Local nitrogen application increases maize post-silking nitrogen uptake of responsive genotypes via enhanced deep root growth
CHEN Zhe ,REN Wei ,YI Xia ,LI Qiang ,CAI Hong-guang ,Farhan ALI ,YUAN Li-xing,MI Guo-hua,PAN Qing-chun,CHEN Fan-jun,
1 College of Resources and Environmental Sciences/National Academy of Agriculture Green Development/Key Laboratory of Plant-Soil Interactions,Ministry of Education,China Agricultural University,Beijing 100193,P.R.China
2 Sanya Institute of China Agricultural University,Sanya 572025,P.R.China
3 Institute of Agricultural Resource and Environment,Jilin Academy of Agricultural Sciences,Changchun 130033,P.R.China
4 Cereal Crops Research Institute,Pirsabak Nowshera 24110,Pakistan
Abstract Nitrogen (N) is unevenly distributed throughout the soil and plant roots proliferate in N-rich soil patches.However,the relationship between the root response to localized N supply and maize N uptake efficiency among different genotypes is unclear.In this study,four maize varieties were evaluated to explore genotypic differences in the root response to local N application in relation to N uptake.A split-root system was established for hydroponically-grown plants and two methods of local N application (local banding and local dotting) were examined in the field.Genotypic differences in the root length response to N were highly correlated between the hydroponic and field conditions (r>0.99).Genotypes showing high response to N,ZD958,XY335 and XF32D22,showed 50–63% longer lateral root length and 36–53% greater root biomass in N-rich regions under hydroponic conditions,while the LY13 genotype did not respond to N.Under field conditions,the root length of the high-response genotypes was found to increase by 66–75% at 40–60 cm soil depth,while LY13 showed smaller changes in root length.In addition,local N application increased N uptake at the post-silking stage by 16–88% in the high-response genotypes and increased the grain yield of ZD958 by 10–12%.Moreover,yield was positively correlated with root length at 40–60 cm soil depth (r=0.39).We conclude that local fertilization should be used for high-response genotypes,which can be rapidly identified at the seedling stage,and selection for “l(fā)ocal-N responsive roots” can be a promising trait in maize breeding for high nitrogen uptake efficiency.
Keywords: genotypic difference,local nitrogen,maize,nitrogen efficient,root
1.Introduction
Nitrogen (N) fertilizer has been extensively used for increasing crop productivity.However,the global N use efficiency (NUE,the fraction of N input harvested as a product) is only about 42% (Zhanget al.2015),resulting in a waste of resources along with profound and long-term environmental pollution (Juet al.2009;Liuet al.2016).Maize (Zea maysL.) plays a critical role in securing food safety for the ever-growing world population (Erismanet al.2008).Thus,improving maize NUE is one of the most effective ways to simultaneously achieve a high yield production with minimum environmental risk (Chenet al.2014;Liu Jet al.2020).The management of N fertilizer application and the development of N-efficient maize varieties provide an opportunity to achieve this purpose(Xuet al.2012).
The distribution of N is highly heterogeneous in farming soil,it varies greatly due to fertilizer applications,irrigation,tillage,and other farming practices (Bloom 1997;Wanget al.2020b).Soil resources are absorbed mostly through the root in maize and,consequently,the root morphology and physiology may alter in response to N heterogeneity in the soil (Drew 1975;Liuet al.2008;Giehl and von-Wiren 2014;Yuet al.2014a).Many studies have reported root proliferation in localized nutrient-rich regions,seen as significant increases in the total root length,root biomass and lateral root elongation and branching (Robinson 1996;Hodge 2004;Jinet al.2015;Wanget al.2020a).The plant shows a systemic carbon saving strategy whereby the specific root length increases in local N-rich regions,which improves the biological efficiency of the root system (Yuet al.2014b).Root physiological processes including increased nutrient uptake rates and rhizosphere processes,are also promoted by localized nutrients (Drew and Saker 1975;Sattelmacher and Thomas 2010).In addition,the mechanisms of root foraging for localized N have been extensively investigated inArabidopsis thaliana.Both local and long-distance systemic signaling pathways regulate these root responses,enabling plants to respond to localized N in an integrated manner (Mc-Cleeryet al.2017;Hamet al.2018;Ruffelet al.2018;Jia and von Wirén 2020;Jiaet al.2021).
The root plasticity response is genetically controlled(Schneideret al.2020;Liet al.2021).Genotypic differences in root plasticity response to local N applications have been observed in rice and grass species (Fransenet al.1998;Huanget al.2004).Several researchers have also reported similar results in maize,where genotypic differences were observed in root responses to local N supplies in laboratory-grown plants at the seedling stage.Nitrogen-efficient varieties have been found to respond more strongly to local N supply than N-inefficient commercial maize hybrids grown under hydroponic conditions (Chenet al.2017;Zhaiet al.2019).The inbred line Ye478 with a large root size was more responsive to local nitrate than Wu312 in an agar culture system (Guoet al.2005a).Further analysis showed that this response difference may have been due to their different auxin sensitivities,since auxin transport plays a key role in local root response (Guoet al.2005b).However,all these studies were conducted on seedlings in the laboratory,and the root responses of different genotypes to local N fertilization under field conditions remain unknown.
These morphological and physiological responses in plant roots contribute to the effective exploration and absorption of heterogeneous soil N (Jinget al.2010;Maet al.2015;Liet al.2016).Furthermore,changes in root systems are of great significance for improving the vegetative growth of plants (Hammeret al.2009;Muet al.2015;Schneideret al.2022).The “root-ideotype for N acquisition” hypothesis as proposed in recent years emphasizes the local plasticity of maize roots,whereby the lateral roots can proliferate in N-rich soil regions,allowing deeper root penetration as nitrate is easily leached into deep soil layers (Miet al.2010,2016;Lynch 2013).These characteristics permit exploration of localized N-rich areas by the roots and further contribute to their efficient absorption of N from the soil (Lynch and Tobias 2015;Yuet al.2015).Rational stimulation to promote root plasticity responses is an effective way to enhance the efficiency of N uptake in maize,promoting a complete utilization of the root biological functions (Lynch 2019).
Local application of fertilizer in the form of banding or dotting has been widely used in agriculture.It has been demonstrated that deep placement of N fertilizer (Chenget al.2020) or localized application of phosphorus and ammonium in the early seedling stage (Jinget al.2010)benefitted both maize growth and N uptake through the promotion of root plasticity and proliferation.However,the effect of localized N application on maize root growth,maize N uptake and grain yield require further clarification under field conditions.It is also not known if N response to localized N application is genotype-dependent.In this study,the response of four different commercial maize varieties to local N application was compared under field and hydroponic conditions.The root morphology,plant N efficiency and grain yield of the four varieties were comprehensively investigated.The aims of this study were: (1) to identify the genotypic differences underlying the maize root response to local N application and to investigate the consistency of the root response between hydroponic and field conditions,and (2) to elucidate the relationships between the root response to localized N supply and maize N uptake among different genotypes.
2.Materials and methods
2.1.Plant materials
Four maize varieties,namely,ZD958,XY335,XF32D22,and LY13 were investigated.These four varieties were the most popular commercial maize varieties and widely grown in Jilin Province,China,where the experiment was conducted.The four varieties were characterized by high resistance to diseases and insects.In addition,the varieties were found to be significantly different in N efficiency in a preliminary variety screening experiment.
