Shugan Jieyu capsule (舒肝解郁膠囊) improve sleep and emotional disorder in coronavirus disease 2019 convalescence patients: a randomized,double-blind,placebo-controlled trial
AN Xuedong,ZHANG Qing,TAO Junxiu,LI Li,CHEN Yun,LI Kejian,HE Jing,LIU Ru,GUO Juan,ZHANG Jia,ZHU Hui,LIAN Fengmei,LI Xiaodong
AN Xuedong,LIAN Fengmei,Endocrinology,Guang'anmen Hospital of China Academy of Chinese Medical Sciences,Beijing 100053,China
ZHANG Qing,Orthopedics,Hubei Provincial Hospital of Traditional Chinese Medicine,Affiliated Hospital of Hubei University of Chinese Medicine,Hubei Institute of Traditional Chinese Medicine,Wuhan 430000,China
TAO Junxiu,LI Xiaodong,Liver Disease branch,Hubei Provincial Hospital of Traditional Chinese Medicine,Affiliated Hospital of Hubei University of Chinese Medicine,Hubei Institute of Traditional Chinese Medicine,Wuhan 430000,China
LI Li,CHEN Yun,LI Kejian,HE Jing,LIU Ru,GUO Juan,Psychology Department,Hubei Provincial Hospital of TCM,Affiliated Hospital of Hubei University of Chinese Medicine,Hubei Institute of Traditional Chinese Medicine,Wuhan 430000,China
ZHANG Jia,ZHU Hui,Clinical College of Traditional Chinese Medicine,Hubei University of Traditional Chinese Medicine,Wuhan 430061,China
Abstract OBJECTIVE: To evaluate the efficacy of the Shugan Jieyu capsule (舒肝解郁膠囊) on improving sleep and emotional disorder during Coronavirus disease 2019(COVID-19) convalescence.METHODS: We conducted a randomized,double-blind,placebo-controlled trial,and recruit 200 COVID-19 convalescence patients and then divide the subjects into two groups respectively: the experimental group (n= 100)and the control group (n= 100).Patients in the control group were given doses as a placebo,while those in the experimental group were given Shugan Jieyu capsule.The investigators mainly observed the differences between the two groups before and after treatment in terms of the rate of reduction and the rate of efficiency in Hamilton Depression Scale (HAMD-17) total scores from baseline,and recorded the scores of Hamilton Anxiety Scale (HAMA),Patient Health Questionnaire-15 (PHQ-15),Insomnia Severity Index (ISI) and Treatment Emergent Symptom Scale at 2 week,the 4 week and the 6 week respectively after treatment,and compared the differences between the groups.And the occurrence of adverse events was recorded.RESULTS: After 6-week treatment,there were statistically significant differences in the rate of reduction as well as efficiency in HAMD-17 scores,HAMA Total Scores,PHQ-15 Score,ISI Score from baseline in the experimental group and control group (P < 0.05).There were 4 adverse events in the experimental group and 1 in the control group.CONCLUSION: Shugan Jieyu capsule could significantly improve sleep and emotional disorder in patients during COVID-19 convalescence.
Keywords: COVID-19;convalescence;sleep quality;emotional disorder;Shugan Jieyu capsule
1.INTRODUCTION
Coronavirus Disease 2019 (COVID-19),in the early stage,presents main symptoms of fatigue,fever,dry cough and so on,accompanied by gastrointestinal symptoms such as diarrhea,nausea,vomiting,and constipation.1,2During the convalescence,the patients can be cancelled out from isolation or can be discharged for COVID-19 convalescence when their body temperatures have been in a normal range for more than 3 d;their respiratory symptoms have been reduced significantly;their lung imaging has showed better absorption of inflammation;and the two consecutive nucleic acid tests of respiratory pathogens should be negative (the sampling intervals should be at least 1 d).
However,given that the development of the pandemic subtly affects the physical and mental health of the public,some patients,even after entering COVID-19 convalescence,still have sleep disorders such as insomnia,or emotional disorders such as depression and anxiety,affecting their quality of life during COVID-19 convalescence.3
For this part,sufficient attention should be paid to further improve the life quality of the discharged patients during COVID-19 convalescence and to speed up the recovery process.The importance of Traditional Chinese Medicine (TCM) in major infectious diseases has been emphasized in the whole-process intervention to patients with COVID-19 fromThe Diagnosis and Treatment Protocol for COVID-19 Patients.4Therefore,in this study,the investigators,having considered the abovementioned symptoms of patients during COVID-19 convalescence,select Shugan Jieyu capsule (舒肝解郁膠囊),a traditional Chinese patent medicine,as the intervention drug,and evaluate its effect on clinical symptoms such as insomnia,anxiety,and depression with a randomized,double-blind,placebo-controlled design,thus forming an effective clinical treatment protocol of TCM intervention during the COVID-19 convalescence.
