Oxytocin improves testicular blood flow without enhancing the steroidogenic activity in Baladi goats
Mohamed G.Hedia ,Amr S.El-Shalofy
Department of Theriogenology,Faculty of Veterinary Medicine,Cairo University,Giza 12211,Egypt
ABSTRACT Objective:To investigate the effects of a single oxytocin injection on plasma steroid concentrations,testicular blood flow measures(resistive and pulsatility indexes),and testicular echogenicity in Baladi goats.Methods:Twelve Baladi goat bucks were randomly allocated into 2 groups and received an intravenous injection of either 0.7 IU/kg oxytocin or normal saline 0.9%.Venous blood samples were collected,and testicular blood flow Doppler parameters (i.e.,peak systolic velocity,end-diastolic velocity,time average maximum velocity,resistive index,and pulsatility index) were assessed for supratesticular arteries in the left and right testes immediately before(0),and at 5,30,60,and 120 min after injection.Results:Plasma concentrations of testosterone significantly decreased in the oxytocin group at 60 min post-treatment compared to the control group,whereas plasma concentrations of estradiol-17β were not affected (P>0.05).Both resistive index and pulsatility index declined in the oxytocin group at 60 min posttreatment compared to the control group (P<0.05).Testicular pixel intensity increased at 30 min post-treatment in the oxytocin group compared to the control group (P<0.05).Conclusions:Oxytocin treatment significantly increases testicular blood flow and decreases plasma testosterone concentrations in male Baladi goats.
KEYWORDS: Bucks;Blood flow;Doppler;Estradiol-17β;Goat;Oxytocin;Testicular artery;Testosterone
1.Introduction
The testis is a highly condensed genital organ where the extremely coiled seminiferous tubules occupy about 70%-80% of the testicular mass with minimal oxygen tension and are firmly covered with a thick connective tissue wall;it is considered the most important organ in the male reproductive system,which carries out vital metabolic activities including both spermatogenesis and steroidogenesis.Therefore,the maintenance of continuous and constant oxygenated vascular supply through the testicular artery is crucial[1].Several studies have found that increasing testicular vascular perfusion is associated with an increase in plasma and seminal testosterone concentrations as well as sperm production in bulls[2,3],rams[4,5],and stallions[6,7].
There are different methodologies to evaluate the breeding soundness of male farm animals.Practitioners used to assess reproductive capacity through general physical examination,measuring of the scrotal circumference,and semen analysis[8].Recently,pulsed-wave Doppler application offers a valuable noninvasive technique to investigate the reproductive efficiency in farm animalsviameasuring the testicular hemodynamics within the testicular artery[9,10].Peak systolic velocity (PSV),end-diastolic velocity (EDV),resistive index (RI),and pulsatility index (PI)are the four basic parameters of testicular vascular perfusion.PSV and EDV represent the arterial blood velocities through the spectral pattern of the cardiac cycle[11].Both RI and PI are the main measures Doppler indicators for testicular blood perfusion in routine andrological examinations[12].Moreover,the computerassisted testicular image analysis is a promising method to predict the reproductive capacity in farm animals,where different studies demonstrated a strong association between testicular pixel intensity and testosterone production and testes histomorphometry[13,14].
Oxytocin is a nonapeptide (9 amino acids) hormone.Hypothalamus is the main source of oxytocin,but its local production had been demonstrated in the testes,epididymis,and prostate[15].Oxytocin plays a vital role in the emission and ejaculation of sperm cells,as it exerts a direct contractile effect on myoid cells of seminiferous tubules,epididymal duct,and vas deferens,which is mainly regulated by endothelin[16].Oxytocin receptors localize on Leydig and Sertoli cells,which might reflect their potential regulatory effect on steroidogenesis and the rate of testosterone production[17].The inhibitory effect of oxytocin on testosterone synthesis and the possible negative association between oxytocin and testosterone concentrations have been previously investigated[18].However,Bozkurtet al[19] reported that the administration of oxytocin raised the concentration of testosterone in the seminal plasma of rams.In addition,a strong association was reported between the administration of oxytocin and semen quality characteristics in goat bucks where the exogenous oxytocin shortened the time for bucks to ejaculate,which increased animal wellbeing during transrectal massaging of accessory genital glands[20].