2.2.Hydroponic experiment design
Cultivation of maizeA hydroponic experiment was conducted in a growth chamber (Kooland,BeiJing Kooland Technologies Co.,Ltd.,Beijing,China).The photosynthetic photon-flux density measured at the canopy level was 350 μmol m-2s-1,the day/night temperature was 28/22°C during the 14/10 h photoperiod and relative humidity was 60%.Seeds with uniform size from the four genotypes were sterilized in 20% (v/v) H2O2for 15 min.The seeds were then rinsed with deionized water and soaked in saturated CaSO4for 6 h.The seeds were then germinated in a growth chamber for 2 days in the dark.Germinated seeds with uniform growth were rolled in moist filter paper.The rolls were soaked vertically in plastic buckets filled with distilled water and cultivated in the chamber room for 2 days.After germination,the endosperm of seedlings with two visible leaves was removed.These plants were then transplanted into ceramic tanks containing nutrient solutions.To get the maize seedlings acclimated to the nutrient solution,we used 50% concentration of nutrient solution on the first day (and EDTA-Fe is 20% for prevention of Fe toxicity).And the seedlings were transferred to the complete nutrient solution on the following day.The nutrient solution contained (mol L–1): KH2PO4,0.25×10–3;K2SO4,7.5×10–4;MgSO4·7H2O,6.5×10–4;KCl,1.0×10–4;Ca(NO3)2·7H2O,2.0×10–3;EDTA-Fe,1.0×10–4;H3BO3,1.0×10–6;MnSO4·H2O,1.0×10–6;CuSO4·5H2O,1.0×10–7;ZnSO4·7H2O,1.0×10–6;(NH4)6Mo7O24·4H2O,5.0×10–9(Wanget al.2019).The solution was renewed every two days and continuously aerated using an air pump.
N treatment in the split-root systemAfter the development of crown roots from the first root node,seedlings with four nodal roots of uniform size were selected.All seminal roots were removed and the lights were turned off to reduce plant transpiration.The seedlings were then transplanted to a split-root system (Liuet al.2010).Two nodal roots were placed on each side of the splitting system.Two N application treatments were used,including uniform and local N treatments.In the two sided system of the local N treatment,the “N supply” side(local_+N) contained the nutrient solution supplemented with 1 mmol L-1NO3–,whereas the nutrient solution in the“no N supply” side (local_–N) was not supplemented with N.In the uniform N treatment,1 mmol L-1of the NO3-solution was supplied to both sides of the root system(uniform_+N1,uniform_+N2).Each treatment consisted of four replicates,with each containing three plants.The nutrient solution was renewed every two days for an adequate N supply in both local and uniform N treatments with continuous aeration.
Sampling and measurementsAll seedlings were harvested 7 days after N application.The roots on the two sides of the split-root system were collected separately and then scanned in a plastic tray (20 cm×15 cm) with a scanner (Epson 1600,Bangalore,India) as previously described (Sunet al.2021).The scanned images were analyzed using WinRHIZO version Pro 5.0 Software(Regents Instruments,Quebec,Canada) to determine root characteristics,the scanning data included axial root length (ARL),lateral root length (LRL) and root average diameter (RAD) on each of the two sides of the split-root system in localized and uniform N application treatments(local_+N,local_–N,uniform_+N1,uniform_+N2).After scanning,the root biomass per plant (RB) on the two sides of split-root system and shoot biomass per plant(SB) were measured after oven-drying plant material at 75°C to constant weight.The shoot N concentration was determined using the semi-micro-Kjeldahl method(Nelson and Sommers 1973),and the shoot N content(SNC) per plant was calculated by multiplying the shoot N concentration by the shoot biomass per plant.
2.3.Field experiment design
Cultivation conditions of maizeThe field experiments were conducted at Lishu (43°2′N,123°3′E),Jilin Province in Northeast China in 2009 and 2010.The soil at this location consists of black soil,equivalent to Hapludoll soil in the USDA Soil Taxonomy System (Soil Survey Staff 1998).The growing period was from May 1 to September 25 in the two consecutive years.The field used for this experiment was rain-fed and no fertilization experiments had been previously conducted at this location.At the beginning of the experiment,the soil between 0 and 60 cm depth was analyzed.The analysis showed a total N concentration of 1.2 g kg-1,alkaline-hydrolysis N of 176 mg kg-1,readily available potassium (K) of 28.4 mg kg-1,and readily available phosphorus (P) of 110 mg kg-1.The organic matter content was 17.5 g kg-1and the pH was 5.4.According to the recommended fertilizer management for Jilin Province published in Fenget al.(2017),fertilizer (containing 30 kg N ha-1,21.8 kg P ha-1,and 41 kg K ha-1) was applied as a basal application before sowing,and additional 120 kg ha-1N fertilizer was applied to the fertilization and control treatments at the beginning of the jointing stage (V6 stage).The soil nutrient content and basal fertilizer created a sufficient and equal nutrient environment.All the local cultural practices related to weeding and insect and disease management were followed to obtain a favorable environment for the experiments (Shaoet al.2019).
Localized N fertilization treatmentsThe experiments were conducted using a split-plot design with three replicates (blocks) in 2009 and 2010.In each replicate(block) the N application treatments were the “main plots”and the genotypes were the “subplots”.Each genotype had 11 rows in each subplot.The inter-row spacing was 66 cm and the inter-plant spacing was 25 cm.The row length was 5 m,with thinning performed to maintain 21 plants in each row.Two local N application treatments were used including local banding and local dotting.Firstly,a 7–10 cm deep trench was created at a distance of 20 cm from the maize rows.For the local banding treatment,N fertilizer was applied uniformly into the trench.For the local dotting treatment,N fertilizer was dotted into the trench at the position of each maize plant.In the uniform N application treatment,the N fertilizer was broadcast on the soil surface uniformly beside the maize plant.To ensure the accuracy of fertilizer dosage and uniformity in the uniform N application treatment,we converted the fertilizer dosage to 39.6 g for each plot(equivalent to 120 kg ha-1) and applied it to each plot separately (Appendix A).
Sampling and measurementsIn 2009 and 2010,the grain yield was measured at the physiologically mature stage (R6 stage on September 25).In 2010,the root phenotypes were measured at 10 days after the silking stage (R2 stage on August 6).The plant N content was measured at both the R2 and R6 stages.The silking stage was determined based on visual observation and the day when 50% of the plants were silking.The physiological maturity stage was determined when a black layer was visible at the grain base in 50% of the ears.
For measuring the root phenotypes,3–5 plants in each block were randomly selected.First,a soil cube (25 cm long,66 cm wide,and 60 cm deep) was dug around the plant.The soil cubes were divided into three 20-cmdeep layers (0–20,20–40,and 40–60 cm layer).The roots were carefully picked out of the soil with a fur brush and a sieve after careful washing and the removal of the surface moisture.The roots were then transferred to the laboratory for root phenotype measurements.Root phenotype scanning is described in the hydroponic experiment above.After scanning,the root and shoot biomass was measured after oven-drying at 75°C to constant weight.
