Effects of polyol excipient stability during storage and use on the quality of biopharmaceutical formulations
Min-Fei Sun,Ji-Ning Lio,Zhen-Yi Jing,Hn Go,Bin-Bin Shen,You-Fu Xu,Wei-Jie Fng,*
aInstitute of Drug Metabolism and Pharmaceutical Analysis,College of Pharmaceutical Sciences,Zhejiang University,Hangzhou,310058,China
bHangzhou Institute of Innovative Medicine,Zhejiang University,Hangzhou Biopharm Town,Hangzhou,310016,China
cZhejiang Haisheng Pharmaceutical Inc.,Taizhou,Zhejiang,318000,China
ABSTRACT
Biopharmaceuticals are formulated using a variety of excipients to maintain their storage stability.However,some excipients are prone to degradation during repeated use and/or improper storage,and the impurities generated by their degradation are easily overlooked by end users and are usually not strictly monitored,affecting the stability of biopharmaceuticals.In this study,we evaluated the degradation profile of polyol excipient glycerol during repeated use and improper storage and identified an unprecedented cyclic ketal impurity using gas chromatography with mass spectrometry(GC-MS).The other polyol excipient,mannitol,was much more stable than glycerol.The effects of degraded glycerol and mannitol on the stability of the model biopharmaceutical pentapeptide,thymopentin,were also evaluated.The thymopentin content was only 66.4% in the thymopentin formulations with degraded glycerol,compared to 95.8% in other formulations after the stress test.Most glycerol impurities(i.e.,aldehydes and ketones)reacted with thymopentin,affecting the stability of thymopentin formulations.In conclusion,this work suggests that more attention should be paid to the quality changes of excipients during repeated use and storage.Additional testing of excipient stability under real or accelerated conditions by manufacturers would help avoid unexpected and painful results.
Keywords:
Excipient stability
GC-MS
Glycerol
LC-MS/MS
Mannitol
Thymopentin
1.Introduction
Biopharmaceuticals generally have complex and fragile structures compared with traditional small-molecule drugs[1,2].Optimization of formulations,that is,the addition of excipients,is a common method for maintaining their storage stability[3].Carbohydrates,polyols,amino acids,buffers,and surfactants are the major excipients used to stabilize proteins in biopharmaceutical formulations[4].These excipients significantly alter the conformational and colloidal stability of proteins and reduce their chemical degradation through interactions with drugs[5-7].For example,carbohydrates and polyols stabilize proteins through various alternative mechanisms to improve their colloidal stability[8,9].
Biocompatible pharmaceutical excipients are generally chemically inert.In practice,they may undergo degradation during production,repeated use,or storage.Owing to the different excipient manufacturing processes of various manufacturers,the quality and stability of the preparation are affected by the purity and contamination level of the excipients[10].Trace metals,for example,are inherent impurities in common excipient production processes and are ubiquitous in almost all excipients[11,12].In addition,carbohydrate and polyol excipients frequently contain reducing impurities during the production process[13,14].Nevertheless,impurities in the production process of excipients can often be traced back to their sources,which can be controlled by optimizing production conditions and purification,and many related studies have been conducted to ensure product quality.
Other impurities in pharmaceutical excipients,such as those produced during storage and use,have not attracted enough attention.For pure excipients that meet the release standard,various degradations may occur during storage and use,leading to potential reactions with drugs[11].For example,since the excipients in chemotherapy are a large part of the dose,accounting for up to 99% of the total formulation mass[15],there is a significant amount of literature about the effect of excipient quality on chemotherapeutic drugs[16-18].In contrast,owing to the small number of excipients used for biopharmaceutical preparations,a bottle of excipients will be stored and used for a long time.During this process,they come into contact with oxygen and moisture,leading to the generation of impurities that are easily overlooked.A vast majority of the existing studies have focused on the effect of polysorbates on the physicochemical stability of biopharmaceuticals such as monoclonal antibodies.Donbrow et al.[19]and Kerwin[20]reported that polysorbates may undergo autooxidation,hydrolysis of the fatty acid ester bond,and cleavage of the ethylene oxide subunits during storage and use.Polysorbate autoxidation generates hydrogen peroxide,leading to oxidation of the protein during preparation[21,22].Its hydrolysis produces free fatty acids,and further studies have revealed that polysorbate-containing biopharmaceutical formulations produce protein particles that contain fatty acids during long-term storage[23,24].These studies suggest that oxygen-free and low-temperature conditions should be maintained during storage and utilization of polysorbate excipients[25].In fact,because of the sensitive physical and chemical stability of biopharmaceuticals,even if the content of excipients in the preparation is small,minor changes in the properties of excipients during storage and use may lead to significant changes in the stability of biopharmaceuticals.Therefore,further studies on the impact of changes in the excipients for biopharmaceuticals during storage and use are needed to increase the industry's awareness of excipient degradation.