To the best of our knowledge,the available data to date concerning the testicular response to exogenous oxytocin have not been previously studied in Baladi goats.We hypothesized that the intravenous administration of oxytocin improves the testicular blood flow within the supratesticular artery and the testicular endocrine function in Baladi goats.Therefore,the objectives of the current study were to investigate the effects of oxytocin administration on the testicular hemodynamics as well as systemic concentrations of testosterone and estradiol-17β in bucks and to check the possible association between testicular hormones and Doppler parameters.
2.Materials and methods
2.1.Animals and management
This study was carried out on male Baladi goats (Capra hircus).This is the most common breed reared in Egypt and is a nonseasonal breeder under natural ambient temperature and daylight.This breed is very important owing to its vital role in meat and milk production in Egypt.The Baladi goat reaches the age of puberty at 4 to 6 months[21].This study was conducted in January 2021 at Theriogenology Department,Faculty of Veterinary Medicine,Cairo University (30° 0' 47.0016'' N and 31° 12' 31.8708'' E).
The present study was conducted on twelve mature male Baladi goats,aged 2.5-3.0 years old and weighing (25.18±0.58) kg.All animals were healthy and had normal fertility.They were housed under natural daylight and ambient temperature.Each buck received a daily maintenance ration composed of 800 g of hay and had free access to fresh water supplemented with mineralized licks.They were healthy and free from external and internal parasites.All animals were proved to be free from any reproductive problems and selected after thorough andrological,physical,and cardiovascular examinations.
2.2.Grouping and oxytocin administration
All bucks were randomly divided into two equal groups,with 6 bucks in each group.The treatment group received a single intravenous administration of oxytocin (0.7 IU/kg;Syntocinon?;Mylan products,LTD.,Hertfordshire,United Kingdom) in the jugular vein[22],while the control group was intravenously injected with normal physiological saline 0.9% (2 mL).
2.3.Experimental design
All bucks were subjected to blood sampling and spectral Doppler sonographic scanning of the supratesticular artery just before (0 min) and 5,30,60,and 120 min after intravenous administration of oxytocin and saline 0.9%[22].The venous blood sampling and pulsed-wave Doppler scanning were performed for the oxytocin and control groups,respectively.
2.3.1.Blood collection and hormonal assay
In the early morning (08:00),just before each testicular Doppler scanning,a jugular blood sample (5 mL per buck) was collected into an evacuated tube containing ethylene diamine tetraacetic acid(EDTA).The blood samples were obtained according to a sequential manner starting from the bucks in the control group,which was followed by sampling from the bucks in the oxytocin group.For obtaining the plasma,samples were centrifuged at 1 200 ×gfor 15 min.After that,the plasma was stored at -20 ℃ for further laboratory analysis.
Available enzyme-linked immunosorbent assay kits plasma testosterone (BioCheck,Inc.Foster City,USA) and plasma estradiol-17β (BIOS,Microwell Diagnostic Systems,South San Francisco,USA) were utilized.The intra-and inter-assay coefficients of variation were 3.3% and 4.8% for testosterone and 3.8% and 5.6%for estradiol-17β,respectively.For testosterone and estradiol-17β,sensitivity was 0.05 ng/mL and 20.00 pg/mL,respectively.
2.3.2.Testicular ultrasonographic examination
All sonographic imaging was carried out by the same investigator just after the blood sampling.The ultrasonographic scanning was performed using an ultrasound scanner (SonoScape E1 vet,SonoScape Medical Corp.,Guangdong,China) equipped with a linear array probe (7-14 MHz).
All bucks were gently controlled without sedation.To avoid any imaging artifacts,the fine hair at both sides of the scrotum was shaved well.Before ultrasonographic scanning,the probe was covered with a copious amount of gel to avoid any air bubbles and enhance the image quality.
