A cyclic effect of cAMP and calcium signaling contributes to jujube growth and development
WANG Li-xin,WANG Lin-xia,ZHANG Meng-ling,QU Ying-yue,YUAN YeEhsan SADEGHNEZHAD,GAO Meng-jiaoZHAO Ruo-yuQl Chao-fengGUO Xiao-xueZHU Wen-huiLl Rui-meiDAl Li,LlU Meng-jun,LlU Zhi-guo#
1 College of Horticulture,Hebei Agricultural University,Baoding 071001,P.R.China
2 Research Center of Chinese Jujube,Hebei Agricultural University,Baoding 071001,P.R.China
3 Key Laboratory of Resource Biology and Biotechnology in Western China,Ministry of Education/College of Life Sciences,Northwest University,Xi’an 710069,P.R.China
4 Department of Plant Biology,Faculty of Biological Sciences,Tarbiat Modares University,Teheran 14115,Iran
Abstract 3′,5′-Cyclic adenosine monophosphate (cAMP) is an important metabolite that is specifically enriched in jujube.However,the effect of cAMP on jujube cellular responses has not been comprehensively studied. Here,we established jujube cell suspension cultures and investigated the calcium influx in response to cAMP treatment through protoplast isolation and fluorescence intensity. Firstly,cAMP treatment could promote jujube growth and increase the content of endogenous cAMP. Using transcriptome analysis with transgenic Arabidopsis plants overexpressing adenylate cyclase(ZjAC) as a positive control,we identified 60 calcium-related differential expressed genes (DEGs) that contributed to the calcium signaling and inter-or intra-cellular responses. Pharmacological treatments such as cAMP and the calcium ionophore A23187 could induce ZjAC expression,the accumulation of cAMP and calcium influx in jujube cells,while ethylene glycol tetraacetic acid (EGTA) or bithionol treatment inhibited these changes. Moreover,the calcium channels and transporters in calcium influx,such as the ZjCNGC2 channel and the mitogen activated protein (MAP) kinase pathway,could be activated by cAMP treatment. In summary,our findings demonstrated that cAMP biosynthesis is dependent on calcium influx and the amplifying effect between calcium and cAMP may be involved in intracellular signal induction,which might contribute to the growth and development of jujube.
Keywords: cAMP,calcium,growth,suspension cells,protoplast,signaling,jujube
1.lntroduction
3′,5′-Cyclic adenosine monophosphate (cAMP) is a cyclic nucleotide monophosphate that is synthesized from adenosine triphosphate (ATP) by adenylate cyclase (AC)viaa one-step pathway,with the release of pyrophosphate (Moederet al.2013). cAMP has been widely shown to participate as a second messenger molecule in intracellular signal transduction in prokaryotes and eukaryotes (Assmann 1995;Gancedo 2013;Gehring and Turek 2017). However,there are some challenges in investigating the role of cAMP in plants. In particular,the main constraint is the low concentration of cAMP in plants,which is at the nanomolar range based on fresh weight,while animals and lower eukaryotes have high concentrations of cAMP at the nano-to micromolar range(Gehring and Turek 2017). Therefore,the regulation of cAMP synthesis in plants has not been comprehensively studied.
Currently,analytical techniques such as highperformance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) allow us to sensitively and accurately measure the cAMP concentration in different plant species. For instance,the concentration of cAMP was determined in BY-2 suspension cells by LC/ESI-MS and radioimmunoassay analysis,and the transient peak of cAMP observed in the S and G1 phases of the cell cycle could be inhibited by prostaglandin treatment,demonstrating that cAMP might function as a signaling molecule in the cell cycle (Ehsanet al.1998;Richardset al.2002). InCupressuslusitanicacell cultures,cAMP signaling was associated with the protein kinase pathway that is involved in the elicitorinduced accumulation of β-thujaplicin (Zhaoet al.2004).
As a second messenger,calcium (Ca2+) acts in plant growth and development and in the responses to various biotic and abiotic stresses (Kudlaet al.2018;Wanget al.2020b),and it can be rapidly transported to the cytosol by Ca2+channels. Cyclic-nucleotide-gated channels(CNGCs) are cation transporting channels that are located on the cell membrane and can transmit sodium,Ca2+,and potassium to the cytosol;and they are regulated by internal second messengers such as cyclic nucleotide monophosphates (3′,5′-cAMP and 3′,5′-cGMP) (Ma and Berkowitz 2011;Charpentieret al.2016;Jhaet al.2016;Duszynet al.2019). cAMP can bind to CNGCs to regulate the cytosolic Ca2+concentration and signal transduction in response to biotic and abiotic stresses(Zelmanet al.2012;Alqurashiet al.2016). For instance,cAMP can be involved in tabtoxinine-β-lactam-induced cell death (Itoet al.2014),potassium homeostasis by CNGC channels (Al-Youniset al.2018),the response to mechanical damage (Swiezawskaet al.2014) and the defense against biotrophic and hemibiotrophic plant pathogens (Bianchetet al.2019). Moreover,the exogenous application of cAMP inArabidopsiscells led to proteomic changes in response to cold and salinity stresses,indicating that cAMP is sufficient to elicit specific stress responses (Alqurashiet al.2016). The relationships between cAMP and either Ca2+or potassium have also been studied in different biological processes(Gehring 2010;Luet al.2016),and cAMP can regulate stomatal closure and potassium exchange (Curvettoet al.1994;Liet al.1994). In addition to cAMP and Ca2+signaling,mitogen-activated protein kinase (MAPK)cascades also play vital roles in signal transduction in response to a wide range of biotic and abiotic stresses (Liu Zet al.2020). However,the complex network of cAMP signaling involved in plant growth and development has not been systematically defined.
Jujube (ZiziphusjujubaMill.),which belongs to the family Rhamnaceae,is the most dominant and characteristic fruit tree species in China. Jujube is stress resistant and rich in nutrients (Liuet al.2014;Liu Met al.2020). An extremely high content of cAMP in the mature fruits of jujube was reported by Japanese scientists (Cyong and Hanabusa 1980). Later studies determined that the content of cAMP in mature jujube fruit (302.50 nmol g-1FW) was the highest among 14 horticultural plants,and it was even more than 10-fold higher than the level in animal livers (Liu and Wang 1991;Liu Met al.2020;Liuet al.2023). Therefore,jujube cells could be an ideal material for studying the biosynthesis and signaling function of cAMP in plants. Suspension cell lines are good model systems for research at the inter-and intracellular levels.These cell lines are also useful systems for efficiently producing metabolites such as alkaloids,proteins,carbohydrates,and natural pigments (Xuet al.2018).Therefore,establishing a jujube suspension cell line and isolating protoplasts are necessary in order to investigate the cAMP-related metabolic pathways,especially the biosynthetic mechanisms and regulatory signaling pathways of cAMP,which remain unclear.
In this study,we examined the effect of cAMP on growth and calcium influx,as well as the activities of calcium transporters and MAP kinase. The suspension cell lineZ.jujuba‘Wuhe 76’ was established,and protoplasts were isolated,providing ideal tools with which to study the relationship between cAMP synthesis and Ca2+signaling. We also conducted a transcriptome analysis of theZjACoverexpressedArabidopsisplants,and identified 60 calcium-related DEGs. Taken together,these results could provide a theoretical basis for the jujube-specific compound cAMP-regulated signaling in the growth and development of jujube.
2.Materials and methods
2.1.Plant materials and cAMP treatment
Sour jujube seeds with uniform size and peel color were selected and planted. After soaking the seeds with water for 18 h,they were transferred into a 28°C incubator for 5 d with a photoperiod of 16 h light and 8 h dark. Then,seedlings with a 2-cm bud gemination were selected for sowing,and when the seedlings grew to the 6-leaf stage,the seedlings were divided into four groups for the treatments. In one treatment group,the plants were irrigated three times once per month with different concentrations of cAMP (25,50,and 100 μmol L-1). In another treatment group,different concentrations of cAMP (50,100,and 200 μmol L-1) were sprayed to the plants six times twice per month. The other two groups were treated with water as negative controls. For each treatment,24 sour jujube plants were treated with three biological replicates. The plant height,fresh weight without roots and shoot diameter were measured in the irrigated plants. The fruiting seedling rate and cAMP content were measured for the sprayed plants.
2.2.Transcriptome analysis of ZjAC1 and ZjAC2 overexpressed Arabidopsis plants
35S::ZjACs-GFPoverexpressedArabidopsisplants were generated for the transcriptome analysis as described in our previous study (Liuet al.2023). Briefly,after growth on 0.5× MS medium for 5 d,theArabidopsisseedlings were transferred into soil,and the transgenic lines and wild type were collected at 13 and 24 d for the transcriptome analysis.
The transcriptome analysis was performed according to Gaoet al.(2021). Briefly,the analysis of differentially expressed genes (DEGs) was performed using the DESeq2 R package. DEGs were selected as those withP-values≤0.05 and |log2FC|≥2. Gene Ontology (GO)enrichment analysis of the DEGs was implemented by Goatools (https://github.com/tanghaibao/Goatools).