Glycerol is a liquid polyol with a wide range of applications in the pharmaceutical industry[26].It can be used not only as an oral or intravenous drug,but also as an excipient in pharmaceutical preparations,such as insulin,thymopentin,and freeze-dried biological drugs[27,28].There are many reports on the oxidation of glycerol[29-32].In addition,Sugiura et al.[33]reported that pure glycerol preparations produced methylglyoxal.However,until recently,there has been no research on the influence of changes in the storage process and the use of glycerol as an excipient for biopharmaceuticals.As an approved drug,thymopentin is the immunoactive center of thymopoietin II,with an amino acid sequence of H-Arg-Lys-Asp-Val-Tyr-OH,and plays an important role as an immune bidirectional regulator[34].Thymopentin shows good clinical results in the adjuvant treatment of chronic hepatitis,respiratory diseases,and malignancies[35].However,like most biopharmaceuticals,it is less stable in aqueous solutions,and the peptide bonds of Asp residues are prone to cleavage,particularly in acidic environments[36].Therefore,for the liquid formulation of thymopentin,polyol excipients,such as glycerol and mannitol(Fig.1),are often added as protective agents to maintain their stability[3,4,10,37].Thymopentin is a low-molecular-weight pentapeptide that facilitates analysis of degradation mechanisms and sites.In this study,thymopentin was used as a model drug to explore the stability of polyol pharmaceutical excipients(glycerol and mannitol)during storage and use,their effects on biopharmaceutical stability,and the potential reactive sites of thymopentin formulations.It is hoped that this study will increase the industry's attention to reactive impurities arising from biopharmaceutical excipients during storage and use,and demonstrate the importance of maintaining low-temperature and oxygen-free storage conditions.
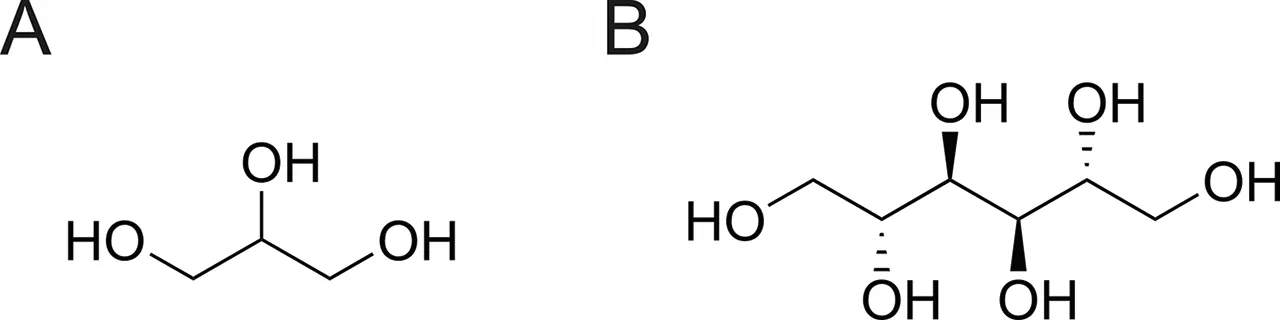
Fig.1.Structures of polyol pharmaceutical excipients:(A)glycerol,and(B)mannitol.
2.Materials and methods
2.1.Materials and chemicals
Thymopentin was purchased from Nantong Feiyu Biological Technology Co.,Ltd.(Nantong,China).Glycerol and ethylenediaminetetraacetic acid disodium salt(EDTA-2Na)of pharmaceutical grade were purchased from Hunan Er-Kang Pharmaceutical Co.,Ltd.(Changsha,China).
Guaranteed reagent-grade mannitol,monobasic sodium phosphate dodecahydrate,dibasic sodium phosphate dihydrate,ferric chloride,sodium hydroxide,and formaldehyde were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).3-methyl-2-benzothiazolone hydrazone hydrochloride was purchased from BioDuly Co.,Ltd.(Nanjing,China).
2.2.Sample preparation
Thymopentin formulations at a concentration of 1mg/mL were prepared in sodium phosphate buffer(pH 7.0,10mM)with 0.02% EDTA-2Na.The excipients mannitol and glycerol before and after oxidative degradation were used as osmoregulators.These formulations were labelled as follows:thymopentin formulations with undegraded mannitol(TP5-M0),thymopentin formulations with degraded mannitol(TP5-M1),thymopentin formulations with undegraded glycerol(TP5-G0),and thymopentin formulations with degraded glycerol(TP5-G1).All formulations were sterile-filtered with a 0.22μm Merck Millipore PES membrane filter(Darmstadt,Germany).The formulations(1.0 mL each)were then aseptically filled into presterilized 2-mL Schott vials(Lishui,China).
2.3.Accelerated stability study
The degraded glycerol and mannitol excipients were obtained by storage at 40°C for 6 weeks.The excipients were oscillated at the maximum speed for 1 min twice a week using a vortex oscillator purchased from Sangon Biotech(Shanghai,China)to simulate the worst condition of excipients during industrial production,that is,the excipients were fully exposed during each use.Different groups of thymopentin formulations(TP5-M0,TP5-M1,TP5-G0,and TP5-G1)were stored at 40°C.At each time point(0,1,2,4,and 8 weeks of storage),three vials of each formulation were used for reversed-phase high-performance liquid chromatography(RP-HPLC)analysis.The 40°C condition was maintained in an LRH-250-II biochemical incubator purchased from Guangdong Medical Instrument Factory(Guangzhou,China).
2.4.Detection of aldehydes and reducing substances
The contents of aldehydes and reducing substances in the polyol excipients and thymopentin formulations were determined by phenol reagent spectrophotometry according to the Chinese Pharmacopoeia(ChP)[34].
Detection of polyol excipients:The total reaction volume was 10mL,and a 0.4 mL of formaldehyde standard solution(5.0μg/mL)was added to the positive control group(ρCH20=0.815 g/cm3).In the experimental group,0.2 g of glycerol and mannitol were added before and after degradation,respectively.Then,0.4mL of the newly prepared 1% phenol reagent was added to each tube and allowed to stand for 5 min.Subsequently,1mL of 0.5% ferric chloride solution was added as the coloring reagent,followed by5 mL of water.Finally,the volume was adjusted to 10mL by using methanol.The colors of the reaction systems of the polyol excipients before and after degradation were observed.