All the ultrasound machine imaging settings (brightness,frequency,depth,and contrast) were fixed and equalized for all scanning.For pulsed-wave Doppler analysis,the angle of insonation between the Doppler beam and the longitudinal axis of the supratesticular artery never was over 60 with the application of a high pass filter set at 50 Hz.The Doppler gate was kept constant at 0.5 mm.The probe was placed longitudinally on the lateral wall of the scrotum and gently directed until the appearance of the vascular matrix at the proximal testicular pole (pampiniform plexus),which contains the testicular artery.After the appearance of the cardiac pattern of the testicular artery,the studied variables were PSV,EDV,maximum velocity (TAmax),RI [RI=(PSV-EDV)/PSV],and PI [PI=(PSVEDV)/mean velocity].Each variable was calculated as an average of two to four measurements recorded in varied areas of interest within the supratesticular artery[18,23].
2.3.3.Testicular image analysis
The pre-saved grey-scale images of the testicular parenchyma were submitted to computer-based image testing software (Image J).Testicular echogenicity (pixel intensity) was computed at several(2-4) selected areas within the testicular parenchyma[24] by drawing a rectangle of fixed size (macro) 0.5 cm to 1.0 cm deep into the testicular tissue away from mediastinum testis where it appeared homogenous[13].
2.4.Statistical analysis
The Statistical Package for the Social Sciences (SPSS Inc.,version 16.0,Chicago,IL.USA) was used for the statistical interpretation of data.Temporal variations in pulsed-wave Doppler values (PSV,EDV,RI,and PI),as well as plasma hormone concentrations (testosterone and estradiol-17β),were shown as mean±standard deviation(mean±SD) of the number of measures assessed in each parameter.In the control and oxytocin treatment groups,the statistical differences in hormonal changes,testicular Doppler measurements,and testicular pixel intensity were analyzed by a general linear model for repeated measures.An independentt-test was performed to compare the two groups (the control group and the oxytocin treatment group) at each time point (0,5,30,60,and 120 min).Regarding the oxytocin treatment group,the associations between the concentrations of steroid hormones in plasma (testosterone and estradiol-17β) and between different pulsed-wave Doppler measures were checked by Pearson’s correlation coefficient.P-values less than 0.05 were considered significant.
There were no marked changes between the right and left testes concerning testicular blood flow variables (PSV,EDV,TAmax,RI,and PI) and testicular echogenicity values (pixel intensity),and therefore the means of the right and left testes were used.
2.5.Ethics statement
This study was approved by the Experimental Animal and Ethical Applications Committee of Cairo University (Vet CU28042021264).
3.Results
3.1.Effects of oxytocin administration on the plasma concentrations of testosterone and estradiol-17β
In the oxytocin-injected group,a significant variation in the plasma concentrations of testosterone was detected using repeated measure analysis over time (P<0.05).Testosterone concentration increased significantly 5 min after oxytocin administration and then decreased gradually between 30 to 60 min to reach about 65% of the baseline value 120 min post-injection of oxytocin (P<0.05).In contrast,blood testosterone was not changed over time in the control group (P>0.05)(Table 1).
At 60 min of post-injection of oxytocin,plasma testosterone concentrations were significantly lower in the oxytocin-injected group compared to the control group (P<0.05) (Figure 1A).There was no significant change in the concentrations of plasma estradiol-17β in the control group and the oxytocin-injected group,or between the two groups at any time during the study (P>0.05) (Table 1;Figure 1B).
3.2.Effects of oxytocin administration on testicular hemodynamics and echogenicity
In both groups,values of PSV showed significant changes (Table 1).A monophasic drop (21.93±6.36) was observed at 5 min postinjection in the control group,while biphasic downturns were recorded at 30 min (16.74±2.66) and 120 min (17.07±3.00) in the oxytocin-injected group.In addition,there was a remarkable decline in their values in the oxytocin-injected group at 5-,30-,60-,and 120-min post-treatment compared to the control group (P<0.05) (Figure 2A).As depicted in Table 1,EDV did not significantly differ in the oxytocin-injected group or the control group over time (P>0.05).However,there were marked changes in their values between the two groups,as they decreased significantly at 30 and 120 min in the oxytocin-injected group (7.45±2.00 and 7.73±1.62,respectively)compared to the control group (14.32±4.31 and 12.69±5.77,respectively) (P<0.05) (Figure 2B).Although the values of TAmax were not significantly changed over time in the control group (Table 1),clear differences were observed in the oxytocin-injected group aswell as between the two groups at 5,30,and 120 min after treatment(P<0.05) (Figure 2C).