2.3.Establishment of the jujube suspension cell line
Jujube (Z.jujubaMill.) ‘Wuhe 76’ was grown in the Jujube Germplasm Resources Nursery of Cangxian,Hebei,China. On July 2nd,2019,young fruits of ‘Wuhe 76’ were collected and taken from the nursery to the laboratory in an ice box. After a series of sterilization steps,the embryos were first cultivated in callus-inducing medium for two months. The medium was prepared using Murashige&Skoog Basal Medium powder (MS,4.414 g L-1),maltose (20 g L-1),agar (5.5 g L-1),1-naphthaleneacetic acid (NAA,0.05 mg L-1,Solarbio,China),and thidiazuron(TDZ,0.3 mg L-1,Solarbio,China),and adjusted to pH 5.8.Then,the calli were transformed for another two months in proliferation medium composed of: MS (4.414 g L-1),maltose (20 g L-1),agar (5.5 g L-1),NAA (0.5 mg L-1),and TDZ (3.0 mg L-1) at pH 5.8. The calli were kept at 25°C in darkness.
To establish the jujube suspension cell culture,we selected vigorous,light yellow,and fragile calli and cultivated them in liquid medium. The medium contained MS salts (4.414 g L-1),maltose (20 g L-1),NAA (0.5 mg L-1),and TDZ (3.0 mg L-1). The pH was adjusted to 5.8 for cell culture. The suspension cells were incubated at 25°C in darkness on an orbital shaker (Bluepart,THZ-98C,China) at 120 r min-1. The cells were subcultured every 7 d by inoculating 7 mL of stationary cells into 23 mL of fresh medium in 100 mL Erlenmeyer flasks.After 5 to 6 subcultures,a stable cell line was established and used for subsequent experiments.
To determine the cell proliferation and growth rates according to the fresh weight,suspension cells (2 mL)were sampled every 2 d after subculturing (Xuet al.2018) and centrifuged at 3 000 r min-1for 5 min. The supernatant was discarded to obtain the fresh weight of cells in at least three biological replicates. For the cell mortality assay,suspension cells (100 μL) were sampled every 2 d after subculturing and stained with 2.5% trypan blue (Wanget al.2019). The cells were counted under a 20× bright light microscope (Zeiss,Germany). For the packed cell volume (PCV) measurement,one flask of suspended cells was aliquoted into Falcon tubes(100 mL) every 2 d and held in a 4°C refrigerator for 3 d.The increase in volume compared to that on d 0 was calculated (Liuet al.2013).
2.4.Pharmacological treatments of the cell line
On the 3rd d after subculturing,the Ca2+ionophore A23187 (Sigma,China) (Wang and Nick 2017;Wanget al.2019) and cAMP (Sigma,China) were applied to the cells at concentrations of 20 and 100 μmol L-1,respectively. The cells were frozen in liquid nitrogen and stored at -80°C for cAMP measurement and RNA extraction. As a negative control,cells were treated with the same concentrations of the solvent dimethyl sulfoxide(DMSO) (Solarbio,China).
2.5.lsolation of protoplasts from the cell lines and viability tests
To obtain highly active protoplasts of jujube,Z.jujubaMill.‘Wuhe 76’ cells were treated with 1.5% cellulase and 1.0%macerozyme for protoplast isolation (Appendix A). The osmotic pressure was adjusted with 0.7 mol L-1mannitol,and enzymatic hydrolysis was performed in a 40 r min-1shaker for 16 h at room temperature. The protoplasts were observed and counted under a microscope. When the concentration of cellulase was 1.5%,the yield number and activity of protoplasts peaked at a macerozyme concentration of 1.0%,with values of 26.89×105and 74.02%,respectively (Appendix B). The fluorescein diacetate (FDA) method was used to determine protoplast viability. According to this method,we pipetted 100 μL of protoplasts into a 1.5-mL tube,added 2.4 μL of FDA reagent (5 mg of FDA dissolved in 1 mL of acetone),and kept the mixture in the dark at room temperature for 5 min.Finally,we observed the green fluorescence under an inverted fluorescence microscope with green fluorescent protein (GFP) excitation (Appendix A) (Proffittet al.1996).
2.6.Calcium staining with fluo-4/AM and fluorescence quantification
To detect changes in the calcium signal in cells,fluo-4/AM dye was applied to the isolated protoplasts. Aliquots of 99 μL of isolated protoplasts were incubated together with 1 μL of fluo-4/AM (Dojindo Laboratories,Kumamoto,Japan) for 30 min at 37°C,with a final fluorescent dye concentration of 5 μmol L-1. Then,the samples were washed three times using basic solution to remove excess fluorescent dye. The fluorescence was observed with an inverted fluorescence microscope with GFP excitation,and fluorescence quantification was performed using ImageJ Software (Media Cybernetics,Inc.,MD,USA). Briefly,we selected the fluorescent cells with the circle drawing/selection tool and measured the area,integrated density and mean gray value. Then,we selected three regions next to the fluorescent cell that had no fluorescence to obtain the related background values.Finally,the corrected total cell fluorescence (CTCF) was calculated by the following formula: CTCF=Integrated density-(Area of selected cell×Mean fluorescence of background readings). For each time point and treatment,at least 20 protoplasts were measured.
To investigate the effect of cAMP treatment on Ca2+signaling,two different concentrations of cAMP (50 and 100 μmol L-1) were added to the isolated protoplasts. The protoplasts were sampled at 5,10,15 and 20 min after treatment and evaluated using the fluo-4/AM staining method and image quantification. To evaluate the effect of A23187 on Ca2+influx,5 and 50 μmol L-1A23187 were added to the isolated protoplasts. The protoplasts were sampled at 5,10,15 and 20 min after treatment and quantified using the fluo-4/AM staining method and ImageJ Software. For the EGTA or bithionol treatment,10 mmol L-1EGTA or 10 μmol L-1bithionol were added to the isolated protoplasts,respectively. The protoplasts were sampled at 5,10,15 and 20 min after treatment and evaluated using the fluo-4/AM staining method and image quantification. Then,for an additional cAMP treatment,the cells were pretreated with either 10 mmol L-1EGTA for 5 min or 10 μmol L-1bithionol for 30 min. Afterward,50 μmol L-1cAMP was added to the isolated protoplasts to perform the fluorescence density test. For each time point and treatment,at least 20 protoplasts were measured.
2.7.Measurement of the cAMP content
To measure the cAMP content in the jujube samples,suspension cells (0.1 g) and fruit samples (1 g) were ground and mixed with sterile water (w/v,1:5). The extracts were incubated at 60°C for 3 h and then centrifuged. The supernatant was filtered through a 0.22-μm filter and injected into an Agilent 1200 Series HPLC system (Agilent,USA) for cAMP detection. An isocratic method was used with methanol-potassium dihydrogen phosphate (v/v,1:9) as the mobile phase on an Agilent XDB-C18 chromatographic column (4.6 mm×250 mm,5 μm) at 30°C and with a detection wavelength of 254 nm.The flow rate and the injection volume were 0.8 mL min-1and 20 μL,respectively. The retention time was 4.6 min.
For the preparation of standard solutions,50 mg of cAMP standard was dissolved in 50 mL of water to yield a final concentration of 1.0 mg mL-1;and then different concentrations of standards of 0.1,0.01,0.02,0.001,0.002,and 0.0003 mg mL-1were prepared to generate the standard curve in order to obtain a regression equation that could be used to calculate the contents of cAMP.
2.8.RNA extraction and quantification of gene expression
Total RNA extraction from the samples and subsequent cDNA synthesis were performed as previously described(Gaoet al.2021). qPCR was carried out on a Bio-Rad iQ? 5 using TransStart Top Green qPCR SuperMix AQ131 (TransGen Biotech,China). The 20 μL reaction system consisted of 10 μL of 2× SYBR Premix ExTaq?,0.4 μL of each primer (10 μmol L-1),1 μL of diluted cDNA,and 8.2 μL of ddH2O. The thermal profile was as follows:preincubation at 94°C for 3 min,followed by 40 cycles of 5 s at 94°C,15 s at 55-63°C,and 15 s at 72°C. The relative expression levels ofZjACwere calculated with the 2-ΔΔCtmethod (Livak and Schmittgen 2001),usingZjActinas an endogenous control for normalization (Buet al.2016). The normalized expression levels ofZjCNGC2,ZjMAPK2,ZjMAPKK2,andZjMAPKK4were calculated with the 2-ΔCtmethod. The nucleic acid sequences are shown in Appendices C and D,and the primer sequences used for qPCR of the genes are shown in Table 1.