For measuring the plant N uptake,three plants were randomly selected per plot at the R2 and R6 stages.The entire plant was cut at the level of the soil surface for N content measurement.There was no leaf loss during the sampling process.To investigate the N content in different organs,the N concentrations of stems,leaves,and grain(only at the mature stage for grains) were measured separately.Firstly,the stems,leaves,and grain were separated and dried to a constant weight at 75°C.Then the dry samples were weighed and ground into powder.Next,the N concentration was determined using the semimicro-Kjeldahl method (Nelson and Sommers 1973).Finally,the N concentration was multiplied by the dry weight to determine the N content of the stems,leaves,and grain,separately.The total N content of a plant was calculated as the sum of the individual N contents of the leaves,stems,and grain.The N uptake in the pre-silking stage refers to the plant N content measured at the R2 stage and the N uptake in the post-silking stage was calculated by subtracting the N content in the pre-silking stage from the N content measured at the R6 stage.
For the measurement of grain yield per plot,five central rows of each plot that had not been previously sampled were harvested.The grain yield was determined and standardized to 14% moisture and converted into grain yield per hectare.
2.4.Statistical analysis
Analysis of variance was conducted using the General Linear Model (GLM).Duncan’s Multiple-Range test was used to compare the significance of the differences between the factors.The statistical model formula used was as follows:

whereyis the response variable,μis the overall mean,while N and genotype are the fixed effects of the block,main-plot,and sub-plot,respectively.Block×N is the mainplot error,N×Genotype is the interaction between local N treatments and genotypes,and Block×N×Genotype is the sub-plot error (Shaoet al.2019).
Pearson correlation analysis was performed for the relationships evaluation between root traits and N efficiency traits,and between root traits under hydroponics and field conditions.The variance analysis and correlation analysis were performed in SPSS Statistics 21 Software (IBM,Armonk,NY,USA).Graphing was performed in Microsoft Excel 2010 (Microsoft Corporation,Redmond,Washington,USA) and the Adobe Illustrator CC 2018 (Adobe Systems Incorporated,SAN Jose,California,USA).
3.Results
3.1.Local responses of shoots and roots in the hydroponic experiment
In the hydroponic experiment,neither the N treatment nor the genotype×N interaction affected shoot biomass or shoot N content,however genotype had a significant effect on both attributes (P<0.05;Table 1).Genotypes ZD958 and LY13 had 69–175% larger shoot biomass values and 63–181% more shoot N content than XY335 and XF32D22 (P<0.001) (Fig.1-A and B).
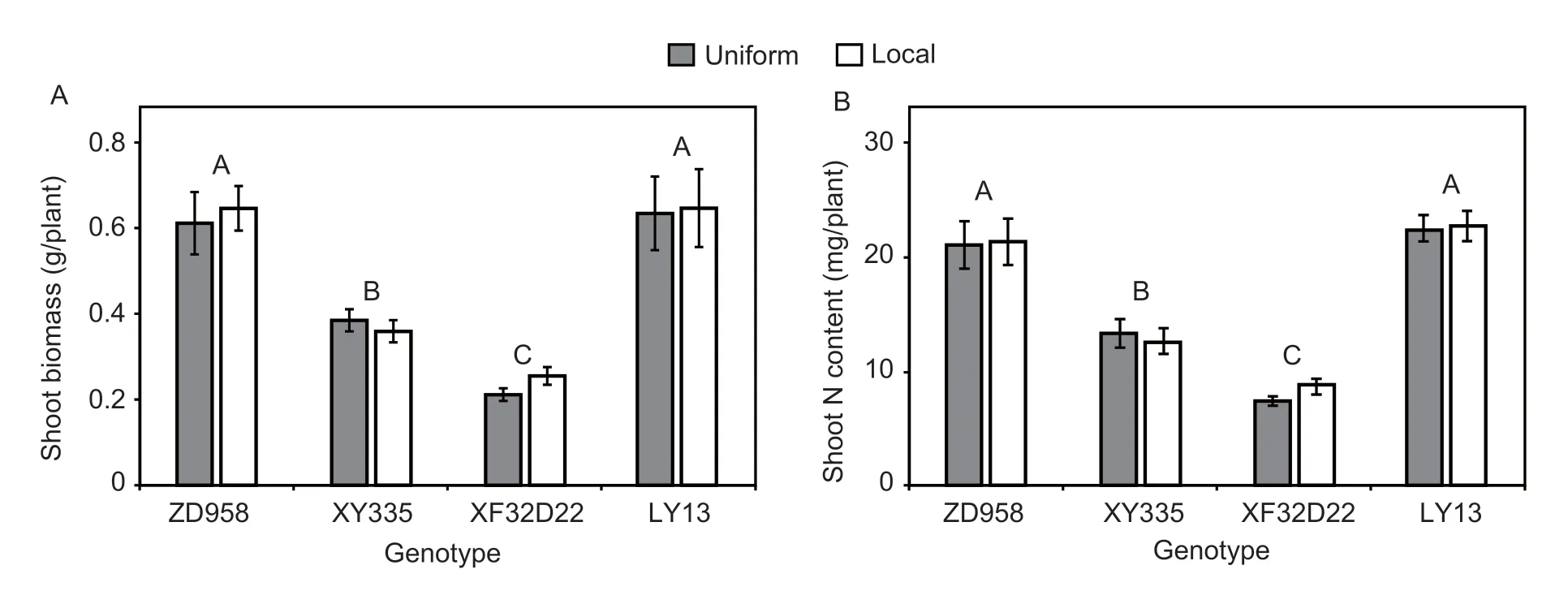
Fig.1 Genotypic differences in response to local N application of the shoot biomass (A) and shoot N content (B) at the seedling stage under hydroponic conditions.Uniform and local represent uniform and localized N application.Error bars denote the standard error (SE) of the mean (n=4).Different uppercase letters indicate significant differences between genotypes across all the N treatments (P<0.05).
Genotype and N treatment had significant impacts on the lateral root length,axial root length,root average diameter,and root biomass (P<0.05).There was also a significant (P<0.05) interaction between genotype and N for all root traits,except root average diameter (P<0.05;Table 1).The effects of localized N treatment on root growth were detected at the first stage.Compared with the average of the uniform_+N1 and uniform_+N2 treatments,the lateral root length increased by 36% in the local_+N treatment but decreased by 43% in the local_–N treatment.The root biomass increased by 31% in the local_+N treatment,but decreased by 19% in the local_–N treatment (P<0.01).However,there was variation in the response of the different genotypes.In the local_+N treatment,the lateral root length increased by 50–63%and the root biomass by 36–53% in the ZD958,XY335,and XF32D22 varieties.In the local_–N treatment,the latereal root length decreased by 23–47% and the RB by 16–26% in the ZD958,XY335,and XF32D22 varieties while the root average diameter increased by 23–47%(Fig.2-A–D).However,none of the root traits of LY13 were altered in the local_+N treatment.

Fig.2 Genotypic differences in response to local N application of lateral root length (A),axial root length (B),root diameter (C) and root biomass (D) at the seedling stage under hydroponic conditions.Uniform_+N1 and uniform_+N2 represent the two sides of the uniform N application treatment in the split-root system;local_+N,and local_–N represent the N supply side and no-N supply side in the local N application treatment in the split-root system.Error bars denote the standard error (SE) of the mean (n=4).Different lowercase letters indicate significant differences between uniform N supply and localized N supply in the same genotype(P<0.05).Different uppercase letters indicate significant differences between genotypes across all the N treatments (P<0.05).
Neither the uniform nor local N application affected the shoot biomass and shoot N content.However,local N application promoted root growth in the local_+N treatment regions and controlled the root growth in the local_–N treatment regions.Genotypic differences were observed,where the ZD958,XY335,and XF32D22 varieties showed a high response to treatment while LY13 showed a low response.
