Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value
Yu-Qi Li, Lei Xin, Yu-Chi Zhao, Shang-Qi Li, Ya-Nuo Li
Abstract
Key Words: Nonalcoholic fatty liver disease; Vascular endothelial growth factor B; "Twohit" theory; "Multiple-hit" theory; Obesity
INTRODUCTION
With the growth of the economy and the increasing change in people's lifestyles, the prevalence and morbidity of nonalcoholic fatty liver disease (NAFLD) are rising rapidly worldwide.NAFLD occurs in one-fourth of the global population, and the highest incidence rate is in South America (31%) and the Middle East (32%), followed by Asia (27%), the United States (24%), and Europe (23%), while is not common in Africa (14%)[1].In the United States, the number of NAFLD cases is expected to increase from 83.1 million in 2015 (approximately 24% of the population) to 100.9 million by 2030[2].Because of its long course and high treatment cost, it has become a global medical and health problem.
NAFLD is an important cause of advanced liver disease, primary liver cancer, and liver transplantation and is also the world's fastest-growing cause of liver-related deaths[3].In the United States, the burden of NAFLD-related cirrhosis is estimated to be twice that of hepatitis C virus (HCV)related cirrhosis, and it is expected to surpass HCV as the main indication for liver transplantation within 5 years[4].In Asia, the incidence rate of hepatocellular carcinoma in patients with NAFLD is 1.8/1000 each year, and the total case fatality rate is 5.3/1000 each year[5].In addition, insulin resistance,upregulation of insulin-like growth factor axis, downregulation of adiponectin expression, and elevated expression of tumor necrosis factor α (TNFα) caused by NAFLD may be potential factors to induce the development of tumors[6].Meanwhile, NAFLD can also promote coronary atherosclerosis, significantly increase the risk of cardiomyopathy (mainly left ventricular hypertrophy), leading to valvular heart disease (mainly aortic valve and mitral valve), cardiac insufficiency, arrhythmia (mainly atrial fibrillation, prolonged QT interval) and some cardiac conduction system defects (such as an atrioventricular block)[7].Therefore, more and more research is focusing on exploring the pathogenesis of NAFLD.
The physiological mechanism of NAFLD is very complex.The pathogenesis of early NAFLD is generally believed to be related to lipid metabolism and inflammatory reactions, which could not systematically and comprehensively explain the molecular mechanism and metabolic changes in NAFLD[8,9].In recent years, studies have confirmed that insulin resistance is closely related to the pathogenesis of NAFLD[10].In 2019, Leeet al[11] reported that NAFLD was related to liver and peripheral insulin resistance, leading to insufficient inhibition of liver insulin resistance, gluconeogenesis, reduced glycogen synthesis, and increased free fatty acid (FFA).Shiet al[12] confirmed that insulin resistance can promote the progression of liver fibrosis and NAFLD, and NAFLD can also accelerate insulin resistance in the liver.
With the deepening of research on NAFLD and the increasing understanding of its pathogenesis, it has been found that the onset of NAFLD is also related to "multiple-hit" such as liver insulin resistance,adipocyte dysfunction, gut microbiota imbalance, immune regulation imbalance, and dietary habits besides the “second-hit”caused by lipid metabolism disorder and inflammation reaction.Adolphet al[13] found that abnormal adiponectin secretion produced by adipocytes can aggravate high-fat diet(HFD)-induced obesity and related metabolic disorders, and the overexpression of adiponectin can hinder the progression of hepatic microsomal steatosis.Bakeret al[14] found that the content of enzymes that can metabolize ethanol in the body of patients with NAFLD and intestinal flora imbalance increased significantly, which increased the permeability of the intestinal wall and was conducive to the entry of reactive oxygen species (ROS), bacterial endotoxins, ethanol and other toxic metabolites into the liver, resulting in increased liver damage andaccelerating the progression of NAFLD (Figure 1).
