Effectiveness of ChAdOx1 nCoV-19 coronavius vaccine in preventing severe disease and mortality during the second wave of pandemic: A case-case analysis from a tertiary care center in South India
Priyanka Rajmohan, Unnikrishnan Uttumadathil Gopinathan, Nada Parvin Ashraf Saudha,Maria Jose, Lucy Raphael, Joe Thomas
1Department of Community Medicine, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India
2Department of Pharmacology, Jubilee Mission Medical College and Research Institute, Thrissur, Kerala, India
ABSTRACT Objective: To determine the real-world effectiveness of the ChAdOx1 nCoV-19 coronavirus vaccine in preventing severe disease and mortality due to COVID-19.Methods: A case-case design was used to estimate the effect of the ChAdOx1 nCoV-19 coronavirus vaccine on severe SARS-CoV-2 outcomes in individuals aged 40 years and above. Cases (n=200)were COVID-19 patients admitted to intensive care unit (ICU) or who died. Controls (n=223) were those with mild COVID-19, fit for home isolation. The logistic regression model was used to estimate adjusted vaccine effectiveness for full vaccination (two doses ≥14 d)and partial vaccination status (one dose ≥14 d or two doses <14 d).Results: The proportion of fully vaccinated individuals was significantly lower among cases (12, 6.0%) compared to controls(30, 13.5%). The adjusted effectiveness of a full dose of ChAdOx1 nCoV-19 coronavirus vaccine in preventing ICU admission or death was 81.9% (95% CI: 61.3%-91.6%, P=0.001). Subgroup analysis restricted to age group, sex, and comorbidities found that ChAdOx1 nCoV-19 coronavirus vaccine had a significant positive effect in all subgroups and categories.Conclusion: COVID-19 vaccination reduces ICU admissions or death. Therefore, increased vaccine uptake may reduce the severity of the pandemic, more so in the elderly and those with comorbidities.
KEYWORDS: Vaccine effectiveness; ChAdOx1 nCoV-19 coronavirus vaccine; Mortality; Case-case analysis; Pandemic
1. Introduction
Since the emergence of the ongoing COVID-19 pandemic which started in December 2019, there have been 606 459 140 confirmed cases including 6 495 110 deaths worldwide, as of 13th September 2022[1]. India ranks second in the world in cumulative total cases of COVID-19 with 44 504 949 cases and 528 185 deaths[1]. This evolving situation is further fuelled by the emergence of new variants of SARS-CoV-2 with increased infectivity in various parts of the world. In India, the variant of concern, B.1617, a double mutant strain, was first reported from Maharashtra in January 2021,causing India to experience a second wave of the pandemic in the middle of March 2021[2].
Currently, ten COVID-19 vaccines have been approved for human use by the World Health Organization[3]. The Government of India has currently authorized twelve vaccines against SARS-CoV-2,including the Covishield [ChAdOx1 nCoV-19 coronavirus vaccine(recombinant)] by AstraZeneca-Oxford University Serum Institute of India and Covaxin (BBV152 vaccine) by Bharat Biotech[4].
Randomized control trials have demonstrated the efficacy of AstraZeneca-Oxford vaccine to be 70.4% in preventing symptomatic disease and 100% in preventing severe disease and death[5]. Results of a phase-2 clinical trial of India’s indigenous vaccine BBV152(Covaxin) also showed improved reactogenicity and enhanced humoral and cell-mediated responses[6].
There are emerging concerns regarding the chance of getting infection after vaccination, more so in the backdrop of emerging variants of SARS-CoV-2[7]. However, vaccination is considered the best defense against SARS-CoV-2 infection because, months after natural infection or vaccination, even if neutralizing antibody levels are insufficient to block viral entry and early replication, it is possible that these low levels of neutralizing antibodies can act to slow viral growth rates and reduce the severity of infection[8].This calls for real-world studies to determine the causal effect of COVID-19 vaccines on post-infection outcomes such as severe disease and mortality and to find the factors associated with vaccine effectiveness. We used a case-case study design, which uses the positive infection status to estimate the net effect of the vaccination on the post-infection endpoint[9], and is considered an appropriate design for this study.