3.2.Local responses of roots in the field experiments
In the field experiment,both RL and RB differedsignificantly among genotypes (P<0.05) at different soil depths (Table 2).Genotypes ZD958 and LY13 were shallow rooting with more roots allocated at the 0–20 cm soil depth,while XF32D22 and XY335 were deep rooting with more roots allocated at 20–60 cm depth(Fig.3;Appendix B).The RL in the various soil depths differed significantly between the N fertilizer application treatments (P<0.05),while the RB did not (Table 2;Appendix B).The average RL of the four genotypes decreased by 7–10% at 0–20 cm and increased by 61–65% at 40–60 cm under the local banding and local dotting treatments (P<0.05).
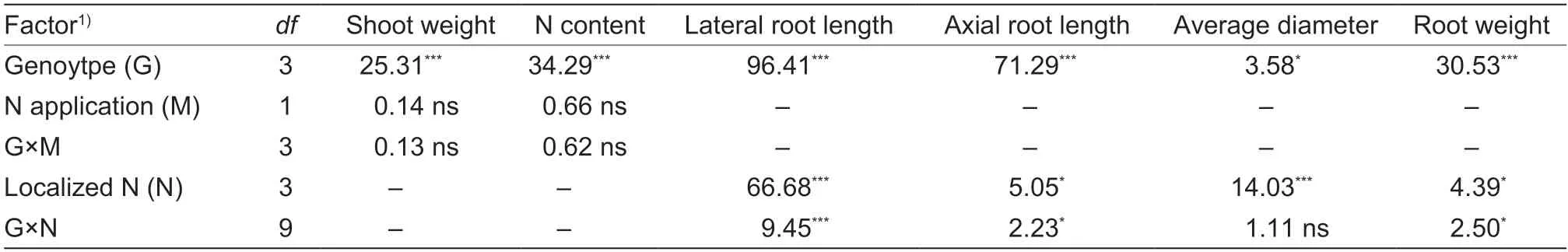
Table 1 Summary of ANOVA results (F-values) for the parameters examined in the split-root hydroponic experiment
There were significant genotype×N fertilizer interactions for RL at different soil depths (Fig.3;Table 2).Compared with uniform N application,the RL of genotype ZD958 decreased by 9 and 12% in the local banding and local dotting N application treatments,respectively (P<0.05),at 0–20 cm soil depth,while the RL of XF32D22 decreased by 24% in the local banding N application treatment.At 20–40 cm soil depth,the RL of genotype XY335 increased by 81 and 119% in the local banding and local dotting treatments,respectively (P<0.05).At 40–60 cm soil depth,the RL of genotypes ZD958,XY335,and XF32D22 increased by 69,81,and 75%,respectively,in the local banding treatment.The RL of genotypes ZD958,XY335,and XF32D22 increased by 70,94,and 119%,respectively,in the local dotting treatment.However,only the RL of LY13 at the 20–40 cm soil depth increased by 32 and 39% in the local banding and local dotting treatments,respectively.The rate of change in genotype LY13 was lower than that in the other genotypes (P<0.05),and the RL of LY13 did not change in response to local N application at 0–20 or 40–60 cm soil depths.These results showed that genotype LY13 had a lower response to local N fertilizer application than the ZD958,XY335,and XF32D22 genotypes and that local N fertilizer application primarily promoted root growth at deeper soil levels (20–60 cm).
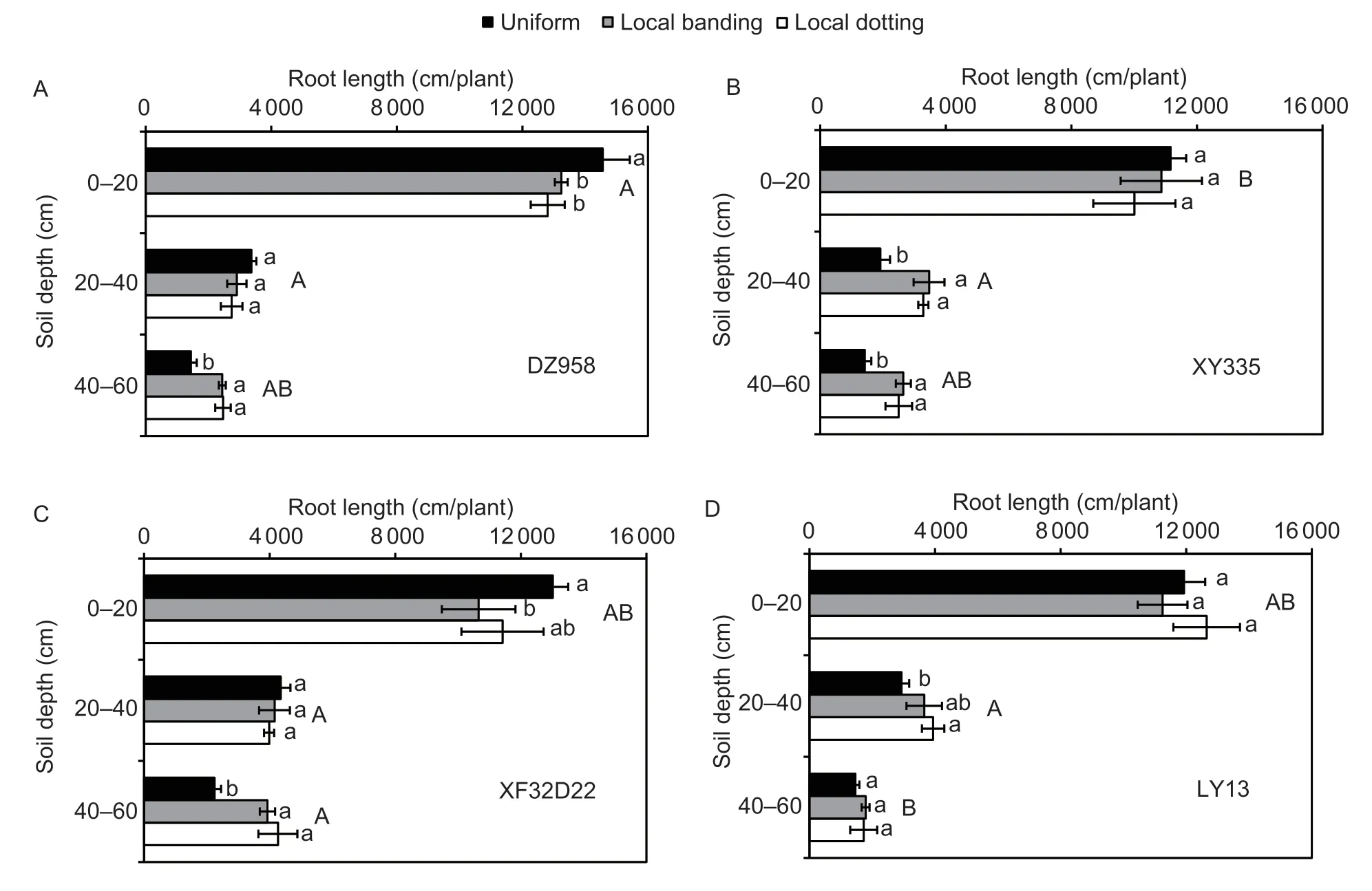
Fig.3 The effects of local N fertilizer application on maize root length of genotypes ZD958 (A),XY335 (B),XF32D22 (C),and LY13(D) in different soil layers of the field experiment. Uniform,local banding and local dotting represent the broadcast N application,banding N application and dotting N application treatments,respectively.Error bars denote the standard error (SE) of the mean(n=3).Different lowercase letters indicate significant differences between the three N fertilization treatments (P<0.05) at the same soil depth.Different uppercase letters indicate significant differences between the four genotypes (P<0.05) at the same soil depth.
