Selection,effective dominance,and completeness of Cry1A.105/Cry2Ab2 dual-protein resistance in Helicoverpa zea (Boddie)(Lepidoptera: Noctuidae)
Tiago SlLVA,Ying NlU,Tyler TOWLES,Sebe BROWN,Graham P.HEAD,Wade WALKER,Fangneng HUANG#
1 Department of Entomology,Louisiana State University Agricultural Center,Baton Rouge,Louisiana 70817,USA
2 Macon Ridge Research Station,Louisiana State University Agricultural Center,Winnsboro,Louisiana 71295,USA
3 Dean Research Station,Louisiana State University Agricultural Center,Alexandria,Louisiana 71302,USA
4 Bayer Crop Science,Chesterfield,Missouri 63017,USA
Abstract In the U.S.,Helicoverpa zea (Boddie) is a major pest targeted by both transgenic maize and cotton expressing Bacillus thuringiensis (Bt) proteins. Resistance of insect to Bt maize and cotton containing cry1A and cry2A genes has widely occurred in the U.S.In this study,two trials were performed to investigate larval survival and development of a Cry1A.105/Cry2Ab2 dual-protein resistant (VT2P-RR),a susceptible,and an F1 heterozygous (VT2P-RS) populations of H.zea on ears of nine Bt and three non-Bt maize hybrids. The Bt maize hybrids evaluated represent five common pyramided traits expressing two or three of the Cry1A.105,Cry1Ab,Cry1F,Cry2Ab2,and Vip3Aa20 proteins. In the laboratory,neonates of the three H.zea populations were inoculated on silks of ears collected from maize at R1-R2 plant stages;and larval survivorship was checked 10 d after neonate release. All three insect populations survived normally on non-Bt maize ears. Varied numbers of VT2P-RR and VT2P-RS survived on ears of Cry1A.105/Cry2Ab2 maize,while all larvae of the three populations died or could not develop on ears of Vip3Aa20-expressing maize. The results demonstrated that the dual-protein resistant H.zea was not cross-resistant to Vip3Aa20-expressing maize,and thus traits with vip3Aa20 gene should be effective to manage Cry1A.105/Cry2Ab2-resistant H.zea. The resistance in VT2PRR was determined to be incomplete on Cry1A.105/Cry2Ab2 maize. The effective dominance levels varied greatly,from recessive to incompletely dominant,depending on maize hybrids and trials,suggesting that proper selection of maize hybrids could be important for mitigating the Cry1A.105/Cry2Ab2 resistance. The data generated should aid in modeling multiple-protein Bt resistance in H.zea.
Keywords: corn earworm,Cry1A/Cry2A,effective dominance,incomplete resistance,Bt maize resistance management
1.lntroduction
Helicoverpazea(Boddie) (Lepidoptera: Noctuidae) is an economically important crop pest in North America (Reay-Jones 2019). It is a polyphagous pest and has more than 200 hosts,including many main crops such as soybean,sorghum,cotton,and maize (Neunzig 1964;Lingrenet al.1994). This moth pest in the New World has several common names based on its host crops;for example,it is named (cotton) bollworm on cotton,sorghum headworm on sorghum,soybean podworm on soybean,tomato fruitworm on tomato,and corn earworm on maize (Porter and Kerns 2020). Traditionally,management ofH.zeaheavily relied on chemical insecticide applications. Since 1996,planting transgenicBacillusthuringiensis(Bt) cotton and maize has become a main approach forH.zeacontrol for the two crops (ISAAA 2019). During the 26 years from 1996-2021,the U.S.planted a total of >518 Mha of Bt maize and Bt cotton (ISAAA 2019;USDANASS 2020,2021). Currently,>80% of maize and >90% cotton planted in the U.S.contain insecticidal Bt transgenes(USDANASS 2021).
To date,five Bt proteins (Cry1Ab,Cry1A105,Cry1F,Cry2Ab2,and Vip3Aa20) have been used in transgenic maize for lepidopteran pest control (DiFonzo 2022).Before 2010,only single-gene maize hybrids expressing either Cry1Ab or Cry1F protein for controlling moth pests were planted.Helicoverpazeais naturally tolerant to Cry1F and thus,single-genecry1Fmaize was ineffective against this species (Buntin 2008;DiFonzo 2022). The single-genecry1Abmaize during its initial years of commercial use could suppressH.zeapopulations (Lynchet al.1999;Horneret al.2003;Buntin 2008). However,because of the long-term and widespread adoption,field populations ofH.zeahave developed field resistance leading to control problems to the single-genecry1Abmaize (Reisig and Reay-Jones 2015;Divelyet al.2016;Guoet al.2021;Niuet al.2021;Linet al.2022).
In the 2010 crop season,a Bt maize event,MON 89034,containing the pyramidedcry1A.105andcry2Ab2genes became commercially available (USEPA 2010;Ghimireet al.2011). Since then,MON 89034 maize hybrids have been planted among the most common pyramided Bt maize in the Americas (Bernardiet al.2017;Grimiet al.2018;DiFonzo 2022). Several studies (Siebertet al.2012;Ruleet al.2014;Yanget al.2014) reported that MON 89034 maize,during its early commercial use,was effective againstH.zea. However,recent studies (Divelyet al.2016;2021;Bilboet al.2019;Kauret al.2019;Yanget al.2019;Niuet al.2021;Yuet al.2021,2022) have documented that field resistance to the pyramided maize hybrids expressing Cry1A/Cry2A proteins has been widespread in the U.S.The prevalent Cry1A/Cry2A resistance inH.zeais a great threat to the continued success of the Bt crop technology in the U.S.,especially for its cotton production in the southern region(USEPA 2018). Thus,effective management measures are urgently needed to mitigate the Cry protein resistance inH.zea(USEPA 2018).