The present study aimed to determine the real-world effectiveness of ChAdOx1 nCoV-19 coronavirus vaccine in preventing severe outcomes,e.g.intensive care unit (ICU) admission and death due to COVID-19.
2. Patients and methods
2.1. Study setting
We conducted a hospital-based, observational case-case epidemiological study at a tertiary care hospital in central Kerala from 1st June to 31st August 2021. The study population was patients aged 40 years and older and SARS-CoV-2 virus-positive.
2.2. Ethical approval
The Institutional Ethics Committee approved the study with the approval number: 76/21/IEC/JMMC&RI.
2.3. Inclusion and exclusion criteria
Patients aged 40 years and older and SARS-CoV-2 viruspositive were included. Patients with symptoms suggestive of COVID-19 were first tested for SARS-CoV-2 virus infectionviareverse transcription polymerase chain reaction (RT-PCR) of the nasopharyngeal or throat swab taken at the fever clinic of our hospital. Based on the clinical severity of COVID-19, the study participants were assigned to the case or control group.
The patients in the case group were hospitalized within 14 d of the positive test, either required ICU care or had died due to the disease.Patients in the control group were tested positive for SARS-COV-2,categorized as category A (mild SARS-CoV-2 infection)[10], and eligible for home isolation and management.
Patients with a previous history of laboratory-confirmed SARSCoV-2 infection and those previously vaccinated with any COVID-19 vaccine other than ChAdOx1 nCoV-19 were excluded from our study.
2.4. Data collection and definition
From the hospital information system database, for all cases that tested positive for SARS-CoV-2 virus in our hospital during the study period (1st June to 31st August 2021), the socio-demographic details, presence of comorbidities, date of onset of symptoms, date of positive test, clinical severity of COVID-19 at hospital admission,and requirement of ICU admission or record of death were collected using a structured case record form.
Information regarding the vaccination status, including type of vaccine and date of first and second doses, was collected from the short message service of the government vaccination portal or the CO-WIN portal, a web portal launched by the India government providing SARS-CoV-2 vaccination-related services to the public,such as vaccination registration and vaccine certificate download.The interval between the first and second doses of vaccine issued by the state government was 84 days at the time of our study.
Participants were defined as unvaccinated if they had not received any dose of SARS-CoV-2 vaccine and as fully vaccinated if at least 14 d had passed since receiving the second dose of SARS-CoV-2 vaccine. Participants were defined as partially vaccinated if 1) they have received two doses of SARS-CoV-2 vaccine but the interval between the second dose and being tested positive for COVID-19 was less than 14 d, or 2) they have received only one dose of SARSCoV-2 vaccine and the interval between vaccination and COVID-19 positive testing was more than 14 d.
2.5. Primary outcomes
COVID-19-related hospitalization, severe or critical hospitalization(ICU admissions), and deaths were primary outcomes.
2.6. Statistical analysis
We estimated sample size based on a study by Lopez Bernalet al. in which the proportion of severe disease among vaccinated and unvaccinated was 7.14% and 15.35% respectively[11]. At 95%confidence level and 5% precision level, we estimated the minimum sample size to be 200 in each group.
Descriptive statistics were used to summarize demographic and clinical data. Qualitative data were presented as frequency and percentage and analyzed by Chi test. Logistic regression model was used to estimate the odds ratio with 95%CIfor severe disease and mortality due to SARS-CoV-2 adjusted for age, sex, and comorbidities. Vaccine effectiveness estimates were calculated as(1-adjustedOR)×100. Vaccine effectiveness estimates were also calculated with the same method for the two groups of partially vaccinated participants.
TheP<0.05 was considered statistically significant for all tests.Statistical analyses were performed using commercially available software Statistical Package for the Social Sciences version 25.0 software (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Vaccination status and demographic characteristics
A total of 423 SARS-CoV-2 positive patients were recruited from our hospital from 1st June to 31st August 2021, which included 200 cases (ICU/death) and 223 controls (non-ICU/death) (Figure 1).
