Physical and emulsifying properties of pea protein: influence of combined physical modification by flaxseed gum and ultrasonic treatment
Ji Yng,Fenghong Hung,Qingde Hung,D M,Yshu Chen,Dengfeng Peng,Xio Yu,c,Qinchun Deng,*,Fng Geng
a Oil Crops Research Institute,Hubei Key Laboratory of Lipid Chemistry and Nutrition,and Key Laboratory of Oilseeds Processing,Ministry of Agriculture,Chinese Academy of Agricultural Science,Wuhan 430062,China
b College of Packaging Engineering,Jinan University,Zhuhai 519070,China
c College of Food and Biological Engineering,Henan Collaborative Innovation Center for Food Production and Safety,Henan Key Laboratory of Cold Chain Food Quality and Safety Control,Zhengzhou University of Light Industry,Zhengzhou 450002,China
d Key Laboratory of Coarse Cereal Processing (Ministry of Agriculture and Rural Affairs),School of Food and Biological Engineering,Chengdu University,Chengdu 610106,China
Keywords:Pea protein Flaxseed gum Ultrasonic treatment Emulsifying properties Physical properties
ABSTRACT This study characterized and compared the physical and emulsifying properties of pea protein (PP) and its modified proteins (ultrasound treated-PP (PPU),flaxseed gum (FG) treated PP (PPFG) and ultrasound treated-PPFG (PPFGU)).The results showed FG triggered the formation of loosely attached complex with PP via physical modification under gentle magnetic stirring at pH 7.0,while ultrasound played an important role in reducing protein size,increasing surface hydrophobicity and molecular fluidity onto oil-water interface.So ultrasound further enhanced the interaction of PP with FG,and produced the PPFGU complex with smaller droplet size,higher ζ-potential and lower turbidity.Further,combination of FG and ultrasound improved the physical properties of PP with higher viscosity,stiffer gels (defined as higher elastic modulus),stronger hydrophobic properties,better thermal stability,and fast protein absorption rate.Therefore,the PPFGU coarse emulsion performed highest emulsifying activity index (EAI) and emulsion stability index (ESI) that the stabilized nanoemulsion obtained smallest droplet size,higher ζ-potential,and longest storage stability.The combination of FG and ultrasonic treatment will be an effective approach to improving the emulsifying property and thermal stability of PP,which can be considered as a potential plant-based emulsifier applied in the food industry.
1.Introduction
Recently,there is an escalating interest in the utilization of protein as an emulsifier since its high biological value,low cost,and multiple functional properties [1].The commercial protein emulsifiers (i.e.whey protein,casein,β-lactoglobulin from egg and milk) possess excellent emulsifying property in stabilizing emulsions with lower interfacial tension on the oil-water interfaces and conferring a protective film around the droplets to prevent them from coalescence [2].However,multiple studies have identified that these animal-derived proteins are common food allergens which can cause a series of immune response such as urticaria,flushing,rhinorrhoea,and diarrhea [3].Apart from these disadvantages,religious beliefs also strict their dietary application in the food industry.Therefore,plant proteins are potential replacement for animal proteins as emulsifier since they are consumer friendly.Pea protein (PP) seems to be a promising replacement of animal origins for its high nutritional value which can provide various essential amino acids for human [4].In addition,good availability,low cost,less connected to GMO questions also improved its application in food processing [5].But PP is sensitive to temperature resulting in low water solubility,poor emulsifying capacity and undesirable product taste [1,5].To overcome these obstacles,various methods for modifying proteins have been reported in recent years which can be divided into three types: physical methods (heat,acid,freezing,mechanical action (ultramicrogrinding,extrusion,etc.),magnetic field,electric field,sound field,low-dose radiation treatments or the addition of other parent substances et al.) [6].Chemical methods (polysaccharide graft modification,organic solvent modification etc.) [7].Enzymatic modification(enzyme-polymerization modification and enzyme-degradation modification) [8].Among them,physical modifications have the benifits of low cost,less time consuming,non-toxic side effects and less damage to the nutritional value of protein which is more acceptable by customers [9,10].Physical ultrasonic modification exists potential on improving emulsifying properties of proteins by reducing protein size,increasing surface hydrophobicity and molecular fluidity.However,ultrasound may promote the denaturation and decrease the thermal stability of protein.The physical interactions of proteins with polysaccharides have been proposed to improve the emulsifying ability,thermal stability,oil solubility,and cover the taste of proteins as emulsifier [11,12].Thus,we designed the combination of physical ultrasound and protein-polysaccharide compounding modification to enhance the thermal stability and emulsifying properties of PP,which would greatly open up the application fields of protein.
Flaxseed gum (FG),an anionic heteropolysaccharide consisting ofD-xylose,L-arabinose,L-rhamnose,L-galactose,D-glucose,L-fucose,andD-galacturonic acid,widely exists in flaxseed epiderm with abundant hydrophilic side chains which shows excellent properties of thickening,foaming,emulsifying,gelling,and water holding ability,etc.[13,14].In addition,FG,as a soluble dietary fiber,can reduce the incidence of diabetes,obesity,and coronary heart disease,prevent colorectal cancer which is considered a beneficial food ingredient [14,15].Previous researches have demonstrated that anionic polysaccharides can form complexes with proteins to decrease the surface hydrophobicity of proteins and increase the emulsifying properties [16-18].However,few studies were carried out to investigate the effect of FG on improving the water solubility,thermostability,and emulsifying properties of PP.In addition,thermodynamic incompatibility can occur for protein -polysaccharide -water system,depending on the concentration of biopolymers,pH,ionic strength and aggregation of one of the biopolymers.When pH is higher than protein pI,negative charged protein and polysaccharide will be repulsive and may form two phases in the solution or emulsion.Thus,extra methods were needed to improve the interaction,co-solubility and complexation of protein with polysaccharide under mild pH to expand the application of protein -polysaccharides [19].
