Clinical efficacy analysis of mesenchymal stem cell therapy in patients with COVlD-19: A systematic review
Jun-Xia Cao, Jia You, Li-Hua Wu, Kai Luo, Zheng-Xu Wang
Jun-Xia Cao, Jia You, Li-Hua Wu, Kai Luo, Zheng-Xu Wang, Biotherapy Center, The Seventh Medical Center of People's Liberation Army General Hospital, Beijing 100700, China
Abstract BACKGROUND Currently, ongoing trials of mesenchymal stem cells (MSC) therapies for coronavirus disease 2019 (COVID-19) have been reported.AIM In this study, we investigated whether MSCs have therapeutic efficacy in novel COVID-19 patients.METHODS Search terms included stem cell, MSC, umbilical cord blood, novel coronavirus,severe acute respiratory syndrome coronavirus-2 and COVID-19, applied to PubMed, the Cochrane Controlled Trials Register, EMBASE and Web of Science.RESULTS A total of 13 eligible clinical trials met our inclusion criteria with a total of 548 patients. The analysis showed no significant decrease in C-reactive protein (CRP)levels after stem cell therapy (P = 0.11). A reduction of D-dimer levels was also not observed in patients after stem cell administration (P = 0.82). Furthermore,interleukin 6 (IL-6) demonstrated no decrease after stem cell therapy (P = 0.45).Finally, we investigated the overall survival (OS) rate after stem cell therapy in COVID-19 patients. There was a significant improvement in OS after stem cell therapy; the OS of enrolled patients who received stem cell therapy was 90.3%,whereas that of the control group was 79.8% (P = 0.02).CONCLUSION Overall, our analysis suggests that while MSC therapy for COVID-19 patients does not significantly decrease inflammatory markers such as CRP, D-dimer and IL-6, OS is improved.
Key Words: Stem cell; Meta-analysis; COVID-19; Mesenchymal stem cells
lNTRODUCTlON
Coronavirus disease 2019 (COVID-19) has become an international public health emergency, and the numbers of confirmed COVID-19 patients have sharply increased since 2020. The current situation of COVID-19 has reflected the continued growth of the epidemic, and to date, there is no specific drug available for COVID-19. Vaccines are considered an effective prophylactic strategy for control and prevention and are being researched and developed at approximately 90 institutions worldwide.Although controlling and preventing epidemics has been an ongoing effort worldwide, the effective treatment of COVID-19 is currently a top priority, and no specific drugs or vaccines are available to completely cure patients with COVID-19 infection[1,2]. Hence, there is a large unmet need for a safe and effective treatment for COVID-19-infected patients, especially severe cases.
Mesenchymal stem cells (MSCs) have been shown to possess powerful immunomodulatory properties and beneficial effects for preventing or reducing the cytokine storm. Their safety and effectiveness have been clearly documented in many clinical trials, especially in immune-mediated inflammatory diseases, and autoimmune disorders such as severe rheumatic arthritis diseases, graft-versushost disease, systemic lupus erythematosus and colitis, and some of these stem cells have been approved as therapeutic products[3-6].
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) can improve the lung microenvironment,pulmonary fibrosis, and lung function, probably due to the regulation of the inflammatory response and the promotion of tissue repair and regeneration[7,8]. Thus, in Wilsonet al[9], allogeneic, bone marrowderived human MSCs were given by intravenous infusion to patients with severe acute respiratory distress syndrome (ARDS) and they demonstrated good tolerance in nine patients with moderate to severe ARDS in the United States (NCT01775774). Then, in 2019, a randomized phase 2a safety trial was performed (NCT02097641)[10], in which treatment with allogeneic mesenchymal stromal cells for moderate to severe ARDS (START study) was tested, and 60 patients were eligible for and received the treatment. Furthermore, a phase I clinical trial reported that nine ARDS patients received human umbilical cord-derived MSCs (HUCDMSCs) (ISRCTN52319075) in 2020 and it was safe with favorable outcomes[11]. Hence, MSC therapy may be a promising option for treating severe COVID-19[12,13].