The dominance level and completeness of resistance are two essential parameters that influence the speed of resistance evolution in pest populations (Carriéreet al.2006;Huanget al.2011,2021a,b;Tabashniket al.2013).The dominance level of resistance is a measure of the phenotypic performance of heterozygotes relative to the corresponding homozygous susceptible and resistant genotypes on treatments containing toxins (e.g.,Bt plants)(Bourguetet al.2000). If the fitness of RS on Bt plants is the same as RR,the resistance is functionally dominant;and if RS on Bt plants has a same fitness as SS,the resistance is recessive. Otherwise,if RS on Bt plants shows a fitness between RR and SS,the resistance is called non-recessive. A functionally non-recessive or dominant resistance means that RS will survive on Bt plants and pass resistance alleles to their offspring,and thus the speed of resistance evolution would be faster than that of the populations associated with recessive resistance. The completeness of resistance is a measure of the phenotypic performance of the homozygous resistant genotypes on Bt plantsvs.on non-Bt plants(Huang 2021b). A complete resistance means that RR on Bt plants has the same fitness as it on the corresponding non-Bt plants;otherwise,if RR on Bt plants has a lower fitness,the resistance is incomplete (Carriéreet al.2010). If other factors are the same,resistance should evolve slower in the field for an incomplete resistance than a complete resistance. Little research has been performed on these parameters for Bt resistance inH.zea,especially for resistance to multiple-Bt proteins.The main reason why few such research has occurred is the difficulty in selecting and maintaining resistantH.zeastrains in the laboratory (Anilkumaret al.2008;Linet al.2022). Recently,we developed aH.zeapopulation highly resistant to both Cry1A.105 and Cry2Ab2 proteinsviacollections of field-selected individuals from MON 89034 maize plants followed by laboratory selections with the two Bt proteins. With the availability of this dualprotein resistantH.zeapopulation,we conducted two independent trials and investigated the larval survival and development of the resistant population,together with a susceptible population and an F1heterozygous population,on common pyramided Bt maize traits,including some recently released traits containing two or three of Cry1A.105/Cry2Ab2,Cry1Ab,Cry1F,and Vip3Aa20 proteins. Additionally,effective dominance level and completeness of the dual-protein resistance inH.zeawere also determined based on the larval survivorship observed in the two trials. Here,we report the results of the study and discuss the implications in resistance management for planting Bt maize to manageH.zea.
2.Materials and methods
2.1.Establishment and confirmation of a Cry1A.105/Cry2Ab2-dual protein resistant H.zea population
During the 2021 cropping season,ears containing naturally-occurred 4th to 6th instars ofH.zea,with ear leaf sheath and husks,were removed from commercial Genuity?VT Double PRO?maize (VT2P) plants near Alexandria,Louisiana,USA. VT2P maize contains the MON 89034 Bt event possessing bothcry1A.105andcry2Ab2genes for moth control includingH.zea(DiFonzo 2022). Field-collected ears containing liveH.zealarvae were brought to the laboratory and placed in 5.7-L plastic boxes (Sterilite Corporation,Townsend,MA,USA) with the dimension of 32 cm long by 19 cm wide by 12 cm high. The insect culturing boxes were arranged in a room at 23-26°C and a 16 h:8 h (L:D) photoperiod for continued larval rearing (Guoet al.2021). After 7 to 10 d,150 mature larvae and pupae were collected from the ears,and the collected mature larvae were transferred to a meridic diet in 30-mL plastic cups (1 larva/cup) for continued rearing to the pupal stage. To synchronize insect development,varied temperatures were used during insect rearing and pupa maintenance. Mature pupae collected from maize ears and diet were placed in a 20-L Seville Classics cage (Torrance,California,USA)in another room at 26°C,>70% RH and a 10 h:14 h (L:D)photoperiod for oviposition as designed in Yuet al.(2022).Offspring of the field-collected individuals were used to establish a dual-protein resistantH.zeapopulation to Cry1A.105 and Cry2Ab2 for this study.
To ensure high homozygosity of the resistance,F1neonates of the field-originatedH.zeafrom VT2P maize were selected again on a meridic diet (Southland Products,Lake Village,Arkansas,USA) treated with a mix of Cry1A.105 and Cry2Ab2 proteins. The two proteins used in the selections were provided in buffers from Bayer Crop Science (St.Louis,Missouri,USA). Detailed information about theEscherichiacoliculture,production,molecular weight,and purity of the two Bt proteins has been described previously in Wuet al.(2009) and Kauret al.(2019). The selections were conducted in eight 8-well bioassay trays (Bio-Smart-8;C-D International,Pitman,New Jersey,USA). Four of the eight trays contained diet over-laid with a mixed Bt solution containing Cry1A.105 at 10 μg cm-2and Cry2Ab2 at 5.0 μg cm-2,and the other four trays had diet with a combination containing Cry1A.105 at 10 μg cm-2and Cry2Ab2 at 10 μg cm-2. The Bt concentrations used in the selections were estimated discriminating concentrations based on our earlier studies(Kauret al.2019;Yuet al.2022). The use of two different concentrations in the selections was to reduce the risk of colony collapse due to potential overdosing. In the laboratory selection,approximately 9 600 neonates of the first generation produced from the VT2P-field originated individuals were placed on the diet surface of the eight trays (~150 neonates/well). Resistance selections were arranged in an incubator at 26°C,~50% RH,and a 16 h:8 h (L:D) photoperiod. After a 7-d selection,20-25 larvae were selected from each tray,which resulted in an approximately selection pressure of 98%. As a result,a total of 180 larvae were selected as the final insect source for the dual-protein resistant population (VT2P-RR) for this study.
To confirm the resistance,diet over-lay bioassays with Cry1A.105 and Cry2Ab2 proteins (Yuet al.2022) was used to determine the dose responses of VT2P-RR and a susceptible population (BZ-SS). BZ-SS was obtained from Benzon Research Inc.(Carlisle,Pennsylvania,USA). Earlier studies (Kauret al.2019;Yuet al.2021)documented that BZ-SS was susceptible to a variety of Bt proteins including Cry1A.105 and Cry2Ab2.