2.MATERIALS AND METHODS
2.1.Study design
A randomized, double-blind, placebo-controlled trial was conducted, with 200 patients during COVID-19 convalescence were recruited from July 2020 to September 2020, presenting clinical symptoms related to sleep and emotional disorders such as depression, anxiety, and insomnia.Totally 200 patients were randomly divided into experimental group (n= 100) and the control group (n= 100) on the basis of random number table method.The experimental group was treated with Shugan Jieyu capsule, while the control group was treated with placebo.The overall intervention course was 6 weeks, and the 0 week, the 2nd week, the 4th week as well as the 6th week respectively were taken as the observation nodes for the follow-up and health management.After the end of the treatment period, the clinical symptoms of the two groups continued to be observed.The protocol was approved by the Ethics Committee of Hubei Hospital of Traditional Chinese Medicine (HBZY2020-C27-01).This study was registered in Chinese Clinical Trial Registry (ChiCTR-2100043108).
2.2.Inclusion criterias
The research included the following patients: (a) those meeting the diagnostic criteria of COVID-19 convalescence;5(b) those aged between 18 and 70 years old, male or female; (c) those simultaneously having 2 symptoms of sleep and emotional disorder of depression, anxiety, poor sleeping quality, fatigue as the main clinical manifestations; (d) those scoring ≥ 17 and ≤ 24 of Hamilton Depression Scale (HAMD 17) total scores from baseline; (e) those scoring ≥ 5 of Patient Health Questionnaire-15 (PHQ-15); (f) those signing an informed consent form.
2.3.Exclusion criterias
Those patients would be excluded: (a) patients who have difficulties in oral medication due to underlying health conditions; (b) Patients with combination of serious primary diseases and dysfunctions of the heart, brain, respiratory, gastrointestinal, endocrine, hematopoietic, liver or kidney; (c) patients with schizophrenia, bipolar disorder, somatoform disorder, mania, anorexia, bulimia, or other types of mental illnesses, as well as histories of mental illnesses; (d) those who cannot cooperate due to their mental state, suffer from mental illnesses, and have difficulty in self-control; (e) those who have serious suicide intention(with HAMD-17 suicide scores ≥ 4) and intended to hurt others; (f) those who have allergies or are allergic to the drug involved in their treatment protocol; (g) pregnant women or women in lactation; (h) those who are participating in other clinical trials; (i) those patients whose participation and enrolment, in the judgement of the investigators, will affect the assessment of efficacy and safety negatively.
2.4.Interventions
The drug volume was allocated and obtained by the central randomization system.The intervention course lasted for 6 weeks, and the patients were not allowed to be given adjustments to their respective doses.The drug used in the research should be placed specifically with full-time pharmacists responsible for the storage and distribution.It was required that the drug must be in a place in consistence with the storage conditions under the specific personnel responsibility.The receipt and distribution of the drug must be clearly recorded with details, including the time, the drug volume, and the information of the receivers and the distributors of the drug and other necessary details.
The experimental group: all the intervention drugs for all subjects were Shugan Jieyu capsule (Batch Number: S200501), with the drug specification: 0.36 g/capsule, 2 capsules once, twice a day per os.The control group: the subjects’ intervention drugs were Shugan Jieyu capsule placebo (Batch Number: S200501-1), with the drug specification: 0.36 g/capsule, 2 capsules once, twice a day per os.
2.5.Outcomes
2.5.1.Primary outcome
The investigators compared differences between the two groups by calculating the rate of reduction and efficiency in HAMD-17 total scores from baseline after the 2nd week, the 4th week, and the6th week of the treatment.The rate of reduction (100%) = (total scores form baseline-total scores after treatment)/ total scores from baseline x 100%; the grading standards for obvious rate of efficiency are: for remission: HAMD total scores ≤ 7;for response rate: the total scores of HAMD reduced ≥ 50%, but the total scores> 7; for treatment inefficiency: the total scores of HAMD reduced < 50%; the obvious effective rate = (remission + response rate) / the number of people in each analysis set 100%.