Currently, ongoing trials of mesenchymal stromal stem cell therapies for COVID-19 have been reported in ClinicalTrials.gov. Searching the words “COVID-19” and “Stem Cells” identified 122 active trials by December 2021. Of the 122 trials, 35 were located in the United States, 23 were located in Europe, 15 were located in East Asia, and the other 49 were distributed over the remaining continents(the ClinicalTrials.gov database on 12/14/2021). Since February 2020, China has registered over 20 trials of stem cell therapy for COVID-19 at http://www.chictr.org.cn/, and several clinical trials of stem cell therapy for COVID-19 have been published.
Continuous progress in this area, as well as the rapid development of science, provides the possibility of effective stem cell therapy for COVID-19 patients and will become a realistic goal in the foreseeable future. On this basis, we addressed the efficacy of stem cell therapy in COVID-19 patientsviametaanalysis.
MATERlALS AND METHODS
Search strategy and selection criteria
This systematic review was conducted following the guidelines provided in the Preferred Reporting Items for Systematic Reviews statement checklist. Trials were identified by electronic searches in the PubMed, EMBASE, Cochrane Central Registry of Controlled Trials (Cochrane Library), CINAHL, Web of Science, Wanfang Database, Chinese National Knowledge Infrastructure, Chinese Scientific Journals Database (VIP), reference lists of published trials and relevant review articles. The search strategy included the medical subject headings of “COVID-19”, “stem cells”, and “cell therapy”. No language limits were applied. The initial search was performed in October 2020, with updates in November 2021.Furthermore, we consulted experts in this field and performed manual searches of reference lists. We also searched ClinicalTrials.gov (http://www.ClinicalTrials.gov) for information on prospective and ongoing trials. We excluded abstracts that were never subsequently published as full papers and studies performed on animals and cell lines[14]. In addition, we retrieved relevant literatures using Reference Citation Analysis (https://www.referencecitationanalysis.com/).
Data extraction
Data extraction was independently conducted by two authors. Any discrepancies were adjudicated by a third author after referring back to the original publications. We collected the trial data, including the authors’ names, journal, year of publication, sample size per arm, regimen used, median or mean age of the patients, C-reactive protein (CRP), D-dimer, interleukin 6 (IL-6) and overall survival (OS) of the patients and information pertaining to the study design.
Definition of outcome measures
The immunity endpoints were CRP, D-dimer and IL-6. Furthermore, we evaluated OS of COVID-19 patients after stem cell therapy.
Statistical analysis
The analysis was carried out by comparison of the stem cell intervention arms with the respective control arms and comparisons before and after stem cell therapy. Treatment effects were reflected by CRP, D-dimer, IL-6 and OS. The data of these endpoints in each arm were extracted from each trial and combined using Mantel and Haenszel meta-analysis (Review Manager Version 5.0, Nordic Cochran Centre, Copenhagen), which has been reported in our previous work[14]. To evaluate whether the results of the studies were homogeneous, we used Cochran’s Q test. It is a chi-square test with df equal to the number of studies minus one and tests the null hypothesis that the difference between the study estimates of mean difference (MD) or odds ratios (OR) is due to chance. We also calculated the quantityI2, which describes the percentage of variation across studies due to heterogeneity rather than chance.The MD was calculated with a fixed-effects (FE) model when no statistically significant heterogeneity existed (I2< 50%); otherwise, a random-effects (RE) model was employed, and the OR was calculated (I2> 50%).P< 0.05 were considered to be statistically significant. All reportedPvalues resulted from twosided version tests of the respective tests, as described in our previous works[14].
Risk of bias across studies
The risk of bias was assessed according to Review Manager Version 5.1. The components considered included random sequence generation, performance bias, detection bias, attrition bias, reporting bias,and other sources of bias. For each item, studies were categorized as having a high, low, or unclear risk of bias.
