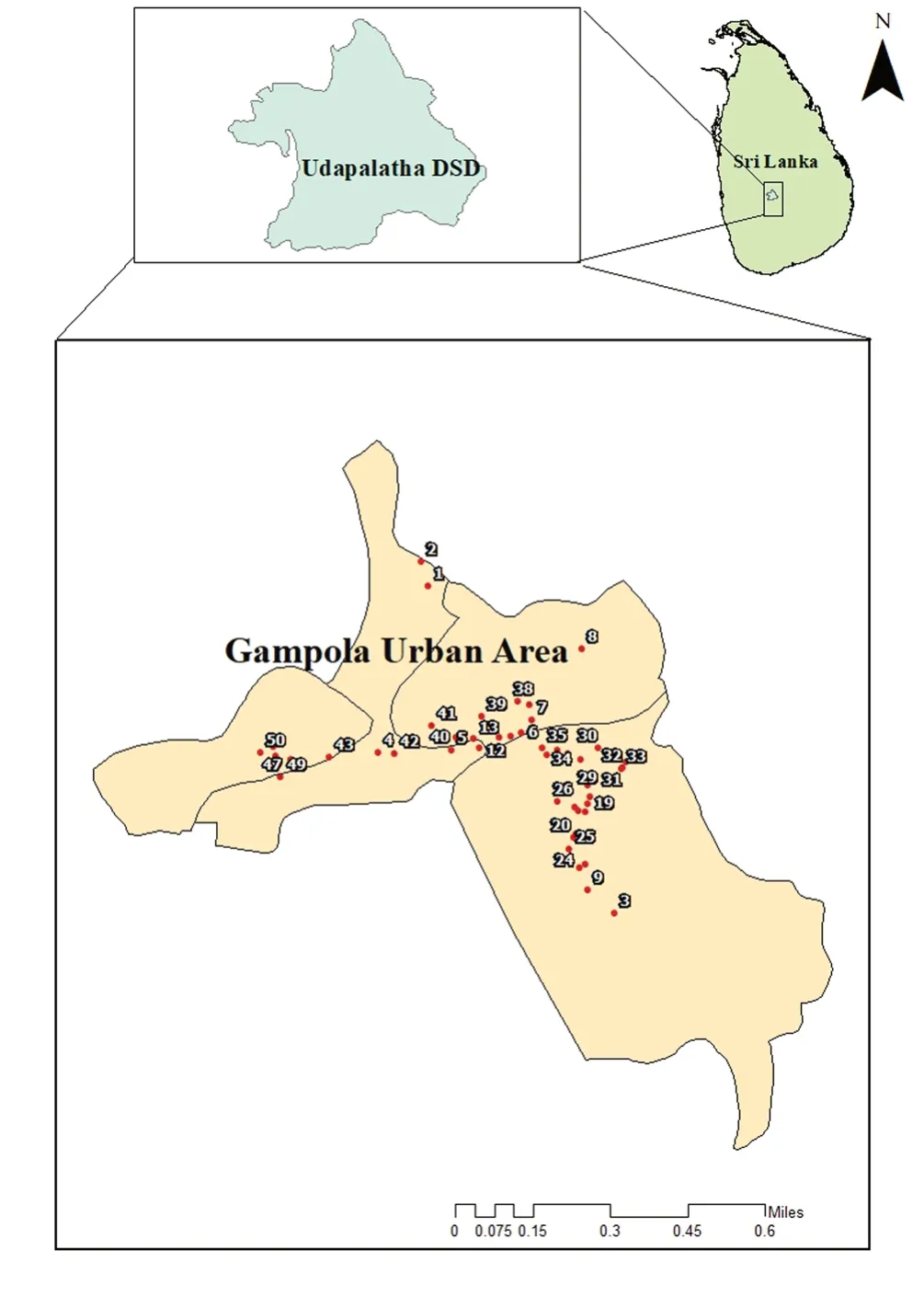
Figure 1.Sampling locations from the selected study site; Udapalatha MOH Division.