2.6.Secondary outcomes
To record the scores of Hamilton Anxiety Scale (HAMA), PHQ-15, Insomnia Severity Index (ISI), and Treatment Emergent Symptom Scale after the 2nd week, the 4th week, and the 6th week of the treatment and then to compare the differences between groups.
2.7.Safety outcomes
The safety outcomes of the experimental group and the control group include the incidence of adverse events and serious adverse events, vital signs, physical examination, laboratory examination and electrocardiogram examination.
2.8.Statistical analysis
We applied the statistical analysis software SAS 9.4 (SAS Institute, Cary, NC, USA) for calculations.For the comparison between the groups, the investigators used a significant level of 0.05 for a two-sided test.P≤ 0.05 would be considered as statistically significant for the differences tested, and a 95% confidence shall be used in the credible interval.Continuous variables shall be summarized by descriptive statistics, including the number of cases, mean ± standard deviation.For measurement data (continuous variable-oriented test or Wilcoxon rank-sum test) shall be generally used.The investigators sum the descriptive statistics, including the number of subjects, percentages, and/or the number of events with categorical variables.If the number of cases is 0, then the percentage shall not be calculated.If no specification, the denominator for the percentage calculated shall be the total number of the subjects in the corresponding group where the analyzed subjects are included.The Cochran-Mantel-Haenszel test (CMH), c2test or Fisher's exact test shall be generally adopted in the comparison between the groups with categorical variables.
3.RESULTS
3.1.Baseline characteristics of the patients
Experimental group (n =100) and the control group (n =100) was given 1 (1.0%) subject without taking drug after the randomization respectively.2 (2.0%) subjects in the placebo group lost to follow-up.Finally, the statistical analysis included 196 patients, as shown in Figure 1.
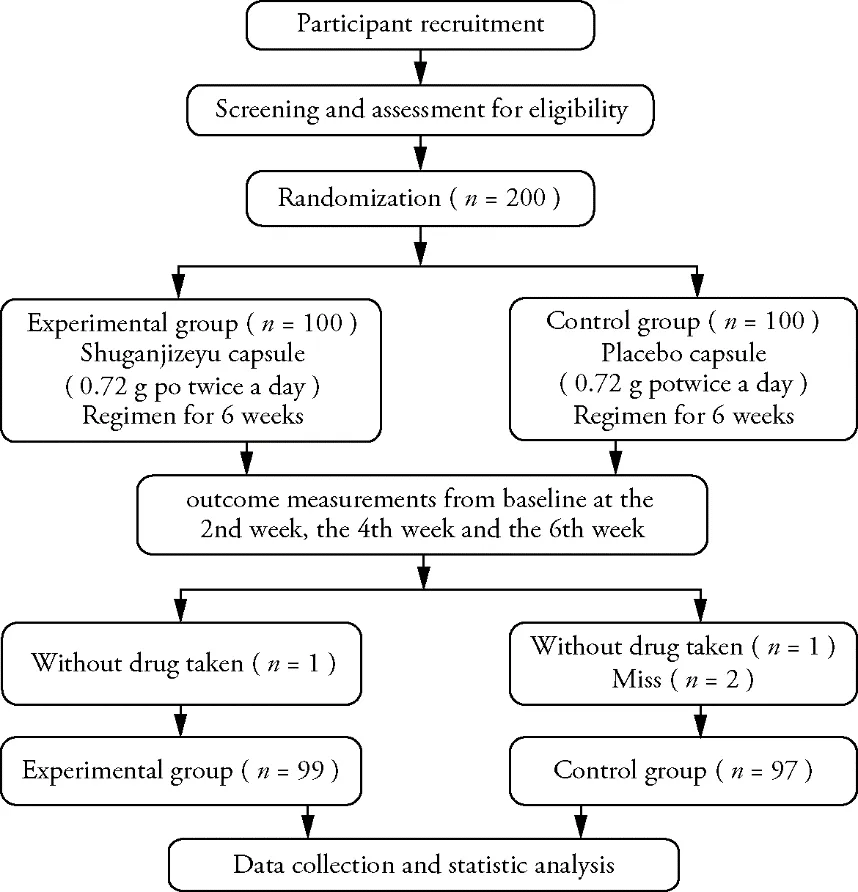
Figure 1 Study design and flow chart
The descriptive demography summary of this study is, as following (Table 1) with the patients’ age, body mass index (BMI) and gender, accompanied somatization symptoms, the time from onset to intervention evenly distributed.There is no statistical difference in the superiority trial between groups (P> 0.05), nor the usage of medication to improve emotion and reduce depression in the past 3 months.The summary results of the exposure and compliance of the drug shall be referred to in the table below (Table 1).The data of drug exposure and compliance of the subjects in this study are generally good.