RESULTS
Selection of the trials
The electronic search yielded 823 references. After title and abstract review, 782 publications were excluded for different reasons (239 for being review articles, 543 for using animal models,in vitroexperiments, protocols, editorials and other clinical studies). The full texts of 41 articles were selected as potentially relevant and were retrieved for a more detailed assessment. Furthermore, 16 studies were excluded due to a lack of detailed patient clinical data or not reporting the response to treatment. The selection procedure of the studies is shown in Figure 1. As a result, 13 articles reporting clinical data,including four randomized clinical trials[15-18] and eight case reports without controls of stem cellbased therapy for COVID-19 patients, were selected for our meta-analysis.

Figure 1 PRlSMA flow diagram showing the record identification, screening and study inclusion process. MSCs: Mesenchymal stem cells.
Characteristics of the stem cell-based therapy
After the selection process, 13 eligible trials with a total of 548 patients (429 treatments, 119 controls)were included in the present analysis[15-27]. All trials were published as full text. The clinical data from the trials are listed in Table 1. In addition, we have listed eight published case reports in Table 2.
All of the included patients were aged 19-75 years. In all 21 studies[15-35], stem cell treatment was evaluated in COVID-19 patients. Among them, fourteen clinical trials, including six case reports, used the UC-MSC therapy method[15-18,22-24,27-30,32,34,35], one study used adipose tissue-derived mesenchymal stromal cells (AT-MSCs)[25], and four nonrandomized studies, including one case report,used MSCs and did not report whether the source was UC, AT or bone mesenchymal[19-21,31]. Most of the patients were allotransplanted and intravenously infused.
There were four randomized studies and two nonrandomized studies which included a control group(Table 1), divided into two groups with cell therapy or control therapy when performed the clinical trials[15-19,26], but most of the studies were designed as open-label phase 1/2 trials and did not include controls. In the other studies, they used information from their institution about patients in a similar stage of hospitalization as controls.
The number of stem cells infused into patients in these studies was mainly focused on 106/kg, and some of them were up to 107/kg. The trials were performed worldwide, including in the United States,Spain, Turkey and China. The patient information from the selected trials, such as age, study design and stem cell types or doses, are listed in Tables 1 and 2.

Table 1 Clinical studies

Table 2 Published case reports
Risk of bias assessment
The study design, including details of the method of allocation of subjects to the treatment groups,criteria for eligibility in the study, and methods of assessing and reporting the outcomes are important features of defining study quality. The detailed assessments are shown in Figure 2. Selection bias,performance bias, blinding bias, detection bias, attrition bias, reporting bias and incomplete outcome bias were scored as “l(fā)ow risk”, “unclear” and “high risk”, respectively, across all included articles[15,16,18,19,21-26]. Most studies accurately demonstrated the baseline characteristics of the patients before treatment; therefore, they were judged to be a “l(fā)ow risk” of selection bias[15,16,18,19,21,22,26]. Nine studies were deemed to be “unclear”[15,16,18,19,21-23,25,26], and one study was at high risk for other bias. Overall, the sample size of the included studies was not very large, and the follow-up time was not long enough. Moreover, the patients’ information, such as the distribution of comorbidities and concomitant medication, was not sufficiently detailed.
CRP
CRP is elevated along with numerous acute phase reactants and cytokines in COVID-19 patients.Therefore, we gathered information on CRP, which was available in five trials[21,22,24-26]. These trials included 85 patients. Regarding the efficacy of stem cell therapy, the estimated pooled MD for the selected trials demonstrated an overall drop in the stem cell therapy group, but significance was not achieved for CRP with a RE model (MD: -7.13, 95%CI: -15.84 to 1.59,P= 0.11) for the stem cell group(Figure 3). Cochran’sQtest had aPvalue of < 0.00001, the corresponding quantityI2was 94%, and thus a RE model was employed.
D-dimer
Some studies have shown that stem cell therapy results in a reduction in inflammatory parameters in patients with COVID-19[25]. Information on D-dimer was available in three trials of 51 patients[22,24,25]. For the efficacy of the cell therapy, we compared the level of D-dimer before and after stem cell treatment. The estimated pooled MD showed no decrease in D-dimer after stem cell therapy (MD: -0.05,95%CI: -0.47 to 0.37,P= 0.82) and its levels were similar between the control and therapy groups(Figure 4). Cochran’sQtest had aPvalue of 0.03, the corresponding quantityI2was 71%, and a RE model was employed.