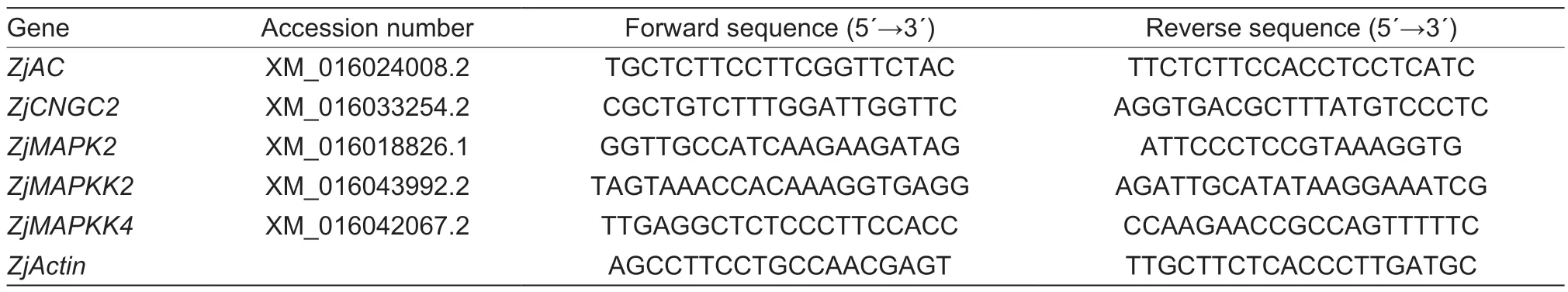
Table 1 The primer sequences of the relevant genes for qPCR
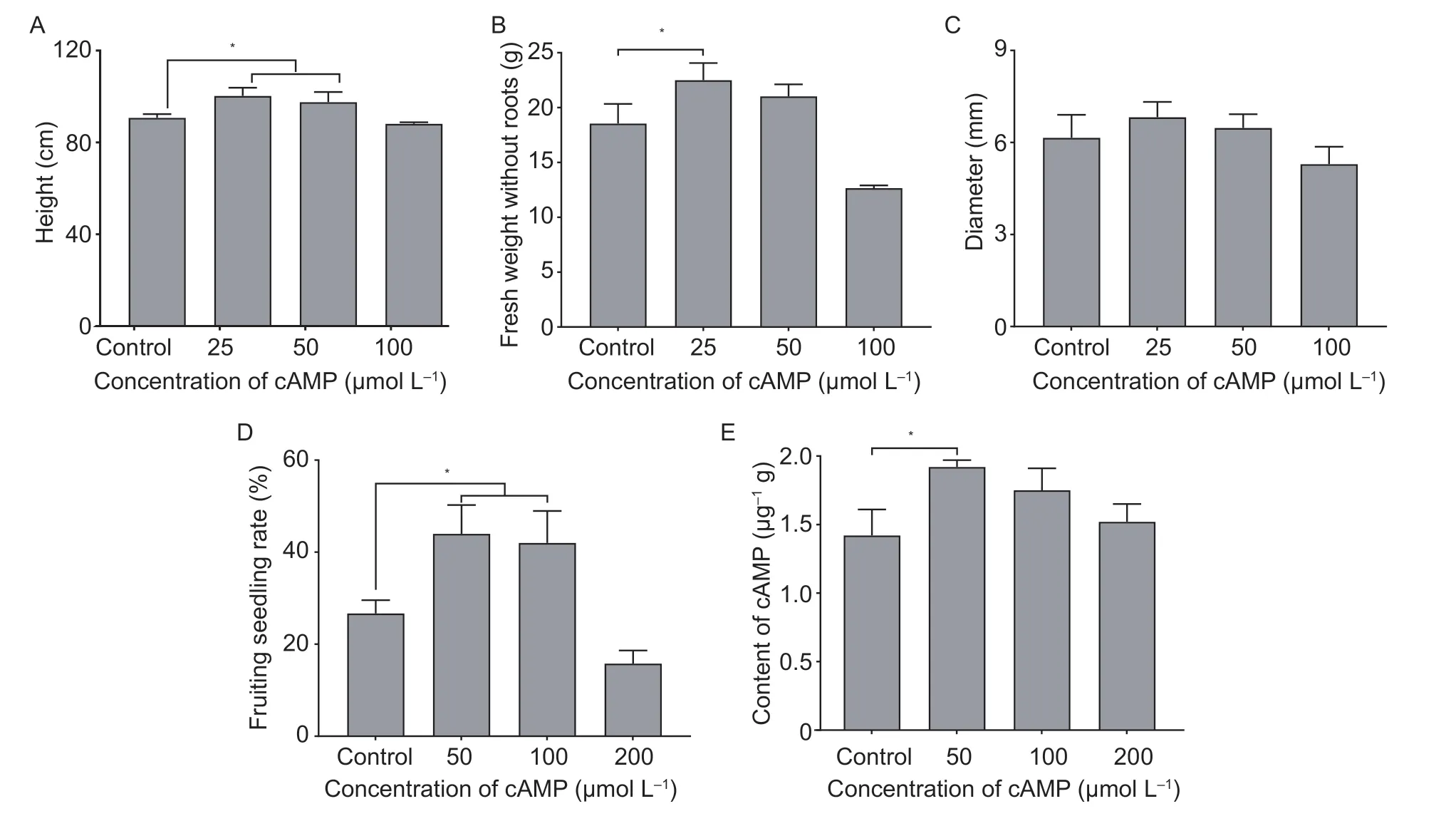
Fig. 1 Effects of the exogenous application of cAMP on plant height (A),fresh weight without roots (B),shoot diameter (C),fruiting seedling rate (D) and content of cAMP (E) in sour jujube. Data represent the mean value±SE from three independent replications.* indicates statistically significant differences at the confidence level of P<0.05.
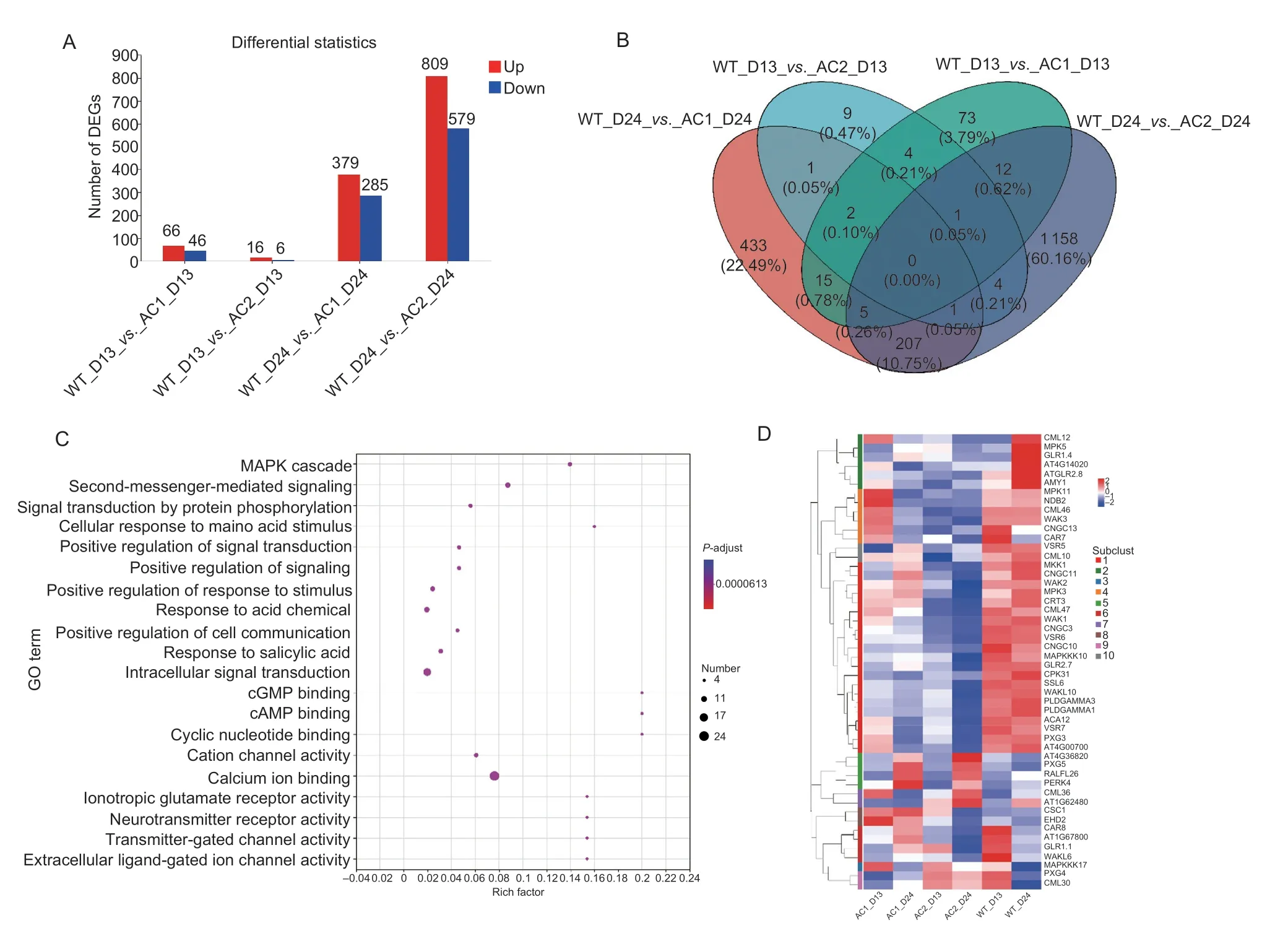
Fig. 2 Differentially expressed genes (DEGs) and heat map analysis in transgenic lines of Arabidopsis overexpressing ZjAC1 and ZjAC2 at the early and late growth stages. A,up-and down-regulated genes in the comparisons of wild type with transgenic lines.B,the Venn diagram. C,the GO enrichment of 20 important terms among 60 DEGs which are involved in calcium signaling. D,heat map analysis of the 60 DEGs according to the K-means clustering method during the developmental time course in ZjAC1/ZjAC2 overexpressed Arabidopsis and WT. The heatmap color scale changes from red to blue for the up-and down-regulated genes,respectively. Different colors indicate the different levels of gene expression based on the log10(TPM+1) values.