Under the above situation,ultrasound stimulates the exposure of internal hydrophobic groups and changes the secondary structure of the protein,leading to the physical and functional properties changes of proteins,such as gel strength,foam stability [10],water holding capacity [20],solubility [21,22],and surface hydrophobicity,immune capacity [10,23].Furthermore,ultrasound can physically degrade polysaccharides through mechanical bond breaking and cavitation effect,thereby changing the spatial conformation,rheological properties,molecular weight etc.,effectively improving the solubility of large molecular weight polysaccharides [24].Therefore,the objective of this work was to study the effect of combined physical ultrasound and FG interaction on the PP composition,particle size,turbidity,ζ-potential,surface hydrophobicity,intrinsic fluorescence,interfacial tension,thermal stability,and rheological characteristics compared with native PP.Furthermore,the emulsifying properties were evaluated through several aspects of emulsifying activity index(EAI),emulsion stability index (ESI),droplets size,ζ-potential,microstructure,and storage stability of flaxseed oil-in-water emulsions prepared with native PP and its modified proteins.This work provides valuable information on physical and emulsifying profiles of PP modified by the combined FG mixing and ultrasound which enlarges the extensive application of plant protein in the food industry as an excellent emulsifier.
2.Materials and methods
2.1 Materials
PP powder was purchased from Shaanxi Ruizi Biological Technology Co.,Ltd.(Xi’an,China).The compositions of PP as stated by manufacturer were 85.40% protein (dry basis),0.30% fat(dry basis),6.76% water,4.70% ash (dry basis).The flaxseed cultivars(No.2 Baya) were donated by the Gansu Academy of Agricultural Sciences.Flaxseed oil was purchased from Hongjingyuan Oil Co.,Ltd.(Xilingol League,China) which composed of 73.20% of polyunsaturated fatty acid.All chemicals were provided by Sigma-Aldrich (Saint Louis,MS,USA).Deionized water was used in all experiments.
2.2 Preparation of samples
2.2.1 Protein purification
The isolation and purification of PP were carried out.Briefly,5 g PP powder was magnetically stirred for more than 5 h in 100 g PBS (5 mmol/L,pH 7.0).Then,the supernatant was collected after centrifugation at 10 000 r/min (Sorvall Biofuge Stratos Centrifuge,Thermo Scientific,Germany) for 30 min at 20 °C.Finally,the supernatant was diluted in PBS and measured by a UV/Vis spectrophotometer (Daojing,Tokyo,Japan) at 280 nm.The PP concentration of the supernatant was calculated as followed [25]:
2.2.2 FG extraction
The protocol of FG extraction was based on previous reports with a slight modification [26].The golden yellow flaxseeds were soaked in deionized water (water/seed ratio: 10:1,m/m) with electric stirring at 2 000 r/min for 2 h at 60 °C.The insoluble flaxseed particles were removed by centrifugation at 4 500 r/min for 10 min.Then,the supernatant was sedimented with pure ethanol (1:3,V/V)at 4 °C overnight and the sediment layer was collected by centrifuge(7 000 r/min for 15 min at 4 °C).Finally,FG powder was obtained after freeze-drying overnight.
2.2.3 Preparation of protein solutions
FG (0.16 wt%) was added in 1 wt% PP solution (pH 7.0) and then stirred for 4 h to ensure complete dissolution and form PPFG solution(pH 7.0).The PPU and PPFGU solutions (pH 7.0) were obtained by ultrasonic treatment of PP and PPFG solutions using a 20 kHz ultrasonic homogenizer (JY92-IIDN,NingbScientz Biotechnology Co.,Ltd.,China) at 400 W for 6 min (1 s:1 s,work/rest cycles).The addictive amount of FG and working condition of ultrasound have been optimized in preliminary study (Fig.S2).
2.3 Composition analysis
2.3.1 Analysis of monosaccharide composition of FG
High performance liquid chromatography (HPLC) along with PMP pre-column derivatization analysis of monosaccharide compositions(Analysis and Testing Center of Jiangnan University) were carried out according to a previous protocol with slight modifications [27].The relative molar percentage of each monosaccharide in FG was calculated based on peak molar area.The HPLC procedures were as follows: a ZORBAX Eclipse XDB-C18(250 mm × 4.6 mm,5 μm) was selected.The type of the detector is G1315B DAD.Mobile phase:0.1 mol/L phosphate buffer (pH 6.7) -acetonitrile (83:17,V/V).Column temperature: 30 °C.Detection wavelength: 250 nm.Flow rate:1 mL/min.Time: 55 min.Injection volume: 20 μL.
2.3.2 Protein composition analysis using electrophoresis
The protein compositions of PP,PPFG,PPU,and PPFGU solutions were measured by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) according to a previous protocol with slight modifications [4].Equal amount of four samples were diluted using loading buffer withβ-mercaptoethanol at 1:1 ratio (V/V),the mixture was heated at 95 °C for 10 min before electrophoresis.10 μL mixture solution was loaded onto a running gel composed of 3% stacking gel and 12% resolving gel.Electrophoresis conditions were 80 V at stacking gel and 120 V at resolving gel.Gel was stained with 2.5 g/L coomassie blue R-250 in 45% alcohol-9% acetic acid and destained with 50% methanol-9% acetic acid.