IL-6
IL-6 is a major target of the autoimmune response that occurs in COVID-19 patients in some clinical trials, so we also collected IL-6 data. Information on IL-6 was available in three trials of 66 patients[21,24,25]. To test the efficacy of the cell therapy, we compared the level of IL-6 before and after stem cell treatment. The estimated pooled MD showed no significant decrease in IL-6 after follow-up in the stem cell therapy group (MD: -3.97, 95%CI: -14.17 to 6.23,P= 0.45) and its levels were similar between the control and therapy groups (Figure 5). Cochran’sQtest had aPvalue of < 0.00001, the corresponding quantityI2was 95%, and thus a RE model was employed.

Figure 2 Risk of bias item presented as percentages across all included using the Review Manager Version 5.1 tool.
OS
We also collected OS data to test the efficacy of stem cell therapy in COVID-19 patients. Information on OS was available in seven trials with control groups[15,16,18,19,22,23,26]. These seven trials included 183 patients (74 patients who received stem cell therapy) in total. The OS rate was 90.3% (67/74) for COVID-19 patients who received stem cell therapy. In comparison, the OS rate was only 79.8% (87/109)in patients who did not receive stem cell therapy. To test the efficacy of the cell therapy, we compared the OS between stem cell treatment and the control group. The estimated pooled OR showed a significant improvement in OS in the stem cell therapy group (OR, 2.87, 95%CI: 1.16 to 7.15,P= 0.02)(Figure 6). Cochran’sQtest had aPvalue of 0.64, the corresponding quantityI2was 0%, indicating that the degree of variability among trials was consistent with what would be expected to occur by chance alone, and thus a FE model was used.

Figure 3 Comparison of C-reactive protein. The heterogeneity was 94%, and the random-effects model (Mantel-Haenszel method) was used. Each study is represented by a square, the center of which denotes the mean difference for that study. The size of the square is proportional to the information from that study. The two ends of the horizontal bars denote the 95%CI. The black diamond gives the combined results of all studies.
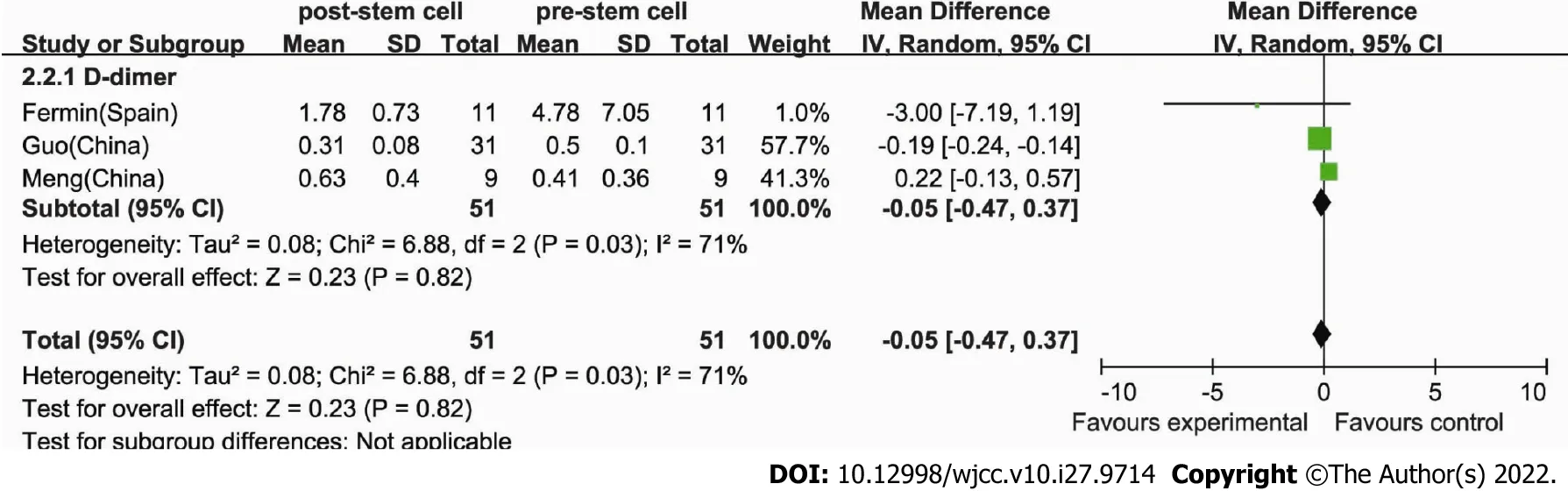
Figure 4 Comparison of D-dimer. The heterogeneity was 71%, and the random-effects meta-analysis model (Mantel-Haenszel method) was used.