2.2.Field planting and sources of maize ears
Two trials,namely Trial-I and Trial-II,were conducted to evaluate the larval survival and growth of threeH.zeapopulations on detached maize ears in the laboratory during 2021. The three insect populations were BZ-SS,VT2P-RR,and an F1hybrid population (VT2P-RS) that was generated by crosses between BZ-SS and VT2PRR. Each trial consisted of 11 commercial maize hybrids,which included eight Bt and three non-Bt hybrids (Table 1).The eight Bt maize hybrids in each trial consisted of five common pyramided traits currently planted in the southern U.S. In each trial,there were two hybrids with the VT2P trait,two hybrids with Genuity?SmartStax?(SMT),two with Trecepta?(TRE),one with Agrisure Viptera?(VPT),and one with Optimum?AcreMax?Leptra? (LEP). SMT expresses three Bt proteins,Cry1A.105,Cry2Ab2,and Cry1F,for control of lepidopteran pests;TRE contains Cry1A.105,Cry2Ab2,and Vip3Aa20;VPT has Cry1Ab and Vip3Aa20;and LEP contains Cry1Ab,Cry1F,and Vip3Aa20 (Table 1). The three non-Bt hybrids were genetically closely related to one or more of the Bt maize hybrids used in the study. One of the two VT2P hybrids evaluated was different between the two trials,while the other 10 hybrids were the same (Table 1). QuickStikTMELSA test strips (EnviroLogix Inc,Portland,Maine,USA)were applied to validate the Bt or non-Bt expressions in each maize hybrid.
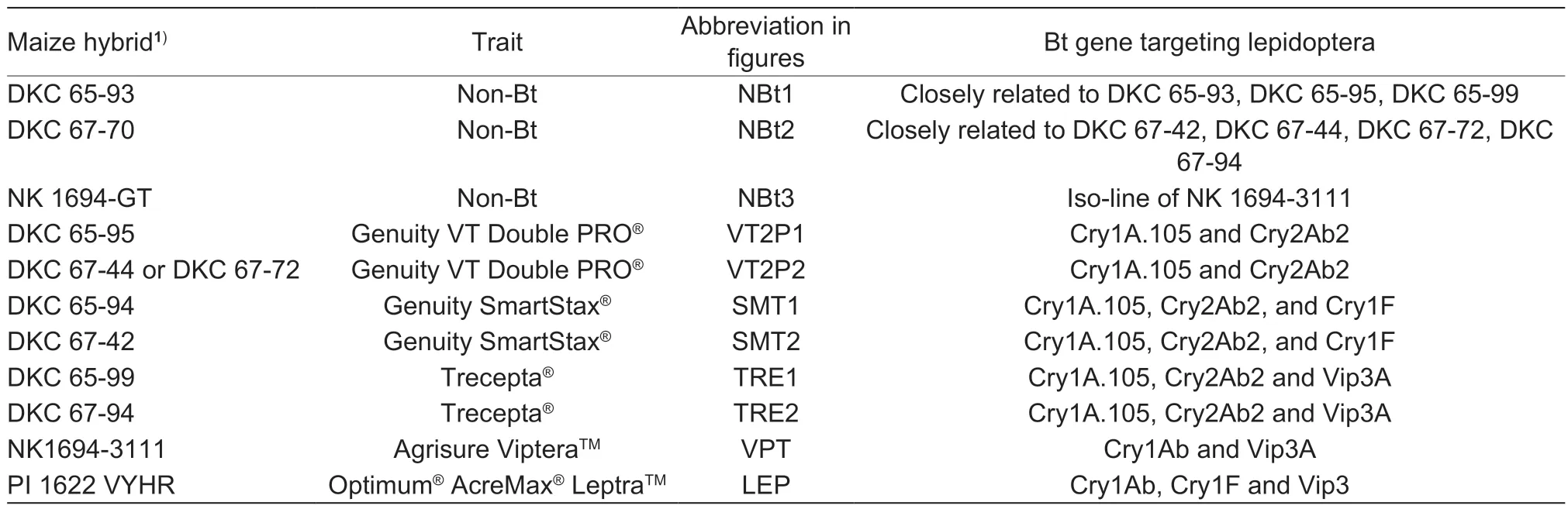
Table 1 Traits and Bt proteins expressed in the 11 maize hybrids evaluated in the two trials of the study
Maize ears used in Trial-I were collected fromfield plots planted at Dean Lee Research Station in Alexandria,Louisiana State University Agricultural Center (LSU AgCenter),Louisiana,USA. The field plot layout in Trial-I was arranged in a randomized complete block design (RCBD) with four 30-ft rows for each plot and four replications of each maize hybrid. Normal field management practices (e.g.,irrigation,herbicide applications) were performed as practiced in other local production fields,but no insecticides were applied for the trial. When plants reached R2 stage,ears with husks and sheaths were removed from the center two rows of each plot and the detached ears were brought to the laboratory for bioassays described below.
Maize ears used in Trial-II bioassays were collected from a hand-planted single-row field plot (~350-ft long)at the LSU AgCenter’s Macon Ridge Research Station in Winnsboro,Louisiana,USA. To minimize crosspollination between Bt and non-Bt maize plots or between different maize traits,there was a two-row alley between non-Bt and Bt maize hybrids,and between different Bt maize traits. Other field management practices were the same as described in Trial-I. When plants reached late R1 stage,ears with husks and sheaths were removed from the plants,and the detached ears were brought to the laboratory for bioassays.
2.3.Detached ear bioassays in the laboratory
The field-collected ears mentioned above were carefully inspected to remove any naturally infestedH.zeaeggs/larvae prior to the laboratory bioassays. In the ear bioassays,four neonates (<24 h old) of eachH.zeapopulations(BZ-SS,VT2P-RS,or VT2P-RR) were inoculated on the silk of each ear. After larval infestation,the ears containing neonates were placed into the 5.7-L plastic boxes mentioned above. There were five ears in each plastic box and each ear was separated with a piece of paper towel as described in Guoet al.(2021). Each trial was organized in a RCBD with four replications (blocks). Ears used in a block in Trial-I were corresponded to the same block of the field planting layout at the Dean Lee Research Station,while ears of a maize hybrid used in Trial-II were randomly assigned among the four blocks. There were 4-6 boxes (20-30 ears) for each replication in Trial-I and two boxes (10 ears) for each replication in Trial-II. In total,Trial-I consisted of 2 699 ears (or 10 796 neonates) and Trial-II contained 1 320 ears (5 280 neonates) for the 11 maize hybrids and three insect populations. The bioassay boxes holding ears infested with neonates were arranged in a room maintained at 23-26°C with a photoperiod of 16 h:8 h (L:D);in which the room was divided into four sections,each section representing one block. After 10 d,the number of live larvae and larval development stages on each ear were checked.