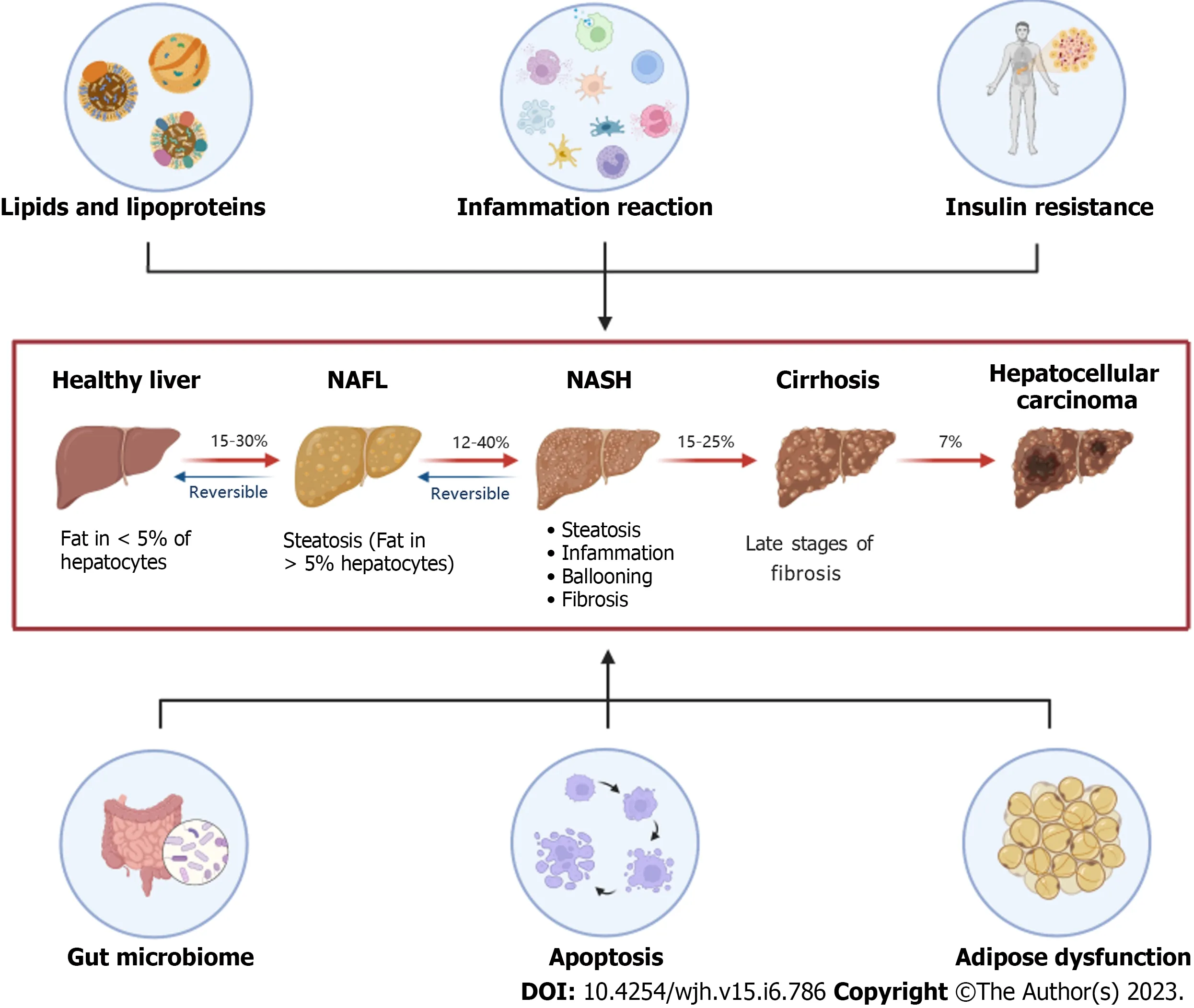
Figure 1 The “multiple-hit”theories are involved in the progress of nonalcoholic fatty liver disease.Lipids and lipoproteins represent the “firsthit”, while the inflammation reaction illustrates the “second-hit”in the development of nonalcoholic fatty liver disease (NAFLD).Six aspects including lipids and lipoproteins, inflammation reaction, insulin resistance, gut microbiome, apoptosis, and adipose dysfunction have a common influence on the pathophysiological mechanism of NAFLD.NAFL: Non-alcoholic fatty liver; NASH: Non-alcoholic steatohepatitis.