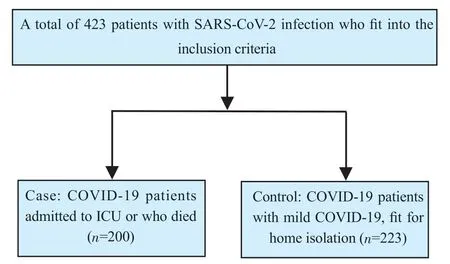
Figure 1. The study flowchart.
Those requiring ICU admission or died due to SARS-CoV-2 were elderly (65.0%vs.44.4%;P<0.001), male-dominated (54.5%vs.41.3%;P=0.006) and had significantly higher proportion of various comorbidities (78.0%vs.56.1%;P<0.001) (Table 1).The proportion of unvaccinated individuals was higher among cases(146, 73.0%) as compared to controls (109, 48.9%). The proportion of fully vaccinated individuals was significantly lower among cases(12, 6.0%) as compared to controls (30, 13.5%) (P<0.001) (Table 1).
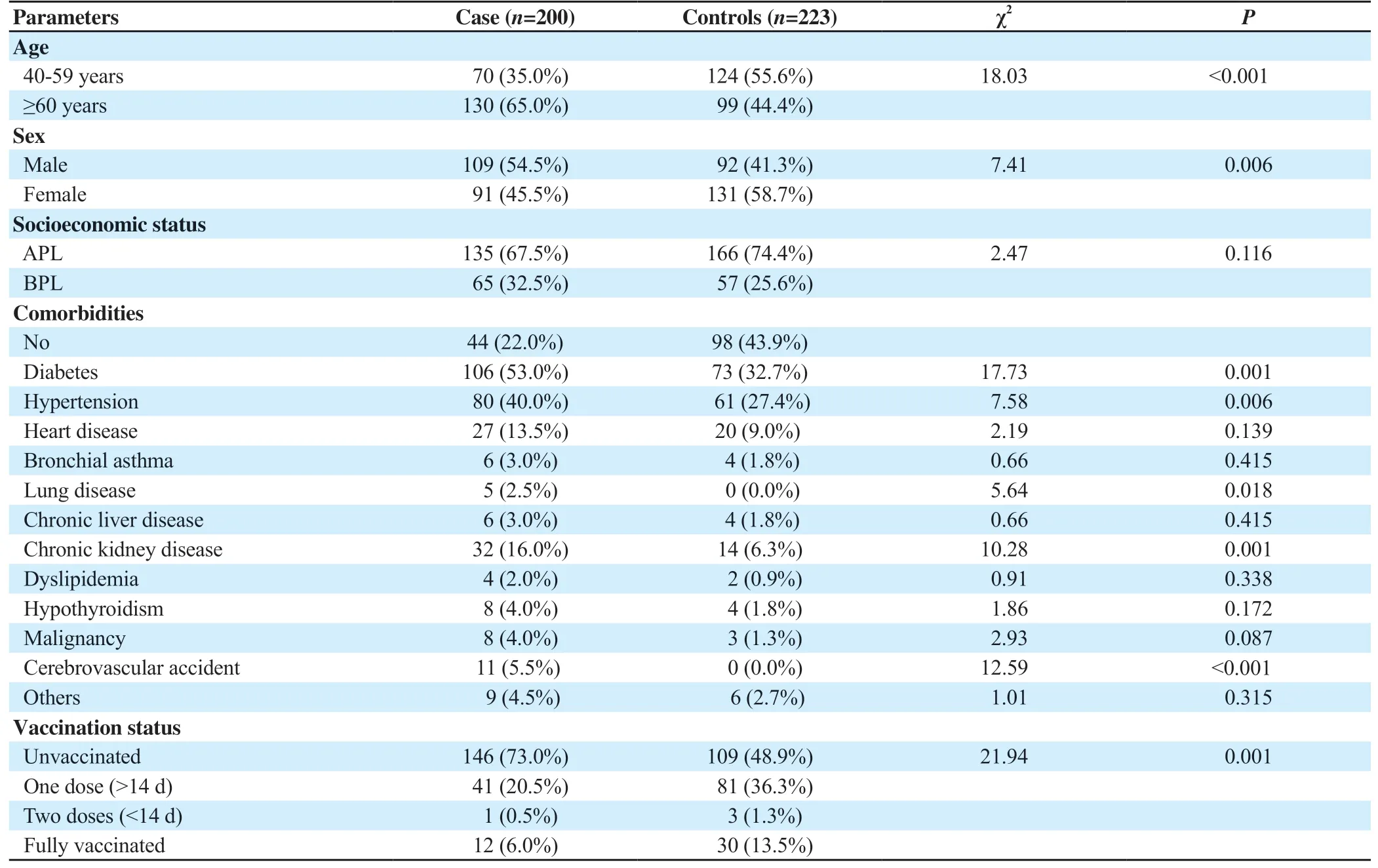
Table 1. Socio-demographic, clinical profile and vaccination status of cases and controls (n, %).