2.4.Data analysis
Larval development stages recorded were converted to a ‘larval development index’: a first instar having a numerical value 1,a 2nd instar having a value 2,a 3rd instar having a value 3,and a 4th instar having a value 4 as described in Yanget al.(2014). Abbott’s method(Abbott 1925) was used to correct the larval survivorship of aH.zeapopulation on a Bt maize hybrid based on the survivorship on its corresponding non-Bt hybrid that was genetically closely related to the Bt hybrid. The corrected larval survivorship and larval development index were transformed using arcsin (x)1/2and log(x+1),respectively,for normality. The transformed data were analyzed using two-way analysis of variance (ANOVA) with insect population and maize hybrid as the two main factors (SAS Institute 2016). LSMEAMS tests at α=0.05 level were used to separate the treatment means. The corrected larval survivorship of VT2P-RR on each of the four Bt maize hybrids containingcry1A.105andcry2Ab2genes(VT2P and SMT) was also considered as a measurement of the completeness of resistance (CR) (Huang 2021b).CR values range from 0 to 1. CR=0 represents resistance with minor genes,CR=1 means complete resistance,while 0<CR<1 represents incomplete resistance. As mentioned above,Cry1F protein in maize plants offers negligible activity against even susceptibleH.zealarvae,and thus CR values were also calculated for the two SMT hybrids. Student’st-test was conducted to determine if the mean CR values measured on the four hybrids (two VT2P and two SMT) were different between Trial-I and Trial-II (SAS Institute 2016).
Additionally,the effective dominance (DML) of the dualprotein resistance in VT2P-RR was computed according to the corrected larval survival rates of the threeH.zeapopulations on each Bt maize hybrid as described in Bourguetet al.(2000):
If DML=0,the resistance is recessive;if 0<DM<0.5,the resistance is incompletely recessive;if 0.5<DM<1,the resistance is incompletely dominant;and if DML=1,the resistance is functionally dominant. As with analyzing RC values,Student’st-test was conducted to examine if mean DMLvalues were different between the two trials(SAS Institute 2016).
3.Results
3.1.VT2P-RR was highly resistant to both Cry1A.105 and Cry2Ab2 proteins
In Cry1A.105 bioassays,VT2P-RR demonstrated an LC50value of 6.75 μg cm-2,which was 519-fold greater than the value (0.013 μg cm-2) for the known susceptible population,BZ-SS. The difference in the LC50between VT2P-RR and BZ-SS was significant according to their nonoverlapping 95% CI (Table 2). In the bioassays with Cry2Ab2 protein,VT2P-RR showed an LC50value of 6.65 μg cm-2,while the value for BZ-SS was 0.105 μg cm-2.The 63-fold resistance ratio of VT2P-RR to Cry2Ab2 was also significant (Table 2).
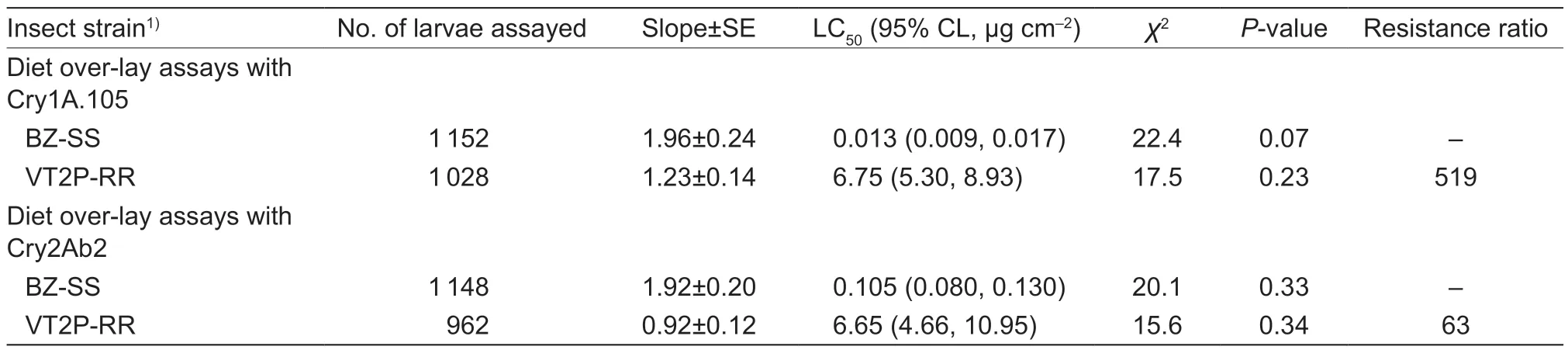
Table 2 Concentration-response of a known susceptible and a dual-protein resistant strains of Helicoverpa zea to Cry1A.105 or Cry2Ab2 protein
3.2.Resistance in VT2P-RR was incomplete on VT2P and SMT hybrids
In both trials,larvae of the threeH.zeapopulations (BZSS,VT2P-RR,and VT2P-RS) survived normally on non-Bt maize ears. Across the three non-Bt hybrids,an average of 1.07 and 1.22 larvae/ear survived in the two trials,respectively. ANOVA showed that the effects ofH.zeapopulation,maize,and the interaction on larval survival were significant for Trial-I (F2,69=19.31,P<0.0001 for insect;F7,69=13.03,P<0.0001 for maize hybrid;andF14,69=3.31,P=0.0005 for interaction),as well as for Trial-II (F2,67=38.58,P<0.0001 for insect;F7,67=27.62,P<0.0001 for maize hybrid;andF14,67=5.90,P<0.0001 for interaction).
Survivorship ofH.zealarvae on the four VT2P and SMT hybrids in Trial-I varied significantly among the insect populations. BZ-SS on DKC 67-44 (VT2P) ears showed 1.7% survivorship,while there were no survivors on the other three hybrids (Fig.1). Survivorship of VT2PRR on the four hybrids ranged from 6.1 to 13.8%;these values were significantly greater (P<0.05) than that of BZ-SS. Significant differences in larval survivorship of VT2P-RR were also observed among the four hybrids;the survivorship (13.8%) on DKC 67-44 (VT2P2) was greater(P<0.05) than the values recorded on DKC 65-95 (VT2P1)and DKC 65-94 (SMT1). VT2P-RS survivorship was also significantly different among the four hybrids,ranging from zero (DKC 65-94) to 5.9% (DKC 67-44) (Fig.1). Based on the corrected survivorship,VT2P-RR exhibited an average CR (completeness of resistance) of 0.088 with a95% confidence interval of 0.031 to 0.145,suggesting that the dual-protein resistance in VT2P-RR was obviously incomplete on the pyramided maize possessingcry1A.105andcry2Ab2genes.