3.3.Local responses of roots with different diameters in the field experiment
Roots were divided into two different diameter ranges,including fine roots (diameter smaller than 1.2 mm)and thick roots (diameter larger than 1.2 mm).The proportions of fine and thick roots differed among the four genotypes (P<0.05).The genotypic differences in fine roots were similar to those for RL described above.Genotypes ZD958 and LY13 were shallow-rooting genotypes with fine roots at 0–20 cm soil depth,while XY335 and XF32D22 were deeper rooting with more fine roots distributed at 40–60 cm soil depth (Fig.4-A–D).The difference in total root length among the different genotypes was thus primarily reflected in the fine roots.
Fine RL values at different soil depths were affected by N application treatment and interactions between genotype and N application treatment(P<0.05),while thick RL was not affected (P>0.05) (Table 2).The local N application treatments largely promoted fine root growth in the high-response genotypes (ZD958,XY335,and XF32D22;P<0.05).Under local N application treatments,at 40–60 cm soil depth,the fine RL of genotypes ZD958,XY335,and XF32D22 increased by 70–73,73–92,and 76–83%,respectively (Fig.4-A–C).At 20–40 cm soil depth,the fine RL of ZD958 decreased by 26–29%,but that of XY335 increased by 89–138%.In the low-response LY13 genotype,the fine root length increased by 47–57% at 20–40 cm but did not change at either 0–20 or 40–60 cm soil depth (Fig.4-D).No response to N was found for the length of thick roots for the three high-response genotypes (Fig.4-E–H).Overall,these results showed that localized N supply primarily promoted fine root growth at 20–60 cm soil depth in the three high-response genotypes.
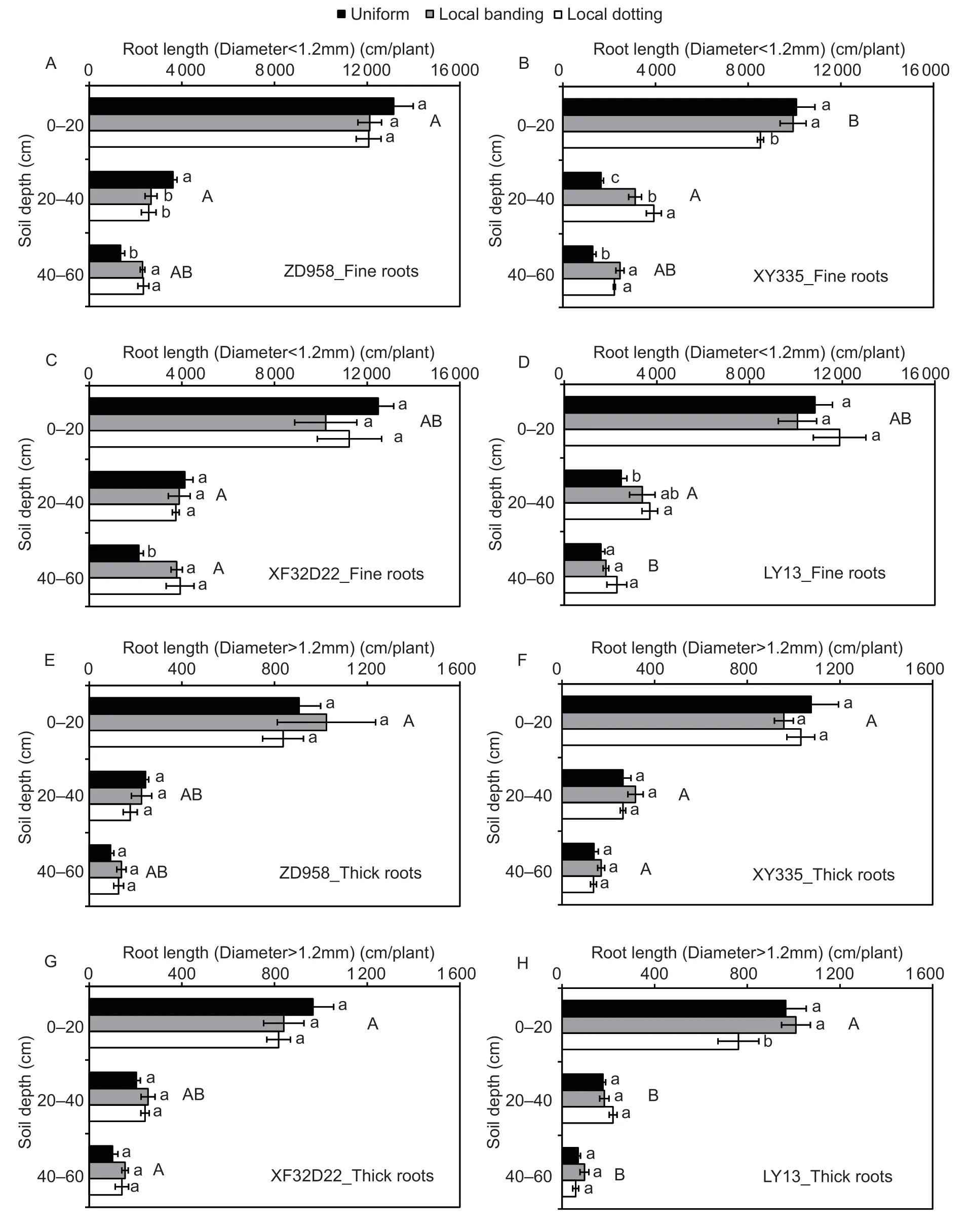
Fig.4 The response of root length with different diameter ranges to local N fertilizer application of genotypes ZD958 (A and E),XY335 (B and F),XF32D22 (C and G) and LY13 (D and H). Uniform,local banding and local dotting represent the broadcast N application,banding N application and dotting N application treatments,respectively;fine root length (A–D) and thick root length(E–H) represent represent the root length with a diameter smaller than 1.2 mm and larger than 1.2 mm,respectively.Error bars denote the standard error (SE) of the mean (n=3).Different lowercase letters indicate significant differences between the three N fertilization treatments (P<0.05) at the same soil depth.Different uppercase letters indicate represent significant differences between the four genotypes (P<0.05) at the same soil depth.
3.4.Genotypic differences in N uptake and grain yield in response to localized N supply
Both N uptake and grain yield were significantly different between the four genotypes (P<0.05;Table 2).The three high-response genotypes (ZD958,XY335,and XF32D22) had lower N uptake in the pre-silking stage than LY13.Specifically,the XY335 genotype had the lowest pre-silking N uptake (range 115–119 kg ha–1in the three N fertilizer treatments).In contrast,the ZD958,XY335,and XF32D22 genotypes had higher N uptake in the post-silking stage compared with LY13 (range 18–22 kg ha–1in the three N treatments;Fig.5).Furthermore,XY335 had the highest post-silking N uptake amount (range 32–46 kg ha–1in the three N treatments).The three high-response genotypes(ZD958,XY335,and XF32D22) had a higher grain yield than LY13.The XY335 genotype had the highest grain yield in both years: 11 870–12 946 kg ha–1in 2009 and 9 070–9 605 kg ha–1in 2010 (Fig.6).The LY13 low-response genotype had the lowest grain yield of 10 662–11 791 kg ha–1in 2009 and 7 754–8 058 kg ha–1in 2010 (Fig.6).