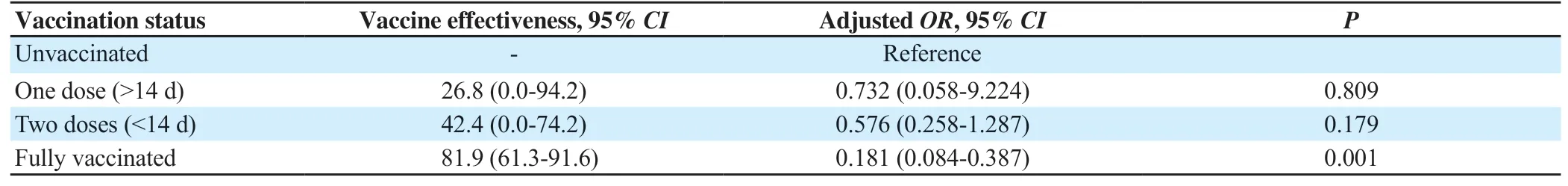
Table 2. Effectiveness of ChAdOx1 nCoV-19 coronavirus vaccine against severe SARS-CoV-2 infection (%).
3.2. Effectiveness of ChAdOx1 nCoV-19 coronavirus vaccine against severe disease or death by dose and duration since vaccination
The adjusted effectiveness of partial vaccination with a single dose (>14 d) of ChAdOx1 nCoV-19 coronavirus vaccine was 26.8% (95%CI: 0.0%-94.2%) in preventing ICU admission or death. Higher vaccine effectiveness, 42.4% (95%CI: 0.0%-74.2%)was noted in partial vaccination of two doses (<14 d) of the same vaccine, however, this was not statistically significant. The adjusted effectiveness of fully vaccinated with ChAdOx1 nCoV-19 (2 doses>14 d) in preventing ICU admission or death was 81.9% (95%CI:61.3%-91.6%) and was found to be significantly higher than the effectiveness of partial vaccination(P=0.001) (Table 2).
We performed subgroup analysis restricted to age group, sex, and comorbidities and found that vaccination with ChAdOx1 nCoV-19 coronavirus vaccine had a significant positive effect in all subgroups and categories, with higher vaccine effectiveness reported among the elderly (71.8%, 95%CI: 50.9%-83.8%), females (71.7%, 95%CI: 47.4%-84.6%) and those with comorbidities (77.8%, 95%CI:73.1%-86.6%) (Table 3).