2.4 Analysis of physical properties
2.4.1 Determination of ζ-potential and particle size
Particle size andζ-potential of PP,PPFG,PPU,and PPFGU solutions were measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern Instruments,Worcestershire,U.K.) [9,10].Z-average diameter (Dz) indicates the intensity-weighted mean hydrodynamic size of the particles.Theζ-potential was measured by DLS in the Folded Capillary Cell DTS107098 at 25 °C.Prior to measuring theζ-potential,all samples were diluted by a PBS solution (1:20,V/V) to circumvent the multiple scattering effects.The refractive indices of water and PP were set at 1.330 and 1.500 in this experiment,respectively.
2.4.2 Turbidity measurements
Turbidity was analyzed based on the absorbance of protein solution at 400 nm in a quartz sample cell with a UV/Vis spectrophotometer (Daojing,Tokyo,Japan).The 5 mmol/L PBS solution (pH 7.0) was used as the control [10].
2.4.3 Determination of protein thermal stability
The thermal stability of PP,PPFG,PPU,and PPFGU solutions (10 mg)was evaluated using a Differential Scanning Calorimeter (Q2000,TA Instrument,USA).The heating program was performed with a slight modification according to the protocol [10].All the samples were heated from 10 to 110 °C at a rate of 5 °C/min,and the nitrogen flow rate was set as 40 mL/min.Peak temperature (Tpeak) and enthalpy (ΔH)of the samples were recorded.
2.4.4 Fourier transform infrared spectroscopy (FTIR)
The structural properties of the freeze-dried PP,PPU,PPFG and PPFGU solutions powder were determined by FTIR.The FTIR spectra were recorded from 400 to 4 000 cm-1using an FTIR spectrophotometer (TENSOR 27,Brucker,USA).All spectra were recorded at room temperature ((23.0 ± 0.5) °C) at a spectral resolution of 4 cm-1.
2.4.5 Determination of rheological properties
The viscosity and viscoelastic properties of the protein solutions was measured using a dynamic shear rheometer (AR 2000 rheometer,TA Instruments,UK).For viscosity measurement,approximately 0.6 mL sample was equilibrated at 25 °C for 2 min in a steal parallel plate geometry (diameter=40 mm,angle=2°) and treated with a constant shearing rate at 1 s-1for 2 min.Apparent viscosity (η) was then measured at different shear rates ranging from 2 to 200 s-1[28].For viscoelastic properties measurement,approximately 0.4 mL sample was placed on a steal parallel plate geometry (diameter=20 mm,angle=0°).The strain was set to 0.5%,the storage modulus(G’) and loss modulus (G’’) over an angular frequency range of 1-100 rad/s were measured [14].The tanδwere calculated based on the following formula (2):

2.4.6 Determination of intrinsic fluorescence
Changes in tryptophane (Trp) residues of the protein solutions were measured using a Multi-Mode Microplate Reader (Spectramax M2,Molecular Devices,Carlsbad,CA).Prior to measuring the intensity of fluorescence,all the samples were diluted to 5 mg/mL by PBS (5 mmol/L,pH 7.0) [29].An excitation wavelength of 290 nm and emission wavelengths from 300 to 500 nm were used.
2.4.7Surface hydrophobicity measurements
1-Anilino-8-naphthalenesulfonate (ANS) was used as a fluorescent probe to mark surface hydrophobic groups of proteins according to previous protocol with slight modifications [30].A series of working solution of PP,PPU,PPFG,PPFGU (0.025,0.05,0.075,0.1 mg/mL)was prepared by PBS buffer (5 mmol/L,pH 7.0).20 μL ANS solution was added into each protein sample and stored in the dark for 8-15 min after vortexed for 5 s.The fluorescence intensity of the samples was detected by a Multi-Mode Microplate Reader(Spectramax M2,Molecular Devices,Carlsbad,CA) at certain excitation (370 nm) and emission wavelengths (470 nm),respectively.The relative fluorescence intensity was plotted against the protein concentrations,and the initial slope of the fluorescence intensity versus protein concentration was obtained as the surface hydrophobicity index (H0) of the protein.
2.4.8 Dynamic interfacial tension (DIFT) measurement
Measurement of DIFT at oil-water interface was based on Joshi’s report [31]by pendant drop method using a drop profile tensiometer(PAT-1TM,Sinterface Technologies,Germany).Throughout the testing process,aqueous phase were PBS and the four protein solutions (PP,PPU,PPFG,and PPFGU).Oil phase was flaxseed oil.The measurement chamber was directly connected to syringe with plunger through a screw thread.Oil droplet with an appropriate shape and size was formed manually at the tip of capillary,and the interfacial tension at different time was recorded.
2.5 Analysis of emulsifying properties
2.5.1 Preparation of emulsions with protein solutions
The flaxseed oil-in-water emulsions were prepared following to previous protocol with some modifications [32].Briefly,10% flaxseed oil mixed with 90% different protein solutions (1 wt% PP,PPU,PPFG,PPFGU) using a high-speed blender (IKA,T25,Germany) at 13 000 r/min for 3 min to formulate the coarse emulsions,respectively.Then the coarse emulsions were subjected to ultrasonic emulsification using ultrasonicator with the power of 400 W for 16 min (1 s/1 s,work/rest cycles) to obtain emulsions in an ice bath.Sodium azide (0.02%,m/m) was added to all emulsions as an antimicrobial preservative during the storage process.EAI,ESI,droplet size,ζ-potential,and microstructure of emulsions were measured as followed.The storage stability of the emulsions at 4 °C was recorded at different time points with a camera.