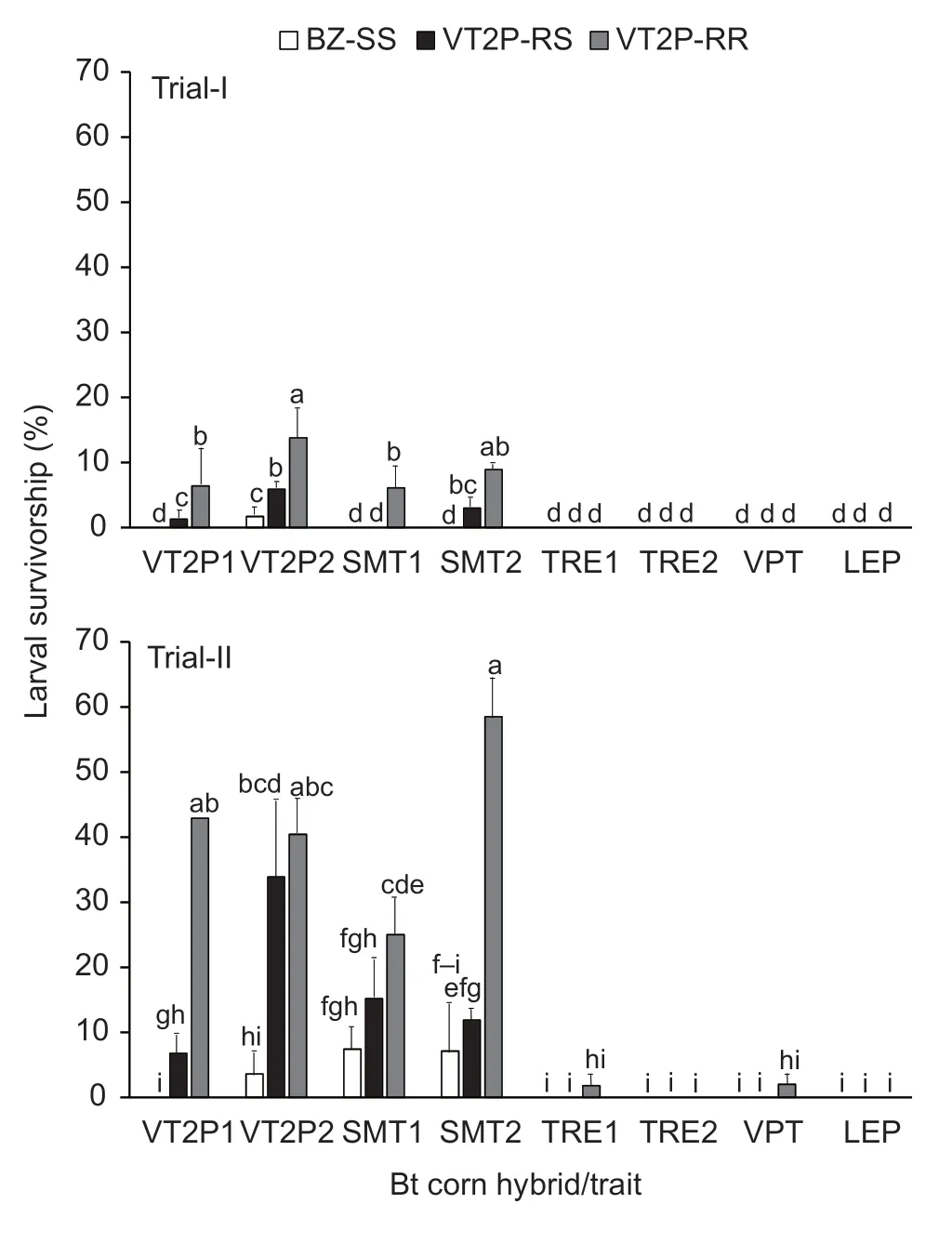
Fig. 1 Survivorship of susceptible,dual-protein heterozygous,and resistant strains of Helicoverpa zea on five common pyramided Bt maize traits. BZ-SS was a known Bt susceptible laboratory H.zea population;VT2P-RR was a Cry1A.105/Cry2Ab2 dual-protein resistant population;and VT2P-RS was a heterozygous resistant population that was generated by reciprocal crosses between BZ-SS and VT2P-RR.Abbreviations for Bt corn hybrids/traits in the figure have been explained in Table 1. Values (mean±SEM) with the same letter above the error bar in a figure are not significantly different(LSMEANS test at α=0.05). If four or more letters are needed for the multiple comparison of a mean,only the first and last letters are presented over the error bar. For example,‘f-i’represents ‘fghi’.
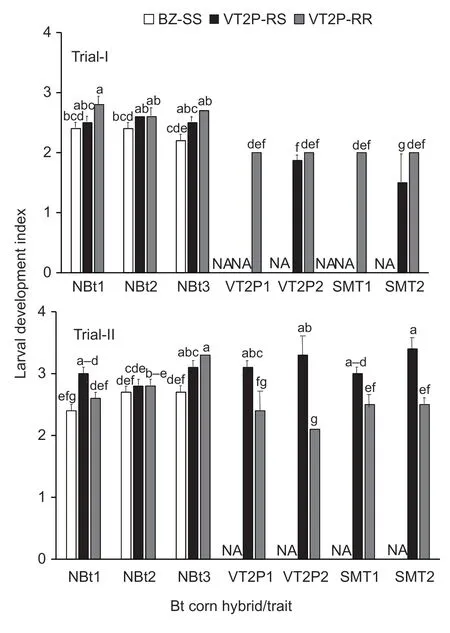
Fig. 2 Larval development index of susceptible,dual-gene heterozygous and resistant strains of Helicoverpa zea on non-Bt and five common pyramided Bt maize traits. BZ-SS was a known Bt susceptible laboratory H.zea population;VT2P-RR was a Cry1A.105/Cry2Ab2 dual-protein resistant population;and VT2P-RS was a heterozygous resistant population that was generated by reciprocal crosses between BZ-SS and VT2P-RR. Abbreviations for Bt corn hybrids/traits in the figure have been explained in Table 1. Values (mean±SEM) with the same letter above the error bar in a figure are not significantly different (LSMEAMS test at α=0.05). NA,data are not available.If four or more letters are needed for the multiple comparison of a mean,only the first and last letters are presented over the error bar. For example,‘a(chǎn)-d’ represents ‘a(chǎn)bcd’.