Both mosquito and microbiota sampling was performed from each habitat.At the sampling time, a collected water sample from the breeding habitat was divided into three plastic containers (6.5 cm width, 12 cm height); 2 for identification of microbiota and 1 for mosquito larval species identification and observing interactions of microbiota with live mosquito larvae.Two of them were immediately preserved in two methods using Rose Bengal stain (5% formalin with 0.04% Rose Bengal stain) solution and 5% Lugols’ solution for microbiota identification.The remaining sample was kept as it is(non-preserved) and covered with a small-sized mesh net for getting live observations.Mosquito species identification was performed using standard identification keys[7,15,16].All samples were labeled and transferred carefully into the laboratory for further processing.
2.3.Identification of microbiota
A total of 1.0 mL aliquot of preserved sample was examined under the compound microscope (×100 magnification) (Olympus ×C21,apan) using a Sedgwick rafter (S-R) cell (50 mm length, 20 mm width, 1 mm deep) and HYDRO-BIOS phytoplankton chamber(dimensions: 33 mm × 33 mm; thickness: 1 mL) for quantifying the microbiota.The sample was well shaken before taking the aliquot for observation.Microbiota species/taxa were identified at taxa/species level using temporary slide mounts.Microbiota were identified using standard identification keys (×400 magnification)[17-19].The nonpreserved sample was observed daily until the pupation of mosquito larvae there.
2.4.Larval rearing and taxonomic identification
The mosquito larvae were first separated to the genus level and classified into instar stages (Ⅰ& Ⅱ and Ⅲor Ⅳ).The larvae were identified under a Binocular light microscope (Olympus C21, Japan)using morphological taxonomic keys[7,15,16,20,21].
2.5.Determination of possible parasitic/pathogenic/epibiont or predatory microbiota against mosquito larvae
Regular daily observations were made, from non-preserved water samples for any significant survival change or reduction of mosquito larval count or any change of motility of live mosquito larvae due to biologically affecting microbiota species/taxa associated.Epibiont/symbiont or predator if any, was observed by micro pipetting 1 mL sample into Sedgwick rafter (S-R) cell and observing under the microscope (×40 and ×100 magnifications).
2.6.Data analysis
Occurrence frequencies of microbiota species were categorized as constant for species found in more than 50% of the collections;common when found between 25% and 50% of the collections;and accidental or rare species when found in less than 25% of the collections[22].Microbiota alpha diversity (α) was calculated for each breeding habitat type as the total number of species in the sampling periods, andαα medium was calculated as the average between the α diversity for the system of the same type; gamma (γ) diversity was estimated using the total number of species from all samples.
Beta diversity (β) was estimated by measuring the species turnover using the β-1 index[23], which measures the amount thatregional diversity exceeds mean alpha diversity.It was calculated by the formula β-1= [(S/αmean)-1]/[N-1]×100, where S is the regional diversity or total richness (the number of species per each sampling site); αmeanis the mean α diversity (mean number of species) for each site in each period; N is the number of sites of the period.Beta-diversity over 50% indicates high heterogeneity in microbiota composition among systems; between 20% and 50% indicates intermediate heterogeneity; and below 20% indicates low heterogeneity[23].The microbiota species diversity was also estimated according to the indices of Shannon and Wiener[24] and evenness[25].
The Chi-square test of independence was used to evaluate the significance of the distribution of different microbiota species among different breeding sites of Aedes (Ae.) aegypti and Ae.albopictus in the study area.
3.Results
3.1.Habitat positivity
A total of 44 breeding habitats were observed with the presence of Ae.aegypti and Ae.albopictus larvae from the total sampled 50 breeding habitats.Moreover, a total of 12 temporary key breeding sites were identified, withAedeslarvae (Table 1).Leaf axils, tree holes and bamboo trees were found in the study area as natural mosquito breeding habitats.The majority of the sampled breeding habitats belonged to the category of man-made temporary microbreeding habitats (Table 1).Such temporary micro-breeding habitat types were positive for Ae.aegypti and Ae.albopictus mosquito immature stages.The highest mosquito larval diversity and abundance were found in metal containers.Plastic containers, metal containers, concrete slabs, leaf axils, coconut shells, and bamboo trees were found positive for bothAe.aegyptiandAe.albopictus.Besides, glassware, tires, ornamental ponds, and tree holes were positive for Ae.aegypti, and discarded roof tiles and clay pots were positive forAe.albopictus(Table 1).