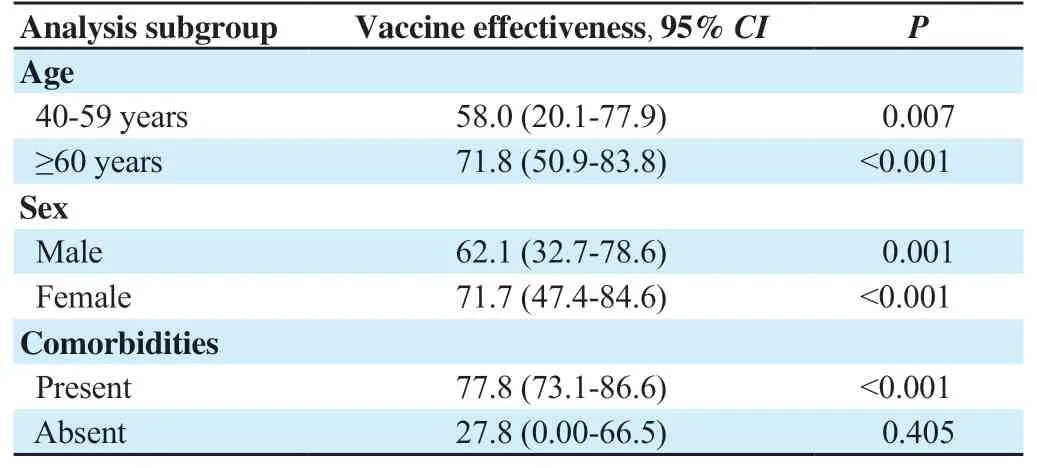
Table 3. Effect of ChAdOx1 nCoV-19 coronavirus vaccination in reducing severity among subgroups (%).
4. Discussion
The present study showed the effectiveness of full immunization with ChAdOx1 nCoV-19 coronavirus vaccine in reducing severe outcomes such as ICU admission or death. Participants who required ICU admission or died were more likely to be unvaccinated compared to those who did not require ICU or did not die. An observational study by Haaset al. used the National Surveillance data on effectiveness of mRNA BNT 162b2 in Israel, also reported a similar finding[12]. The proportion of unvaccinated individuals hospitalized due to severe SARS-CoV-2 infection was higher than that of vaccinated individuals (71.4%vs. 8.1%). In addition, the proportion of unvaccinated individuals who died due to COVID-19 was also higher than their vaccinated counterparts (64.2%vs.12.4%).
The vaccine effectiveness estimated in our study (81.9%) was comparable to the efficacy estimates in an exploratory analysis of a clinical trial by Emaryet al., with a reported clinical vaccine efficacy against symptomatic Nucleic Acid Amplification Test positive infection of 81.5% (95%CI: 67.9%-89.4%) for non-B.1.1.7 lineage,and 70.4% (95%CI: 43.6%-84.5%) for B.1.1.7 lineage[13].
In the phase-3 study conducted by Falseyet al. on the safety and efficacy of ChAdOx1 nCoV-19 coronavirus vaccine, the overall estimated vaccine efficacy was 74.0% (95%CI: 65.3%-80.5%,P<0.001)[14], which was lower than the effectiveness estimated in our study. This could probably be because of that our study compared the vaccine effectiveness against severe outcomes, using a case-case design, in a real-world scenario. It is understood now that,in the backdrop of emerging variants of SARS-CoV-2, COVID-19 vaccines might not prevent infection but it has effectiveness in preventing severe outcomes. Most clinical trials compared the infection end-point between the vaccinated and unvaccinated group,whereas, in our study, we used the positive infection status to estimate the vaccine effectiveness on post-infection endpoints such as severe disease or death.
Bobdeyet al. in a prospective study on effectiveness of ChAdOx1 nCOV-19 coronavirus vaccine reported the overall vaccine effectiveness to be 88.6% (95%CI: 81.55%-92.37%) in completely vaccinated and 44.1% (95%CI: 4.55%-67.3%) in partially vaccinated individuals[15], which was similar to the findings from our study. A study by Bhatnagaret al. reported the effectiveness of full vaccination with AZD1222/Covishield to be 80% (95%CI:73%-86%)[16], which is also concordant with our estimate.
A study on AZD1222 vaccine effectiveness in prevention of COVID-19-related deaths found that the vaccine effectiveness was 98.53% (95%CI: 0.00%-99.99%)[17], which is well above our estimate. This might be due to the inclusion of a younger age group [mean age (27.6±6.2) years] and individuals with minimum comorbidities in the above study, whereas our study included patients aged ≥40 years and also those with comorbidities.
A study done in Qatar on the effectiveness of mRNA-1273 COVID-19 vaccine against severe SARS-CoV-2 reported the effectiveness to be 81.6% (95%CI: 71.0%-88.8%) ≥14 d after the first dose but before receiving the second dose, and was 95.7% (95%CI: 73.4%-99.9%) 14 d or more after the second dose[18], which is higher than our estimates.