3.2.Depression symptoms--HAMD-17 score
The results of changed in the 6th week of HAMD-17 total scores relative to the baseline with MMRM model analysis were as followed: from the comparison between the experimental group and control group (P< 0.001), the superiority trial between the groups was statistically significant, namely Shugan Jieyu capsule could significantly improve the patient's depressive symptoms with Table 2 for details.From the 2nd week, compared with the placebo group, the experimental group witnessed the decreased total scores of HAMD-17, while the linearly increased growth with follow-up examinations.
3.3.Depressive symptoms—effective rate of HAMD-17 score
The results of the obvious rate of efficiency in HAMD-17 scores are shown in Table 3 below.HAMD total scores ≤ 7; response rate: HAMD total scores reduction ≥ 50%, but total scores > 7; ineffective treatment: HAMD total scores reduction < 50%; the obvious rate of efficiency = (remission + response rate)/the number of people in the analysis set with the follow-up examinations in each group x 100%.The comparativePvalue between the groups in the 6th week is < 0.001, and the superiority test result is statistically significant.
3.4.Anxiety symptoms—HAMA total scores
The results of the changes of HAMA total scores from baseline in the 6th week analyzed by the MMRM model (P< 0.001), and the superiority test between the groups was statistically significant.It means, Shugan Jieyu capsule could significantly relieve the anxiety symptoms of patients, as shown in the Table 4 below.From the 2nd week, compared with the placebo group, the experimental group witnessed the faster decrease in total HAMA scores, while the linear growth with the increased follow-up examinations.
3.5.Physical symptoms--PHQ-15 score
The results of changes in the PHQ-15 score from baseline with the MMRM model in the 6th week showed that the comparisonP< 0.001, the superiority test between the two groups was statistically significant.That is, the Shugan Jieyu capsule could significantly relieve patients’ physical symptoms (Table 5).From the 2nd week, compared with the placebo group, the experimental group presented the faster decreased PHQ-15 total scores, while the linear growth with the increased follow-up examinations.

Table 1 Baseline characters [n (%)]

Table 2 Analysis on the 6-week changes in HAMD-17 total scores from baseline with MMRM model
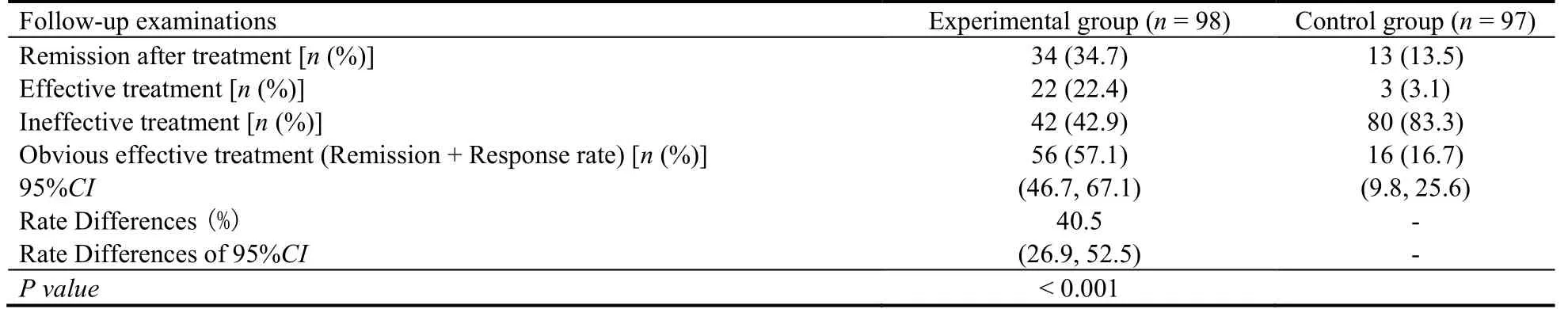
Table 3 Obvious rate of efficiency in HAMD-17 score on the 6th Week

Table 4 Analysis on 6-week changes in HAMA total scores from baseline with MMRM model

Table 5 Analysis on 6-week changes in PHQ-15 Score from baseline with MMRM model

Table 6 Analysis on 6-week changes in ISI Score from baseline with MMRM model
3.6.Sleep disorder symptoms-ISI score
The results of changes of the ISI score from baseline in the 6th week analyzed by MMRM model (P< 0.001), and the superiority test between the groups was statistically significant.That is, Shugan Jieyu capsule could significantly relieve the symptoms of sleep disorders in patients (Table 6).From the 2nd week, compared with the placebo group, the experimental group showed a faster decrease in total ISI scores, while the linear growth with more follow-up examinations.