In Trial-II,the overall 10-d survivorship of VT2P-RR and VT2P-RS populations on VT2P and SMT hybrids was much greater than observed in Trial-I. BZ-SS survivorship ranged from zero for DKC 65-93 (VT2P1) to 7.4% for DKC 65-94 (SMT1). The differences were significant (P<0.05)among the four hybrids (Fig.1). Survivorship of VT2PRR on the four hybrids also varied significantly (P<0.05)and ranged from 25.0% for DKC 65-94 (SMT1) to 58.5%for DKC 67-42 (SMT2). VT2P-RR on the four hybrids had a significantly greater survivorship than BZ-SS in all cases. The average CR values of VT2P-RR based on the corrected survivorship on VT2P and SMT hybrids in Trial-II was 0.417 with a 95% confidence interval of 0.200 to 0.653,which again suggests that the dual-protein resistance in VT2P-RR was incomplete on VT2P and SMT hybrids,but the mean CR value measured in Trial-II was significantly greater than that observed in Trial-I(equality of variance:F3,3=14.83,P=0.0529;Student’st-test:tdf=6=-4.64,P=0.0035).
3.3.VT2P-RR was highly susceptible to Bt maize hybrids containing Vip3Aa20 protein
In Trial-I,no survivors of the threeH.zeapopulations were recovered from ears of the four hybrids expressing both Cry and Vip3Aa20 proteins (Fig.1). Similarly in Trial-II,no survivors were observed in ears of the four Cry/Vip3Aa20 hybrids that were infested with BZ-SS or VT2PRS (Fig.1). There were also no VT2P-RR survivors from ears of two of the four Cry/Vip3Aa20 hybrids,DKC 67-94(TER2) and PI 1622 VY HR (LEP). However,unlike in Trial-I,one 2nd instar and one 3rd instar VT2P-RR larvae survived in Trial-II on the other two Cry/Vip3Aa20 hybrids,DKC 65-99 (TRE1) and NK 1694-3111 (VPT),respectively,though the survivorship was not significant(P>0.05) from zero (Fig.1).
3.4.Larval development indices of VT2P-RR and VT2P-RS varied in the two trials
As mentioned above,across insect strains and trials,only two live larvae (one 2nd and one 3rd instar of VT2P-RR) were recovered from the four maize hybrids expressing both Cry and Vip3Aa20 proteins,and thus,these four hybrids were excluded in the ANOVA of larval development indices. Similarly,few larvae (1st or 2nd instars) of BZ-SS in the two trials survived on ears of the four hybrids containing VT2P or SMT trait,and larval development data of BZ-SS on the four VT2P and SMT hybrids were also not included in the data analysis.
ANOVA was performed on the remaining data in Trial-I,and the results showed that the effects on larval development indices were significant for the two main factors (F2,35=9.28;P=0.0006 for insect andF6,35=17.11;P<0.0001 for maize),but the interaction was not significant (F6,35=1.11;P=0.3763). The development index (2.8) of VT2P-RR on DKC 65-95 (VT2P1) was greater (P<0.05) than the indices of BZ-SS on non-Bt hybrids,and the index (2.2) of BZ-SS on NK 1694-GT non-Bt hybrid (NBt3) was significantly less than most of the indices of VT2P-RR and VT2P-RR on non-Bt hybrids (Fig.2). Relative to the larvae observed on non-Bt maize ears,development of the larvae on VT2P and SMT hybrids in Trial-I was delayed (P<0.05). All surviving larvae recovered from the four Bt hybrids were at the 2nd instar stage for the ears infested with VT2P-RR or 1st and 2nd instars for ears infested with VT2P-RS (Fig.2).
ANOVA for Trial-II showed that the effects of insect,maize,and interaction on insect development were significant (F2,44=24.73,P<0.0001 for insect;F6,44=3.25,P=0.0098 for maize hybrid;andF8,44=4.77;P=0.0003 for interaction). On non-Bt maize,development indices of VT2P-RR (3.3) and VT2P-RS (3.1) on NK 1694-GT (NBt3)were greater (P<0.05) than those of BZ-SS on non-Bt hybrids and VT2P-RR on DKC 65-93 (NBt1) (Fig.2).As shown in Trial-I in most cases,larval development of VT2P-RR on VT2P and SMT hybrids in Trial-II was delayed significantly (P<0.05). However,unlike in Trial-I,development indices of VT2P-RS larvae on VT2P and SMT hybrids in Trial-II were similar to the indices of the population on non-Bt maize ears (Fig.2).
3.5.Effective dominance of the dual-protein resistance in VT2P-RR on maize ears of VT2P and SMT hybrids varied from recessive to incompletely dominant
In Trial-I,DMLvalues of the dual-protein resistance in VT2P-RR on VT2P and SMT hybrids ranged from 0 on DKC 65-94 (SMT1) to 0.459 on DKC 67-44 (VT2P2)with an average of 0.234±0.095 (mean±SEM),indicating recessive to incompletely recessive resistance (Table 3).DMLvalues measured in Trial-II on VT2P and SMT hybrids varied from 0.078 on DKC 67-42 (SMT2) to 0.814 on DKC 67-72 (VT2P2) with an average of 0.394±0.195,indicating that the dual-protein resistance in VT2P-RR was incompletely recessive to incompletely dominant on these hybrids (Table 3). However,Student’st-test showed that the difference in the mean DMLvalues measured in the twotrials was not significant (equality of variance:F3,3=2.78,P=0.4230;t-test:tdf=6=-0.87,P=0.4159). As mentioned above,across insect populations and trials,only a total of two larvae (VT2P-RR) survived on the four hybrids containing both Cry and Vip3Aa20 proteins,and thus,the dual-protein resistance in VT2P-RR was functionally recessive (DML=0) on these pyramided hybrids expressing Cry and Vip3Aa20 proteins.