2.5.2 EAI and ESI
EAI and ESI were measured according to previous protocol with slight modifications [33].The absorbance values of freshly prepared coarse emulsions stabilized by PP,PPU,PPFG and PPFGU solutions at 0 (A0) and 30 (A30) min were obtained at 500 nm,following the introduction of 100 μL of samples into 10 mL of 0.1% sodium dodecyl sulfate (SDS).Then,EAI and ESI of emulsions were measured and calculated as follows:

Wheredis the dilution factor,Cis the initial concentration of protein (g/mL),Φrefers to oil volume fraction of the emulsion,A0andA30are the absorbance at 0 and 30 min,respectively.
2.5.3 Emulsion microstructure
Confocal laser scanning microscopy (CLSM,Nikon DEclipse C1 80i,Nikon,Melville,NY) equipped with a 600 × magnification was conducted to test the microstructure of different proteins-stabilized emulsions.Briefly,mixture of emulsion and PBS (1:1,V/V) was dyed with Nile red-ethanol solution (1 mg/mL) to highlight the location of the flaxseed oil droplets within the emulsions [28].Then,5 μL emulsion was located on the microscope slide and covered with a coverslip to observe the microstructure of the emulsion.All images were taken and analyzed using the instrument software program(NISElements,Nikon,Melville,NY).
2.5.4 Particle size and ζ-potential measurement of the emulsions
Particle size andζ-potential of emulsions were measured by Malvern Mastersizer 3000 (Mastersizer 3000,Malvern Instruments,Westborough,MA) and Zetasizer Nano ZS (Malvern Instruments,Worcestershire,U.K.),respectively.To circumvent multiple scattering effect,all samples were diluted 250-fold with PBS solution beforeζ-potential measurement.Refractive indexes of the flaxseed oil and aqueous phases used in the calculations were 1.490 and 1.330 [34].
2.6 Statistical analysis
All data were reported as mean ± standard deviations.Significant differences (P<0.05 at 95% level of confidence) among the results were calculated by one-way analysis of variance (ANOVA) and Tukey’s test using SPSS 21.0 software (SPSS,Inc.,Chicago,IL,USA).
3.Results and discussion
3.1 Monosaccharide composition of FG
The gas chromatogram of monosaccharide composition of the extracted FG showed in gas chromatogram (Fig.S1) including rhamnose,galacturonic acid,glucose,galactose,xylose,arabinose,and fucose,which was consistent with previous reports [35].The molar percentage of each monosaccharide was shown in Table 1.Among them,the content of xylose was highest accounting for 41.56% .Arabinose,galactose,and rhamnose were 15.84%,14.83%,and 10.08%,respectively.The total content of the three acid monosaccharides of fucose,rhamnose and galacturonic acid was 23.92%,which would negatively charge the FG in the aqueous solution.

Table 1 Molar percentages of the monosaccharide compositions in FG.The average and standard error were obtained from two replicates.
3.2 Effects of FG and ultrasound on protein compositions and FTIR spectra of PP
The protein compositions of PP,PPU,PPFG and PPFGU were investigated by SDS-PAGE for analyzing the effect of ultrasound (400 W for 6 min,1 s:1 s,work/rest cycles) or FG (0.16 wt%),which has been optimized by preliminary experiment,on subunit compositions and structures of PP (Fig.1A).PP consist of legumin (11S),a hexameric protein including Lαacidic subunit (8-40 kDa) and Lβbasic subunit (19-22 kDa),and vicilin (V),a 7S trimeric protein composed of 51,70,and 19-12.5 kDa heterogeneous subunits [4].The SDS-PAGE results showed that the fractions of Lα,Lβ,and V of four protein solutions were similar indicating that ultrasound and FG treatment didn’t affect the subunit compositions and structures of PP.Compared to PP,no extra bands were detected with either ultrasonic treatment or additional FG which was congruence with the previous report [11].Generally,addition of polysaccharides into protein belongs to chemical modification of protein when polysaccharides interact with protein producing new substance [36].Here we found that interaction of PP and FG was physical binding without new substance forming.Therefore,in this system,we can infer that the effect of FG (0.16%,m/m) and ultrasound (400 W,6 min,1 s:1 s,work/rest cycles) on PP belongs to physical modification.

Fig.1 The protein compositions pattern of PP,PPU,PPFG and PPFGU solutions based on SDS-PAGE under reducing conditions (A).In the lanes of PP,PPU,PPFG and PPFGU solutions,V=bands from vicilin proteins,Lα=legumin acidic subunit and Lβ=legumin basic subunit.FTIR of freeze-dried PP,PPU,PPFG and PPFGU powders (B).