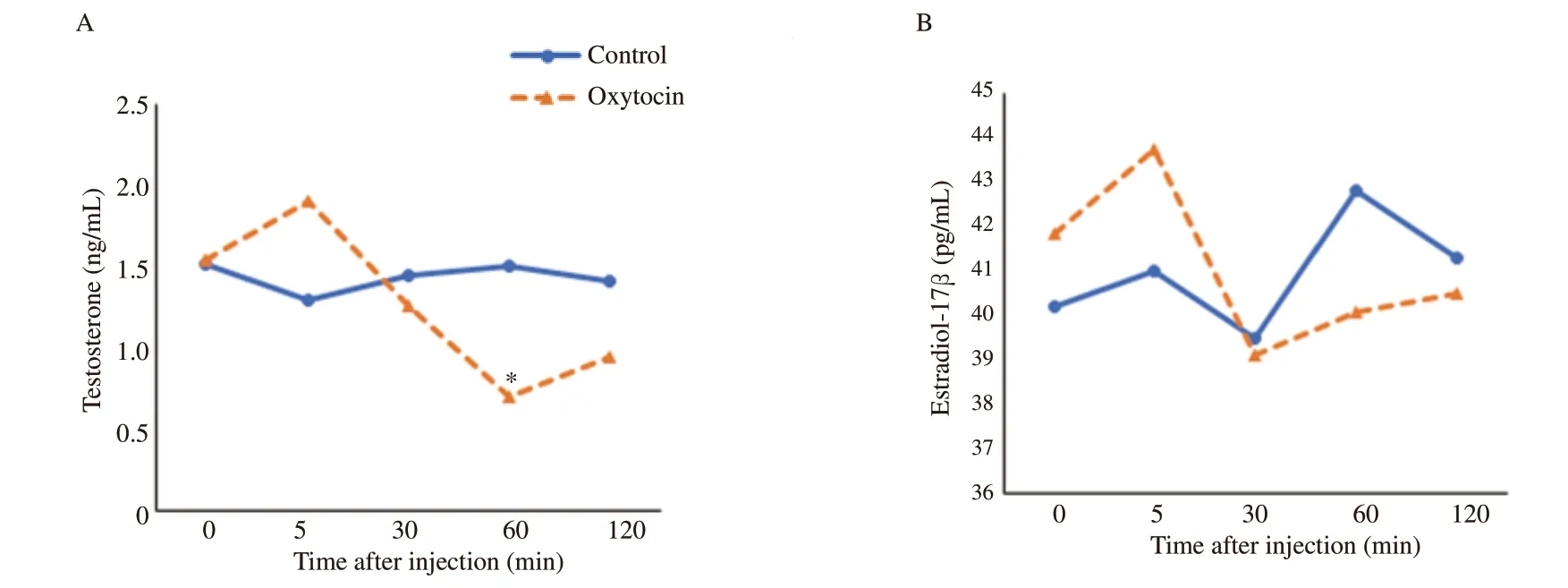
Figure 1.Temporal changes in the plasma concentrations of testosterone (A) and estradiol-17β (B) in male Baladi goats after injection of saline (control) or oxytocin.* Values are significantly different between the two groups at the indicated time during the study (P<0.05).
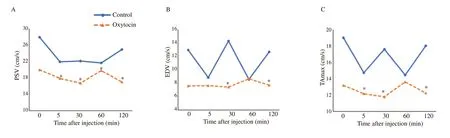
Figure 2.Temporal changes in blood flow velocities within the supratesticular artery in male Baladi goats after injection of saline (control) or oxytocin.*Values are significantly different between the two groups at the indicated time during the study (P<0.05).A: peak systolic velocity (PSV);B: end diastolic velocity (EDV);C: maximum velocity (TAmax).