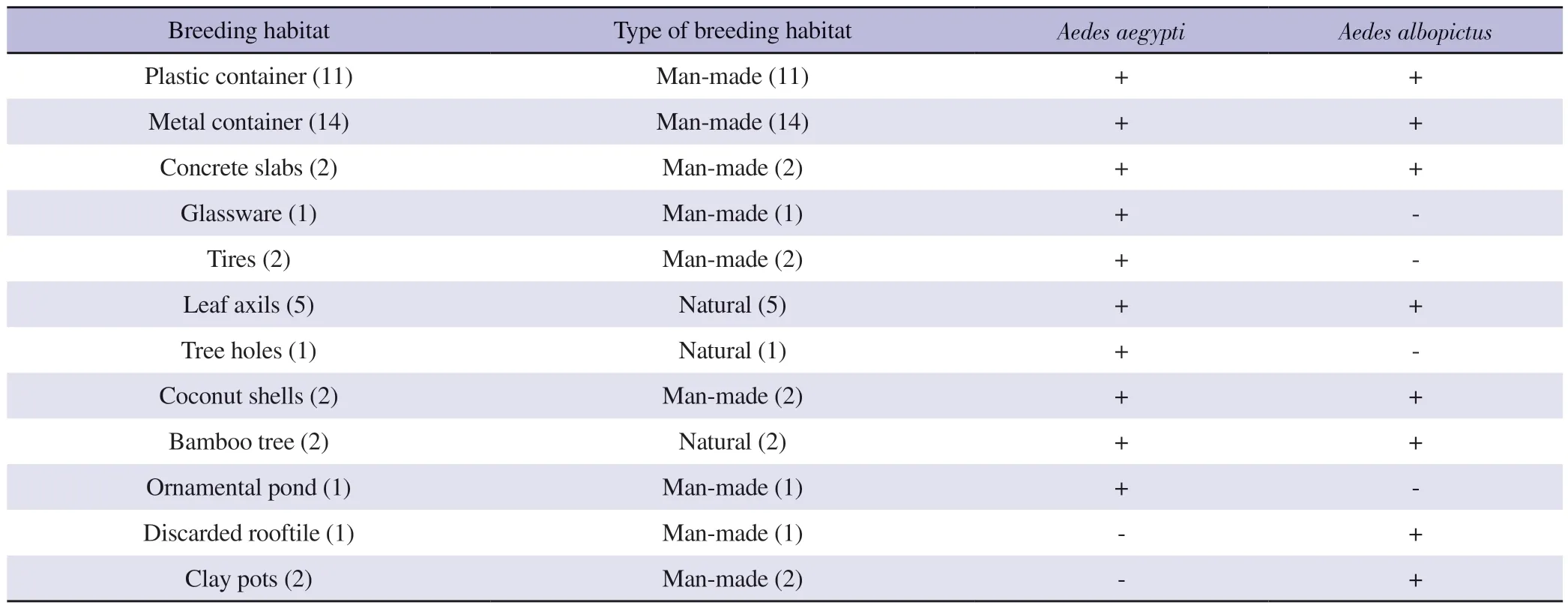
Table 1.List of breeding habitats positive for Aedes aegypti and Aedes albopictus mosquito immature stages encountered from selected area in Udapalatha MOH Division, Gampola, Sri Lanka.
Ae.albopictus showed a relatively higher distribution and abundance over Ae.aegypti.No co-existing of Ae.aegypti and Ae.albopictus was found in the samples during the present study.
3.2.Diversity and occurrence of microbiota from different mosquito breeding habitats of Ae.aegypti and Ae.albopictus
A total of eleven (11) microbiota species belonging to four (4)phyla (Arthropoda, Rotifera, Euglenozoa, and Ochrophyta)were identified from 20 different mosquito breeding habitats of Ae.aegypti; while 8 microbiota species belonging to four phyla(Arthropoda, Rotifera, Euglenozoa, and Ochrophyta) were identified from 20 breeding habitats of Ae.albopictus (Figure 2).