FTIR spectroscopy helps to reveal the microstructural difference between the four protein samples [37].The FTIR spectra of these freeze-dried powders were shown in Fig.1B.Two characteristic peaks were recognized at 1 649-1 650.93 cm?1and 1 548.71 cm?1,which were related to C=O stretching vibration in the amide I area,C-N stretching and N-H bending vibration in amide II area,respectively.The characteristic peak at 1 649 cm?1were shifted to 1 650.93 cm?1when FG bound to PP,which was possibly due to the structural changes in the functional groups as a result of molecular hydrophobic and hydrogen bonding interactions.In addition,the broad band from 3 200 cm?1to 3 500 cm?1was due to the overlapping of hydroxyl group and amino group stretching vibrations [5,37].Ultrasound and/or FG influenced the peak position that interpreted a large number of hydrogen bonds formed during the fabrication of the complexes.Among them,the combination of FG and ultrasound minimized peak intensity of the above three typical peaks,which indicated the strong molecular hydrophobic and hydrogen bond interaction between PP and FG [5,38].Therefore,PP and FG is physical binding by molecular hydrophobic and hydrogen bonding interactions.
3.3 Effects of FG and ultrasound on particle size distribution and ζ-potential of PP
The particle size distribution (Dz) andζ-potential of protein solutions (PP,PPU,PPFG and PPFGU) were shown in Fig.2.Compared with PP and PPFG solutions,theDzof PPU and PPFGU solutions decreased from 285 nm to 226 nm,and 905 nm to 788 nm,respectively (Fig.S3).It was attributed to the cavitation effect of ultrasound that created turbulence and high shear forces that broke protein molecules into small molecules [29,39].Hence,ultrasound treatment decreased theDzof PP and further produced a small peak at around 5 000 nm for PPU and PPFGU solutions revealing some aggregation of particles [40,41](Fig.2A).After the addition of FG,the proportion of PP with larger size was bigger indicating the formation of PP and FG complex.The SDS-PAGE results showed that the interaction of PP and FG was not chemical reaction.The PP and FG complex formed via physical interaction.
Theζ-potential of PP,PPU,PPFG and PPFGU was (-18.17 ± 0.41),(-20.13 ± 0.83),(-21.07 ± 0.60) and (-22.33 ± 0.75) mV,respectively (Fig.2B).The higher value ofζ-potential indicates greater electrostatic repulsion leading to the formation of a more stable colloidal system with soluble protein particles [42].Therefore,we can conclude the PPFGU showed the best ability to form stable colloidal system.ζ-Potential increase of protein was due to negatively charged amino acids exposure via ultrasonic cavitation [11,43].Theζ-potential of PPFGU was not significantly changed compared to the PPFG solution.The possible reason was the FG interaction with PP after ultrasonic treatment leading to the electrostatic screening effect on PP.Taken together,the complex of FG with PP,and cavitation effect of ultrasound decreased the particle size and increased theζ-potential value of PP.
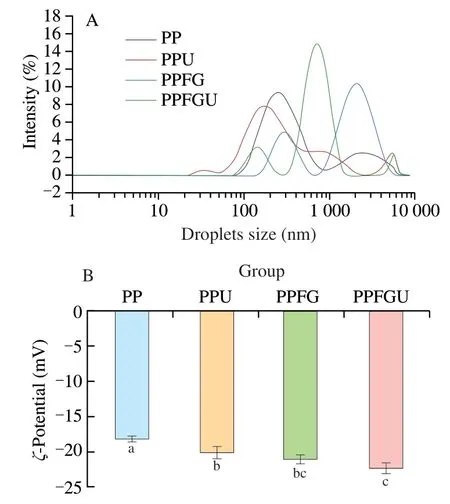
Fig.2 Particle size distribution (A) and ζ-potential (B) of PP,PPU,PPFG and PPFGU solutions.Data points and error bars represent means ± standard deviations (n =3).Different superscript letters indicate significant differences at the P <0.05 level.
3.4 Effects of FG and ultrasound on turbidity of PP
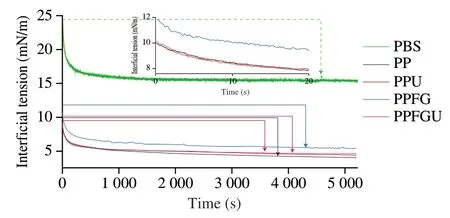
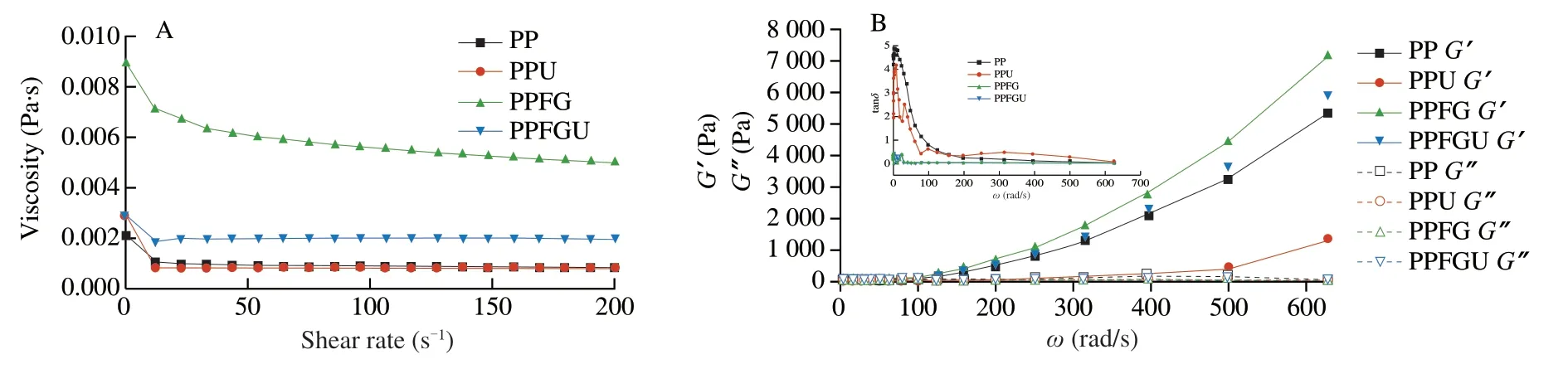
The turbidity and appearance of the four protein solutions were shown in the Fig.3.Ultrasound significantly decreased the turbidity of PP while FG increased it which is congruence with the results of particle size (Figs.2A,3).Similar phenomenon of sunflower protein and casein micelle solutions was also observed due to ultrasonic treatment [44].The turbidity of four protein solutions ranked with order of PPU Fig.3 Turbidity of PP,PPU,PPFG and PPFGU solutions.Insert picture is the visual appearance of PP,PPU,PPFG and PPFGU solutions.Data points and error bars represent means ± standard deviations (n =3).Different superscript letters indicate significant differences at the P <0.05 level. Exposure of tryptophan residues buried in PP may alter the intrinsic fluorescence intensity which can reflect the conformational changes of PP tertiary structure [29,45,46].Fluorescence intensity of PP,PPU,PPFG and PPFGU at pH 7.0 was shown in Fig.4A.Compared to PP,the fluorescence intensity at peak of PPFG,PPU and PPFGU were enhanced indicating ultrasound and FG urged the exposure of PP tryptophan residues.The effect of ultrasound on improving PPU intrinsic fluorescence was the same with PPFGU which showed the highest peak value compared to PP and PPFG.This result suggested the exposure of tryptophan residues by ultrasound treatment.In addition,we investigated the fluorescence intensity of PPFG and PPFGU solutions at pH 2.0-10.0 (Figs.S4A,B).The fluorescence intensity of PPFGU was higher than PPFG solution at the same pH,demonstrating the more internal hydrophobic groups exposed of