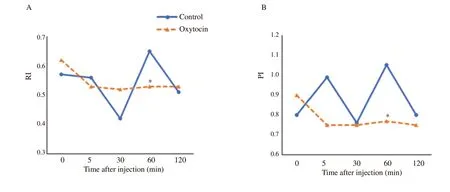
Figure 3.Temporal changes in pulsed-wave Doppler indices within the supratesticular artery in male Baladi goats after injection of saline (control) or oxytocin.*Values are significantly different between the two groups at the indicated time during the study (P<0.05).A: resistive index (RI);B: pulsatility index (PI).
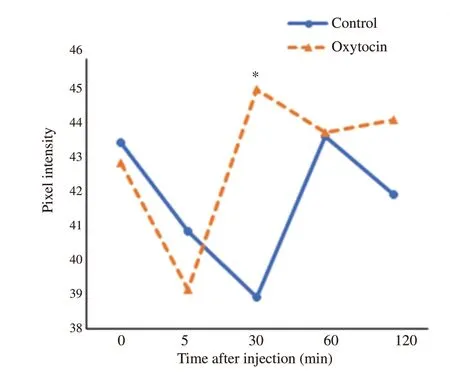
Figure 4.Temporal changes in testicular echogenicity (pixel intensity) in male Baladi goats after injection of saline (control)or oxytocin.* Values are significantly different between the two groups at the indicated time during the study (P<0.05).
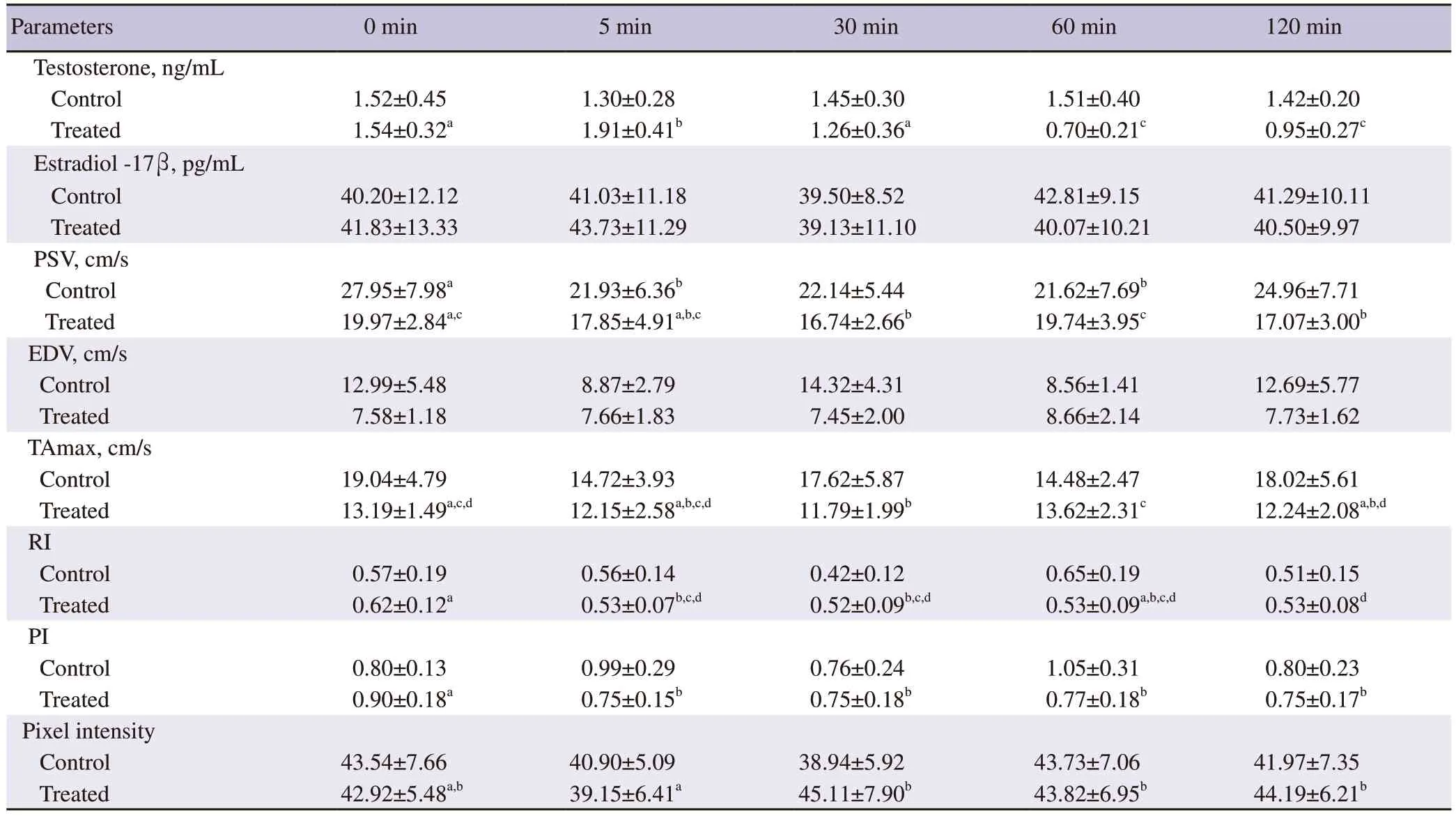
Table 1. Plasma hormonal profile,testicular blood flow measures,and testicular pixel intensity in the control and oxytocin treatment groups of male Baladi goats.
Regarding RI and PI,unlike the control group,their values significantly decreased at 5 min after administration of oxytocin(Table 1).For comparison between the two groups,the RI and PI significantly declined in the oxytocin-injected