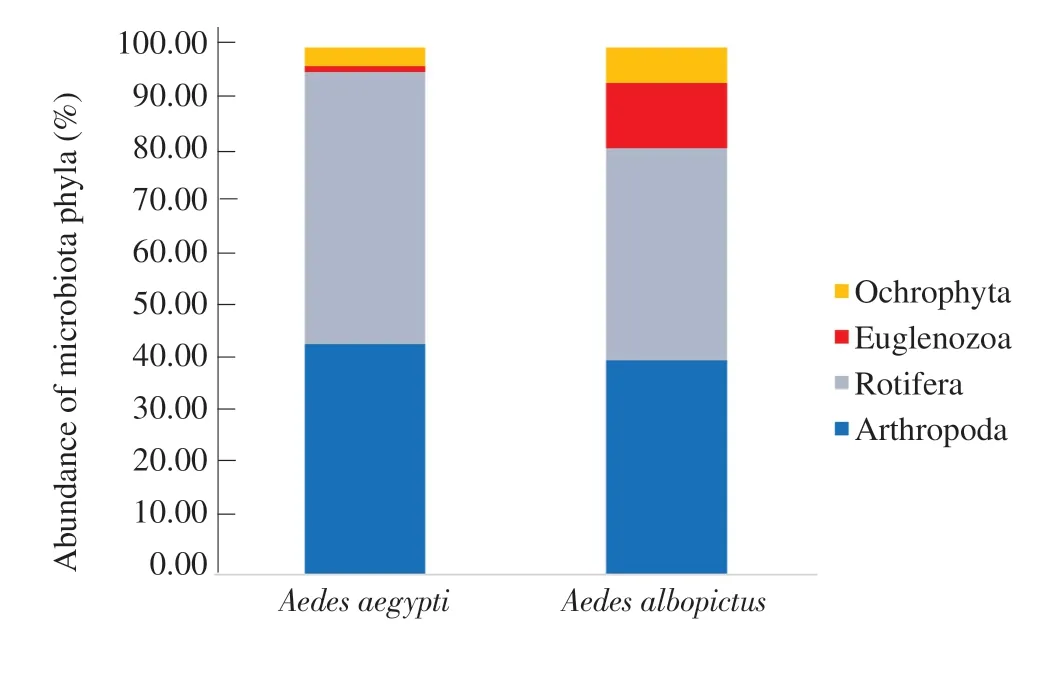
Figure 2.Percentage abundance of microbiota phyla encountered from different mosquito breeding habitats of Aedes aegypti and Aedes albopictus.
The phylum Rotifera gave the highest percentage abundance(51.66%) of total microbiota and the phylumEuglenozoa gave the lowest percentage abundance (1.02%) for Ae.aegypti breeding habitats.Meanwhile for Ae.albopictus breeding habitats, phylum Arthropoda showed the highest percentage of abundance (40.64%),followed by phylum Rotifera (40.27%) and the phylum Ochrophyta had the lowest percentage of abundance (6.72%) (Figure 2).
Mosquito breeding habitat types occupied with Ae.aegypti larvae,exhibited a diversity of microbiota belonging to four phyla, with a higher abundance of phylum Rotiferain plastic containers and glassware.Rotifers exhibited a wide range of morphological variations within breeding habitats.Rotaria rotatoria had the highest abundance of the total rotifers found fromAe.aegyptibreeding habitats (Suppementary Table 1).The abundance of the phylumArthropoda was found to be dominant in metal containers and leaf axils (Figure 3).

Figure 3.Occurrence of microbiota phyla encountered from mosquito breeding habitats.(A) Aedes aegypti (B) Aedes albopictus.
Considering the breeding habitats occupied byAe.albopictus,phylum Arthropoda showed the highest abundance.Phylum Arthropoda was dominant in plastic containers and leaf axils.Further, metal containers were identified as the breeding habitat with the highest number of microbiota species occurrence with Ae.albopictus larvae (Figure 3).Phylum Rotifera was found as dominant in metal containers.Discarded roof tiles exhibit the highest abundance of microbiota from the phylum Euglenozoa.Bamboo trees, coconut shells and concrete slabs had the presence of only one microbiota phyla; Arthropoda, Ochrophyta, and Rotifera,respectively (Figure 3).
3.3.Occurrence frequencies of microbiota species in different types of breeding habitats of Ae.aegypti and Ae.albopictus
Canthocamptus staphylinushas existed as a common microbiota species in many breeding habitat types such as concrete slab(100.00%), plastic containers (50.03%), associating with Ae.aegypti larvae and in bamboo trees (100.00%) and clay pot (58.83%)associating withAe.albopictuslarvae (Supplementary Table 1).Interestingly it has existed as an accidental and rare species also in other breeding habitat types.Therefore, Canthocamptus staphylinus has been identified as a species that shows all three possible occurrences in different types of breeding habitats (Supplementary Table 1).