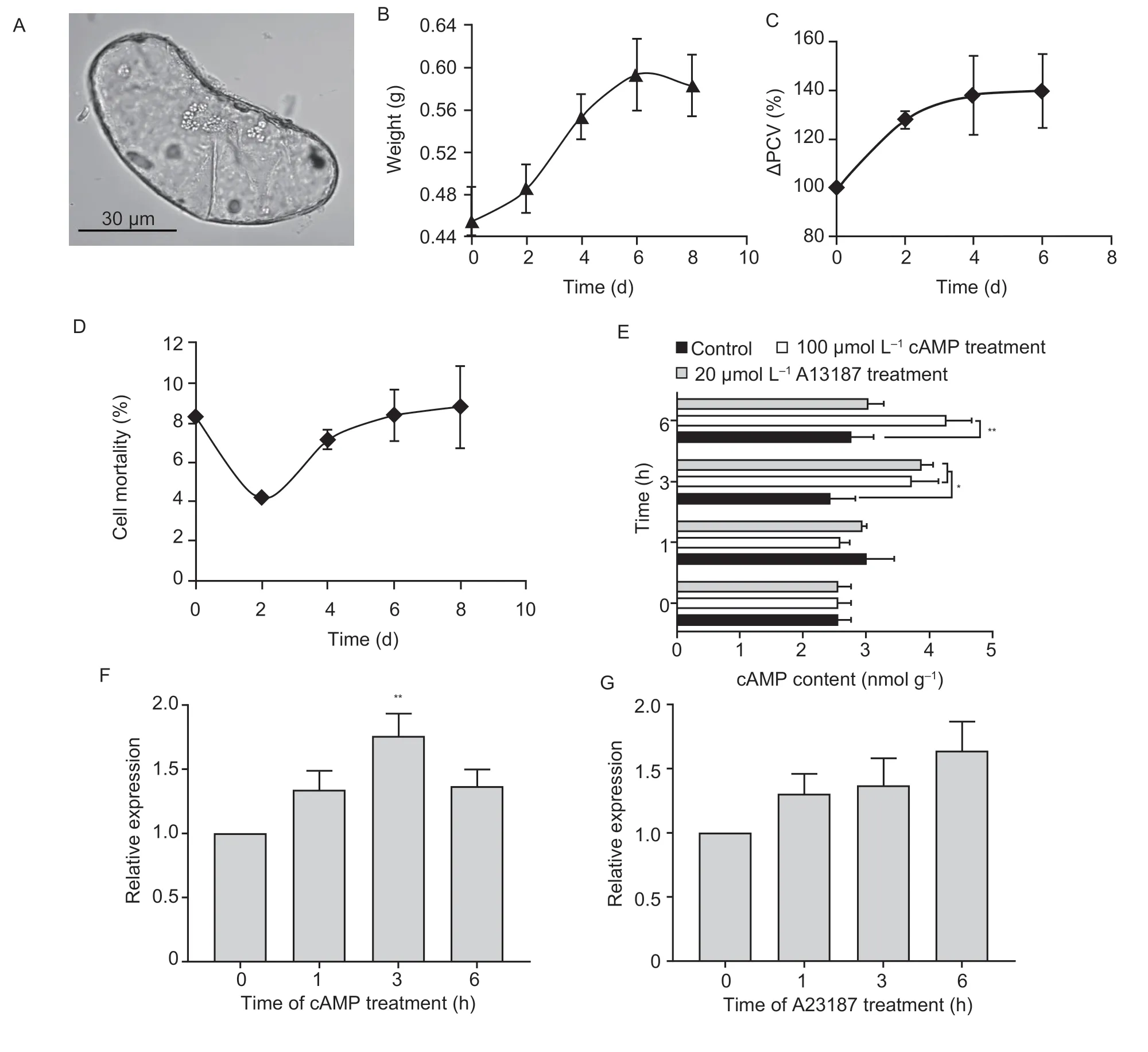
Fig. 3 Establishment of a jujube suspension cell line,and cAMP or A23187 treatment for ZjAC expression analysis. A,the shape of a single cell of the suspension cell line;bar=30 μm. B,time course of cell line growth indicated by changes in fresh weight. C,time course of ΔPCV relative to that on d 0 (100%). D,time course of cell mortality during the growth and development of the cell line.E,time course of changes in the cAMP content after cAMP or A23187 treatment. F,relative expression level of ZjAC in response to cAMP treatment. G,relative expression level of ZjAC in response to A23187 treatment. Three independent replications were performed for the measurement of each parameter,and the error bars represent the standard errors (SE) (n=9). * and ** indicate statistically significant differences at confidence levels of P<0.05 and P<0.01,respectively,by a one-sided t-test.
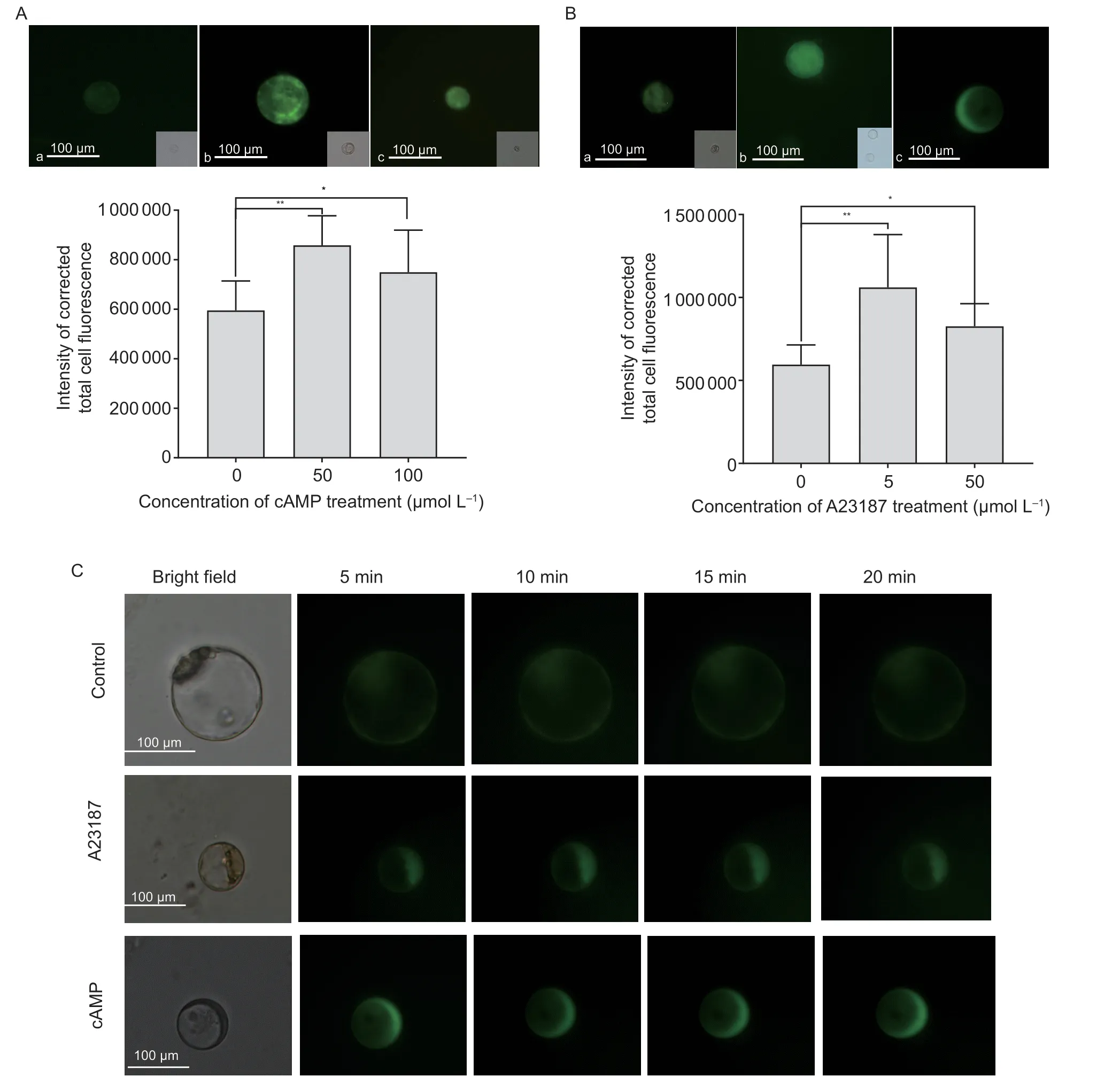
Fig. 4 Effects of different concentrations of cAMP or A23187 on calcium changes in protoplasts. A,protoplast loading with 5 μmol L-1 fluo-4/AM after 0 (a),50 (b) and 100 (c) μmol L-1 cAMP treatments for 15 min. B,protoplast loading with 5 μmol L-1 fluo-4/AM after 0 (a),5 (b) and 50 (c) μmol L-1 A23187 treatments for 15 min. The fluorescence intensity was analyzed by ImageJ Software. Data represent the mean value±SE from three independent replications with at least 20 protoplasts each. * and **indicate statistically significant differences at confidence levels of P<0.05 and P<0.01,respectively,by a one-sided t-test. C,time course of fluorescence changes in a single cell after cAMP and A23187 treatments. Upper row: intact protoplast under the control condition loaded with 5 μmol L-1 fluo-4/AM. Middle row: protoplast loaded with 5 μmol L-1 fluo-4/AM after treatment with 5 μmol L-1 A23187. Lower row: protoplast loaded with 5 μmol L-1 fluo-4/AM after 50 μmol L-1 cAMP treatment. At least 20 protoplasts were observed for each treatment.