Early in 2008, Karpanenet al[15] unexpectedly found that vascular endothelial growth factor B(VEGFB) has a weak role in the vascular system but has a significant advantage in regulating lipid metabolism.In 2012, Hagberget al[16] proved that targeting VEGFB as a novel treatment for insulin resistance and type 2 diabetes.In 2016, Robciucet al[17] also found that transferring the VEGFB gene into HFD-induced obese mice can improve lipid metabolism and increase insulin supply and signal transduction.Huet al[18] confirmed that VEGFB recombinant protein can reduce lipid accumulation and improve hyperlipidemia in NAFLD.
The regulatory role of VEGFB in the occurrence and development of metabolic diseases has attracted many scholars’attention.In this review, we mainly focus on the underlying mechanism of VEGFB in the onset of NAFLD and analyze how VEGFB participates in the "multiple-hit" of NAFLD by regulating lipid metabolism, inflammatory reactions, adipocyte dysfunction, and cell apoptosis.First, we introduce the positive regulatory effect of VEGFB on lipid metabolism and discuss how it affects fatty acid oxidation and lipid synthesis under the mediation of Adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) signaling.Then, we summarize the role of VEGFB in anti-inflammation in NAFLD and further discuss the current mechanism of VEGFB in insulin resistance and the impact of targeted therapy.Finally, we also explain the controversial role of VEGFB in metabolic diseases and estimate whether VEGFB-mediated signal transduction could provide a theoretical and experimental basis for the pathogenesis of NAFLD and help identify potential treatment targets.
THE NOVEL ROLE OF VEGFB IN NAFLD
VEGFB is a special type of vascular endothelial growth factor.The total length of the VEGFB gene is 1197 bp, with 7 exons, and the total length of the CDS region is 566 bp, with two subtypes, VEGFB167and VEGFB186[19].The VEGFB167homotype has a similar effect to that of VEGFB186[17].VEGFB is a glycoprotein that forms a homodimer through the covalent binding of disulfide bonds.It needs to combine with a high-affinity tyrosine kinase receptor to exert biological effects[20].The VEGF family includes seven members, VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor[21].The VEGF receptor family includes VEGFR1, VEGFR2, VEGFR3, and neuropilin 1/2 (NRP1/2).VEGFA can combine with VEGFR1 or VEGFR2 to play a role in promoting angiogenesis[22].Unlike other members of the VEGF family, the effect of VEGFB on vascular endothelial growth is not obvious.The biological function of VEGFB is exerted by forming a complex with VEGFR1.Moreover, the combination of VEGFB and NRP1 can also induce a series of reactions through a paracrine mechanism[23].In recent years, studies have shown that the VEGFB/VEGFR1 pathway has therapeutic potential for obesity, type 2 diabetes, and other lipid metabolism disorder-related diseases[17].
VEGFB mainly exists in the heart, skeletal muscle, brown adipose tissue, and other tissues with high metabolic activity and plays a role in regulating blood vessel distribution and lowering blood lipids[24].The level of VEGFB in the liver is also significantly higher than that in tissues with general metabolic activity.Shanget al[25] found that cardiac-specific overexpression of VEGFB can reduce the activity of lipoprotein lipase and improve the metabolic level of myocardial cells.Wagenmakerset al[26] showed that VEGFB can control the expression of fatty acid transport protein (FATP) in the capillary endothelium and connect the uptake of endothelial FFA with the oxidation ability of the skeletal muscle to potentially prevent the accumulation of skeletal muscle lipotoxic FFA.Robciucet al[17] confirmed that the complex of VEGFB and VEGFR1 can reshape the vascular distribution in adipose tissue and improve the insulin function of obese mice.