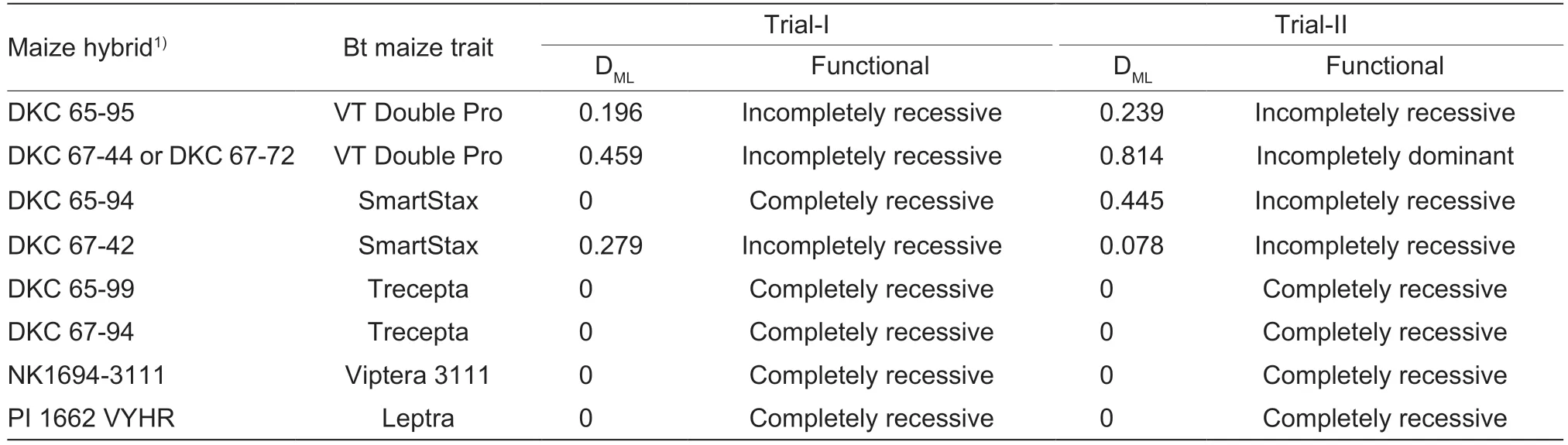
Table 3 Effective dominance levels (DML) of the dual-protein Bt resistance in Helicoverpa zea on pyramided Cry1A/Cry2A maize traits
4.Discussion
The high resistance levels of VT2P-RR to both Cry1A.105 and Cry2Ab2 exhibited in the current study demonstrated that the survival of fieldH.zeapopulations on the pyramided Cry protein maize was caused by resistance development. The Cry1A/Cry2A dual-protein resistant(VT2P-RR) and heterozygous (VT2P-RS) populations were virtually unable to survive on ears of the hybrids containing Vip3Aa20 protein. Prior to this study,at least three studies(Kauret al.2019;Yanget al.2019;Divelyet al.2021) had reported that maize hybrids expressing Vip3Aa20 protein were effective in controlling fieldH.zeapopulations where hybrids expressing Cry1A/Cry2A protein without Vip3Aa20 had control problems. Results of the current study with the known Cry protein resistant population,VT2P-RR,further showed that no cross-resistance existed between Vip3Aa20 and Cry1A.105/Cry2Ab2 inH.zea,and thus maize traits with Vip3Aa20 can be used to control the Cry resistance.However,because of the already widespread Cry1A and Cry2A resistance in the southern U.S.,the ‘pyramided’maize containing multiple genes ofvip3A,cry1Aand/orcry2Amay function just like a single-gene Bt trait in the region (Kauret al.2019). Consequently,resistance to Vip3A could evolve rapidly if effective measures for mitigating Cry protein resistance are not executed (USEPA 2018;Kauret al.2019;Niuet al.2021).
Results of a previous F2screen (Linet al.2022)showed that the frequency of major resistance alleles to Vip3Aa20 inH.zeapopulations in the southeastern U.S.was low,but minor Vip3Aa20 resistance alleles may be more common. Maize hybrids containing Vip3Aa20 protein in the U.S.are still effective for controllingH.zeain the field (USEPA 2018;Dimaseet al.2020);resistance ofH.zeato Vip3Aa20 maize with control problems has not been documented in the field. However,unexpected injury (UXI) in Vip3A cotton and maize fields caused byH.zeahas been observed on several occasions in recent years (USEPA 2018;Yanget al.2019;Huanget al.2022).Our on-going studies (Huanget al.2022) have shown thatH.zeapopulations collected from UXI plants in Louisiana,USA in 2021 did not show reduced susceptibilities to Vip3Aa protein and could not survive on ears of Vip3Aa20-expressed maize in laboratory bioassays. Linet al.(2022) indicated that minor Vip3Aa20 resistance alleles in field populations ofH.zeacould be involved in the UXIs observed in Louisiana. In the current study,maize hybrids expressing Vip3Aa20 were highly toxic toH.zea;all larvae of BZ-SS and VT2P-RS on ears of such hybrids were killed,while two VT2P-RR larvae(one 2nd and one 3rd instar) survived in Trial-II. The low survivorship of VT2P-RR in Trial-II was not statistically significant,but such a low survivorship (to 3rd instar)might still have important implications for resistance evolution. We believe thatH.zeaindividuals carrying major Cry1A/Cry2A resistance alleles,plus minor Vip3Aa resistance alleles might survive (at least to early instars)on pyramided maize traits expressing Cry and Vip3A proteins,and thus,to cause UXI in maize fields.
