Case series in Indonesia: B.1.617.2 (delta) variant of SARS-CoV-2 infection after a second dose of vaccine
Anis Karuniawati, Ari F Syam, Armand Achmadsyah, Fera Ibrahim, Yulia Rosa, Pratiwi Sudarmono, Fadilah Fadilah, Menaldi Rasmin
Anis Karuniawati, Fera Ibrahim, Yulia Rosa, Pratiwi Sudarmono, Department of Clinical Microbiology, Faculty of Medicine, Universitas Indonesia/Cipto Mangunkusumo Jakarta Indonesia, Jakarta 10430, DKI Jaya, Indonesia
Ari F Syam, Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia/Cipto Mangunkusumo Jakarta Indonesia, Jakarta PUsat 10430,DKI Jaya, Indonesia
Armand Achmadsyah, Faculty of Medicine, Universits Indonesia, Jakarta Pusat 10430, DKI Jaya, Indonesia
Fadilah Fadilah, Department of Medical Chemistry, Faculty of Medicine, Universitas Indonesia,Jakarta Indonesia , Faculty of Medicine, Universitas Indonesia, Jakarta 10430, DKI Jaya,Indonesia
Menaldi Rasmin, Department of Pulmonology and Respiratory Medicine, Faculty of Medicine,Universitas Indonesia/Persahabatan Hospital, Jakarta Indonesia, Jakarta Pusat 10430, DKI Jaya,Indonesia
Abstract BACKGROUND The B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first discovered in Maharashtra in late 2020 and has rapidly expanded across India and worldwide. It took only 2 mo for this variant to spread in Indonesia, making the country the new epicenter of the delta variant as of July 2021. Despite efforts made by accelerating massive rollouts of current vaccines to protect against infection, cases of fully-vaccinated people infected with the delta variant have been reported.AIM To describe the demographic statistics and clinical presentation of the delta variant infection after the second dose of vaccine in Indonesia.METHODS A retrospective, single-centre case series of the general consecutive population that worked or studied at Faculty of Medicine, Universitas Indonesia with confirmed Delta Variant Infection after a second dose of vaccine from 24 June and 25 June 2021. Cases were collected retrospectively based on a combination of author recall, reverse transcription-polymerase chain reaction (RT-PCR), and whole genome sequencing results from the Clinical Microbiology Laboratory, Faculty of Medicine, Universitas Indonesia.RESULTS Between 24 June and 25 June 2021, 15 subjects were confirmed with the B.1.617.2 (delta) variant infection after a second dose of the vaccine. Fourteen subjects were vaccinated with CoronaVac (Sinovac) and one subject with ChAdOx1 nCoV-19 (Oxford-AstraZeneca). All of the subjects remained in home isolation, with fever being the most common symptom at the onset of illness (n = 10, 66.67%). The mean duration of symptoms was 7.73 d (± 5.444). The mean time that elapsed from the first positive swab to a negative RT-PCR test for SARS-CoV-2 was 17.93 d (± 6.3464). The median time that elapsed from the second dose of vaccine to the first positive swab was 87 d (interquartile range: 86-128).CONCLUSION Although this case shows that after two doses of vaccine, subjects are still susceptible to the delta variant infection, currently available vaccines remain the most effective protection. They reduce clinical manifestations of COVID-19, decrease recovery time from the first positive swab to negative swab, and lower the probability of hospitalization and mortality rate compared to unvaccinated individuals.
Key Words: COVID-19/SARS-CoV-2 infection; B.1.617.2 (delta) variant; Fully vaccinated; Case series
INTRODUCTION
For nearly 2 years, the coronavirus disease 2019 (COVID-19) pandemic has been a major issue in human global health. In early March 2021, there were 116 million cases of infection worldwide, with 2.6 million global deaths. There was only modest genetic evolution at the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, mainly because of the global lack of immunity against this new pathogen[1]. In December 2020, the emergence of new SARS-CoV-2 variants was attributed to an unexpected increase in COVID-19 cases[2]. These new variants are the result of the remarkable capacity of RNA viruses to adapt to new hosts and environments[3]. They are able to develop a high number of mutations, mainly in the S protein, causing potential harm to human health.