PPFGU.Fluorescence intensity of the PPFG and PPFGU solutions significantly increased from pH 2.0 to pH 7.0,and tended to be stable during pH 7.0-10.0.In acid condition,the intrinsic tryptophan residues of PPFG and PPFGU aggregated resulting in low fluorescence intensity.However,the fluorescence intensity was relatively higher during neutrality or alkaline conditions (pH 7.0-10.0),resulting from the more intrinsic tryptophan residues exposed to the polar surface at higher pH due to the unfold of protein molecules and electrostatic repulsion [29].Particularly,the fluorescence intensity increased by ultrasound may attribute to the stronger interaction of PP with FG by electrostatic interaction force,hydrophobic interaction force,hydrogen bonding force that absorbed the tryptophan residues onto the polar surface [47].The fluorescence intensity difference of PPFGU and PPFG in different pH suggested the interaction of PP with FG was enhanced under ultrasound treatment which was consist with the particle size,ζ-potential and turbidity of PPFGU compared to PPFG.Therefore,we can conclude that ultrasound treatment increased the exposure of tryptophan residues PP or PPFG. Fig.4 Intrinsic fluorescence (A) and surface hydrophobicity (B) of PP,PPU,PPFG and PPFGU solutions at pH 7.0.Data points and error bars represent means ± standard deviations (n =3).Different superscript letters indicate significant differences at the P <0.05 level. Changes in surface hydrophobicity (H0) can be used to measure changes in the spatial structure of proteins.TheH0of PP,PPU,PPFG and PPFGU were determined and ranked with the order of PP DIFT of flaxseed oil -protein solutions interface at pH 7.0 was presented in Fig.5.Compared with PBS solution,proteins in the four PP solutions were able to adsorb onto the oil/water interface.The unfolded protein molecules acquired negative charge at pH 7.0 leading to simply and quickly adsorption onto the oil/water interface and decreased the interfacial tension [51].These proteins exhibited two distinct regimes on DIFT curve: a sharp decrease in DIFT (first phase) immediately after the formation of the protein-adsorbed interface,followed by a slow decrease (second phase) before reaching the equilibrium value.The interficial tension of oil in PP solution decreased from~10.02 to~4.09 mN/m.Ultrasound promoted the adsorption capacity of proteins (PPU) that the initial interfacial tension (9.96 mN/m) reduced more quickly at the first phase than PP.Ultrasound can decrease the protein particles size and stimulate protein molecules to unfold resulting in easier adsorption of PP onto the oil-water interface (Fig.2A) [31,33].However,the plateau value(4.51 mN/m) of PPU was slightly higher than PP,it may due to the protein aggregation by ultrasound.The addition of FG changed the property of oil/water interface,and significantly increased the initial interfacial tension (~11.83 mN/m) when compared with PP and PPU.It may be attributed to the increase of solution viscosity or the competitive adsorption effect between FG and PP that caused shielding effect on PP adsorption [52].However,ultrasonic treatment decreased the whole interfacial tension from the beginning than PPFG,and accelerated the decreasing rate.Therefore,ultrasound treatment effectively enhanced the interaction of PP with FG and accelerated the absorption of PP-FG complex onto the interface with less energy [24,31]. Fig.5 DIFT of samples with PP,PPU,PPFG and PPFGU solutions at flaxseed oil/water interface.Inset graph is the magnified figure of DIFT curves in the first 20 s. In addition,the interfacial behaviour of proteins at oil-water interface is an important factor associated with their emulsifying property.During homogenization or emulsification process,oil phase would rapidly break into numerous oil droplets within a timescale of milliseconds.Evidence showed that the quicker of protein adsorbed onto the surface of droplets during homogenization or emulsification process,the smaller droplets and better stability of emulsions will be formed [31].In this work,although the physical mixing of FG with PP increased the whole interfacial tension,ultrasound significantly decreased the DIFT,and showed obviously faster reducing rate that was beneficial to produce smaller droplets and better stability of emulsion. The viscosity behaviors and viscoelastic properties of protein solutions were investigated (Fig.6).PP is a globular protein that inner structures are easily broken under low shear rates resulting in low viscosity [41].Both PP and PPU showed the lower viscosity compared to PPFG and PPFGU (Fig.6A).FG exerts thickening and gelling properties which can sharply increase the viscosity of protein solution.Therefore,the viscosity of PPFG and PPFGU solutions was higher than that of PP and PPU solutions.Due to the effect of ultrasonic shearing action,the viscosity of PPFGU decreased compared to PPFG.The rheological property of protein solutions is also related with particle size.Addition of FG increased particle size of PP resulting in higher