VEGFB also plays a biological role in the liver by forming a complex with VEGFR1.Cordeiroet al[27]showed that targeting VEGFB can effectively prevent lipid deposition in peripheral tissues in animal models.Huet al[18] observed that the complex of VEGFB and VEGFR1 can increase the oxidation level of fatty acids in liver tissue and hepatocytes and reduce obesity-related hyperlipidemia and fatty liver disease in HFD-induced liver.Liet al[28] found that inhibiting VEGFB gene expression in liver tissue not only increased the weight and body fat rate of obese mice but also led to pathological changes, such as hepatocyte steatosis and liver fibrosis.These studies suggest that VEGFB is involved in the onset and development of simple steatosis and liver fibrosis in NAFLD (Figure 2).

Figure 2 The vascular endothelial growth factor family and its receptors, and the biological function of vascular endothelial growth factor B.The vascular endothelial growth factor (VEGF) family includes VEGFA, vascular endothelial growth factor B (VEGFB), VEGFC, VEGFD, and so on.The VEGF receptor family includes VEGFR1, VEGFR2, VEGFR3, and neuropilin 1/2 (NRP1/2).VEGFA combines with VEGFR1, VEGFR2, or NRP1/2.VEGFB and placental growth factor combine with VEGFR1 or NRP1/2.VEGFC and VEGFD combine with VEGFR2 or VEGFR3 to exert their biological functions.VEGFB can prevent the accumulation of lipotoxic free fatty acid (FFA) in skeletal muscle, reduce lipoprotein activity in the heart, increase adipose tissue vascularity, and increase the oxidation level of FFA in the liver.PIGF: Placental growth factor; FA: Fatty acid; NRP1/2: Neuropilin 1/2; VEGF: Vascular endothelial growth factor; VEGFB: Vascular endothelial growth factor B.
VEGFB PARTICIPATES IN REGULATING THE "FIRST HIT" IN NAFLD
In 1998, Dayet al[29] first proposed the "two-hit" theory of the pathogenesis of NAFLD.The "first hit" of NAFLD mainly involves lipid metabolism disorder caused by various factors.Hepatotoxicity is caused by FFA, which leads to an increase in the permeability of the cell membrane, destruction of mitochondrial function, and inhibition of related enzymes to produce genotoxicity.As the disease progresses, excess FFA undergoes β oxidation in mitochondria.When the capacity of the mitochondria to β oxidize FFA is overloaded, excess FFA accumulates in the liver and aggravates the steatosis of hepatocytes.Meanwhile, the triglyceride (TG) synthesized by excess FFA in the liver cannot be converted into very low-density lipoprotein for transport to the peripheral adipose tissue for storage.Therefore, TG can only be stored in the liver and eventually aggravate the onset of liver steatosis.Reducing lipid accumulation and restoring the balance of lipid metabolism are the key methods to improve the "first hit" of NAFLD.
The research findings on the role of VEGFB in improving the lipid disorder of the heart, skeletal muscle, and brown adipose tissue provide the theoretical and experimental basis for VEGFB to participate in the regulation of hepatic lipid metabolism in NAFLD.Shanget al[25] observed that rat heart lipoprotein lipase activity and lipid accumulation were decreased and insulin function was improved after cardiac-specific overexpression of VEGFB.Liet al[30] found that VEGFB can enhance the expression of FATP1 and FATP4 in C2C12 cells, promote the oxidation of FFA and the decomposition of TG in C2C12 myotubes, and inhibit the re-esterification of FFA to reduce lipid accumulation in myotubes.Chenet al[31] found that after inhibition of adipose-specific VEGFB, mice increased in size with more white adipose tissue, and the form and function of fat changed from those of brown adipose tissue to those of white adipose tissue, which indicated that VEGFB was the main regulator of the growth and function of fat.