The only microbiota species recorded from the study area that belonged to phylum Ochrophyta was Euglena capucina and it was encountered in plastic containers, tires, leaf axils and coconut shells withAedeslarvae (Supplementary Table 1).Ae.albopictuslarvae were more frequently occupied with two common microbiota species: Canthocamptus staphylinus (42.15%) and Rotaria rotatoria(32.35%) in metal containers.All other microbiota species had a rare occurrence in metal containers (Supplementary Table 1).
Considering the microbiota abundance in plastic containers associated with Ae.aegypti, Rotaria rotatiria was recognized as a constant species with a 51.73% occurrence, and all other microbiota recorded belonged to common or rare categories (Supplementary Table 1).
The highest Shannon Weiner diversity index and species richness/gamma (γ) diversity of microbiota associated with Ae.aegypti larvae were recorded from leaf axils, while it was from metal containers forAe.albopictus(Table 2).Further, the highest heterogeneity of microbiota associated with Ae.albopictus was recorded from plastic containers as it gave the highest beta diversity value of 0.8.Tires forAe.aegyptihad the highest beta (β) diversity value of 1(Table 2).Therefore, tires forAe.aegyptiand plastic containers for Ae.albopictus indicated a higher heterogeneity of microbiota composition which is associated with their biology.
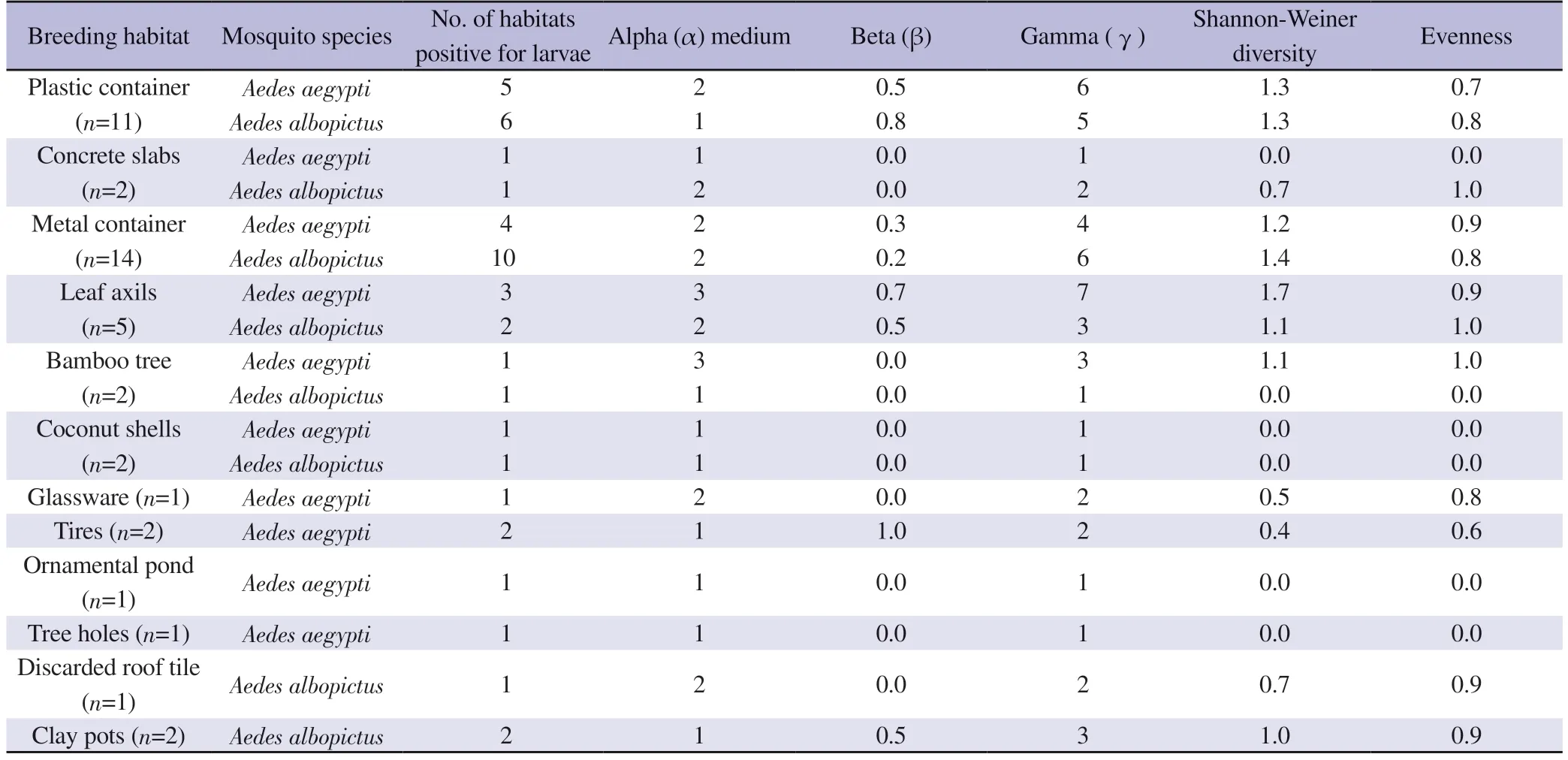
Table 2.Evenness, Shannon diversity, alpha (α) medium, beta (β), and gamma (γ) diversities of type of habitats.