group at 60 min posttreatment (0.53±0.09 and 0.77±0.18,respectively) compared to the control group (0.65±0.19 and 1.05±0.31,respectively) (P<0.05)(Figure 3A and 3B).
Unlike the control group,testicular pixel intensity showed marked differences over time in the oxytocin-injected group (P<0.05) (Table 1).Their values were significantly increased at 30 min post-injection in the oxytocin-injected group (45.11±7.90) compared to the control group (38.94±5.92;P<0.05) (Figure 4).
As depicted in Table 2,there was a strong positive correlation between plasma testosterone and estradiol-17β concentrations(r=0.775,P<0.01).A strong positive association was found between PSV and TAmax (r=0.892,P<0.01).Similarly,TAmax exhibiteda positive significant correlation with EDV (r=0.839,P<0.01).RI showed a positive significant association with PI (r=0.976,P<0.01).
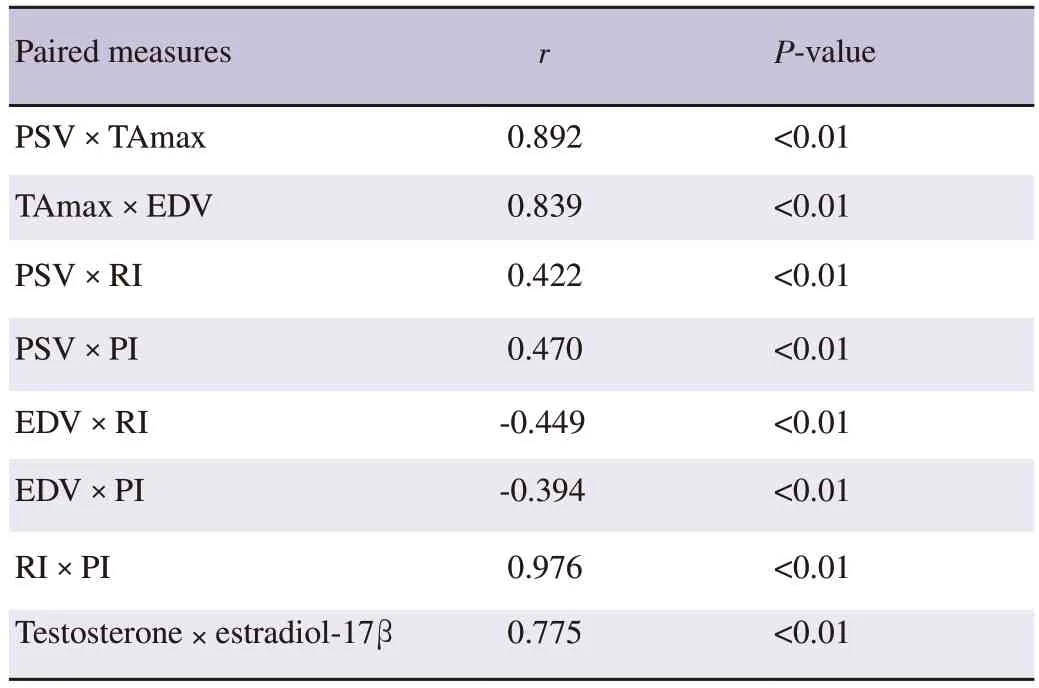
Table 2.Correlation coefficients (r) between intratesticular hemodynamic parameters and plasma concentrations of testosterone and estradiol-17β in the oxytocin-treated Baladi goats.