Some scholars have proposed that the signaling pathway triggered by the combination of VEGFB with its receptor can promote lipid flow in the body, which may become a promising target to prevent the accumulation of ectopic lipids.In 2020, Tonget al[32] showed that VEGFB can reduce the levels of TG and FFA in the liver to prevent HFD-induced fatty liver disease by producing E.coli-expressed recombinant tPep-VEGFB.In 2021, Huet al[18] found that recombinant VEGFB protein reduced the increase in high-density lipoprotein and low-density lipoprotein in the liver caused by HFD and reduced liver hyperlipidemia.
The mechanism of NAFLD involves multiple signaling pathways, of which the AMPK signaling pathway plays a key role in de novo synthesis and fatty acid oxidation[33].Harjeset al[34] confirmed that the combination of VEGFB with its receptor VEGFR1 can activate AMPK, FATP3, and FATP4 to potentially promote the usage of FFA.AMPK activation is regulated by its upstream molecule Ca2+/Calmodulin-dependent protein kinase β (CaMKKβ), which responds to increased intracellular calcium content[35].Extracellular calcium ions enter the cell through a calcium channel carrier.The elevated intracellular calcium level causes the conformational change of CaMKKβ and the phosphorylation of AMPK[36].Jiaet al[37] showed that a high concentration of VEGFB recombinant protein can increase the level of calcium ions in MIN6 cells to increase insulin secretion.Liet al[28] suggested that inhibiting the expression of VEGFB in the liver can reduce the expression level of CaMKKβ and then affect the phosphorylation level of AMPK induced by CaMKKβ.
AMPK can control lipid metabolism by regulating its downstream molecules after its phosphorylation[38].AMPK can directly phosphorylate and inhibit the activation of acetyl coenzyme A carboxylase (ACC), the rate-limiting enzyme that inhibits the synthesis of fatty acids, thereby activating carnitine palmitate transferase (CPT1) and transferring fatty acids into mitochondria for β oxidation[39,40].AMPK can also negatively regulate the expression of sterol-regulatory element-binding protein-1c(SREBP1c), downregulate the level of desaturase [stearoyl-CoA desaturase-1 (SCD1)], and inhibit the synthesis of fatty acids and TG[41].Huet al[18] showed that VEGFB recombinant protein can upregulate the AMPK/ACC/CPT1 signaling pathway in the liver by binding to VEGFR1, promoting FFA oxidation and reducing lipid deposition.That study also found that VEGFB can simultaneously upregulate the expression levels of the lipid oxidation-related genes PPARα, PGC-1α, HSL, ACO, and CPT1 and that the downregulation of lipid synthesis can inhibit weight gain under HFD conditions and improve obesity-related hyperlipidemia and fatty liver disease[18].Liet al[28] also found that VEGFB knockout can downregulate the CaMKKβ-mediated AMPK/ACC/CPT1 signaling pathway to inhibit fatty acid oxidation and activate the AMPK/SREBP1/SCD1 signaling pathway to promote lipid synthesis, thus affecting the level of lipid metabolism (Figure 3).