Moreover, the plastic containers and metal containers forAe.aegyptihad beta (β) diversity between 0.2 and 0.5, indicating intermediate heterogeneity of microbiota composition among the systems (Table 2).This observation corresponded to metal container,leaf axil, and clay pot breeding habitats for larvae ofAe.albopictus.The rest of the habitats were with a β diversity below 0.2, indicating low heterogeneity of microbiota within the breeding habitat (Table 2).
3.4.Variation of microbiota communities across mosquito species
The relative distribution of microbiota associated with mosquito species differed significantly among the Ae.aegypti and Ae.albopictus(χ2=486.091;P<0.001).
3.5.Identified microbiota parasitic/ pathogenic/ epibiont to mosquito larvae
Natural population of mosquitoes was kept under check by the activities of parasites/epibionts and pathogens on mosquito larvae,no such microbiota was identified from the present sampling site.
4.Discussion
The present study identified naturally occurring microbiota species associated with a variety of vector mosquito breeding habitats.The present study findings address the knowledge gap regarding information, recording a total number of 11 microbiota species from a variety of mosquito breeding habitats in the study area.
Although some vector-borne diseases like malaria and filariasis have been eliminated from Sri Lanka through successful control strategies, some vector-borne diseases, such as dengue, have increasing trends each year[26].It has become a major public health and socio-economic concern in Sri Lanka.Two major vector mosquito species are responsible for dengue transmission in Sri Lanka; Ae.aegypti and Ae.albopictus.Ae.aegypti is known to be the primary vector, which is the predominant vector in urban areas, while Ae.albopictus is considered as the secondary vector which is the predominant vector in rural areas[27].Thus, the present study considered microbiota associated with both species of Aedes mosquito breeding waters.Ae.aegypti were mostly encountered from plastic containers whileAe.albopictuswere from metal containers.Containers that keep water for extended periods, such as artificial containers, build better or excellent mosquito breeding environments[28-31].Present study findings revealed, both Ae.aegypti andAe.albopictuswere occupied in different types of breeding habitats, especially in temporary microhabitats, indicating the high risk of dengue vector distribution through increased accumulation of artificial containers.Further, there was a higher percentage (54.54%)of larval habitats positive for the secondary vector Ae.albopictus than through the primary vectorAe.aegyptiin the Gampola urban area indicating higher possibility of transmitting the dengue virus through the secondary vector.
Previous larval surveys carried out in the Gampola study area also revealed that the Ae.albopictus was the most abundant species in the area, compared toAe.aegypti[32].
Previous studies on microbiota inhabiting mosquito breeding habitats in different districts also reported a wide range of microbiota with some parasitic species.Ranasingheet al[12] reported some pathogenic or parasitic ciliates, includingVorticella microstoma,Zoothamnium spp., and Chilodinella sp., from rice field habitats in Gampaha District.Cyanobacterial diet items for mosquito larvae like Spirulina (from plastic containers, tree holes, etc.), Anabaena affinis(from irrigation canals),Scenedesmus armatus(from drainage and tree holes) and Scenedesmus bijuga (from ponds, plastic containers,etc.) were also recorded[12].