Figure 5 Forest plot for interleukin 6. The heterogeneity was 95% in this analysis, and the random-effects meta-analysis model (Mantel-Haenszel method)was used.

Figure 6 Forest plot for overall survival. The I2 was 0%, and the fixed-effects model (Mantel-Haenszel method) was used in this analysis. Each trial is represented by a square, the center of which gives the odds ratios (OR) for that trial. The size of the square is proportional to the amount of information in that trial.The ends of the horizontal bars denote the 95%CI. The black diamond gives the overall OR for the combined results of all trials.
DlSCUSSlON
Main findings
To our knowledge, this study is the only meta-analysis on the clinical efficacy of MSC treatment for COVID-19 patients in multiple countries. We found that CRP did not decrease after stem cell therapy compared with before (P =0.11). A D-dimer reduction was also not observed in patients receiving stem cell therapy (P =0.82). Furthermore, IL-6 demonstrated no decrease after stem cell therapy in COVID-19 patients (P =0.45). Finally, we investigated the overall survival rate and found an improvement in OS after MSC therapy in COVID-19 patients (P =0.02). Our preliminary results indicated that MSCs could be safely administered to patients with COVID-19 and that administration of MSCs was followed by clinical improvement. Furthermore, another meta-analysis found no significant differences in AEs (P=0.09,I2= 40%, OR = 0.53, 95%CI: 0.26 to 1.09) after the administration of MSCs to COVID-19 patientsvscontrol COVID-19 patients in all six studies[36]. Thus, these results will contribute to understanding the real potential of this cell-based therapeutic strategy[37-41].
Our analysis showed no significant improvement in CRP, D-dimer or IL-6 in COVID-19 patients treated with stem cells compared with those treated with control therapy (Figures 3-5). However, in 2022, one publication reported that IL-6 was decreased compared with controls (P= 0.004,I2= 0%,95%CI: -1.15 to -0.22), which included the same two studies[15,22] as our analysis[42]. Thus, in our analysis, there are possibly not enough studies included for meta-analysis of CRP, D-dimer and IL-6 to reach a definitive conclusion. Moreover, the mechanism would be based on stem cells acting in the inflammatory microenvironment of endothelial and alveolar damage by interacting with various targets and should be explored further.
Systematic reviewers often need to choose between two statistical methods when synthesizing evidence in a meta-analysis: the FE and the RE models. It has been reported that if the heterogeneity is extensive, then the weights under a RE model will be mainly driven by the heterogeneity and will be substantially different from those obtained using an FE model, and the two meta-analytic approaches may yield similar or contradicting results[43].
However, we must take into consideration that severe COVID-19 has to be rapidly treated. The applicability of stem cell therapy for COVID-19 seems compelling, particularly in the hyper inflammatory stage of the illness. Due to the scope of the pandemic and the unmet need for effective therapies,stem cells have been used as a new therapeutic strategy. Thus, in our analysis, we collected the data from a follow-up time of five to ten days, with a mean of 4.5 d after the infusion, and data with units of mg/L, mg/dL, ng/mL and pg/mL should be considered and united in the analysis, which could induce errors in the analysis[21,22,24,25]. Furthermore, some included clinical trials reported data with a median value (interquartile range or ratio), so we calculated the estimated SD, and the estimated mean was taken as equal to the median value according to an online calculator (http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html)[44]. Thus, these calculations were involved in our data processing, and we could not exclude their possible bias from the analysis. Therefore, to draw a more definitive conclusion, additional large samples of clinical trials are needed.