viscosity,while ultrasound significantly decreased the viscosity of PPFGU resulting from ultrasonic effect on reducing the particle size of complexes (Fig.2A).For viscoelastic properties of protein solutions,G’ were all higher thanG’’ for the four solutions (Fig.6B),suggested that these protein solutions were mainly solid and has weak gel properties.FG significantly increased theG’,this could reveal a tendency toward a network arrangement.While ultrasound decreasedG’ which could be attributed to the ultrasonic degradation effect that produced more molecular aggregation.So theG’ was ranked in PPFG >PPFGU >PP >PPU.In addition,the tanδfor PP and PPU were higher than 1 at low frequency (80 rad/s for PP,50 rad/s for PPU),and lower than 1 at higher frequency.But the tanδfor PPFG and PPFGU were lower than 1 at the test frequency.These suggested that PP and PPU performed liquid behavior at low frequency while PPFG and PPFGU both performed solid-like behavior at the any test frequency.Taken together,smaller viscosity,good solid-like network structure and smaller particle size of PPFGU would show better emulsifying ability.Therefore,ultrasonic treatment is an effective approach to improving the PP emulsifying property [41]. Fig.6 (A) Viscosity as a function of shear rate for PP,PPU,PPFG and PPFGU solutions.(B) Storage modulus (G’,solid line) and loss modulus (G’’,dash line)of PP,PPU,PPFG and PPFGU solutions as a function of frequency.Inset graph is the value of tanδ (tanδ=G’’/G’) for PP,PPU,PPFG and PPFGU solutions. Thermal stability of protein emulsifiers is a pivotal index for its application when considering product shelf life [53].Thermal properties of the PP solutions were evaluated by DSC thermograms(Fig.S5).The estimated enthalpy (ΔH) and transition temperature(Tpeak) were summarized in Table 2.TheTpeakof PP,PPU,PPFG and PPFGU were (70.56 ± 0.35),(68.75 ± 0.33),(72.05 ± 0.38) and(69.49 ± 0.31) °C,respectively,indicating that the addition of FG enhanced the thermal stability of PP.Similar results were reported by Chen et al.[54]who observed that the addition of FG slightly increased the transition temperature of meat protein by possible interaction of FG with meat protein.This phenomenon might result from the rearrangement of the PP and FG,and the formation of a new network structure [55].Ultrasound significantly decreased theTpeakof PPU and PPFGU compared to PP and PPFG.TheTpeakof PPFGU was slightly lower than PP,which was also attributed to the significant effect of ultrasound.Interestingly,theTpeakof PPFGU was higher than PPU,demonstrating the effect of FG on improving the thermal stability of protein.In addition,the slope curve of heat flow in PPFG and PPFGU also changed slower than that of PP and PPU,indicating FG slowed down the denaturation of PP (Fig.S5).The ΔHof PP,PPU,PPFG and PPFGU were (1 791 ± 15),(1 918 ± 18),(1 957 ± 23) and (1 914 ± 20) J/g,respectively.Ultrasound promoted the aggregation of protein,and more heat was needed to denature the aggregates of PPU.After the addition of FG,the ΔHof PPFG and PPFGU was significantly increased than native PP.Taken together,the addition of FG can promote the thermal stability of PP with higherTpeak,and the combination of ultrasound and FG can reduce the denaturation speed of protein that were both beneficial for the protein thermal stability. Table 2 Protein denaturation temperature (Tpeak) and enthalpy (ΔH) of PP,PPU,PPFG and PPFGU solutions. The EAI and ESI of freshly prepared coarse emulsions stabilized with PP,PPU,PPFG and PPFGU solutions were determined and represented in Fig.7A,B.The EAI of the coarse emulsions ranked with order of PP(29.85 m2/g) Fig.7 EAI (A) and ESI (B) of PP,PPU,PPFG and PPFGU solutions stabilized coarse emulsion (insert picture is the visual appearance of the four coarse emulsions).Droplets size (C),ζ-Potential (D) of PP,PPU,PPFG and PPFGU emulsions.Data points and error bars represent means ± standard deviations (n =3).Different letters on the columns indicate significant difference (P <0.05).Microstructure (E) of PP,PPU,PPFG,PPFGU emulsions at day 0.The scale bar is 10 μm.The visual appearance (F) of PP,PPU,PPFG and PPFGU emulsions during 0-46 days of storage at 4 °C. The droplet sizes,ζ-potential,microstructure and visual appearance stability of emulsions prepared with the four protein solutions were investigated (Figs.7C-F).The droplet sizes of emulsions were ranked as the order of PP-emulsion >PPU-emulsion ≥PPFG-emulsion >PPFGU-emulsion (Fig.7C),which was consistent with the size and dispersion of the droplets in the micrograph(Fig.7E).Ultrasonic treatment didn’t affect theζ-potential of emulsions,while FG significantly increased theζ-potential (Fig.7D),which could be explained by the highζ-potential value of PPFG complex (Fig.2B)that absorbed onto the oil-water interface.Smaller droplet size and higher negativeζ-potential are beneficial to the stability of emulsions.Therefore,PP-emulsion appeared creaming on the top of the emulsion after stored for 18 days at 4 °C,presented oil released and water