3.7.Safety analysis
Safety Set will be used as the primary population for the safety analysis in this study.The safety analysis includes adverse events, laboratory tests, vital signs, physical examinations and electrocardiograms.During the treatment period, the adverse events among the 4 subjects in the treatment period of Shugan Jieyu capsule group were mild diarrhea, Abnormal liver function, cervical polyp, and stomach pain” respectively.All these abovementioned events except for cervical polyp were adversereactions.One adverse event in the placebo group was mild gastrointestinal discomfort, which was not judged as an adverse reaction.The laboratory examinations, vital signs, physical examinations, and electrocardiograms of the two groups showed no change from baseline or abnormality without clinical significance turned to those with clinical significance.The overall safety results were good in both groups (Table 7).
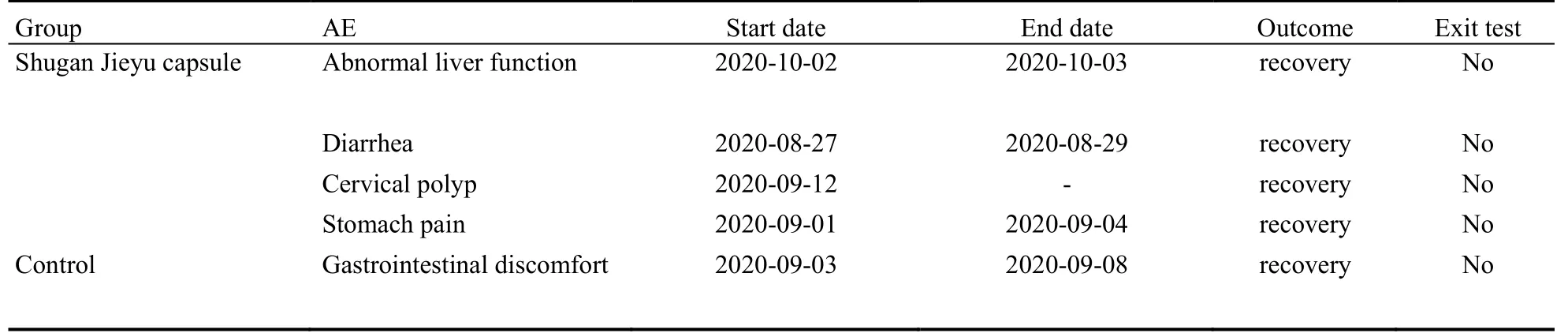
Table 7 Adverse events during treatment
4.DISCUSSION
As the sequelae are difficult to completely be eradicated after the patients’ virology turns negative when they enter COVID-19 convalescence.Bad emotions affect sleep disorders and aggravate the physical discomfort of the patients.A timely psychological adjustment will be conducive to the recovery of social order as well as the health of people's lives after the pandemic.
After 6 weeks of treatment, the scores depression and anxiety level of the patients were significantly improved, and the accompanying physical symptoms and insomnia were also significantly relieved.As Liet al6found in the study of Shugan Jieyu capsule in the treatment of patients with rheumatoid arthritis and depression, the Shugan Jieyu capsule has the same effect as paroxetine, which can reduce the depression and anxiety level of patients.Although western medicine can improve depression to some extent, they also have many adverse reactions or side effects, such as gastrointestinal reactions, dizziness, drowsiness, weight gain and so on.7It is suggested that given the curative effect is quite the same, TCM can be used in combination to better the curative effect.During the observation period of the curative effect of Shugan Jieyu capsule in the treatment of perimenopausal women with insomnia, we found that the symptoms of perimenopausal women, such as hot flashes, sweating, irritability and insomnia, can be well improved, as well as reducing the adverse drug reactions and relieving drug dependence concerns.