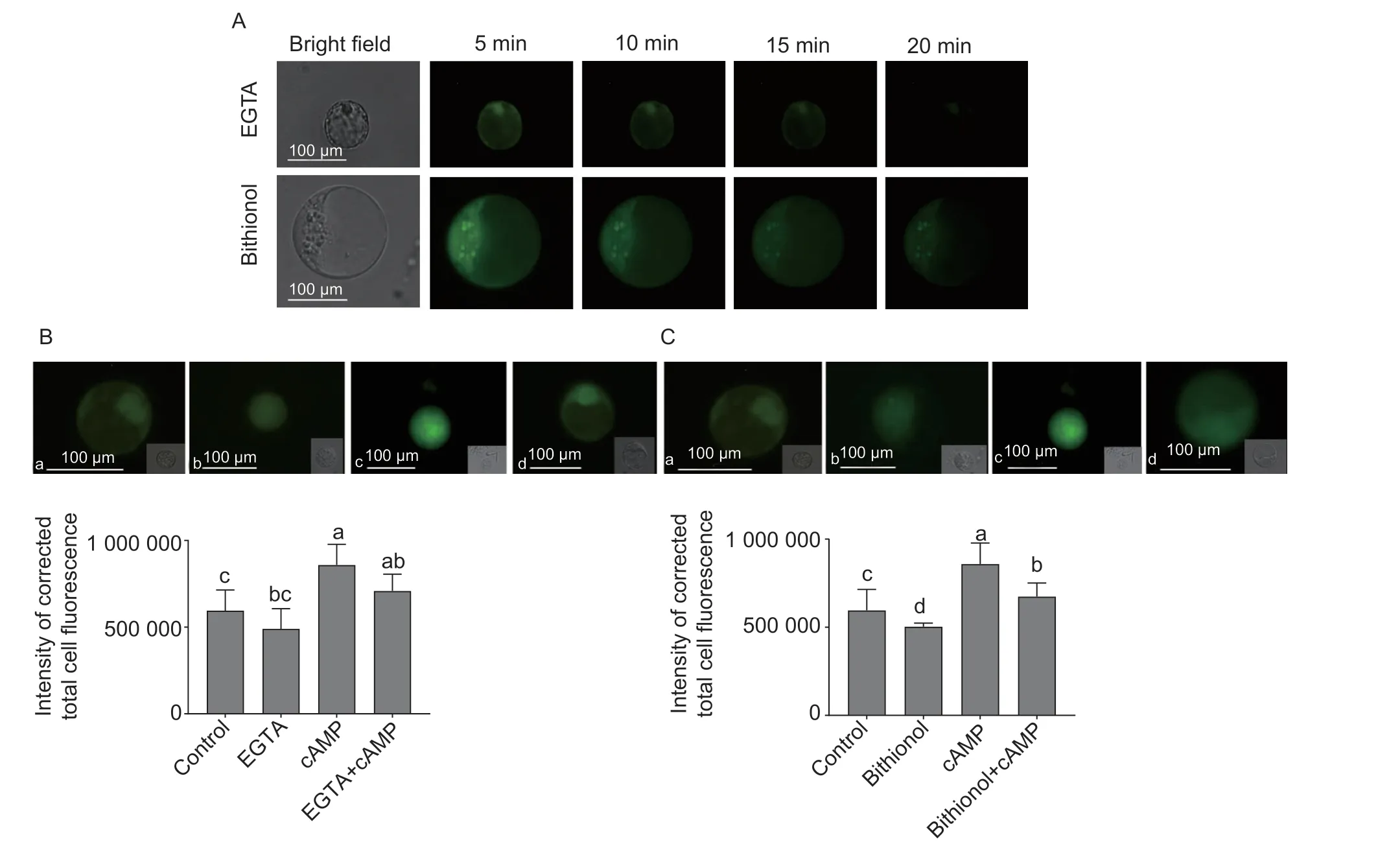
Fig. 5 Effects of EGTA,EGTA+cAMP,bithionol,bithionol+cAMP,and cAMP treatments on calcium changes in protoplasts. A,time course of fluorescence changes in a single cell after EGTA or bithionol treatment. B,protoplast loading with 5 μmol L-1 fluo-4/AM after 10 mmol L-1 EGTA (b),50 μmol L-1 cAMP (c) and EGTA+cAMP (d) treatments for 15 min. C,protoplast loading with 5 μmol L-1 fluo-4/AM after 10 μmol L-1 bithionol (b),50 μmol L-1 cAMP (c) and bithionol+cAMP (d) treatments for 15 min. a,negative control. The fluorescence intensity was analyzed by ImageJ Software. Data represent the mean value±SE from three independent replications with at least 20 protoplasts in each treatment. Values followed by different letters in a column represent significantly different levels at P<0.05.

Fig. 6 Expression of ZjCNGC2 in response to treatments with cAMP (A) and A23187 (B),and expression levels of ZjMAPK2,ZjMAPKK2 and ZjMAPKK4 in response to either treatment (C). Data represent the mean value±SE from three independent replications. * and ** indicate statistically significant differences at confidence levels of P<0.05 and P<0.01,respectively,by a onesided t-test.
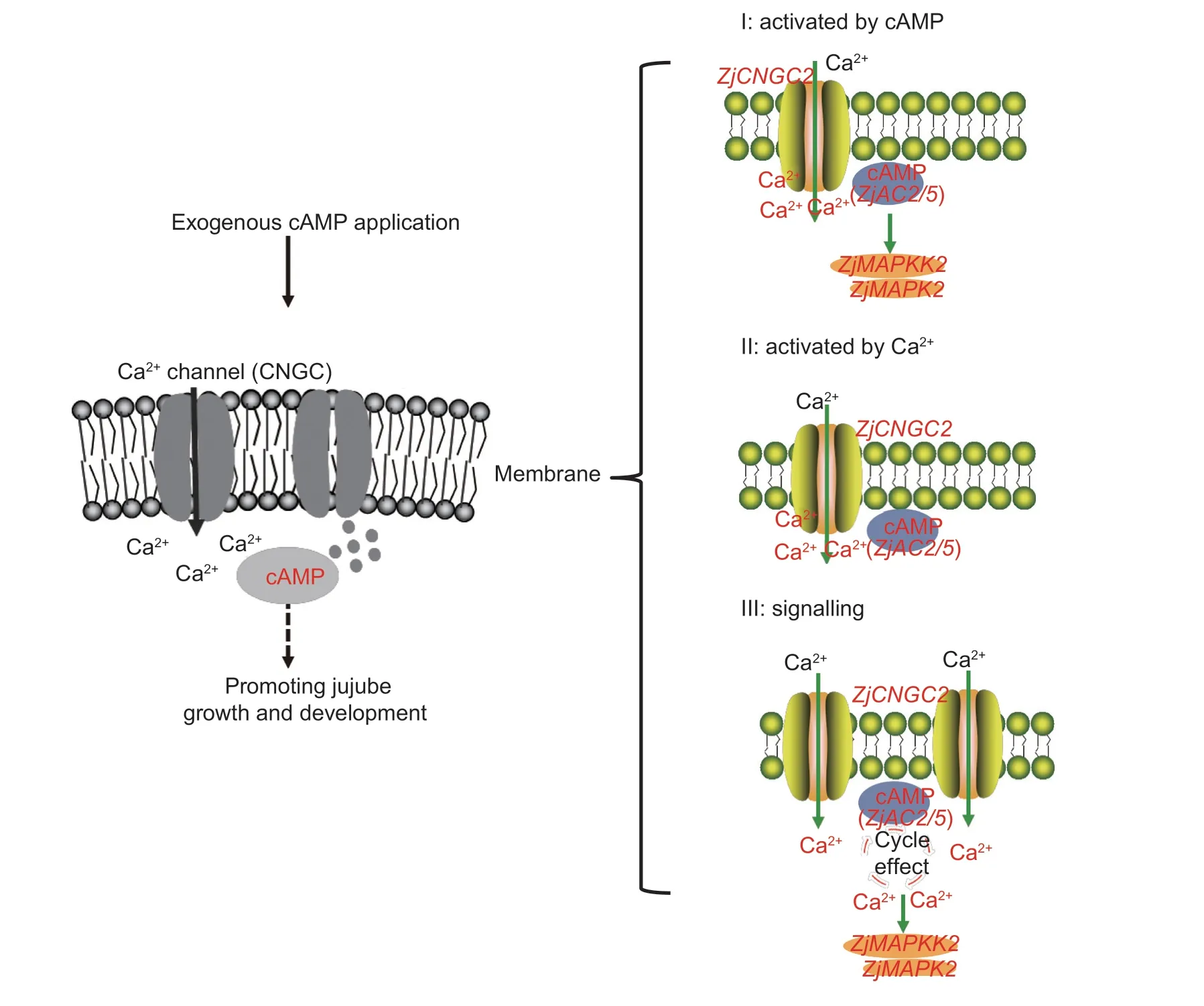
Fig. 7 Schematic models of the contributions of cAMP-Ca2+ signaling to jujube growth. Model I,cAMP treatment could activate ZjCNGC2 to induce the rapid influx of Ca2+ into the cytosol and it could induce an increase in cAMP in the cytosol by activating the expression of ZjAC. In addition,it could also induce the expression of downstream genes,such as ZjMAPKK2 and ZjMAPK2,to transfer the signals to the nucleus. Model II,calcium treatment could significantly induce the synthesis of cAMP with the rapid induction of ZjAC,and it could induce the expression of ZjCNGC2 but have no effect on MAPKs. Model III,under the effect of cAMP in the process of jujube growth,rapid calcium signaling could be observed first,and the influx of this ion into the cytosol might occur via the ZjCNGC2 channel. Then,rapid calcium accumulation in the cytosol could induce the expression of ZjAC to induce the synthesis of cAMP,which could then further transfer the signals to MAPKs,such as ZjMAPKK2 and ZjMAPK2. Calcium treatment could also induce an increase in the cAMP level,demonstrating that cAMP and Ca2+ together have multiple cycle-amplifying effects and function as important signaling molecules to transduce the signal. cAMP,3′,5′-cyclic adenosine monophosphate;CNGC,cyclic-nucleotide-gated channel;Ca2+,calcium.