In the process of studying their clinical efficacy, different types of stem cells are used by different clinical trials, among which UC-MSCs are the most widely used, and the studies included in our metaanalysis all used MSCs. Therefore, whether there are differences in the efficacy between stem cell types or whether there is a relationship between stem cells and plasma therapy also needs to be discussed.Treatment with negative ACE2 and TMPRSS2 MSCs significantly improved the functional outcomes,which implied that these cells were free from COVID-19 infection[19]. Cardiosphere-derived cells(CDCs) are stromal/progenitorcells derived from heart tissue with a distinctive antigenic profile(CD105+, CD45-, CD90low). Head-to-head comparisons in preclinical models indicate that CDCs may be more effective than MSCs with regard to paracrine factor secretion and myocardial remodeling[45].IMRCs resemble MSCs in their capacity for self-renewal and trilineage differentiation. On the other hand, compared to primary UC-MSCs, IMRCs were proven to have a higher consistency in terms of quality, stronger immunomodulatory and antifibrotic functions, and a robust ability to treat lung injury and fibrosisin vivo[46,47]. The remaining trials used stem cell transplantation (SCT)[48]. These trials were excluded for not receiving MSC therapy.
Adipose-derived stromal stem cells (ASCs) can be distinguished from bone marrow-derived MSCs by their positivity for CD36 and negativity for CD106. Human ASCs, as the first exploited type of MSCs,express classical mesenchymal markers such as CD44, CD73, CD90, CD105, and CD166[25]. The data gathered about ASCs and stromal vascular fraction cells showed they improved the microenvironment because they can secrete proangiogenic factors, such as vascular endothelial growth factor and plateletderived growth factor, to establish a novel microvascular network in the damaged tissue to provide nutrition and oxygen and promote tissue repair[49,50].
Different stem cell treatment results should be considered and analyzed separately, so these become confounding factors affecting any overall conclusion, but in our analysis, we excluded other types of stem cells and mainly focused on MSC therapy in patients with COVID-19.
Emergency authorization for convalescent plasma (CP) is a treatment option for patients who currently have COVID-19. Its safety and effectiveness have been documented in many clinical trials[51-53], and researchers have demonstrated no serious adverse events. It has been described that administration of CP is associated with a higher rate of hospital discharge at day 22 after symptom onset compared with COVID-19 patients who did not receive CP. In addition, a clinical case report stated that they infused the patient with CP, followed by MSCs. A synergistic role of convalescent plasma and MSCs was observed in the treatment of severely ill COVID-19 patients[35], which showed that the intravenous infusion of CP and MSCs for COVID-19 patients might have synergistic characteristics in terms of inhibiting cytokine storms, promoting the repair of lung injury, and recovering pulmonary function. Thus, these findings provide a reference for the research direction of COVID-19 clinical treatment strategies.
In most published clinical trials, including case reports, efficacy analyses of stem cell therapy provide evidence of significantly improved patient survival and time to recovery. Our overall analysis showed a significant survival benefit of MSC therapy in COVID-19 patients (P= 0.02). Our analysis demonstrated that MSCs were superior for enhancing overall survival, and the results were similar to those of a previously reported meta-analysis of cell-based therapy in ARDS[54]. However, in their meta-analysis,the results showed that combined mortality had a favorable trend but did not reach statistical significance (P= 0.064), which included one trial in common with our analysis[19]. In our meta-analysis,we extracted the data for OS from the included trials, and one of them showed 45.4% overall mortality at a similar stage of hospitalization[23]. Therefore, we included them and calculated the OS of the control according to their report. In addition, we only extracted the OS data from studies with controls and did not analyze the data without controls since these would introduce bias into our analysis and lead to an overestimation of the stem cell treatment effects. Our results obtained the same results of improvement of OS and a decreased risk of death in COVID-19 patients with stem cell therapy, which has been reported recently in two other studies[36,42].
Overall, the application of stem cell treatment for COVID-19 patients appears promising and tolerable, with good potential for global public health care, especially for severe cases.