Detection of thymopentin formulations:Quantitative reactions was performed in 96-well plates.The detection principle was the same as described above,and the total reaction volume was 200μL.First,a standard curve was obtained from a standard solution of formaldehyde(1μg/mL),as shown in Fig.S1,and the absorbance wavelength was 655 nm.The absorbance of TP5-M0,TP5-M1,and TP5-G0 formulations before degradation and after the 8 week stability test was detected at 655 nm.The absorbance of the TP5-G1 formulations under the 40°C stress test at weeks 0,1,2,4,and 8 was also detected at 655 nm under the same conditions(n=3).Finally,the aldehyde content was calculated according to the standard curve and absorbance values.
2.5.Gas chromatography with mass spectrometry(GC-MS)
The structures of impurities in degraded glycerol were detected using Waters GCT Premier GC-TOF-MS(Milford,USA).GC conditions:type of injection,split;injection time,0.5min;capillary column,Wax column(30mm×0.32 mm,0.25μm;Thermo Fisher Scientific Inc.,USA).The column temperature started at 60°C,was held for 2min,and then increased at a rate of 40°C/min up to 120°C without retention,and then at a rate of 40°C/min to 300°C,which was held for 7min.The temperature of the inlet was 240°C,the column flow was 1.84mL/min,the split ratio was 10:1,and the carrier gas was helium.MS conditions:ionization mode,EI;electron energy,70eV;the temperature of the ion source,200°C;the temperature of quadrupole,250°C;scanning range,35-450 amu.
2.6.Reversed-phase high-performance liquid chromatography(RP-HPLC)
The contents of thymopentin in different formulations were monitored using an Agilent 1260 HPLC(Agilent Technologies Inc.,Karlsruhe,Germany)on a Supersil ODS column(4.6 mm×250 mm,2.5μm;Dalian Elite Analytical Instruments Co.,Ltd.,Dalian,China)equipped with a DAD G4212B detector.A gradient elution method was used for chromatographic separation,with 0.1%(V/V)formic acid(FA)in water as mobile phase A and 0.1%(V/V)FA in acetonitrile as mobile phase B.The initial ratio of mobile phase B was 2%,which was increased to 25% over 35min to elute thymopentin,followed by 0.9min of elution with 90% mobile phase B.Before use,the mobile phases were filtered and degassed.The injection volume was 10μL and the flow rate for the analysis was 0.5mL/min.The column temperature was maintained at 30°C.The thymopentin and its degradation products were monitored at 214,220,254,and 280 nm.The method validation data are presented in Fig.S2 and Tables S1 and S2.
2.7.Liquid chromatography coupled with tandem MS(LC-MS/MS)
The LC(1290 HPLC,Agilent Technologies,Inc.,Karlsruhe,Germany)conditions for the detection of degradation products were consistent with those of the RP-HPLC described in Section 2.6,except that the injection volume was changed to 2μL.The mass conditions(6460 Triple Quad LC/MS,Agilent Technologies Inc.,Karlsruhe,Germany)were as follows:ion source,ESI positive mode;ion spray voltage,5.0kV;temperature,350°C;curtain gas,5.0L/min;nebulizer gas,45.0 psi;sheath flow rate,10mL/min;sheath temperature,350°C.First-order mass spectrometry was performed using total ion chromatography(TIC)full-ion scanning with a scanning range of 50-1000amu.Based on LC-MS,a relatively large number of degradation peaks(mainly the degradation products with peak areas>1%),which require structural confirmation,were selected for tandem mass spectrometry detection.The collision gas was nitrogen,the collision energy was set to 35 or 40V,and the fragmentor was set to 135V to obtain fragment ion peaks.
3.Results and discussion
3.1.The color reaction results of degraded polyol excipients
The results of the qualitative phenol reagent color reaction of the polyol excipients indicated that glycerol before degradation(G0)and mannitol before and after degradation(M0 and M1)did not show a blue-green color.However,the degraded glycerol(G1)was blue-green in the color reaction,which suggested that there were aldehydes and reducing substances in the degraded glycerol sample,as shown in Fig.2.The content of aldehydes and reducing substances in the degraded glycerol exceeded the requirement of no more than 10 ppm in the ChP and European Pharmacopoeia(EP);thus,this excipient was unqualified after the stress test[34,38].
Glycerol and mannitol are polyol excipients with similar functional groups,as shown in Fig.1.However,they showed a dramatic difference in stability during storage and repeated use.The principal reason why the stability of the two excipients differed so dramatically is probably that glycerol is a liquid excipient,while mannitol is a crystalline solid,which shows much higher stability owing to the restricted mobility and retarded exchange of oxygen on the surface.In addition,glycerol is highly hygroscopic,and its oxidation is accelerated when the moisture content is increased.Thus,ChP and EP require strict control of the moisture content of glycerol[34,38].Mannitol,a crystalline sugar alcohol,is chemically stable and exhibits no hygroscopicity even under extremely high relative humidity[39].The implication for formulation development is that when excipients with similar protective effects on biopharmaceuticals are selected,the more stable solid excipients should take precedence over the liquid excipients owing to stability issues in long-term manufacturing storage and use.
3.2.Impurities in degraded glycerol
To investigate the impurities produced by glycerol after degradation and their potential influence on biological drugs,GC-MS was used to detect glycerol and its degradation products.The reactive impurities associated with thymopentin degradation are listed in Table 1.The representative mass spectrum of glycerol impurity 4(mean molecular weight=164 Da)is shown in Fig.3,and the other spectra of the glycerol impurities are shown in Fig.S3.