2.9.Statistical analysis
The figures were drawn using GraphPad Prism 7.0 Software (GraphPad Software,Inc.,La Jolla,CA,USA).Student’st-test was employed to analyze the significance of differences among the experimental groups.
3.Results
3.1.cAMP promotes growth indexes and seedling fruiting of sour jujube in a concentration-dependent manner
In order to understand whether cAMP could regulate plant growth,different concentrations of cAMP were applied to sour jujube and the plant height,fresh weight without roots and shoot diameter were measured. As shown in Fig.1-A-C,after cAMP treatment,the height of sour jujube increased significantly at the concentrations of 25 and 50 μmol L-1,and the fresh weight without roots also increased at the concentration of 25 μmol L-1,while the shoot diameter was not significantly affected. These results indicated that cAMP has important functions in the growth process of sour jujube.
The effect of cAMP on fruit and its endogenous cAMP were also determined. More seedlings with fruit were observed under the 50 and 100 μmol L-1cAMP treatments compared to the control group. The fruiting seedling rate was improved by about 15% (Fig.1-D). In addition,exogenous cAMP treatment could increase the content of cAMP in the fruit of sour jujube at the full red stage(Fig.1-E). Thus,the exogenous application of cAMP has positive effects on the fruiting seedling rate and cAMP content in sour jujube.
3.2.Transcriptome analysis of ZjAC overexpressed Arabidopsis confirmed the contribution of adenylate cyclase to calcium signaling in Chinese jujube
AC is the only key enzyme of cAMP synthesis,and fourACswere identified in Chinese jujube. Overexpression of theseZjACsin jujube fruit andArabidopsiscan increase the content of cAMP and promote the growth ofArabidopsis(Liuet al.2023),which were consistent with the effects of exogenous cAMP application in sour jujube. Therefore,in order to understand the underlying molecular mechanism,we obtained the transgenic lines ofArabidopsisplants overexpressingZjAC1andZjAC2,and performed transcriptome analysis at the early (13 d)and late growth stages (24 d). As shown in Fig.2-A,the total number of DEGs in groups WT_D13_vs._AC1_D13,WT_D24_vs._AC1_D24,WT_D13_vs._AC2_D13 and WT_D24_vs._AC2_D24 were 112,664,22 and 664,respectively. Among them,the up-regulated genes in the four groups outnumbered the down-regulated genes during the growth process ofArabidopsis. The number of DEGs increased significantly,especially at the d 24 stage.In addition,the pairwise comparison analysis showed that 90 and 642 unique DEGs were found in the WT_D13_vs._AC1_D13 and WT_D24_vs._AC1_D24 groups,respectively;while 16 and 558 unique DEGs were found in the WT_D13_vs._AC2_D13 and WT_D24_vs._AC2_D24 groups,respectively (Fig.2-B). This differential and unique number of DEGs might contribute to the molecular mechanism that drives the cAMP increment in the plants and promotes plant growth and development.
As calcium signaling plays an important role in cAMP regulation,the 60 DEGs involved in calcium signaling were identified in the above DEGs. GO enrichment analysis revealed that these DEGs were involved in calcium ion binding (GO:0005509),intracellular signal transduction (GO:0035556),second messenger mediated signaling (GO:0019932),cAMP binding (GO:0030552),and other processes (Fig.2-C). Moreover,the expression levels of these DEGs are shown in Fig.2-D,and someCNGCs(cyclic nucleotide-gated channel),CMLs(calcium-binding protein),CARs(Protein C2-DOMAIN ABA-RELATED) were down-regulated in theZjAC1andZjAC2overexpressedArabidopsis,while the expression ofCSC1(Hyperosmolality-gated Ca2+permeable channel) andCML36were up-regulated. Thus,we speculated that the cAMP increment inArabidopsiscould regulate the calcium signal in the process of growth and development.
3.3.cAMP and A23187 increase the intracellular content of cAMP in jujube suspension cell line
In order to further elucidate the relationship between cAMP and calcium signaling,we established the jujube suspension cell line. Firstly,embryos of youngZ.jujuba‘Wuhe 76’ fruit were used to induce calli. MS medium containing 20 g L-1maltose,0.5 mg L-1NAA,and 3.0 mg L-1TDZ was used as the appropriate suspension medium for the optimum growth of cells. The fragile calli were transferred to liquid medium after several subculturing steps. The suspension cell line was composed mainly of individual cells and small cell clusters. The mitosis of the cells was vigorous,and most of the cells were spherical in shape,although a few were elongated or rod shaped(Fig.3-A).
To understand how the suspension cell line grew over time after subculturing,we evaluated various growth parameters,such as fresh weight,packed cell volume(PCV),and cell mortality. The cells grew slowly during the first 2 d after subculture. The cells entered the logarithmic growth phase from d 2 to 6. Then,they entered the stationary phase,and cell growth gradually decreased(Fig.3-B). Therefore,the growth curve of the ‘Wuhe 76’suspension cells was a typical S-shaped growth curve.Furthermore,the relative ΔPCV showed the same growth pattern,and the volume of the suspension cells peaked at d 6 (Fig.3-C). In addition,the cell mortality remained below 10% from d 0 to 8 (Fig.3-D),indicating that the cell activity was strong and that most of the cells exhibited good growth characteristics. Finally,all the growth parameters demonstrated that the subculture cycle of the suspension cells lasted for 7 d.
To understand how the application of cAMP and Ca2+affects the cAMP content in the cell line,we measured the content of cAMP by HPLC (Appendix E) and compared it to the control. In the control treatment,the content of cAMP in the cells showed no changes over 6 h. However,with calcium ionophore A23187 treatment,the content of cAMP increased significantly at 3 h and then it decreased at 6 h. The same change in cAMP content was observed after cAMP treatment,which culminated at 6 h (Fig.3-E).These results demonstrated that the exogenous application of either cAMP or Ca2+could significantly increase the levels of cAMP in jujube cell culture.
AC is the key enzyme that synthesizes cAMP through a one-step pathway,catalyzing the conversion of ATP to cAMP with pyrophosphate release (Moederet al.2013). In our previous study,we identified fiveZjACsin the jujube genome. Among them,the amino sequence of ZjAC has the CYTH-like_AC_IV-like conserved domain,and its expression was found to be positively correlated with high cAMP accumulation (Appendix C). Thus,the expression level ofZjACwas measured after the exogenous application of either cAMP or A23187 in this study. As shown in Fig.3-F and G,the expression ofZjACwas significantly induced at 3 h after cAMP treatment,but the expression level ofZjACgradually increased within 6 h after calcium ionophore A23187 treatment. These results demonstrated that the exogenous application of either cAMP or A23187 could induce the expression ofZjACin order to increase the cAMP content in the suspension cells.