In this study, the Shugan Jieyu capsule is mainly composed of Guanyelianqiao (Herba Hyperici Perforati, GYLQ) and Ciwujia (Radix et Caulis Acanthopanacis Santicosi) (CWJ).GYJST is relatively cold constitution in nature and pungent in taste.It contributes to the liver.It has the effects of soothing the liver and reducing depression, helping calm down, reducing swelling and promoting lactation.CWJ is pungent, slightly bitter, heat constitution in nature, contributing to the spleen, kidney and heart.It has the functions of invigoratingQi, strengthening kidney and making people calm and sedative.Given these functions, it can be used for patients with spleen-kidney deficiency, physical weakness, loss of appetite, sores or pains in the waist and knee, insomnia and dreaminess.Modern studies have shown that GYLQ can activate central nervous systems, increase the concentration of neurotransmitters in the synaptic cleft, decrease the density of adrenergic receptors in the presynaptic membrane, regulate the thalamus-pituitary-adrenal axis, and help antidepression.8CWJ contains non-aromatic unsaturated organic acids and a variety of trace minerals.It has sedative and anti-fatigue effects which can help establish normal sleep cycles, and have greater advantages in the treatment of sleep disorders.The treatment effect is the same as that of the western medicine Fluoxetine, but with fewer adverse drug reactions and more safety.
The outbreak of the pandemic not only deprives us of lives and property, but also causes severe psychological trauma to humanity.With the extreme infection conditions, most COVID-19 related patients need longterm isolation treatment.Negative emotions are thus easily generated to a certain degree as they suddenly have to cut their connections to the outside world, facing physical burdens independently in relatively isolated space.9The treatment of COVID-19 should not only focus on physical diseases, but also psychological intervention.“Body mind” is an important principle in the holistic view of TCM to improving liver and spleen.Applying Shugan Jieyu capsule will promptly intervene psychological stress accumulated in the context of the pandemic.10,11
There may be some limitations in this study.COVID-19 is a newly emerging infectious disease in the world, and there is no data from previous studies and no therapeutic effect value of intervention measures for reference.Meanwhile, the sample size estimated lacks data support from previous studies, the source of sample is limited and the size of sample is relatively small.Shugan Jieyu capsule has shown obvious curative effect since 2 weeks, but according to the characteristics and treatment scheme of depression, it can avoid the recurrence and resurgence of symptoms because it is taken persistently.12Considering the special background of the disease at that time and referring to the previous data of Shugan Jieyu capsule, the research scheme of this project is only designed for the treatment period of 6 weeks, so the follow-up after drug withdrawal is not carried out.In order to ensure the scientific accuracy of this study as much as possible,the opinions of statistical experts were used as the main reference for sample size consideration,and strict quality control was carried out on the research process and results,including strengthening the training of investigators,checking the integrity,logic and extreme values of data,and using various statistical methods to control the possible deviation as little as possible.
In conclusion,the findings showed that the potential effect of Shugan Jieyu capsule on COVID-19 convalescence patients with depression,anxiety and insomnia suggests the role of TCM in mental health of COVID-19 patients during convalescence.13The positive effect should be further investigated in trials with a greater number of patient groups and longer follow-up duration.
 Journal of Traditional Chinese Medicine2022年5期
Journal of Traditional Chinese Medicine2022年5期
- Journal of Traditional Chinese Medicine的其它文章
- Application value of Qisexingtai hand diagnostic method in diagnosis of coronary artery disease
- An infrared thermographic analysis of the sensitization acupoints of women with primary dysmenorrhea
- Preliminary single-arm study of brain effects during transcutaneous auricular vagus nerve stimulation treatment of recurrent depression by resting-state functional magnetic resonance imaging
- Effect of treatment with Fufang Huangqi decoction (復(fù)方黃杞湯劑)on dose reductions and discontinuation of pyridostigmine bromide tablets,prednisone,and tacrolimus in patients with type I or II myasthenia gravis
- Wenshen Jianpi recipe (溫腎健脾方) induced immune reconstruction and redistribution of natural killer cell subsets in immunological nonresponders of human immunodeficiency virus/acquired immune deficiency syndrome: a randomized controlled trial
- Network pharmacology and molecular docking study on the effect of Kaempferol in treatment of metabolic associated fatty liver disease