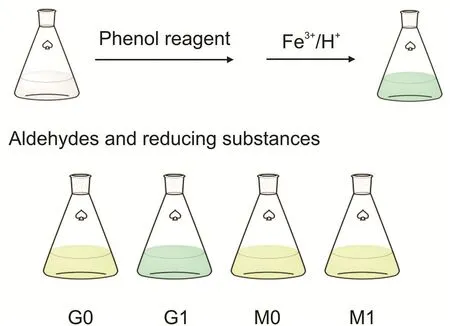
Fig.2.Spectrophotometry with phenol reagent.
Impurity 1 is hydroxyacetone,which is presumed to be obtained from the dehydration of glycerol and subsequent keto-enol tautomerization,as shown in Table 1.Impurity 2 is 2,3-epoxy-1-propanol(glycidol),which is most likely formed by epoxidation via the SN2 reaction between the vicinal diol of glycerol.
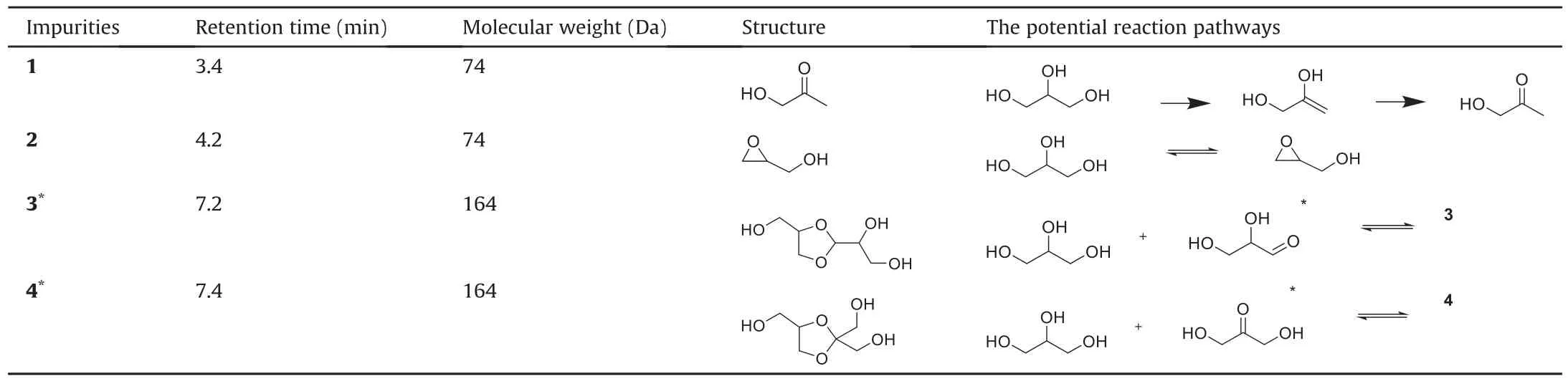
Table 1Gas chromatography with mass spectrometry(GC-MS)results of the main degradation products of glycerol and their potential reaction sources.
1,2-dihydroxypropionaldehyde (GLAD) and 1,3-dihydro xyacetone(DHA)are common oxidation products in glycerol[29],and our thymopentin stability studies(see below)also suggest that these two degradation compounds exist in degraded glycerol.However,they were not detected by the GC-MS analysis.Instead,we identified two impurities,impurity 3(a cyclic acetal)and impurity 4(a cyclic ketal),presumably formed by the reactions of GLAD and DHA with glycerol,respectively.Owing to the large excess of glycerol,the reaction for GLAD and DHA could be driven nearly to completion.Impurity 3(CAS#112401-29-3)has only been reported by Hibbert and Whelen[40]and Fisher and Smith[41],but it was first reported to result from the storage of glycerol in our study.Impurity 4 has not been reported previously in the literature.It has been reported that glycerol easily reacts with acetone to form stable five-membered or six-membered ring compounds,depending on the hydroxyl groups involved[42].The stability of the cyclic acetal/ketal depends on strain energy.The lower the strain energy,the more stable is the structure.Three types of strains contribute to the overall energy of a cyclic acetal/ketal:torsional strain,angle strain,and steric strain [43].Although the six-membered ring is slightly more stable than the five-membered ring when considering the cycloalkane ring strain,the product rings are not always suitable models of the transition states for some ring-closure reactions[44].For the reaction of glycerol and acetone,the selectivity for the formation of the five-membered ring is better than that for the sixmembered ring under most catalytic conditions because of steric hindrance.The number of six-membered rings in the reaction products did not exceed 2%-3% under the most common acidic catalytic conditions[42,45,46].The reaction was reversible,and the acetal and ketal could be easily hydrolyzed back to GLAD and DHA.Therefore,the color reaction and thymopentin stability results suggested that GLAD and DHA were present in the oxidized glycerol.
Among the several new substances produced by glycerol degradation,hydroxyacetone(impurity1),GLAD (hydrolysis product of impurity 3),and DHA(hydrolysis product of impurity 4)have carbonyl groups,which are presumed to react directly with free amino groups in thymopentin through a Maillard reaction[47].