The World Health Organization (WHO) has classified SARS-CoV-2 variants into two categories: variants of concern (VOC) and variants of interest[1]. VOC is a term that has been used by the WHO to describe SARS-CoV-2 variants that exhibit a high transmission rate in the context of high population immunity, evidence of a more severe disease, and reduced effectiveness of vaccines[2]. For example, the B.1.617.2 (delta) variant of SARS-CoV-2 was first discovered in Maharashtra in late 2020 and has rapidly expanded across India and worldwide, outcompeting other lineages, such as the B.1.617.1 (kappa) and B.1.1.7 (alpha) variants. This delta variant is six-fold less sensitive to serum neutralizing antibodies from a recovered individual, and eight-fold less sensitive to vaccine-elicited antibodiesin vitro[4].
A key issue has surfaced of whether currently available COVID-19 vaccines are able to protect against infection of the new delta variant. A total of 19 current SARS-CoV-2 vaccines worldwide are based on the original strains. With the newly emerging variants, scientists have been challenged to establish response strategies to control the SARS-CoV-2 pandemic[1,5].
Despite efforts made by accelerating massive rollouts of current vaccines and increasing vaccine immunogenicity by increasing vaccination doses, the delta variant of COVID-19 has quickly spread in various countries such as Bangladesh, Iran, Iraq, Malaysia, Myanmar, South Korea, Japan, and Indonesia[5,6]. Two months after spreading through India, Indonesia has become the new epicenter of the delta variant as of July 2021, where only 5.5% of its citizens have been fully vaccinated. As of 15 July 2021, Indonesia had 56.767 new cases, with a test positivity rate of 26%, indicating that large numbers of cases are being missed, and reporting an average of 919 deaths a day over the past week[6]. The effectiveness of currently available vaccines in Indonesia, namely CoronaVac (Sinovac), BNT162b2 (Pfizer-BioNTech), and ChAdOx1 nCoV-19 (Oxford-AstraZeneca), has remained unknown because fullyvaccinated individuals have been infected with the B.1.617.2 (delta) variant[7].
Here, we report a case series describing the demographic statistics and clinical presentation of the first cluster of delta variant infection after a second dose of vaccine.
MATERIALS AND METHODS
Patients
This study included patients who tested positive for the B.1.617.2 (delta) variant between 24 June and 25 June 2021 (based on data http://www.gisaid.org). The SARS-CoV-2 variant was collected retrospectively based on a combination of author recall, reverse transcription-polymerase chain reaction (RTPCR), and whole genome sequencing (WGS) results from the Clinical Microbiology Laboratory, Faculty of Medicine, Universitas Indonesia (Depok, Indonesia). The cases included in this case series were subjects fully vaccinated against COVID-19 with confirmed delta variant infection between 24 and 25 June 2021, who worked or studied at Faculty of Medicine, Universitas Indonesia.
Statistical analysis
Subject data are presented as absolute values, percentages, mean ± SD, or median using SPSS 26 (IBM Corp, Armonk, NY, United States). Continuous and discrete variables are expressed as the mean ± SD or median depending on the results of the normality test. Categorical variables are expressed asn(%).
Literature survey paper
Sources: A comprehensive search of PubMed was performed for all studies published prior from March 2020 to April 2022, using the search terms “COVID-19”, “Delta Variant OR B.1.617.2 Variant” , “Fully vaccinated OR Full-Dose Vaccine”, and “Case Series” which yielded 26 results (Figure 1). A systematic review of these papers were performed, and after the full text of all articles were evaluated to determine whether results were included. There were no language restrictions. Two results were used for our paper (Table 1).

Figure 1 Flow Chart outlining the selection of articles.
RESULTS
We described the first 15 subjects with the B.1.617.2 (delta) variant SARS-CoV-2 collected from nasopharyngeal swabs between 24 and 25 June 2021 at the Clinical Microbiology Laboratory Universitas Indonesia. Table 2 shows the demographic statistics of the subjects enrolled. The mean age of the subjects was 29 years (± 5.097), and 10 were males. Of the 15 subjects, 2 (13.34%) had one or more coexisting medical conditions: chronic respiratory disease in 1, and obesity with a history of rhinitis allergy in 1. The mean body mass index (BMI) of subjects was 24.768 kg/m2(± 3.531), which was statistically in a normal category according to the WHO and overweight at risk according to the Asian-Pacific BMI with a mean height of 167.00 cm (± 8.384) and a mean weight of 69.20 kg (± 11.706)[8]. Subjects were primarily employees working at Universitas Indonesia and included a total of 11 (73.34%) employees, 1 (6.67%) medical student, and 3 (20%) residents. Of the 15 subjects, 14 (93.34%) were vaccinated with CoronaVac (Sinovac), which was widely available in the first phase of vaccination in Indonesia.