As a cross-crop pest,H.zeais targeted by both Bt cotton and Bt maize in the southern U.S.(Yanget al.2014;USEPA 2018). In the region where maize and cotton are planted together,H.zeaadults emerge from overwintering pupae in early spring and usually oviposit on weed hosts. The first-generation moths developing from larvae feeding on weed hosts prefer to lay eggs on young silks of ears at the R1 silking stage,and eggs on silks hatch in a few days. Thus,newly hatched larvae (neonates) can feed on silks that are still tender.Approximately 7-10 days after the silking stage,the silks start to dry and are no longer suitable forH.zeaoviposition. At that stage,young larvae (2nd-3rd) like to feed on ear tips and more developed larvae move down to eat ear kernels. Adults emerging from maize fields usually move to other crops,especially grain sorghum,soybean,and cotton,for 2-3 more generations (USEPA 2010;Yanget al.2014). Studies have shown that the sources ofH.zeapopulations in cotton fields mainly originate from adults emerging from maize fields (USEPA 2001;Jacksonet al.2008;Headet al.2010). Because of the short time window for oviposition on maize ears,early planting of maize is an effective cultural practice to reduceH.zeapopulations in the region (Reay-Jones 2019). In the current study,the overall survivorship of the threeH.zeapopulations on non-Bt maize in Trial-I (1.07±0.06 larvae/ear) was less than that in Trial-II (1.22±0.07),while the overall survivorship of VT2P-RR and VT2P-RS on VT2P and SMT was considerably greater in Trial-II than those in Trial-I. We believe that the use of ears at different plant stages between the two trials could be the reason for the observed differences in larval survivorship. As mentioned above,ears used in Trial-I were collected at the R2 plant stage,while ears used in Trial-II were approximately 1-week younger. If the observed differences were indeed caused by using different aged ears,it suggests that early planting of maize not only can reduce overallH.zeapopulations but also can enhance incompleteness of the Bt resistance. As a result,if susceptible populations are present,resistance alleles inH.zeapopulations moving from maize fields could be further diluted in cotton fields.
Incomplete resistance is a common phenomenon for Bt resistance and has been considered an important factor in delaying evolution of resistance to Bt crops (Carriéreet al.2006). Considering the delayed larval development of VT2P-RR on VT2P and SMT ears relative to non-Bt ears,the completeness of the dual-protein resistance could be even lower if survivorship was measured as neonate-to-pupation or to adult emergence. However,despite the resistance being noticeably incomplete,H.zeain the U.S.still evolved resistance to Cry1A/Cry2A maize in the field rapidly.
We believe that the non-recessive inheritance of the dual-protein resistance inH.zeahas been an important cause of the widespread Cry1A/Cry2A resistance. The dominance level of resistance has been documented to be an essential parameter affecting resistance evolution to Bt crops (Huanget al.2011;Tabashniket al.2013;Huang 2021a). For example,Huang (2021a) analyzed the dominance levels of 17 cases of major resistance to single-protein Bt crops which included six cases of field resistance with control problems. The results showed that all six cases with field resistance were associated with non-recessive resistance (or in other words with the use of non-high dose Bt maize) and their DMLlevels were significantly greater than the values of the 11 cases where field resistance has not occurred. Prior to the current study,DMLs to dual/multiple proteins on pyramided Bt crops had been investigated for only four cases (Huang 2021a),all associated withSpodoptera frugiperdato Bt maize in the Americas to Bt maize traits expressing Cry1A.105/Cry2Ab2,Cry1Ab/Vip3A,or Cry1A.105/Cry2Ab2/Cry1F proteins. The resistance in all four cases had not yet caused field control problems.Studies showed that the resistance in three of the four cases was completely recessive (DML=0) and one was incompletely recessive with a DMLof 0.20. In addition,a recent independent study (Santiago-Gonzálezet al.2022)reported that the effective dominance level of a Cry1A/Cry2A resistantH.zeapopulation from Texas was also incomplete with a DMLof 0.37. As shown in the current study and the study by Santiago-Gonzálezet al.(2022),the DMLvalues (averaged 0.31-0.37) for the dual-Cryprotein resistance inH.zeawere considerably greater than the values for the four cases withS.frugiperda.Additionally,many other factors may also be important for the rapid evolution of resistance to the Cry1A/Cry2A maize in the southern U.S.,such as prior selection,refuge non-compliance,cross-resistance,limited and similar modes of action in Bt cotton and Bt maize,sequential introduction of single Bt transgenes in pyramids,and the cross-cropping system in the region (Kauret al.2019).
5.Conclusion
The general conclusions of the two trials in the current study were consistent. Both trials showed that the resistance in VT2P-RR on ears of VT2P and SMT possessingcry1A.105andcry2Ab2genes was noticeably incomplete,and DMLvalues of the resistance varied among Bt maize hybrids.The Bt resistantH.zeapopulation,VT2P-RR,derived from collections from pyramided Cry1A/Cry2A maize,was highly resistant to the two Bt proteins,but survivorship of VT2P-RR larvae on ears of VT2P and SMT hybrids was considerably lower than on ears of non-Bt maize plants. Additionally,development of VT2P-RR larvae on VT2P and SMT ears was postponed relative to on non-Bt plants. Varied numbers of VT2P-RS larvae survived on ears of VT2P and SMT,but all larvae of the three populations (BZ-SS,VT2P-RS,and VT2P-RR) were virtually killed on the ears of maize plants expressing both Cry and Vip3Aa20 proteins. The results further validate that the field control problems of the maize plants containingcry1Aandcry2Agenes were caused by resistance evolution inH.zea. VT2P-RR was not crossresistant to maize hybrids with Vip3Aa20 protein,and thus,Vip3Aa20 maize traits can be used to control the Cry protein resistantH.zea. Effective dominance levels of VT2P-RR varied from recessive to incompletely dominant,depending on maize hybrids and trials,suggesting that proper selection of maize hybrids could be important for mitigating the Cry1A/Cry2A resistance. Data from this study should aid in modeling multiple-protein Bt resistance inH.zea.
Acknowledgements
This article is published with the approval of the Director of the Louisiana Agricultural Experiment Station as manuscript No.2022-234-37238. This project represents work supported by Bayer Crop Science (St.Louis,MO,USA),the Hatch funds from the USDA National Institute of Food and Agriculture,and the USDA Regional Research Project NC-246.
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2023年7期
Journal of Integrative Agriculture2023年7期
- Journal of Integrative Agriculture的其它文章
- Understanding changes in volatile compounds and fatty acids of Jincheng orange peel oil at different growth stages using GC-MS
- Untargeted UHPLC-Q-Exactive-MS-based metabolomics reveals associations between pre-and post-cooked metabolites and the taste quality of geographical indication rice and regular rice
- A double-layer model for improving the estimation of wheat canopy nitrogen content from unmanned aerial vehicle multispectral imagery
- The potential of green manure to increase soil carbon sequestration and reduce the yield-scaled carbon footprint of rice production in southern China
- lmprovement of soil fertility and rice yield after long-term application of cow manure combined with inorganic fertilizers
- A novel short transcript isoform of chicken lRF7 negatively regulates interferon-β production