Figure 3 Vascular endothelial growth factor B regulates lipid metabolism in the “first hit”of nonalcoholic fatty liver disease via the activated protein kinase signaling pathway.Vascular endothelial growth factor B (VEGFB) performs its biological function by combining with VEGFR1.Once it enters the cell, it activates Calmodulin-dependent protein kinase β (CaMKKβ), which is induced by an increase in intracellular Ca2+ content.VEGFB activates the CaMKKβ-mediated activated protein kinase (AMPK)/A carboxylase (ACC)/carnitine palmitate transferase (CPT1) signaling pathway and related genes, such as CPT1 and long-chain acyl coenzyme A dehydrogenase, which regulate FFA oxidation in mitochondria.VEGFB activates AMPK/SREBP1/SCD1 and related genes, such as ACC1 and FAS, to inhibit lipid synthesis in the endoplasmic reticulum.CaMKKβ: Calmodulin-dependent protein kinase β; AMPK: Adenosine 5’-monophosphate(AMP)-activated protein kinase; ACC: A carboxylase; CPT1: Carnitine palmitate transferase-1; FFA: Free fatty acid; NRP1/2: Neuropilin 1/2; SCD1: Stearoyl-CoA desaturase-1; SREBP1: Sterol-regulatory element-binding protein-1; VEGFB: Vascular endothelial growth factor B; VEGF: Vascular endothelial growth factor;VEGFB: Vascular endothelial growth factor B; FAS: fatty acid synthase; ACO: Acyl Coenzyme A Oxidase; HSL: hormone-sensitive lipase; LCAD: long-chain acyl-CoA dehydrogenase; PPARα: proliferator-activated receptor-α; PGC-1α: peroxidase proliferator activator receptor γ coactivator 1α.
VEGFB PARTICIPATES IN REGULATING THE "SECOND HIT" IN NAFLD
Liver lipid accumulation induces overloaded lipid catabolism, causing lipid peroxidation.Excessive lipid peroxidation leads to oxidative stress, making it the "second hit" to the progression of NAFLD,which can accelerate inflammation and hepatocyte damage.Nuclear factor-kappa B (NF-kB) signaling plays an important role in the macrophage-mediated liver inflammatory response[42].Research has confirmed that NF-kB can be activated by FFA in patients with NAFLD, and as the severity of NAFLD increases, the activity of NF-kB increases[43].Moreover, liver mitochondrial dysfunction also accelerates the occurrence and development of NAFLD.The compensated acceleration of β oxidation in mitochondria can produce a large number of ROS[44].When the antioxidant system mainly composed of reduced glutathione fails to eliminate ROS in time, oxidative stress develops[45], and a large number of peroxides are generated, which aggravate hepatocyte damage[46].The apoptotic bodies produced by hepatocyte apoptosis are engulfed by Kupffer cells to decrease the activity of endothelial nitric oxide synthase (eNOS), which affects Mitochondrial function.
VEGFB can induce cell proliferation and differentiation, tumor immunity, and other biological effects through the signaling pathway mediated by the tyrosine-protein kinase receptor[47].Kusuharaet al[48]observed that the VEGFR1 signal in monocytes and macrophages was significantly affected by the upregulation of VEGFB under inflammatory conditions.Akiyoshi U confirmed that VEGFR1 can regulate AKT signaling and affect the activity of NF-KB and eNOS respectively to regulate macrophage migration and mitochondrial function[49].Mehlemet al[50] found that VEGFB signaling is involved in regulating pathological lipid accumulation in diabetes, obesity, and cardiovascular disease and mainly affects mitochondrial genes related to the regulation of fatty acid intake.Caoet al[51] showed that VEGFB/IL-17 inhibits the expression of fatty acid transporters to reduce the accumulation of renal lipids and inhibit renal oxidative stress and mitochondrial dysfunction, thus improving the inflammatory response.Shenet al[52] also found that VEGFB/IL-22 can not only regulate glucose and lipid metabolism but also reduce inflammation and ROS accumulation.Robciucet al[17] transduced the VEGFB gene into mice to inhibit obesity-related inflammation and improve metabolic health.
The lipid deposition caused by the "first hit" can lead to an inflammatory cascade, causing the "second hit" to hepatocyte damage and accelerating pathological changes in NAFLD.VEGFB can affect inflammatory response by regulating lipid metabolism in NAFLD, thereby affecting the "first hit" and improving the "second hit" in NAFLD.