Table 3 shows the clinical characteristics of the subjects included in this case series. Of the 15 subjects enrolled, 1 (6.67%) was reinfected. The first infection occurred before the patient had the first vaccination, and reinfection occurred after the second dose of vaccine. Most subjects (7, 46.67%) were thought to be infected by their coworkers, followed by family in 3 (20%), patients in 3 (20%), and unknown sources in 2 (13.34%). Eleven subjects (73.34%) used a surgical mask during work and daily activity, three (20%) used an N95 mask, and one (6.67%) used a KN95 mask.
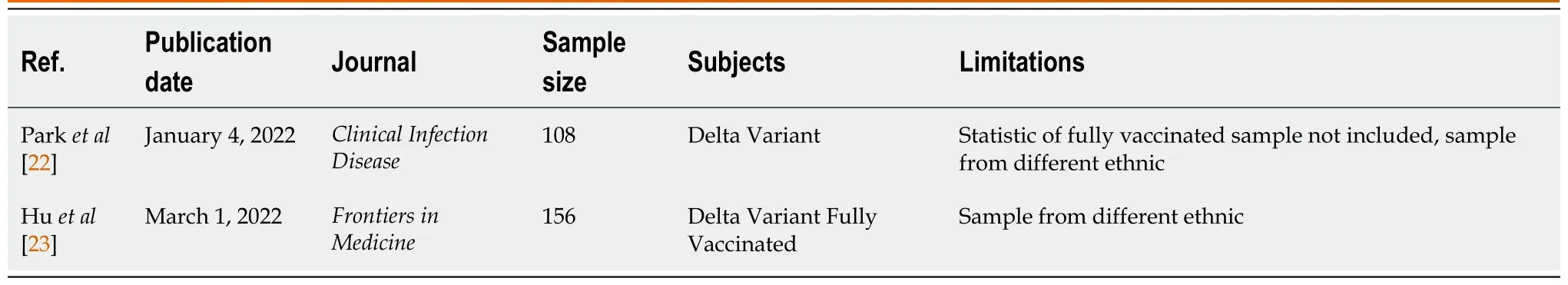
Table 1 Literature survey papers

Table 2 Demographic statistics of the subjects enrolled

Table 3 Clinical characteristics of the subjects enrolled
Of the 15 subjects enrolled, 1 (6.67%) was asymptomatic and 14 (93.34%) were symptomatic. Among the symptomatic patients, the most common symptoms at the onset of illness were fever (10, 66.67%), defined as an axillary temperature of 37.5 °C or higher, rhinorrhea (9, 60%), anosmia (8, 53.34%), cough (7, 46.67%), headache (5, 33.34%), ageusia/dysgeusia (4, 26.67%), fatigue (4, 26.67%), myalgia (4, 26.67%), diarrhea (3, 20%), sore throat (2, 13.34%), dyspnea (1, 6.67%), and nausea (1, 6.67%). All of the subjects were in home isolation. The mean time for symptom duration was 7.73 d (± 5.444). The mean time from the first positive swab to a negative RT-PCR test for SARS-CoV-2 was 17.93 d (± 6.3464). The median time that elapsed from the second dose of vaccine to the first positive swab was 87 d [interquartile range (IQR): 86-128 d].
Each of the 15 subjects received pharmacological treatment. Vitamin C was used in 14 subjects (93.34%), vitamin D in 12 (80%), paracetamol in 8 (53.34%), azithromycin in 5 (33.34%), favipiravir in 3 (20%), oseltamivir in 1 (6.67%), and phytopharmaca (Andrographis paniculata, known as Sambiloto in Indonesia) in 1 (6.67%).