3.3.The contents of aldehydes and reducing impurities in formulations and monitoring of thymopentin contents by RP-HPLC
To explore the influence of glycerol before and after degradation on the stability of biopharmaceutical formulations,an accelerated stability test of thymopentin formulations was carried out.In early excipient tests,we found that the aldehyde content in glycerol was high after degradation.Therefore,the aldehyde content of the thymopentin formulations was determined during the stress test(Fig.4A).RP-HPLC results indicated changes in thymopentin content in each formulation from 0 to 8 weeks,as shown in Fig.4B.First,the effects of different excipients on thy-mopentin stability were compared.No significant difference between TP5-M0 and TP5-G0(P>0.05)was observed,indicating that the protective effects of mannitol and glycerol on the stability of thymopentin preparations were similar,and there were no obvious aldehyde impurities in the formulations even after degradation.Regarding the effects on the stability of thymopentin in the same excipients before and after degradation,no significant difference was observed between the TP5-M0 and TP5-M1 formulations(P>0.05),which utilized undegraded and degraded mannitol,respectively.
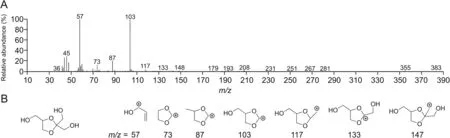
Fig.3.(A)Mass spectra of impurity 4 eluting at 7.4 min in gas chromatography with mass spectrometry(GC-MS).(B)The structure of impurity 4 and its mass spectrum fragments.
However, thymopentin in the TP5-G1 formulations was significantly degraded(P<0.0001)compared to TP5-G0,and the aldehyde content showed an unusual decrease.In the eighth week,the thymopentin content was only 66.4% in the TP5-G1 formulations.The contents of intact thymopentin(red)and aldehydes(blue)in the TP5-G1 formulations are shown in Fig.4B.It was found that the trends of both thymopentin and aldehyde contents were surprisingly consistent.First,the contents of intact thymopentin and aldehydes in the TP5-G1 formulations were highest at week 0.Both the thymopentin and aldehyde contents in TP5-G1 formulations showed a rapid decline during weeks 0-2,from which it can be inferred that the aldehyde impurities in degraded glycerol reacted with thymopentin.Finally,the aldehyde content tended to be stable at weeks 4-8,while the thymopentin content decreased.This result indicated that during weeks 4-8,the decrease in thymopentin content was primarily caused by the self-degradation of thymopentin during the 40°C stress test.