3.4.Calcium influx in jujube cell protoplasts
After establishment,protoplasts can serve as an ideal material for studying the effects of cAMP and A23187 on the changes of Ca2+in the cytosol using Ca2+-binding chemical dyes. For example,fluo-4/AM can pass through the membrane to the cytosol without inhibition by the cell wall. As shown in Fig.4,slight green fluorescence could be observed in the control treatment after fluo-4/AM staining for 15 min. With 50 or 100 μmol L-1cAMP treatment after fluo-4/AM staining,fluorescence could also be observed,and it significantly increased at 15 min.Quantification of the images indicated that the change in fluorescence intensity was the same as in the figures after cAMP treatment (Fig.4-A). In addition,a time course study of the 100 μmol L-1cAMP treatment showed that the highest fluorescence and density could be observed at 5 min (Fig.4-C). Fluorescence could also be observed with 5 or 50 μmol L-1A23187 treatment after fluo-4/AM staining,and the intensity increased significantly at 15 min(Fig.4-B). Quantification of the images indicated that the change in fluorescence intensity was the same as in the figures after A23187 treatment (Fig.4-B). A time course study of the 5 μmol L-1A23187 treatment indicated that the highest fluorescence and density could be observed at 5 min (Fig.4-C). These results demonstrated that cAMP could trigger the Ca2+influx into the cytosol.
3.5.lnhibition of adenylate cyclase diminishes calcium fluorescence in jujube cells
To further confirm the relationship between cAMP and the effects of calcium signaling,EGTA (a calcium chelator) and bithionol (an inhibitor of adenylate cyclase)were applied to the protoplasts. As shown in Fig.5-A,a 10 mmol L-1EGTA treatment slightly reduced the fluorescence intensity within 10 min,and the green signal became very weak at 20 min. Quantification of the images indicated that the change in fluorescence intensity was the same as in the figures after EGTA treatment (Fig.5-B). In addition,a 10 μmol L-1bithionol treatment reduced the fluorescence intensity within 20 min (Fig.5-A),which was consistent with the changes observed in the quantification of the images (Fig.5-C).Moreover,after pretreatment with EGTA or bithionol,the fluorescence intensity could be rescued with an additional cAMP treatment,but it did not increase as much as after the cAMP treatment alone (Fig.5-B and C).These results further confirmed that cAMP could trigger the Ca2+influx into the cytosol.
3.6.Calcium influx occurs by ZjCNGC2 and induces MAPK cascades in a time-dependent manner
Plant CNGCs are important cation transport channels that allow sodium,calcium,and potassium to cross cellular membranes,although they can be regulated by internal second messengers such as cyclic nucleotide monophosphates (Charpentieret al.2016). To determine whether theCNGCgene could be activated by cAMP or A23187,the normalized and relative expression levels ofZjCNGC2were determined. As shown in Fig.6-A and B,the normalized expression level ofZjCNGC2with activation by cAMP was higher than that induced by A23187. However,the expression ofZjCNGC2was rapidly induced by A23187 at 1 h,while it was significantly up-regulated at 3 h after treatment with cAMP. These relative expression patterns were consistent with the changes in normalized expression levels within these two treatments (Appendix F).
Moreover,MAPK cascades also play important roles in signal transduction. Whether these cascades are regulated by cAMP and A23187 treatments was tested here. As shown in Fig.6-C,the normalized expression level ofZjMAPK2was higher than the normalized expression levels ofZjMAPKK2andZjMAPKK4with either treatment. The normalized expression level ofZjMAPKK4was the lowest,but its expression level could be suppressed by the A23187 treatment. The normalized expression level ofZjMAPKK2showed significant upregulation within 1 to 6 h after cAMP treatment. Under A23187 treatment,the normalized expression levels ofZjMAPKK2decreased significantly from 1 to 6 h,while the expression level ofZjMAPK2did not change significantly. The relative expression patterns of these three genes were consistent with the changes in their normalized expression levels within these two treatments(Appendix F). Therefore,cAMP might be an important signaling molecule that inducesZjMAPKK2andZjMAPK2expression during cell growth.
4.Discussion
cAMP functions as an important signaling molecule in eukaryotic cells. Jujube plants have a high content of cAMP in their mature fruit,so they are an ideal candidate plant material for systematically and comprehensively investigating the biological functions,biosynthesis and signaling functions of cAMP. Thus,the effect of exogenous cAMP application on jujube growth and the endogenous cAMP content were analyzed for the first time in this study. Transcriptome analysis revealed that the calcium signal might be involved in the fast growth ofZjACoverexpressedArabidopsis. We then established a jujube suspension cell culture to investigate the function of cAMP at the cellular level. Moreover,using pharmacological treatments,we manipulated and examined various physiological,chemical,and molecular indicators to reveal the relationships of Ca2+and cAMP in signal transduction in jujube cells,which could lay the foundation for the further elucidation of cAMP-regulated signaling in the growth and development of jujube.
4.1.Biological functions of cAMP in jujube
cAMP has been detected in microorganisms and plants,although the signaling function of cAMP in higher plants is still under debating. However,recent developments indicate more and more functions of plant cAMP,which compels us to gradually accept that cAMP could also function as an important messenger for some biological functions in plant physiological processes. For instance,auxin receptor proteins TIR1/AFB have important functions in auxin signal transduction (Suet al.2022;Wanget al.2022). However,a recent study showed that the AC activity ofTIR1regulated cAMP signaling functions in auxin-induced root growth inhibition and root gravitropism,which revealed a new function ofTIR1and cAMP functions as a second messenger in plants (Qiet al.2022). Moreover,studies on cAMP have shown that it is involved in abiotic stresses,especially in heat stress,implying that cAMP could function as a chemical regulator that improves plant thermotolerance (Yanget al.2021;Zhaoet al.2021;Lianget al.2022). In our current study,we found that the exogenous application of cAMP could promote the growth and development of sour jujube plants,which can enrich the known biological functions of cAMP in plants. Interestingly,the exogenous application of cAMP could increase the content of endogenous cAMP in jujube fruit,which might indicate that the exogenous application of cAMP during jujube growth and development might act by regulating the endogenous cAMP involved in this process. Then,through transcriptome analysis,we found calcium related genes involved in this process. The same mechanism has been demonstrated in pollen growth ofAgapanthus umbellatus,in which cAMP activator treatment could induce a transient increase in the cytosolic Ca2+level(Moutinhoet al.2001). Thus,we then established jujube suspension cells and protoplasts in order to further elucidate the relationship between calcium and cAMP.
4.2.Calcium could activate the expression of ZjAC for cAMP synthesis
Plant suspension cells,as dedifferentiated cultures,are a valuable tool with which to study biological processes at the cellular and molecular levels. Suspension cells have many advantages compared to whole plants,including rapid and uniform access to nutrition,precursors,growth hormones and signaling compounds,with a relatively homogenous cell population in a liquid medium (Mustafaet al.2011;Santoset al.2016;Kobylinskaet al.2018).In addition,natural production from cell suspensions requires cell line selection,biotransformation,product secretion,cell permeabilization,extraction and purification(Ochoa-Villarrealet al.2016). Plant cell suspensions that are widely used for scientific research have been derived from tobacco (Nicotianatabacum),rice (Oryzasativa),and carrot (Daucuscarota) (Trexleret al.2005;Rosales-Mendoza and Tello-Olea 2015). Among horticultural plants,watermelon cell suspensions have been established and used to study glycinebetaine biosynthesis in response to osmotic stress (Xuet al.2018). However,the successful establishment of a plant cell suspension requires the consideration of several other parameters,such as the fresh and dry weights,packed cell volume,cell count,nutrient and metabolite concentrations,and cell viability (Schripsemaet al.1990;Mustafaet al.2011). These indicators can guide the application of cell lines for either scientific experiments in the laboratory or industrial production at the pilot and semi-pilot levels.Our establishment of a jujube cell suspension in this study,developed with the aim of evaluating cAMP as an important second messenger,can address some of the challenges in the investigation of cAMP biosynthesis that arise from its lower abundance in other plants. Jujube fruit has a relatively high abundance of cAMP (Liu and Wang 1991),so a cell line established from this plant could be an alternative tool for studying the biosynthesis of cAMP and its role in signal transduction. In the current study,embryogenic calli from the ‘Wuhe 76’ jujube cultivar were used to establish a cell suspension. After subculturing,the cells entered the logarithmic growth phase for 2 to 4 d.The growth curve of the cell suspension showed a typical‘S’ shape over 7 d of observation. During the growth cycle,the cell mortality ranged between 4 and 10%,which indicated that the cell activity was very strong and that most of the cells exhibited good growth characteristics.Thus,the successful establishment of this suspension cell line provides a simplified model system for the study of cAMP biosynthesis and the evaluation of its role in biological reactions.