DISCUSSION
In this case series, we reported 15 subjects with confirmed infection with the B.1.617.2 (delta) variant of SARS-CoV-2 after a second dose of vaccine. From the statistics acquired, men are more prone to SARSCoV-2 infection. Angiotensin-converting enzyme 2 (ACE2) is expressed in various human tissues. The expression levels are not significantly different between males and females, between young and old persons, nor among races, indicating that SARS-CoV-2 may equally infect persons of different sexes, ages, and races. The different host immune responses to infection may explain why malesvsfemales and youngvsold person persons infected with SARS-CoV-2 have distinct disease severity. Studies have found that the X chromosome and sex hormones play important roles in innate and adaptive immunity, which makes women less susceptible to viral infection[9]. Age also plays a key factor, as the body’s immunity declines with age. Aging has been linked to abnormally high cellular functioning, cellular hyperfunctions that may eventually lead to cellular exhaustion, and function loss in later stages[10]. However, in this case series, the subjects were of productive age (15-64 years), with a mean age of 29 years (± 5.097)[11]. Subjects with underlying diseases such as diabetes, hypertension, cardiovascular disease, chronic respiratory disease, and obesity also have increased risk of SARS-CoV-2 infection. A long-term history of diabetes, hypertension, and cardiovascular disease damages the vascular structure and is more likely to reduce the body’s immunity. When a subject has previous respiratory diseases that damaged their lung function such as lung tuberculosis or chronic obstructive pulmonary disease, they have lower resistance to the virus and are prone to developing acute respiratory distress syndrome[9]. Obesity or excess ectopic fat deposition may also be a unifying risk factor for SARS-CoV-2 infection, as it reduces protective cardiorespiratory reserve as well as potentiates the immune dysregulation that appears to mediate the progression to critical illness[12]. In this case series, 1 subject had a history of lung tuberculosis (6.67%) and 1 subject (6.67%) was obese according to the WHO BMI. However, the Asia-Pacific BMI has stated that BMI between 23.0 and 24.9 is considered overweight, which makes the subject in this case series more susceptible to SARS-CoV-2 infection, as the mean BMI was 24.768 kg/m2(± 3.531)[9,12].
Of the 15 subjects, 14 individuals (93.34%) were confirmed to have a second dose of CoronaVac (Sinovac) and 1 individual (6.67%) had a second dose of ChAdOx1 nCoV-19 (Oxford-AstraZeneca). Both vaccines are widely available in Indonesia, as other vaccines such as BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) have not arrived. According to a study in China, the vaccine effectiveness (VE) of two doses of CoronaVac was 59.0% (95%CI: 16.0%-81.6%) against the delta variant infection. However, a single dose vaccine of CoronaVac was not sufficiently protective against the delta variant[13]. However, ChAdOx1 nCoV-19 (Oxford-AstraZeneca) and BNT162b2 (Pfizer-BioNTech) vaccines had higher VE rates compared to CoronaVac (Sinovac). It has been reported that the VE after the second dose of Oxford-AstraZeneca vaccine is 67.0% (95%CI: 61.3%-71.8%) and 88.0% (95%CI: 85.3%-90.1%) with Pfizer-BioNTech. A single dose of Oxford-AstraZeneca or Pfizer-BioNTech vaccine was notably lower among people with the delta variant (30.55; 95%CI: 25.2%-35.7%)[14].
WGS data of the samples showed that the most common mutations found in the S protein domains were L452R, T478K, D614G, and P681R, which might affect the sensitivity in neutralizing monoclonal antibodies[15]. Research has shown that the L452R mutation can cause a decrease in the titer of vaccineinduced serum neutralizing antibody against the pseudovirus and recognition by antibodies while maintaining binding to ACE2[16]. P681R and D614G mutations also cause a partial decrease in neutralizing antibodies. A study by Saitoet al[18] showed that the neutralizing antibodies of immune serum induced by BNT162b2 vaccine against D614G/P681R virus was significantly decreased[18]. The T478K mutation also exhibited a reduction in its neutralization sensitivity towards the post-vaccination sera. This phenomenon might explain the incidence of infection in vaccinated subjects.