VEGFB PARTICIPATES IN REGULATING THE "MULTIPLE-HIT" IN NAFLD
“Multiple-hit”theory believes that the pathological mechanism of NAFLD involves insulin resistance,adipocyte dysfunction, gut microbiota disorder, aggregation of inflammatory factors, mitochondrial dysfunction, lipotoxicity, endoplasmic reticulum stress, and so on[53].These factors collaborate and overlap with each other, accelerating hepatocyte damage and ultimately developing into cirrhosis, liver cancer, and end-stage liver failure.Insulin resistance is a common metabolic abnormality in patients with NAFLD and is considered the first step in the development of NAFLD[54].Studies have shown that the activation of insulin receptor substrate 1 (IRS1) protein is downregulated and SREBP-1c is upregulated when insulin resistance occurs, which ultimately increases the expression of de novo synthesis of lipids, thus increasing the transport of FFA to the liver[55].Meanwhile, hyperinsulinemia can inhibit the β-oxidative of FFA to further promote lipid accumulation in the liver.Excessive lipid accumulation in the liver can disrupt the homeostasis of glucose metabolism[56].Hepatic insulin resistance participates in the inhibition of forkhead box protein 1 (FOXO1) and serine/threonine kinase(GSK-3) through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), reduces Phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6 phosphatase (G6Pase) levels in the liver, which promotes gluconeogenesis and inhibits glycogen synthesis[57] (Figure 4).Recent studies have shown that VEGFB plays an active role in regulating metabolic diseases related to insulin resistance.Robciucet al[17] found that VEGFB can increase the sensitivity of peripheral insulin and improve insulin resistance.Huet al[18] found that VEGFB can reduce insulin resistance by reducing the content of FFA and total cholesterol, thus improving the disorder of lipid metabolism in NAFLD.

Figure 4 Vascular endothelial growth factor B participates in the “multiple-hit”of nonalcoholic fatty liver disease.Vascular endothelial growth factor B (VEGFB) regulates lipid metabolism, inflammation reaction, and glucose metabolism, which co-exist in the nonalcoholic fatty liver disease progression.VEGFB activates the AMPK phosphorylation to regulate free fatty acid oxidation and lipid synthesis.Long-term lipid metabolism disorders will cause inflammatory reactions and glucose metabolism disorders.VEGFB promotes the phosphorylation of protein kinase B (AKT) via combining with the VEGFR1 to affect macrophage migration and mitochondrial inflammation reaction.Meanwhile, VEGFB/VEGFR1 also plays an important role in inhibiting gluconeogenesis and promoting glycogen synthesis by activating the phosphorylation of AKT to regulate glucose metabolism.CaMKKβ: Calmodulin-dependent protein kinase β; VEGFR1: Vascular endothelial growth factor receptor-1; VEGFB: Vascular endothelial growth factor B; IR: Insulin receptor; IRS1: Insulin receptor substrate-1; PI3K: Phosphoinositide 3-kinase; AKT:Protein kinase B; AMPK: Adenosine 5‘-monophosphate (AMP)-activated protein kinase; ACC: A carboxylase; CPT1: Carnitine palmitate transferase-1; Lcad: longchain acyl-CoA dehydrogenase; SREBP: Sterol-regulatory element-binding protein; FAS: fatty acid synthase; NF-kB: Nuclear factor-kappa B; NF-AT: Nuclear factors of activated T; eNOS: endothelial nitric oxide synthase; FoxO1: Forkhead box protein-1; GSK-3: Serine/threonine kinase-3; PEPCK: Phosphoenolpyruvate carboxykinase; G6Pase: Glucose 6 phosphatase;
Insulin resistance has been proven to be an activator of cell apoptosis[58].Cell apoptosis, an injury factor in the "multiple hits", is a common and important mechanism of NAFLD lesions and liver injury.Liet al[59] believe that the decrease in the number of hepatocytes may be due to apoptosis, and excessive apoptosis of hepatocytes is an important sign of NAFLD/NASH patients.As a member of the vascular growth factor family, VEGFB participates in many angiogenesis-dependent diseases, and its pathogenesis is related to cell apoptosis.Williamset al[60] showed that inhibition of VEGFB leads to increased apoptosis in cardiomyocytes of patients with diabetes.Daiet al[61] also demonstrated that VEGFB plays an antiapoptotic role in the context of tumors.