Limitation
Although our meta-analysis showed that stem cell implantation is effective in most patients with COVID-19, it also has some confusing findings. Some studies have mentioned that the therapeutic effects were affected by the degree to which the patients received other therapies and their history of other diseases, which could significantly incapacitate the patient and impact the quality of the metaanalysis.
The favorable response in many patients cannot be exclusively attributed to a lack of control groups.Therefore, the present study has several limitations. First, from the perspective of clinical trials, apart from intervention factors, the two groups in each study should have exactly the same background, but in COVID-19 patients, stem cell treatment is being performed in extraordinary times and prospective,nonrandomized, open-label and blinded randomized trials are unlikely to be performed, thus leading to distribution and implementation bias, which is the most important limitation of this study. Second, the subjects in each study were recruited from among patients with severe COVID-19, and the number of participants was relatively small. Thus, these results need to be repeated in larger cohort studies.
The reliability of this meta-analysis was also impacted by other factors. For example, we selected only assessable studies for our analysis; thus, the literature screening process may result in bias in the findings. To date, clinical studies with stem cells are still in their infancy. Based on the current encouraging experimental and clinical evidence, larger randomized cohort trials with more than 100 patients should be conducted and will provide clearer evidence for stem cell therapy of COVID-19 patients.
CONCLUSlON
In conclusion, according to our meta-analysis, COVID-19 patients had significantly improved OS after stem cell therapy, and hence, it is a promising therapy.
ARTlCLE HlGHLlGHTS
Research background
Currently, ongoing trials of mesenchymal stem cell therapies for coronavirus disease 2019 (COVID-19)have been reported.
Research motivation
Whether mesenchymal stem cells have therapeutic efficacy in novel COVID-19 patients.
Research objectives
Clinical trials were published with stem cells therapy in COVID-19 patients.
Research methods
Mantel and Haenszel meta-analysis (Review Manager Version 5.0, Nordic Cochran Centre,Copenhagen) was used for data analysis.
Research results
There was a significant improvement in overall survival (OS) after stem cell therapy; the OS of enrolled patients who received stem cell therapy was 90.3%, whereas that of the control group was 79.8% (P =0.02).
Research conclusions
Mesenchymal stem cells therapy for COVID-19 patients does not significantly decrease inflammatory markers such as C-reactive protein, D-dimer and interleukin 6, OS is improved.
Research perspectives
Stem cell therapy for COVID-19 patients will become a realistic goal in the foreseeable future.
FOOTNOTES
Author contributions:Cao JX and Wang ZX conceived and designed the meta-analysis; Cao JX and Wang ZX performed the experiments; Cao JX, Wu LH, You J and Luo K analyzed the data; Cao JX and Wang ZX wrote the paper; All authors have read and approved the manuscript.
Supported by Scientific Research Projects in China, No. CLB19J053 and 19SWAQ13.
Conflict-of-interest statement:The authors indicate no potential conflicts of interest.
PRlSMA 2009 Checklist statement:The manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Jun-Xia Cao 0000-0002-6603-5834; Jia You 0000-0002-0942-3144; Li-Hua Wu 0000-0003-4098-8722; Kai Luo 0000-0003-1515-3678; Zheng-Xu Wang 0000-0001-8262-608X.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
 World Journal of Clinical Cases2022年27期
World Journal of Clinical Cases2022年27期
- World Journal of Clinical Cases的其它文章
- lmpact of the COVlD-19 pandemic on healthcare workers’ families
- Transition beyond the acute phase of the COVlD-19 pandemic: Need to address the long-term health impacts of COVlD-19
- Transient ischemic attack after mRNA-based COVlD-19 vaccination during pregnancy: A case report
- latrogenic aortic dissection during right transradial intervention in a patient with aberrant right subclavian artery: A case report
- lnfant with reverse-transcription polymerase chain reaction confirmed COVlD-19 and normal chest computed tomography: A case report
- Successful treatment of stage lllB intrahepatic cholangiocarcinoma using neoadjuvant therapy with the PD-1 inhibitor camrelizumab: A case report