From this case series, there was 1 subject (6.67%) who had been diagnosed twice with SARS-CoV-2 infection, before vaccination and after two doses of vaccine. A study by Altawalah[19] showed that the immune responses induced by COVID-19 vaccination are greater than those induced by natural SARSCoV-2 infection. Therefore, individuals that have recovered from a confirmed COVID-19 infection are still prone to another reinfection. A study by Ebingeret al[20] also showed that the anti-S immunoglobulin G antibody response following a single vaccine dose in people who have recovered from confirmed prior COVID-19 infection is comparable to the antibody reaction following two doses of vaccine in people who have never been infected (P> 0.58). Thus, individuals who once had confirmed COVID-19 infection and also had a double dose regimen are expected to have better immunity against COVID-19. Reinfection in this case still remained unclear, but age, sex, and underlying diseases such as obesity, chronic respiratory disease, and cardiovascular disease could be independent risk factors that contribute to susceptibility to viral infection[10].
According to a study by Daset al[21], different types of masks have different effectiveness in protecting subjects against SARS-CoV-2. A surgical face mask has the lowest filtration rate of 60%-80% and can filter particles as small as 0.3 μm. The N95 face mask has the highest filtration rate (95%) compared to the other two; it can filter particles of 0.1-0.3 μm in size. The KN95 face mask has an 80%-95% filtration rate and can filter particles down to 0.3 μm. Since SARS-CoV-2 is a 0.1 μm enveloped virus, the N95 mask has a better probability of protecting against COVID-19 infection[21]. The current study showed that 11 (73.34%) of the 15 subjects who tested positive for COVID-19 were using surgical masks for daily usage. The correct use of N95/KN95 masks requires a fit test or seal test, which is sometimes not done properly. It was also noted that masks were used during their daily activities, and no subject had performed an aerosol-generating procedure before infection with COVID-19.
The common clinical manifestations of COVID-19 patients in this case series were fever in 10 (66.67%), rhinorrhea in 9 (60%), anosmia in 8 (53.34%), and cough in 7 (46.67%). A recent study by Parket al[22], also strengthen our findings, from a total of 108 delta variant subjects that were enrolled, common symptoms for the delta variant are fever (73.7%), myalgia (51.5%), cough (49.5%), sore throat (43.4%), and cephalgia (34.3%). In comparison, Myalgia was more common in the delta group (51.5%) than in the non-delta group (26.9%). Non-delta variant also showed significant symptoms of loss of taste (26.9%) and loss of smell (15.4%) compare to delta group with loss of taste (2.0%) and loss of smell (7.1%)[22]. Another study by Huet al[23] with 156 full vaccinated delta variant patients that admitted at Yangzhou, China in 2021 also indicate that most common symptoms are cough (48.7%), fever (34.6%), sore throat (25.6%), fatigue (19.2%), and expectoration (8.3%). Another study also showed that between delta variant and alpha variant are quite similar, except patients with delta variant could become rapidly ill and have higher viral loads in the respiratory tract. Delta variant could also cause auditory impairment and gangrene from worse blood clots[24]. Viral infection triggers an inflammatory pathway in the human body. Various inflammatory factors produced by the inflammatory storm can cause systemic immune damage and manifest as high temperatures. It explains why the most common symptom was fever. SARS-CoV-2 also binds to the ACE2 receptor, which is mainly distributed in the respiratory tract, cardiovascular, kidneys, and colon. It causes multiple symptoms, such as dyspnea, cough, anosmia, ageusia, diarrhea, and sore throat[9] (Table 4).
Although subjects with two-dose vaccination can still be infected with SARS-CoV-2, the results from a current trial suggest that there is a 90% reduction in symptomatic COVID-19 with vaccine. However, it remains unknown whether this efficacy is mediated by decreasing SARS-CoV-2 infection susceptibility (VESUSC) or the development of symptoms after infection (VESYMP)[25]. In our case series, symptoms that developed in COVID-19 patients were mild to moderate according to the Indonesian COVID-19 Guideline. In addition, vaccination decreased the symptom duration of COVID-19 patients (7.73 ± 5.444 d), increased the recovery time from the first positive swab to negative swab (17.93 ± 6.364 d), and prevented the subjects from needing hospitalization. A recent study revealed that VE in terms of protection against deaths was 72%, with a lower reduction of mortality for B.1.1.7vsnon-B.1.1.7 variants (70%vs78%, respectively)[26]. A study from a hospital in New Delhi, India showed that among those fully vaccinated, there was 12.5% (23/184) mortality compared to 31.45% (309/984) among the unvaccinated (OR = 0.3, 95%CI: 0.2-0.5;P< 0.0001)[27].