Adipocyte dysfunction has also been confirmed to be closely related to the pathogenesis of NAFLD[62].The degree of adipocyte dysfunction is consistent with abnormal metabolism in NAFLD.Martina Rudnicki's study confirms that male mice fed with HFD exhibit adipocyte dysfunction[63].Robciucet al[17] has shown that the VEGFB/VEGFR1 pathway can be used to enhance vascular distribution in adipose tissue, which improves metabolic health and obesity.The VEGFB gene may affect the occurrence and development of NAFLD by affecting the expansion and loss of adipose tissue.
CONCLUSION
The role of VEGFB in regulating metabolic diseases, such as NAFLD, has attracted increasing attention from scholars.Research has shown that VEGFB can reduce lipid accumulation and restore insulin sensitivity in NAFLD.VEGFB activates the AKT signaling pathway by combining with VEGFR1,inhibits FOXO1 and GSK3 genes, blocks gluconeogenesis, accelerates glycogen synthesis, and improves insulin resistance.VEGFB not only improves liver insulin resistance, but also activates the AMPK signaling pathway, thereby activating the ACC signal to inhibit the expression of SREBP protein,improving fatty acid oxidation, inhibiting lipid synthesis, and restoring lipid metabolism balance.The activated AKT protein inhibits nuclear factors and proteins such as NF-kB or eNOS after phosphorylation, regulates inflammatory factors such as macrophages and liver mitochondrial function,reduces the occurrence of inflammatory reactions in hepatocytes, and prevents the progression of NAFLD[43].
Although more and more studies support that VEGFB can be a new target for the treatment of NAFLD and type 2 diabetes, some studies have shown that VEGFB has not played a positive role in regulating lipid metabolism and insulin resistance.Ninget al[64] confirmed that the changes in VEGFB did not affect glucose metabolism or lipid uptake.Hagberget al[65] suggested that VEGFB gene deletion can prevent ectopic lipid deposition and ameliorate dyslipidemia.Falkevallet al[66] showed that inhibition of VEGFB signaling can target liver steatosis by inhibiting lipolysis and preventing the development of NAFLD.
At present, the understanding of the role of VEGFB in regulating NAFLD and its mechanism remains controversial and is not completely clear.So more research focuses on the mechanism of VEGFB in the occurrence and development of NAFLD, which will provide a new idea for the study of pathophysiological mechanisms and therapeutic targets of NAFLD.
FOOTNOTES
Author contributions:Li YQ prepared and drafted the manuscript; Xin L made the critical revision; Zhao YC edited the manuscript; Li SQ collected the literature review; Li YN approved the final version.
Conflict-of-interest statement:All the authors have declared no conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yu-Qi Li 0000-0003-0236-8581; Lei Xin 0000-0003-0084-6498; Yu-Chi Zhao 0000-0002-4776-1419; Shang-Qi Li 0009-0000-1702-9406; Ya-Nuo Li 0000-0002-6441-5024.
S-Editor:Liu JH
L-Editor:A
P-Editor:Cai YX
 World Journal of Hepatology2023年6期
World Journal of Hepatology2023年6期
- World Journal of Hepatology的其它文章
- Management of sepsis in a cirrhotic patient admitted to the intensive care unit: A systematic literature review
- Liver injury from direct oral anticoagulants
- Randomized intervention and outpatient follow-up lowers 30-d readmissions for patients with hepatic encephalopathy, decompensated cirrhosis
- Lower alanine aminotransferase levels are associated with increased all-cause and cardiovascular mortality in nonalcoholic fatty liver patients
- Acute pancreatitis in liver transplant hospitalizations: Identifying national trends, clinical outcomes and healthcare burden in the United States
- Tumor budding as a potential prognostic marker in determining the behavior of primary liver cancers