The chromatograms of all thymopentin formulations after 8 weeks of the accelerated stability study are shown in Fig.4C,and the peak at approximately about 13.0 min is the peak of thymopentin.In general,the degradation of the TP5-M0,TP5-M1,and TP5-G0 formulations was similar,and only three degradation peaks were observed.These three degradation products are caused by the instability of thymopentin in aqueous solutions and are assigned to the self-degradation peak.However,a large number of degradation product peaks were observed in the TP5-G1 formulations,indicating that oxidized glycerol had a great influence on the stability of thymopentin formulations during storage.At the same time,the three common self-degradation peak areas in the TP5-G1 formulations were significantly higher than those in other formulations,indicating that degraded glycerol promoted the production of these degradation products.
Analysis of the degradation of thymopentin by HPLC further proved that some reactive impurities produced in the degraded glycerol led to severe instability of the thymopentin formulations.Therefore,researchers should pay more attention to changes in excipients during storage and use because they may lead to a significant decline in the stability of biopharmaceutical preparations.In addition,TP5-M1,after the accelerated stability test,had no such issues,suggesting that mannitol was more stable than glycerol as an excipient.
3.4.Characterization of the degradation products of thymopentin formulations by LC-MS/MS
Because more degradation peaks were produced in the thymopentin formulation(TP5-G1)containing degraded glycerol,this formulation was analyzed by LC-MS/MS to explore the degradation mechanism of thymopentin.The structures of thymopentin and its major degradation products(Table 2)were obtained using LC-MS/MS analysis,as shown in Figs.5 and S4.
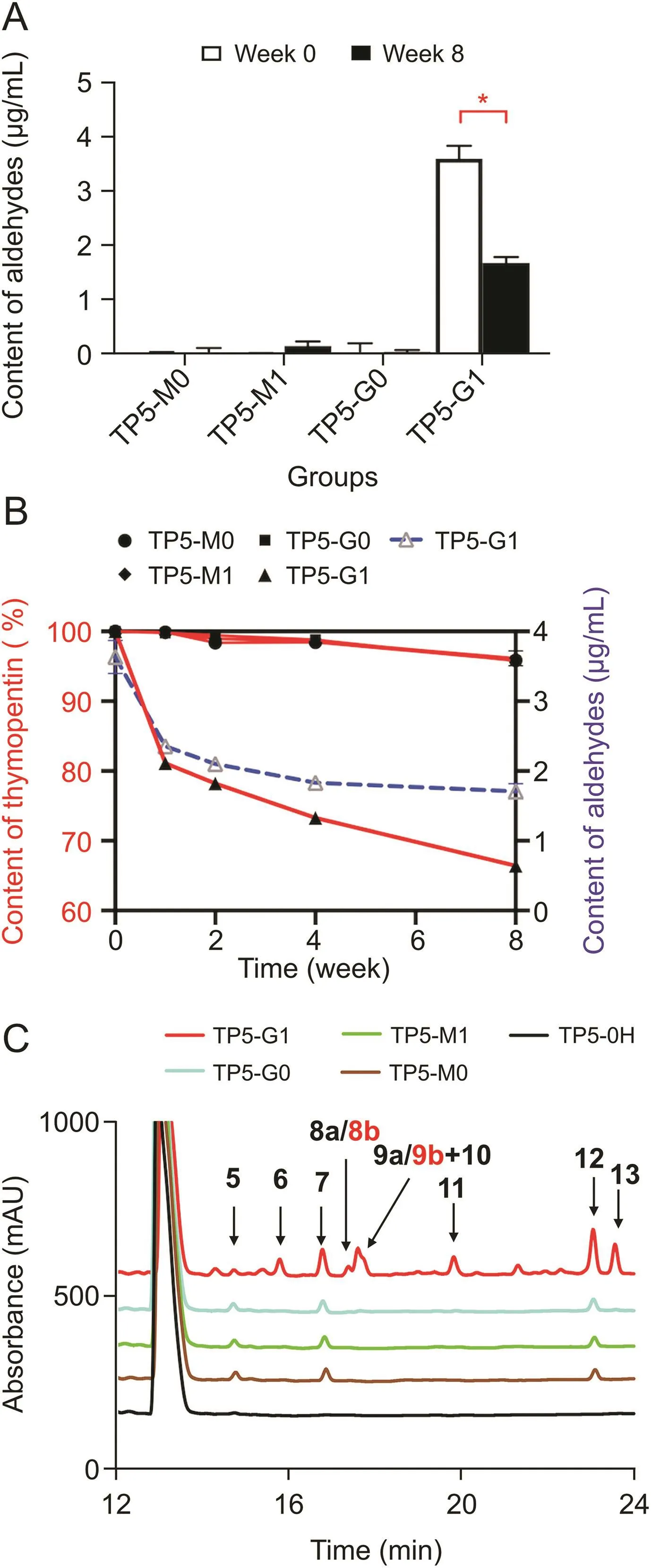
Fig.4.(A)The contents of aldehydes at 0 and 8 weeks of each formulation group.*P<0.0001.(B)Degradation of thymopentin in different formulations at weeks 0-8 by reverse phase high performance liquid chromatography(RP-HPLC)(red);changes in aldehyde content in TP5-G1 formulations at weeks 0-8(blue).(C)HPLC chromatogram of thymopentin formulations at the 8th week.(TP5-M0 refers to thymopentin formulations with undegraded mannitol;TP5-M1 refers to thymopentin formulations with degraded mannitol;TP5-G0 refers to thymopentin formulations with undegraded glycerol;TP5-G1 refers to thymopentin formulations with degraded glycerol;TP5-0H refers to untreated thymopentin formulations;mean±SD,n=3).
Table 2 shows that products 5 and 7 are formed by the degradation of thymopentin itself.Product 5 is an isoAsp-containing variant of thymopentin and this impurity was also observed in other formulations.Therefore,this degradation product is due to the degradation of thymopentin in solution and has no direct correlation with the degraded glycerol.The Asp isomerization reaction has been widely reported previously[48-50].The specific process of Asp cyclization occurs through the attack of N+1 main chain amide nitrogen on the Asp side chain carbonyl,resulting in a dehydrated aspartic succinimide(Asu)intermediate,which is degradation product11.Then,the hydrolysis of the Asuintermediate