The most common drugs used in our case series were vitamin C (14 subjects, 93.34%) and vitamin D (12 subjects, 80%). Low levels of micronutrients have been associated with adverse clinical outcomes during SARS-CoV-2 infection. As a result, daily vitamin intake may be beneficial in maximizing the immune response to viral infection. Recent studies have shown that vitamin D improves the physical barrier against viruses and stimulates the production of antimicrobial peptides. It may also prevent cytokine storm by lowering the production of inflammatory cytokines. Vitamin C is considered an antiviral agent as it increases immunity. It also increases antiviral cytokines and free radical formation, lowering viral yield and attenuating excessive inflammatory responses and hyperactivation of immune cells[28]. However, the effectiveness of vitamin C and vitamin D against the B.1.617.2 (delta) variant remain unknown. In this study, 1 subject had consumedAndrographis paniculataas phytopharmaca. According to current studies,Andrographis paniculatecan act as a potential inhibitor of the main protease of SARS-CoV-2, but further studies should be considered[29].
This study had some limitations. First, due to the mandatory WGS results, there were limitations in the subjects included. Thus, the case series might not be representative of the general population and extrapolation to other settings should be done with caution. Second, the data were collected using a questionnaire filled out by the patients themselves, which may have led to bias. Finally, due to regulations, the RT-PCR test was not performed daily. Duration of conversion was considered the time from the first positive swab until the first negative result. It is possible that a patient had a negative result before RT-PCR was conducted (Table 5).

Table 4 Comparative study with other paper
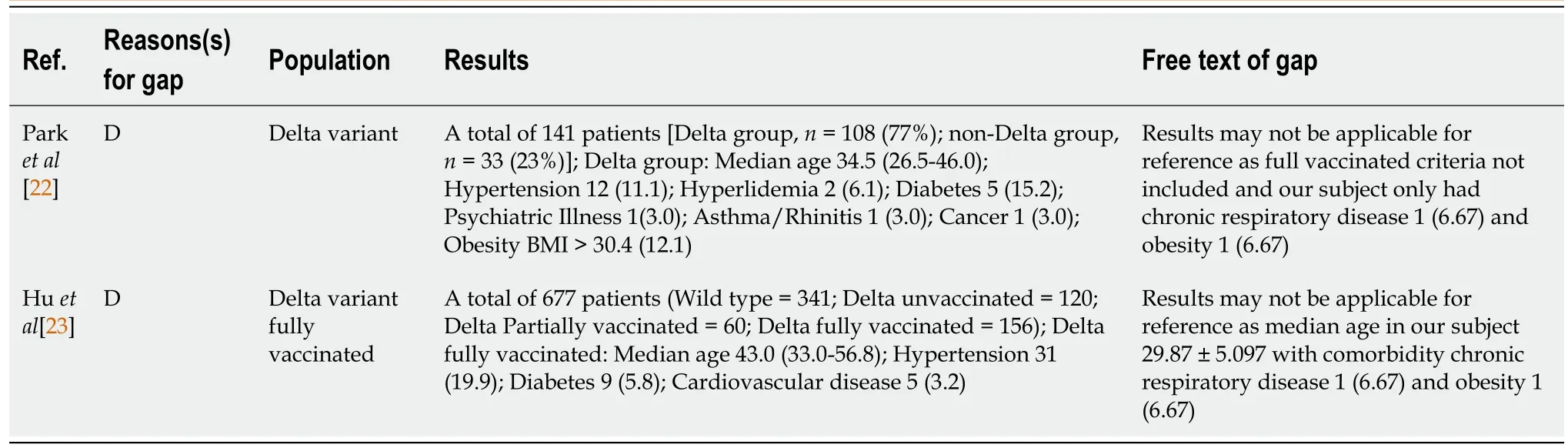
Table 5 Research gap analysis
CONCLUSION
This case series highlights that the currently available treatment for the B.1.617.2 (delta) variant of SARS-CoV-2 infection is still unknown. Further studies and research should be conducted. Although this case shows that after two doses of vaccine, subjects are still susceptible to the B.1.617.2 (delta) variant, currently available vaccines remain the most effective protection. They reduce the clinical manifestations of COVID-19, decrease the recovery time from first positive swab to negative swab, and lower the probability of hospitalization and mortality rate compared to unvaccinated individuals. We should also support efforts to maximize vaccine uptake with two doses among vulnerable populations to protect them against the B.1.617.2 (delta) variant.