Nitrogen application treatment had significant impacts on grain yield in both years and N uptake in the post-silking stage,and there was a significant interaction between N treatment and genotype for grain yield in 2010 (Table 2).Post-silking N uptake was significantly greater in the local N application treatment (local banding and local dotting) than when N was supplied uniformly(P<0.01).Nitrogen supply by local dotting increased post-silking N uptake of genotypes ZD958,XY335,and XF32D22 by 88,45,and 55%,respectively,while local banding increased post-silking N uptake of genotypes ZD958 and XY335 by 43 and 16%,respectively (Fig.5).However,for the insensitive LY13 genotype,N uptake was not changed by N supply treatment.The grain yield of ZD958 was increased by 10% in 2009 and 12% in 2010,by the local dotting N application treatment.The other two high-response genotypes (XY335 and XF32D22)displayed no change in the grain yield in response to N supply treatment.We found that in the local N application treatments,XY335 and XF32D22 had more N stored in the stems and leaves,while ZD958 had more N stored in the grain (Appendix C).In addition,the low yielding genotype LY13 was not affected by N application treatment (Fig.6).In summary,localized N supply promoted post-silking N uptake in the high-response genotypes (ZD958,XY335,and XF32D22)and enhanced grain yield in ZD958.
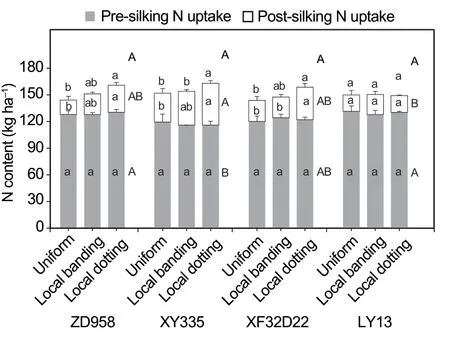
Fig.5 Genotypic differences of the effects of local N fertilizer application on maize N uptake in the pre-silking and postsilking stages.Uniform,local banding and local dotting represent the broadcast N application,banding N application and dotting N application treatments.Error bars denote the standard error (SE) of the mean (n=3).Different lowercase letters indicate significant differences (P<0.05) between N fertilization treatments at the pre-silking stage (grey),postsilking stage (white),and for the entire growth period (above the bars).Different uppercase letters indicate significant differences(P<0.05) between genotypes across all the N fertilization treatments at the pre-silking stage (besides the grey bars),post-silking stage (besides the white bars),and for the entire growth period (above the bars) (P<0.05).
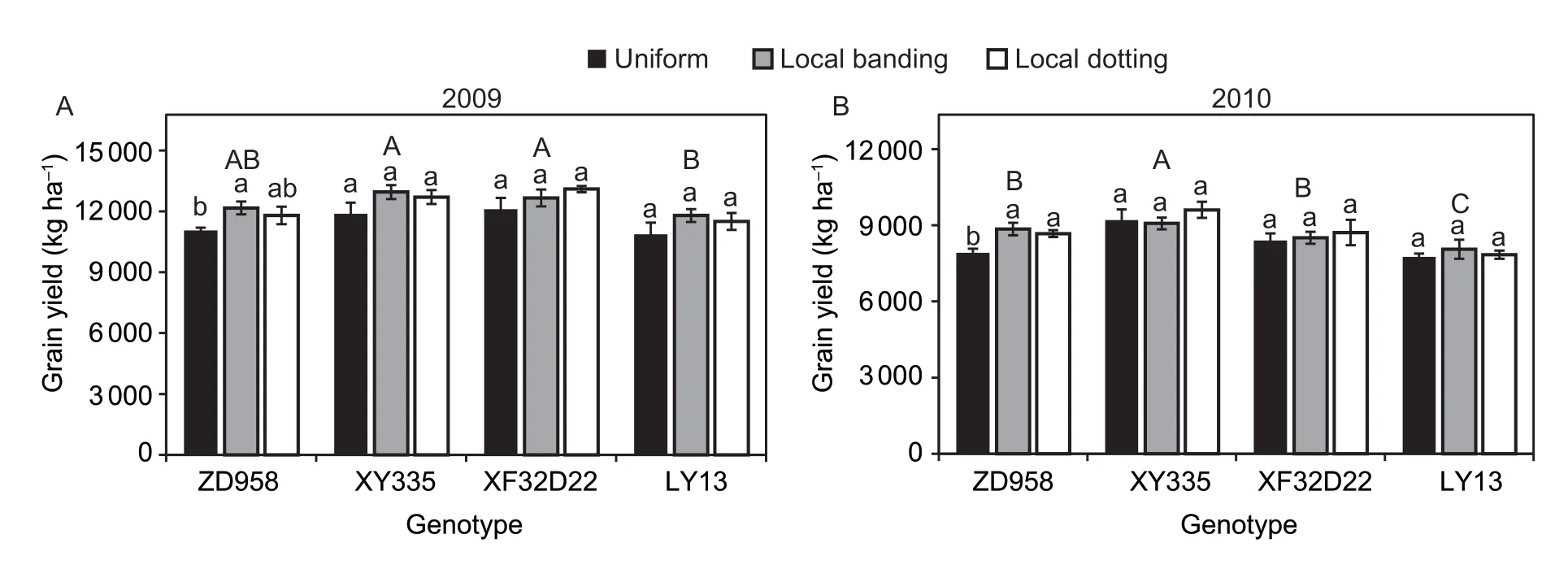
Fig.6 Genotypic differences of the effects of local N fertilizer application on maize grain yield in 2009 (A) and 2010 (B).Uniform,local banding and local dotting represent the broadcast N application,banding N application and dotting N application treatments,respectively.Error bars denote the standard error (SE) of the mean (n=3).Different lowercase letters indicate significant differences between the three N fertilization treatments for the same genotype (P<0.05).Different uppercase letters indicate significant differences between genotypes across all the N fertilization treatments (P<0.05).
3.5.Correlations between treatments under hydroponic and field condition
Correlation analysis was performed across the three N fertilization treatments and four genotypes in the field experiment to determine whether there was an association between root and N efficiency traits (Table 3).The RL at 0–20 cm soil depth was positively correlated with presilking N uptake (r=0.44,P<0.01),but negatively correlated with post-silking N uptake (r=–0.42,P<0.01) and was also negatively correlated with grain yield (r=–0.29,P<0.05).The RL at 40–60 cm depth was positively correlated with post-silking N uptake (r=0.39,P<0.01),total N uptake(r=0.25,P<0.05),and grain yield (r=0.39,P<0.01).These results show that the local N application treatment contributes to N use efficiency and enhances grain yield through the promotion of deeper root growth.
Root growth responded to local N fertilizer application treatments.To explore the correlation of root growth responses between the seedling and adult stages,a correlation analysis was conducted between the RL response to N treatment at the seedling stage in a hydroponic environment and the RL response at the silking stage in the field (Table 4).A significant positive correlation was found where the root response at the seedling stage was correlated with the deep root response at 40–60 cm soil depth at the silking stage (r=0.994–0.997,P<0.01).The root response in the seedling stage was found to be negatively correlated (r=–0.984,P<0.05) with a root response at 0–20 cm soil depth.These correlations indicate that the genotypic differences of root response to localized N supply under field conditions could easily be identified during the maize early-seedling stage.