product 11 produces either isoAsp-containing product 5 or the Asp-containing starting material thymopentin.The relative amounts of the isoAsp and Asp products formed depend on the pH of the solution.Under neutral conditions,the isoAsp product is usually favored[51].Therefore,a certain amount of product 5 was detected in the thymopentin preparation in this study.Theoretically,the succinimide intermediate product 11 is unstable in aqueous solution;however,it was still detected by LC-MS/MS.Product 7 is obtained by the cleavage of the peptide bond between the Arg1and Lys2residues.Products 5 and 7 further verify that the degradation peaks in all formulations are caused by the degradation of thymopentin itself.Helm and Müller[52]reported that the liquid preparation of thymopentin is unstable under acidic conditions,and the peptide bond of the Asp residue easily breaks to form dipeptides and tripeptides.This study showed for the first time that the peptide bond between Arg and Lys in a thymopentin liquid preparation broke easily under neutral conditions.
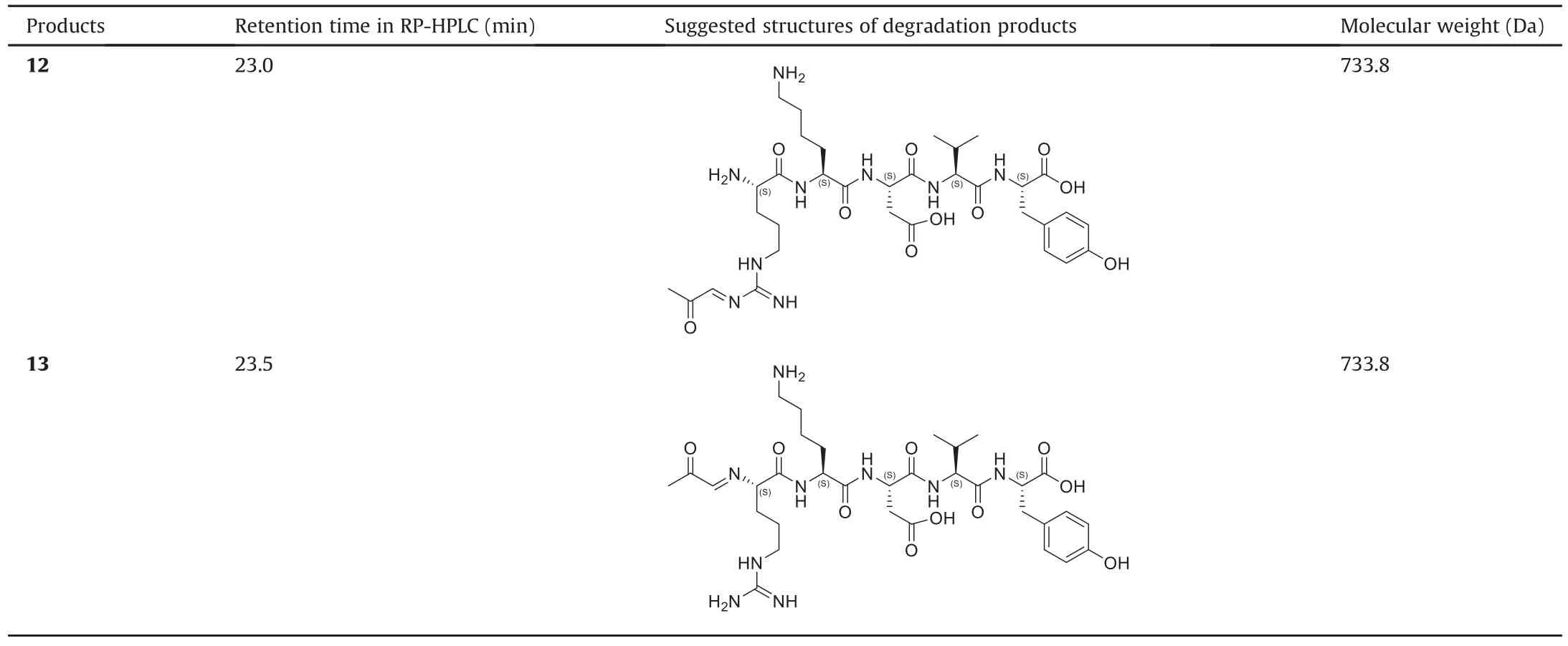
Table 2(continued)

Table 2Suggested structures of thymopentin degradation products in TP5-G1 formulations at week 8.
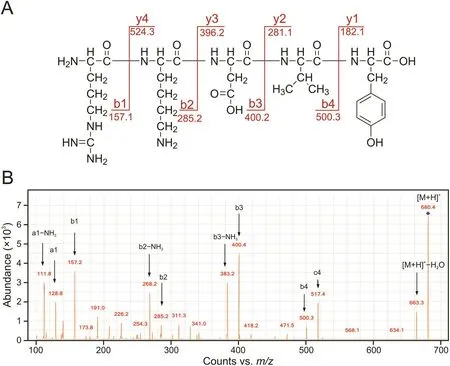
Fig.5.(A)MS/MS analysis of thymopentin as a representative.(B)MS/MS spectrum of thymopentin.
Other thymopentin degradation products were generated via glycerol oxidation.Thymopentin reacts directly with glycerol oxidation products,such as GLAD and DHA,to form products 8 and 9.GLAD and DHA were not detected in degraded glycerol but were probably the reversible hydrolysis products of impurities 4(an acetal)and 5(a ketal)in the degraded glycerol,respectively.The other degradation products that contain glycerol impurities are dehydration products,specifically,products 10,12,and 13.Among them,product 12 was formed by the dehydration of product 9a,and similarly,product 13 was obtained by the dehydration of product 8a.Changes in the peak areas of products 8a and 13 and their conversion mechanisms are shown in Figs.6 and 7,respectively.The main impurities in glycerol oxidation products involved in the degradation of thymopentin are hydroxyacetone,GLAD,and DHA.The products from the Maillard reaction of hydroxyacetone with the free amino group of thymopentin were unstable and further dehydrated to produce the degradation product 10.The health risks of hydroxyacetone impurities in glycerol after dermal exposure have also been evaluated[53].In this study,it was found that this impurity could also react with free amino acids in biopharmaceuticals,resulting in a decrease in the stability of the drug preparation,which affects its biological efficiency.
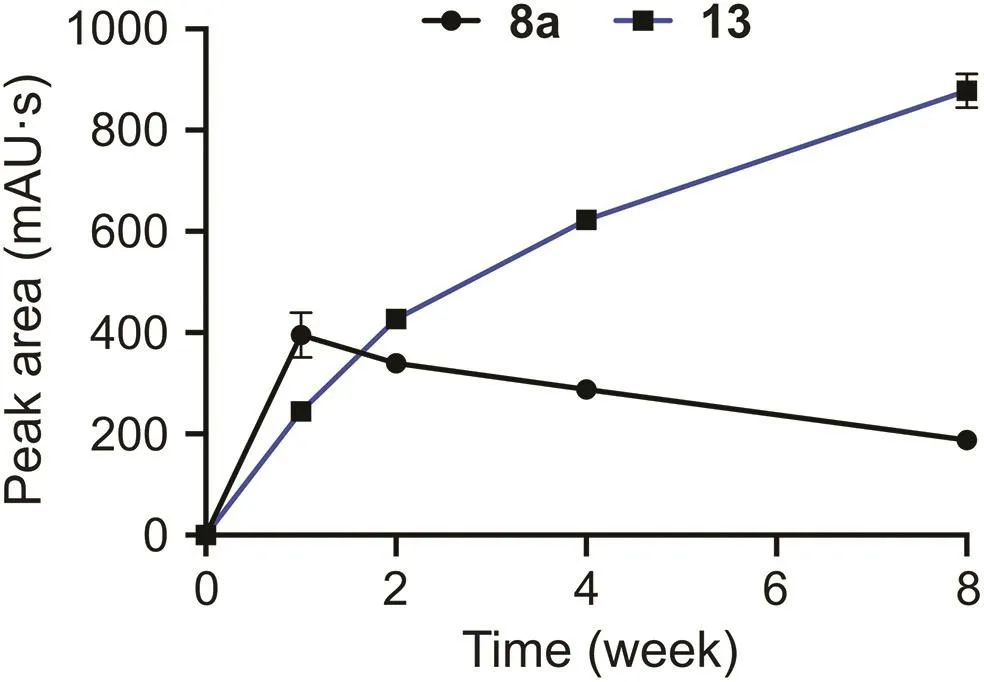
Fig.6.Changes in peak area of products 8a and 13 in weeks 0-8 of thymopentin formulations with degraded glycerol(mean±SD,n=3).