Further, Ranasingheet al[33] reported mosquito larval mortalities associated with high densities of Vorticella sp.and Zoothamnium sp.attached to the siphon and thoracic cuticular areas.Anyhow such pathogenic or parasitic ciliates were not encountered in the present study.A previous study carried out in the Kandy district recorded several algae species associated with bothAe.aegyptiand Ae.albopictus larvae[12].Most algae that inhabit breeding water serve as food sources, while some algal species like blue-green algae and cyanobacteria have lethal effects on mosquito larvae[34-36].However, such algae species were not identified from the present study site.Those findings may be due to sampling from temporary container-type breeding habitats mainly, during the present study.For the development of microbiota in a breeding habitat, abiotic and other biotic factors associated play a key role.Further, there is comparatively less diversity of microbiota in a temporary containertype habitat, compared to the diversity recorded in literature from a natural habitat[12].The present study confirms the above findings.
Recently conducted experiments have shown the adverse impacts of cladocerans such as competitors and cyclopoid copepods like predators on mosquito survival[37].Competitors and predators are efficient as biocontrol agents against mosquito larvae[10,38,39].Antagonistic crustaceans such asMesocyclops aspericornisand Daphnia magna cause the late development of mortality of early instar larvae of Aedes by predatory effects and interspecific competition for resources[3].From the present study, the highest species richness of microbiota for Ae.aegypti was recorded from Phylum Rotifera includingLepadella ovalis, Lepadella patella,Rotaria rotatoria, Rotaria sp., Asplanchna brightwelli and Trichocercarattusin a range of breeding habitats.Several prior studies have highlighted the significance of some rotifers against mosquito larvae survival[40].The species Asplanchna brightwelli and Trichocercarattuswere competitors or predators on mosquito larvae[40].The present study has not revealed such an association may be due to less abundance of rotifers in breeding waters in container habitats.Overall present study findings revealed that temporary-container breeding habitats harbor less diversity and abundance of naturallyoccurring microbiota associated with mosquito larvae, compared to a natural breeding habitat.
A total of eleven microbiota species belonging to four phyla(Arthropoda, Rotifera, Euglenozoa, and Ochrophyta) and 8 microbiota species belonged to four phyla (Arthropoda, Rotifera,Euglenozoa, and Ochrophyta) were identified fromAe.aegyptiandAe.albopictusbreeding habitats respectively.The relative distribution of microbiota associated with mosquito species differed significantly among theAe.aegyptiandAe.albopictus.The presence of dengue vectors in the study area in considerable numbers can cause public health concerns as dengue is one of the major challenges in these areas.Therefore, a study of this nature would be useful to identify the entomological potential for disease transmission and an update on microbiota associated withAedesmosquito larvae would be facilitated for implementing appropriate future vector control interventions.Morphological identification of some microbiota that was too small, up to the species level served as a limitation of the study.
Conflict of interest statement
We declare that there is no conflict of interest.
Funding
The present research received funds from Department of Zoology and Environmental Management, University of Kelaniya, Sri Lanka.
Acknowledgments
This work was supported by the Department of Zoology and Environmental Management, University of Kelaniya, Sri Lanka.
Authors’ contributions
Deepika Amarasinghe did the designing of the research, overall supervision and guidance of the research work and final editing and reviewing the manuscript.Yasoda Kumari performed sampling and data collection, data analysis and writing the manuscript.Koshila Ranasinghe did the supervision of the research work, data analysis,writing and reviewing of the manuscript.All authors read and approved the final manuscript.
 Asian Pacific Journal of Tropical Medicine2023年8期
Asian Pacific Journal of Tropical Medicine2023年8期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Addressing the needs and rights of sex workers for HIV healthcare services in the Philippines
- Healthcare-associated Staphylococcus aureus infections in children in Turkey: A sixyear retrospective, single-center study
- Cutaneous anthrax associated with facial palsy: A case report
- Molecular evidence and phylogenetic delineation of spotted fever group Rickettsia species in Amblyomma ticks from cattle in Gauteng and Limpopo Provinces, South Africa
- Clinical characteristics and outcomes of nosocomial COVID-19 in Turkey: A retrospective multicenter study
- Clinical profile, etiology, management and outcome of empyema thoracis associated with COVID-19 infection: A systematic review of published case reports