Due to the relatively low level of cAMP in plants,whether cAMP functions as an important second messenger in plant cells remains controversial and continues to be debated (Kaplanet al.2007;Blancoet al.2020). However,cyclic nucleotides,mainly through CNGCs,have a direct effect on the influxes of Ca2+,potassium and sodium (Maet al.2019). Moreover,cAMP-triggered calcium signaling during plant growth and development has been studied in several plants.InPyruspyrifolia,treatment with the cAMP inhibitor alloxan inhibited pollen tube growth,while cAMP treatment rescued pollen growth when the Ca2+current was changed (Moutinhoet al.2001;Wuet al.2011). In addition,cAMP could induce phytoalexin accumulation through Ca2+influx in carrot and alfalfa cell suspension cultures (Kurosaki and Nishi 1993;Cookeet al.1994). In bean cell suspension cultures,early induction of reactive oxygen species occurs in response to fungal elicitors involved in cAMP and calcium signaling (Bindschedleret al.2001). To determine the relationships between Ca2+and cAMP,protoplasts can be used to follow the influx of Ca2+under cAMP treatment. Therefore,we first obtained viable protoplasts from the ‘Wuhe 76’ cell suspension by enzymatic hydrolysis. Jujube protoplasts could be a useful biological system for investigating the synthesis and function of cAMP and the effect of Ca2+,as these protoplasts satisfy several key criteria. First,these protoplasts are homogenous as pollen tubes,so uniform concentrations of cAMP can be uniformly and rapidly applied. Second,the cAMP content in cell suspensions or protoplasts can be easily detected. Finally,a rapid Ca2+influx can be detectedinvivoby small-molecule fluorescent probes. We treated the protoplasts with cAMP or the calcium ionophore A23187 following fluo-4/AM staining and observed fluorescence when Ca2+influx into the cytosol was triggered. In addition,with the A23187 treatment,the content of cAMP increased significantly at 3 h and an increase in protoplast fluorescence was induced after fluo-4/AM staining. Moreover,the expression level ofZjAC4/5could be activated by A23187,and exogenous cAMP treatment could rescue the fluorescence intensity that was inhibited by either EGTA or bithionol treatment.Both results demonstrated that Ca2+influx into the cytosol was sufficient to trigger the synthesis of cAMP. However,whether calcium is necessary for cAMP synthesis is an interesting question that could be investigated by treatment with flagellin,which is a generic elicitor,or cold stress (which is known to induce calcium influx) (Wang and Nick 2017;Guanet al.2021). These treatments could be conducted on suspension cells or protoplasts to determine whether they can induce cAMP and whether this effect can be disrupted by gadolinium or EGTA.
4.3.A cycling effect of cAMP and calcium signaling contributed to jujube growth
Biochemical and molecular biology studies have demonstrated that cAMP exists in higher plants and plays a role in a variety of biological processes in conjunction with potassium and Ca2+currents. For example,cAMP is involved in the accumulation of antitoxins in cypress cells. cAMP and its analogs could stimulate the production of antitoxins in cells under the effect of cAMP activators and inhibit the production of antitoxins in response to potassium and Ca2+inhibitors,indicating that cAMP may participate in the accumulation of antitoxins through alterations of the Ca2+and K+contents (Zhaoet al.2004). Through the identification ofCNGCgenes in Chinese jujube,ZjCNGC2was found to act as a mediator in signaling cascades under abiotic stress. An analysis of the jujube CNGC family showed that the expression ofZjCNGC2may be regulated by cAMP as a signaling molecule in response to cold stress(Wanget al.2020a). InArabidopsis,cAMP analogs could stimulate an increase in cytosolic Ca2+viaa plasma membrane-localized Ca2+channel (Lemtiri-Chlieh and Berkowitz 2004),while the Ca2+current triggered by cAMP could be impaired by the loss of function ofCNGC2(Aliet al.2007). In the current study,we found thatZjCNGC2could be activated by cAMP and A23187,while the expression levels ofZjMAPK2andZjMAPKK2could be regulated by cAMP treatment but not by A23187 treatment. However,the expression times and levels of these genes were totally different,which suggested a model in which both Ca2+and cAMP signaling contribute to jujube growth (Fig.7).
The cAMP treatment activatedZjCNGC2(expressed at 3 h),which is located at the cell membrane,and the expression level was stronger than that induced by A23187. In addition,the cAMP treatment induced the rapid influx of Ca2+into the cytosol (5 min) and could also induce an increase in the cAMP level in the cytosol,especially by activating the expression ofZjACat 3 h,demonstrating that the elevated synthesis of cAMP after the 3 h treatment is positively correlated with theZjCNGC2-mediated calcium influx. Moreover,cAMP treatment also induced the expression of downstream genes,such asZjMAPKK2andZjMAPK2,to transduce the signals to the nucleus (Fig.7,Model I). The A23187 treatment significantly induced the synthesis of cAMP (at 3 h) through the rapid induction ofZjAC(at 1 h),while it induced the expression ofZjCNGC2more rapidly (at 1 h)than the cAMP treatment but had no effect onZjMAPKs.This finding demonstrated that the rapid Ca2+influx into the cytosol mediated by the ZjCNGC2 channel was sufficient for cAMP synthesis (Fig.7,Model II). Taking all these results together,we found that our constructed Model III (Fig.7) was superior,as this model represents the effect of cAMP or even other stimuli. Rapid calcium signaling could be observed first,and the influx of this ion into the cytosol might occurviatheZjCNGC2channel.Then,rapid Ca2+accumulation in the cytosol could induce the expression ofZjACand lead to the synthesis of cAMP,which could further induce the expression of MAPKs,such asZjMAPKK2andZjMAPK2. In addition,the Ca2+treatment could also induce an increase in the cAMP level,and EGTA or bithionol treatment could inhibit the calcium signal,suggesting that the inhibition of endogenous cAMP synthesis by AC inhibitors could also reduce the calcium signal. These results demonstrated that cAMP and Ca2+together have multiple cycle-amplifying effects on signal transduction. However,it would be interesting to further clarify MAPK signaling in response to a PD98059 treatment,which could block MAPK activity. It would also be useful to determine whether cAMP treatment has any effects of the expression ofZjMAPKK2andZjMAPK2after PD98059 treatment.
Altogether,this study comprehensively revealed that there is a link between Ca2+and cAMP in signal transduction in the process of jujube growth. However,the more critical molecular mechanism of how cAMP regulates jujube growth should be further deciphered,especially by identifying the downstream signaling,clarifying whether hormone levels such as auxin or cytokinin are involved in this process,and whether there is a relationship between cAMP and those hormones.Further understanding of the molecular mechanism behind them could greatly enrich our knowledge of how cAMP functions as an important signal molecule involved in various biological processes in plants.
5.Conclusion
Exogenous cAMP treatment could promote jujube growth and increase the endogenous cAMP content.Transcriptome analysis of theZjACsoverexpressedArabidopsisplants showed 60 calcium related DEGs and demonstrated that the cAMP increment inArabidopsiscould be regulated by calcium signaling. The suspension cell line ‘Wuhe 76’ was established,and the cAMP content in this cell line was determined. The exogenous application of cAMP and the calcium ionophore A23187 triggered an increase in the cAMP level in the cell line by activating the main cAMP biosynthesis gene (ZjAC). In addition,exogenous cAMP treatment induced calcium influx into the cytosol within 5 min,as determined by the fluo-4/AM protoplast staining method,while EGTA or bithionol treatment inhibited the calcium fluorescence in the cells. Furthermore,both the cAMP and A23187 treatments activated theZjCNGC2channel,but only the cAMP treatment triggered the expression of downstream genes,such asZjMAPKK2andZjMAPK2.
Acknowledgements
This research was funded by the Provincial Supporting Program of Hebei for the Returned Oversea Scholars,China (C20210114),the Science and Technology Project of Hebei Education Department (QN2022017),the Fundamental Scientific Research Fund of Universities in Hebei Province (KY2021059),the China Agriculture Research System (CARS-30-2-07),the National Key Research and Development Project of China(2019YFD1001605),the Natural Science Foundation of Hebei Province (C2020204082),the Funds for Hebei Jujube Industry Technology Research Institute after Operation Performance (205676155H),and the Young Talent Project of Hebei Agricultural University Foundation(YJ201853).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available at https://doi.org/10.1016/j.jia.2023.04.039
 Journal of Integrative Agriculture2023年7期
Journal of Integrative Agriculture2023年7期
- Journal of Integrative Agriculture的其它文章
- Understanding changes in volatile compounds and fatty acids of Jincheng orange peel oil at different growth stages using GC-MS
- Untargeted UHPLC-Q-Exactive-MS-based metabolomics reveals associations between pre-and post-cooked metabolites and the taste quality of geographical indication rice and regular rice
- A double-layer model for improving the estimation of wheat canopy nitrogen content from unmanned aerial vehicle multispectral imagery
- The potential of green manure to increase soil carbon sequestration and reduce the yield-scaled carbon footprint of rice production in southern China
- lmprovement of soil fertility and rice yield after long-term application of cow manure combined with inorganic fertilizers
- A novel short transcript isoform of chicken lRF7 negatively regulates interferon-β production