sedimentation 36 days later (Fig.7F).After stored for 42 days,PPU-and PPFGemulsions appeared water sedimentation,while the PPFGU emulsion was still stable.Ultrasonic treatment produced smaller particle size that accelerated molecular mobility to adsorb onto the oilwater interfaces and show better ability to prevent oil droplets from aggregation and floating up [56].In addition,the hydrophobic protein residues inside the untreated aggregates were further exposed by ultrasound resulting in the increase of hydrophobicity and improvement of the interface accumulation at the droplet interface.FG owns the ability of gelling and emulsifying,which can limit the mobility of oil and water from separation and sedimentation.PPFGUemulsion performed best storage stability indicating the synergistic effect of ultrasound and FG on enhancing the stability of emulsion.Due to the better physiochemical and emulsifying properties of PPFGU with higher negativeζ-potential,smaller droplet size,higher viscosity,good gel property,stronger hydrophobic properties,better thermal stability,fast protein absorption rate,the PPFGU-emulsion showed the most stable compared to other three emulsions. The overall findings of this study are summarized in Fig.8.Ultrasound can decrease the particles size,increase the ability of protein emulsifier absorbing onto the interface with less energy and higher surface area coverage leading to the enhancement of emulsifying property (Figs.7F,8).PP and FG showed co-solubility in PBS which acted as hydrocolloids of the same type.However,without ultrasound effect,loosely attached complex of PPFG stabilized coarse emulsion showed the worst stability due to the energy difference of PP and FG absorbing onto the interface.Depletion flocculation appeared when a high amount of FG presented in the continuous phase during the storage period [19],so phase separation occurred in PPFG stabilized coarse emulsion (Fig.7B).PP and FG became strongly attached under the treatment of ultrasound for the improved surface hydrophobicity by the exposure of hydrophobic tryptophan residues.Therefore,PPFGU coarse emulsion exhibited better stability compared to PPFG coarse emulsion.Furthermore,ultrasound can change the spatial conformation of FG that may increase the chance of FG colliding with PP.The smaller protein particles of PPFGU compared to PPFG showed a better ability to adsorb onto the oilwater interface,and PPFGU obtained higherζ-potential and thicker membrane than PP in preventing droplet flocculation (Fig.7F).Overall,our results suggested that combination of two mild physical modification methods of FG mixing and ultrasonic treatment,effectively improved the physical properties of PP,especially emulsifying ability as emulsifier which can be extensively applied in food industry. Fig.8 Diagrammatic illustration of particles structure hypothesis in PBS solutions and emulsions. In this work,FG and ultrasonic treatment significantly improved the physical and emulsifying properties of PP through noncovalent interactions of the intermolecular hydrophobic and hydrogen bonding interactions.PPFGU exhibited larger particle size,bigger turbidity and higherζ-potential.After ultrasonic treatment,the particle size of PP-FG complex decreased via cavitation effect.The intrinsic fluorescence intensity andH0were enhanced resulting in the decreased rate of interfacial tension and increase of the protein absorption rate in PPFGU.Ultrasound and FG also reduced the denaturation speed of PP,while increased the solution viscosity.Hence,PPFGU showed the best performance on the improvement of emulsifying properties.The flaxseed oil-in-water coarse emulsion stabilized by PPFGU exhibited the highest EAI,ESI.The PPFGU-emulsion performed best stability during storage compared to emulsions stabilized by PP,PPU,and PPFG.In all,the combination of ultrasound and anionic polysaccharide can significantly improve the emulsifying properties of plant proteins,which can contribute to their extensive application as functional emulsifiers in the food industry. Conflict of interest The authors have declared no conflicts of interest. Acknowledgements This work was financially supported by grants from the Key Scientific Research Projects of Hubei Province (2020BCA086),the National Key Research and Development Program of China(2017YFD0400200),Wuhan Application Fundamental Frontier Project of China (2020020601012270),the National Natural Science Foundation of China (31771938),the China Agriculture Research System of MOF and MARA,and the Wuhan Achievement Transformation Project (2019030703011505). Appendix A.Supplementary data Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.1016/j.fshw.2022.07.045.
3.5 Effects of FG and ultrasound on hydrophobic properties of PP

3.6 Effects of FG and ultrasound on DIFT
3.7 Effects of FG and ultrasound treatment on rheological properties of PP
3.8 Effects of FG and ultrasound treatment on thermal properties of PP

3.9 Effects of FG and ultrasound treatment on emulsifying properties of PP


4.Conclusions