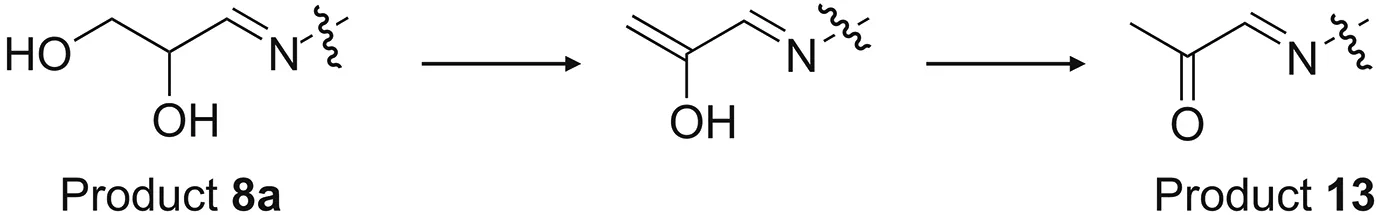
Fig.7.Conversion mechanism of products 8a and 13.

Fig.8.Effect of polyol excipient stability during storage and use on the quality of the thymopentin formulations.GC-MS:gas chromatography with mass spectrometry;LC-MS/MS:liquid chromatography coupled with tandem mass spectrometry.
Degradation product 6 was speculated to react with formaldehyde in air.The guanidine group of arginine has been reported to capture formaldehyde[54,55].This is probably caused by impurities with catalytic activity in degraded glycerol,which promoted the arginine guanidine group of thymopentin to capture formaldehyde in the air,leading to further degradation of the thymopentin formulation.
In general,glycerol generates oxidation impurities,such as aldehydes and ketones(impurities 1,3,and 4),during repeated use and/or improper storage.These impurities react with thymopentin to produce various related thymopentin degradation products,such as products 8,9,and 10,as shown in Fig.8.There are two main reaction sites in thymopentin:the guanidine group on the arginine residue and the free N-terminal group.These two sites are prone to Maillard reactions with carbonyl impurities,dramatically reducing the stability of thymopentin formulations in TP5-G1 and affecting their biological activities.Therefore,researchers should raise awareness regarding the stability of excipients during storage and use,especially for labile liquid excipients.
4.Conclusions
Overall,excipients are considered chemically inert and often fail to attract sufficient attention.The trace active impurities produced in the process of repeated use and storage of excipients have a great impact on biopharmaceuticals and deserve more attention.In this study,two active impurities that have not yet been reported were found during glycerol storage.We then explored the mechanism by which the degradation products of glycerol lead to a decrease in the stability of thymopentin preparations.Furthermore,the physical state of polyol excipients with similar chemical properties determines their stability during storage and use,with mannitol being much more stable than glycerol.
In summary,this study focused on impurities produced during the storage and use of biopharmaceutical excipients.For manufacturers,more attention should be paid to the storage and use environment(i.e.,low temperature and anaerobic conditions).Moreover,the impurities and stability of frequently used excipients should be regularly evaluated to ensure consistency between batches of biopharmaceutical products.Finally,the results of this study indicate that when choosing excipients with similar protective effects for biopharmaceuticals,solid excipients may be preferred over liquid excipients owing to stability concerns during long-term manufacturing storage and use.
CRediT author statement
Min-Fei Sun:Methodology,Investigation,Formal analysis,Writing-Original draft preparation,Reviewing and Editing;Jia-Ning Liao:Methodology,Investigation;Zhen-Yi Jing:Investigation;Han Gao:Investigation;Bin-Bin Shen:Investigation;You-Fu Xu:Investigation;Wei-Jie Fang:Conceptualization,Methodology,Writing-Reviewing and Editing,Funding,Supervision.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Funding for this work was generously provided by the National Natural Science Foundation of China(Grant No.:81741144)and Ministry of Science and Technology of China(Grant No.:2018ZX09J18107-002).The authors would also like to thank Mr.Jiankai Zou and Ms.Haihong Hu of Zhejiang University for their help with the GC-MS analysis and laboratory management,respectively.We also thank Elsevier Language Editing Services for improving the language.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.03.003.
 Journal of Pharmaceutical Analysis2022年5期
Journal of Pharmaceutical Analysis2022年5期
- Journal of Pharmaceutical Analysis的其它文章
- Caenorhabditis elegans deep lipidome profiling by using integrative mass spectrometry acquisitions reveals significantly altered lipid networks
- Effective extraction of fluoroquinolones from water using facile modified plant fibers
- Rapid fabrication of zwitterionic sulfobetaine vinylimidazole-based monoliths via photoinitiated copolymerization for hydrophilic interaction chromatography
- Evaluation of the metabolism of PEP06,an endostatin-RGDRGD 30-amino-acid polypeptide and a promising novel drug for targeting tumor cells
- Multimodal integrated strategy for the discovery and identification of quality markers in traditional Chinese medicine
- The dynamic metabolic profile of Qi-Yu-San-Long decoction in rat urine using UPLC-QTOF-MSEcoupled with a post-targeted screening strategy