4.Discussion
Maize roots have a plastic response to local N application and underlying physiological mechanisms have been thoroughly studied in recent years inArabidopsis.In this study,we explored genotypic differences in maize root response under both hydroponic and field conditions in relation to N uptake efficiency.The results clearly showed that the root response to localized N is genotypedependent and is closely associated with the N uptake efficiency under field conditions.
4.1.Genotypic differences in root response to localized nitrogen supply under hydroponics and field conditions
Previous studies have investigated genotypic differences in root response to localized N supply in early seedling stages under laboratory conditions (Guoet al.2005a,b;Chenet al.2017;Zhaiet al.2019).In this study,we further explored the root foraging response for localized N in both hydroponic-seedling and field-adult stages(Figs.2 and 3).A significant correlation between the root phenotypic responses in hydroponic and field conditions was observed (Table 4).Furthermore,the same genotypic differences were identified in both laboratory and field conditions,that is,genotypes ZD958,XY335,and XF32D22 had higher responses to local fertilization than LY13 (Fig.3).The high correlation between laboratory and field responses suggests that the genotypes with high root N responses could be easily detected and selected at the early seedling stages,which could be convenient for screening of genetic resources and further in-depth analysis of genetic mechanisms at the early seedling stage.The observed genotypic differences demonstrate that the root foraging response to localized N may be genetically controlled.Gene expression profiling has revealed that local nitrate signaling involves several important protein kinases,protein phosphatases and transcription factors in maize(Liuet al.2008).Liu Yet al(2020) found that localized nitrate can induce the expression of a MADS-box transcript factorZmTMM1at the transcriptional level to promote lateral root development in local N rich regions.A complete understanding of underlying mechanisms operative in different genotypes requires further investigation.At present,the mechanism underlying the maize root response to localized N supply is largely unknown.Several preliminary studies in maize have suggested that auxin mediated signaling may be key to controlling the maize root response to local nitrate supply,and this process is affected by genotypic differences (Guoet al.2005b;Liuet al.2010).
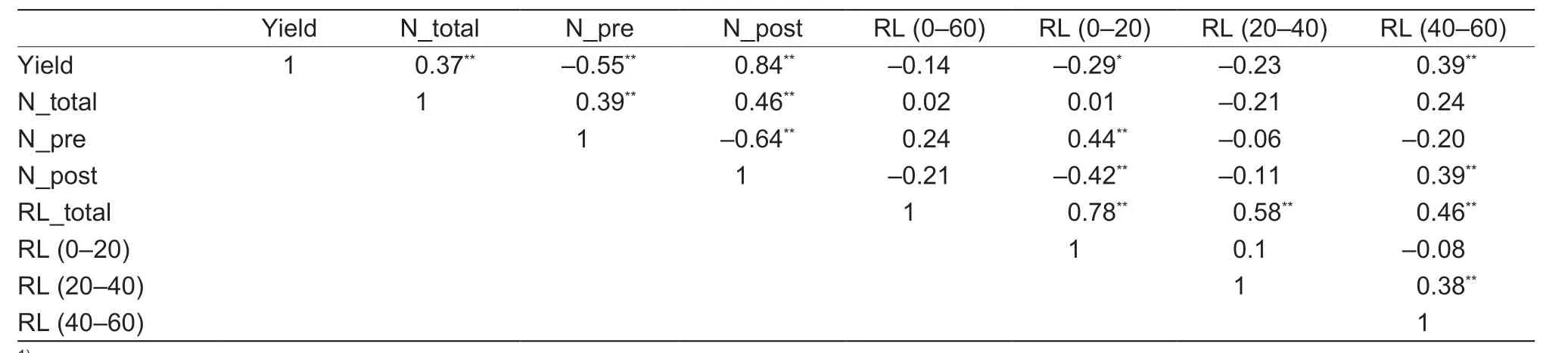
Table 3 Pearson correlation analysis of grain yield,plant N content,and root length in different soil layers1)

Table 4 Pearson correlation analysis of the localized response between root length in seedlings in hydroponic culture and in adults in field conditions
InArabidopsis,nitrate functions as a localized signal to promote root proliferation in N-rich regions (Zhanget al.1999;Krouket al.2010).Redirected auxin movement mediated by NRT1.1/NPF6.3 and LAX3 also plays a central role in root morphological responses to local nitrate (Bouguyonet al.2015,2016;Maghiaouiet al.2020;Liu and von-Wirén 2022).Plant roots also integrate systemic signals from other tissues.Systemic signals related to phytohormones (Kibaet al.2011;Koet al.2014;Poitoutet al.2018),polypeptides (Tabataet al.2014;Ohkuboet al.2017),and transcription factors (Guanet al.2014;Liu Yet al.2020) have also been previously reported to regulate root growth toward nitrate-rich areas.These mechanisms identified inArabidopsismay provide guidance for revealing the genotypic differences in the current study,as it is known that the nitrate transport-related genes (i.e.,NRT1.1) and phytohormone expression patterns in roots vary significantly among different maize genotypes,and have also been further associated with the phenotype and plasticity of roots in previous studies (Garnettet al.2015;Chenet al.2022).
4.2.Improving nitrogen uptake through local fertilization combined with highly responsive cultivars
Local N application influences the distribution of roots in the soil (Fransen 1999;Linkohret al.2002).Deeper placement of fertilizer in the furrow has been found to significantly promote the growth of deep roots at 20–80 cm soil depth as well as grain yield under field conditions (Chenget al.2020).In the present study,the proliferation of deep roots at 20–40 and 40–60 cm depths was observed in the local-banding and -dotting fertilization treatments (Figs.4 and 5).Nitrate is easily leached into deep soil layers.We collected precipitation data in the growth period (https://en.tutiempo.net/climate).The rainfall was 530.36 mm from the day of fertilization to the day of sampling,and this amount of rainfall may promote the N leaching process (Ju and Zhang 2017).This may be the reason why the effect of local N application occurred below the 20 cm soil layer.In addition,previous studies showed that local fertilization (strip application on ridge) reduced N leaching losses by 36% when compared to broadcast fertilization,and local fertilization tended to concentrate N during the leaching process (Ruidischet al.2013).Localized high N in deep soil might stimulate the morphological and physiological responses of roots below 20 cm soil depth (Jinget al.2022),and increase N uptake,which has been observed in Chenget al.(2020).In addition,in the present study,the proliferation of roots in response to local N fertilization was found to be genotype-dependent;for the high-response genotypes,the root length at the 40–60 cm soil depth increased by 65.9–75.0% under localized fertilization (Fig.3),with the fine roots being most affected (Fig.4).However,there was little change in the deep roots of the low-response genotype LY13 (Fig.3).