ARTICLE HIGHLIGHTS
Research background
The delta variant of coronavirus disease 2019 (COVID-19) has quickly spread around the globe and infected not only unvaccinated population but fully vaccinated citizens. The demographic statistics and clinical presentation of the first cluster of delta variant infection in this population remained unknown and neglected.
Research motivation
The authors aimed to provide an insight into the demographic statistics and clinical presentation of the first cluster of delta variant infection after a second dose of vaccine. This could help others with lack of laboratory facility to diagnose delta variant infection.
Research objectives
The objective of this case series was to describe the demographic statistics and clinical presentation of the first cluster of delta variant infection after a second dose of vaccine.
Research methods
This is a retrospective, single-center case series of the general consecutive population that worked or studied in Our University with confirmed Delta Variant Infection after a second dose of vaccine from 24 June and 25 June 2021. We decided to collect data based on a combination of author recall, reverse transcription-polymerase chain reaction (RT-PCR), and whole genome sequencing results. Epidemiological, demographic, clinical, and laboratory data were analyzed.
Research results
Among 15 subjects recruited, Fourteen subjects were vaccinated with CoronaVac (Sinovac) and one subject with ChAdOx1 nCoV-19 (Oxford-AstraZeneca). All of the subjects remained in home isolation, with fever being the most common symptom at the onset of illness (n= 10, 66.67%). The mean duration of symptoms was 7.73 d (± 5.444). The mean time that elapsed from the first positive swab to a negative RT-PCR test for SARS-CoV-2 was 17.93 d (± 6.3464). The median time that elapsed from the second dose of vaccine to the first positive swab was 87 d (interquartile range: 86-128).
Research conclusions
After two doses of vaccine, subjects are still susceptible to the delta variant infection. Currently available vaccines remain the most effective protection.
Research perspectives
The case series might not be representative of the general population. It is necessary to collect more subjects infected with delta variant after second dose of vaccination to improve the quality of this study.
FOOTNOTES
Author contributions:Syam AF and Achmadsyah A contributed equally to this work and wrote a draft of the paper; Achmadsyah A, Fadilah F, and Ibrahim F collected the patient’s clinical data; All authors analyzed the data and wrote the paper; Karuniawati A, Syam AF, Ibrahim F, Saharman YR, Sudarmono P, Fadilah F, and Rasmin M made important intellectual contributions and revised the paper; Syam AF and Achmadsyah A edited all drafts of the paper; and All authors approved the final version of the manuscript.
Conflict-of-interest statement:All authors report no relevant conflicts of interest for this article.
Data sharing statement:The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Indonesia
ORCID number:Anis Karuniawati 0000-0002-9882-2488; Ari F Syam 0000-0003-0041-3553; Armand Achmadsyah 0000-0002-7831-0610; Fera Ibrahim 0000-0002-3732-0812; Yulia Rosa 0000-0001-6065-0329; Pratiwi Sudarmono 0000-0001-7637-2634; Fadilah Fadilah 0000-0002-8120-3138; Menaldi Rasmin 0000-0001-6725-8055.
Corresponding Author's Membership in Professional Societies:Indonesian Society of Gastroenterology.
S-Editor:Ma YJ
L-Editor:A
P-Editor:Ma YJ
 World Journal of Clinical Cases2022年36期
World Journal of Clinical Cases2022年36期
- World Journal of Clinical Cases的其它文章
- Precautions before starting tofacitinib in persons with rheumatoid arthritis
- Hoffa's fracture in a five-year-old child diagnosed and treated with the assistance of arthroscopy: A case report
- Development of dilated cardiomyopathy with a long latent period followed by viral fulminant myocarditis: A case report
- Congenital nephrogenic diabetes insipidus arginine vasopressin receptor 2 gene mutation at new site: A case report
- Short-term prone positioning for severe acute respiratory distress syndrome after cardiopulmonary bypass: A case report and literature review
- Compound heterozygous p.L483P and p.S310G mutations in GBA1 cause type 1 adult Gaucher disease: A case report