When Susan came back into the living room, there was a strange mist in her eyes. She handed Jessica back to Dad before taking my hand and quietly leading me into the room.
Haaset al. reported a higher estimates of mRNA BNT 162b2 vaccine effectiveness against all the SARS-CoV-2 outcomes such as hospitalization 97.2% (95%CI: 96.8%-97.5%), critical hospitalization 97.5% (95%CI: 97.1%-97.8%) and death 96.7%(95%CI: 96.0%-97.3%)[12]. However, Liuet al. in a retrospective analysis of COVID-19 mRNA vaccine breakthrough infections found a significant reduction in death among individuals infected with COVID-19 (adjustedHR=0.20, 95%CI: 0.08-0.49)[19], which was similar to our finding.
On subgroup analysis, we found significant differences between vaccine effectiveness against severe outcomes in the older age group ≥60 years compared to 40-59 years. The vaccine effectiveness against death or critical hospitalization was also significantly different between males and females with females reporting higher effectiveness as compared to males. Haaset al. reported a similar finding of adjusted vaccine effectiveness against all COVID-19 outcomes to be higher among those aged ≥75 years[12]. Our finding indicated that individuals without comorbidities had lower vaccine effectiveness 27.8% (95%CI: 0.0%-66.5%) is not valid enough to conclude since it could be a biased estimate that a higher number of participants with comorbidities were included in our study, and vaccination uptake could be higher in them as they were prioritized for vaccination.
The reports from various follow-up studies across the world reveal a reduction in the antibody titers within months after COVID-19 vaccination[20,21], which indicates a waning humoral immunity, with the impending risk of SARS-CoV-2 infection among vaccinated individuals, more so in the current emergence of mutant strains of the virus. However, severe outcomes of infection could still be prevented by the administration of COVID-19 vaccines, since protection against severe COVID-19 is thought to be mediated through a cell-mediated immune response.
Our study included patients aged above 40 years since they were prioritized for vaccination. More numbers patients with comorbidities were included in our study, which could be a limitation against generalization of the findings. There could be the influence of confounders associated with vaccination and severe SARS-CoV-2,though we have included the known variables in the multivariate logistic regression model.
Since our study used a case-case analysis of patients diagnosed to have laboratory-confirmed SARS-CoV-2 infection, we could attribute the vaccine effectiveness to the ability of ChAdOx1 nCoV-19 coronavirus vaccine to reduce critical hospitalization and death after SARS-CoV-2 infection had occurred.
We utilized the post-infection outcomes to estimate ChAdOx1 nCoV-19 coronavirus vaccine effectiveness during the second wave of the pandemic. We found that the majority of critically hospitalized or dead COVID-19 patients were above 60 years old.The COVID-19 vaccination reduced ICU admissions or deaths.Therefore, increased vaccine uptake may reduce severity of the pandemic, more so in the elderly and those with comorbidities.
Conflict of interest statement
The authors report no conflict of interest.
Funding
This study received no extramural funding.
Authors'contributions
P.R., U.U.G., N.P.A.S., M.J., L.R., and J.T. all contributed to concept and design development, defined intellectual, conducted literature search and clinical study, acquired and analyzed the data,prepared, edited, and reviewed the manuscript.
 Journal of Acute Disease2022年5期
Journal of Acute Disease2022年5期
- Journal of Acute Disease的其它文章
- Congestive heart failure masquerading as acute abdomen: A case report
- Symmetrical peripheral gangrene triggered by Escherichia coli sepsis
- Successful management of depression skull fracture in a boy with dog bite injury: A case report
- COVID-19 presentation as acute pancreatitis: A case report
- Effect of pH, lactate, electrolyte, and strong ion difference variability on prediction of intensive care unit mortality: A retrospective study
- Comparative efficacy of ketamine, lidocaine, acetaminophen, and dexmedetomidine combined with morphine patient-controlled analgesia in treating opium-addicted patients undergoing tibia fracture surgery: A randomized clinical trial