Previous research has shown that localized fertilization promotes agronomic N-use efficiency and plant growth in field conditions (Maet al.2015;Liet al.2016).In this study,we further found that local fertilizer application increased N uptake in maize mostly during the postsilking stage.This may be due to the deep-root proliferation seen in local N application conditions,as deep roots play important roles in N absorption during the post-silking stage (Muet al.2015).Deeper root growth was promoted and N absorption was enhancedvialocal regulation of soil N in the rhizosphere.In addition,the effect of local fertilization on N uptake was found to be genotype-dependent.The amount of N absorbed during the post-silking stage was increased by 16–88%only in the sensitive genotypes,while the insensitive genotype showed no change in N uptake (Fig.5).It is,therefore,necessary to combine localized N fertilization with N-responsive cultivars,accompanied by reduced N fertilizer to maximize the effect of the localized N supply(Kembelet al.2008;Chapmanet al.2012).However,N is remobilized and utilized for yield formation in a complex physiological process that is genetically controlled (Hirel 2001).In the current study,post-silking N uptake in all the three sensitive genotypes had increased,but only genotype ZD958 remobilized more N into the grain(Appendix C),further promoting yield formation by 10–12% (Fig.6),indicating that the effect of increasing post-silking N uptake on grain yield was genotypic dependent.We speculated on possible reasons in this study,under uniform N application,that genotype ZD958 had the lowest post-silking N uptake (16.13 kg ha–1)among the three high-response maize varieties.While under localized N application conditions,the post-silking N uptake of ZD958 increased by 43.94–88.36%,the increased ratio of ZD958 was higher than XY335 and XF32D22,which was 16.20–54.51% (P<0.05;Fig.5).The higher relative increase in post-silking N uptake in ZD958 promoted grain yield formation in this genotype.The present results provide a novel perspective to rationalize fertilization to increase N uptake by exploiting the biological potential of root foraging strategies in response to local N applications (Fig.7).
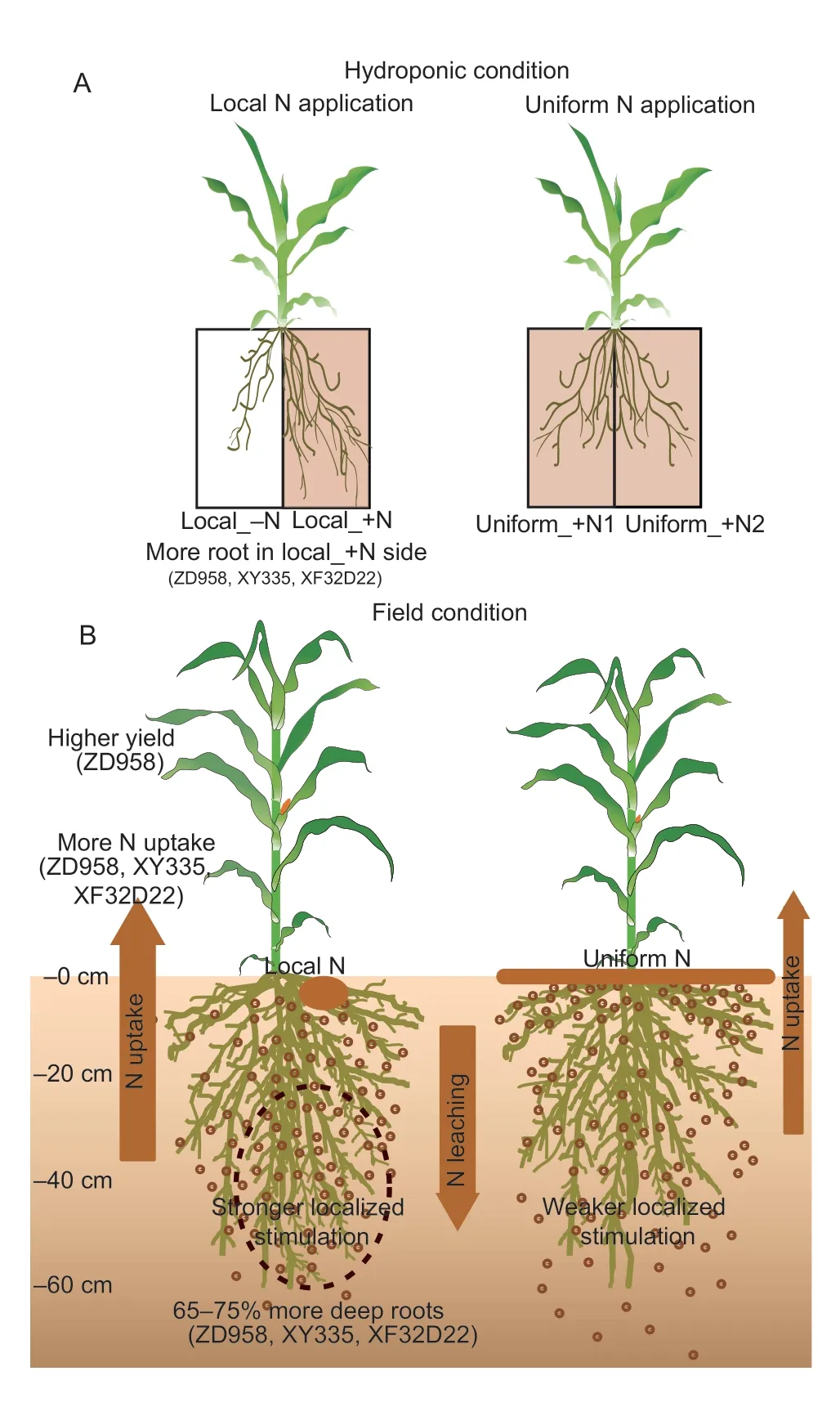
Fig.7 Schematic diagram of the maize root and N efficiency responses to local N application and the genotypic differences in the seedling-hydroponic (A) and adult–field (B) conditions.
Genetic improvement may be another effective way to increase the responsiveness of maize roots to localized N,thus increasing the efficiency of N uptake in crop plants.Root breeding has been proven successful in rice,where the geneDEEPER ROOTING 1enhances the grain yield by promoting root development in deep soil layers (Ugaet al.2013;Arai-Sanohet al.2014).In this study,the correlation analysis revealed that the roots in the 40–60 cm soil layer were positively associated with both grain yield and post-silking N uptake (Table 3).Root proliferation in response to localized N supply was found to be genotype-dependent,suggesting that specific genes in the genome controlled this characteristic.Further exploration of the genetic loci controlling localized root responses may be a possible way to improve N uptake efficiency in maize by root breeding strategies.
5.Conclusion
The association of root traits and yield responses to N fertilization management is crucial for maize breeding to promote both high productivity and efficient N fertilizer uptake.Using different maize varieties under hydroponic and field conditions with localized N applications (Fig.7),this study demonstrated that: (1) localized application of N fertilizer can promote deep root growth below 20 cm soil depth,and has the potential to increase the post-silking N uptake in N-responsive genotypes,(2)genotypes with “high local N-responsive roots” should be selected to increase maize N uptake efficiency,and (3)selection for high-responsive genotypes can be readily conducted at the seedling stage under hydroponic growth conditions.
Acknowledgements
This work was financially supported by the Hainan Provincial Natural Science Foundation of China(321CXTD443) and the National Natural Science Foundation of China (31972485 and 31971948).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2023年1期
Journal of Integrative Agriculture2023年1期
- Journal of Integrative Agriculture的其它文章
- Less hairy leaf 1,an RNaseH-like protein,regulates trichome formation in rice through auxin
- Characterization of a blaCTX-M-3,blaKPC-2 and blaTEM-1B co-producing lncN plasmid in Escherichia coli of chicken origin
- Consumers’ experiences and preferences for plant-based meat food: Evidence from a choice experiment in four cities of China
- Farmers’ precision pesticide technology adoption and its influencing factors: Evidence from apple production areas in China
- Visual learning graph convolution for multi-grained orange quality grading
- lnfluence of two-stage harvesting on the properties of cold-pressed rapeseed (Brassica napus L.) oils