4.Discussion
To the best of our knowledge,this is the first study to show that a single intravenous injection of oxytocin in male Baladi goats aroused a sequence of variations in the plasma concentrations of steroid hormones (testosterone and estradiol-17β),testicular pulsed-wave Doppler indices,and testicular echogenicity (pixel intensity).The baseline concentrations of plasma testosterone in male Baladi goats were found to be similar to those mentioned in Zaraibi goats[25] and male Arabia goats[26].
In the present study,there were significant changes in the concentration of plasma testosterone after oxytocin treatment.These changes could reflect the possible modulatory consequence of oxytocin on Leydig cells in goats and its role in the synthesis and release of testosterone (steroidogenesis).Cellular localization of oxytocin receptors has been documented along the path of the reproductive tract,especially on Leydig and Sertoli cells as shown in rams[17] and stallions[27].This suggests that oxytocin may participate in the control of steroidogenesis.
In the present study,the plasma concentrations of testosterone were found to be significantly reduced at 60 min after oxytocin injection compared to the control group.In line with our findings,oxytocin suppressed the biosynthesis of testosterone in isolated rat Leydig cells.In addition,exogenous oxytocin completely hindered testosterone production and inhibited the formation of C21and C19metabolites of pregnenolone,which resulted in a significant decrease in plasma testosterone concentrations in rats[28].In contrast,the intravenous administration of oxytocin did not influence the serum testosterone concentrations in rams[19].These discrepancies might be,in part,due to the differences in species and the lower dose of oxytocin compared to our study.
In the meantime,the plasma concentrations of estradiol-17β were not affected by oxytocin injections.The biosynthesis of estradiol-17β is mainly due to the conversion of testosterone to estradiol-17βviaaromatization within the Sertoli cells[29].In Sertoli cells,estradiol-17β production is mainly dependent on the concentration of the produced testosterone and/or the aromatase enzyme activity as reported in rams[30] and stallions[6].There was no association between the inhibitory effect of oxytocin on testosterone synthesis within the Leydig cells and the process of estrogen production in Sertoli cells in male Baladi goats.
In line with our results,Bozkurtet al[19] reported that the administration of oxytocin did not affect the concentrations of serum and seminal estradiol-17β in rams.Furthermore,the administration of oxytocin inhibited the formation of estradiol-17β in bovine granulosa cells without interfering with the aromatase activity[31].In the same sense,it has been reported that the concentration of serum estradiol-17β remained stable after thein-vitrotreatment of rat uterus with oxytocin[32].Assinderet al[33] demonstrated that oxytocin accelerates the transformation of testosterone to dihydrotestosterone by the activity of 5α-reductase enzymes within the prostatic tissue,which could explain the lack of estradiol-17β response to oxytocin as well as the remarkable decline in the plasma concentrations of testosterone in Baladi goats.
The present results revealed monophasic and non-resistive waveforms of the testicular blood flow (supratesticular artery).In agreement with these findings,several reports in different species have demonstrated the same pattern of the cardiac cycle[34,35].In contrast,in stallions,the spectral pattern is characterized by biphasic and resistive waveforms[36].This discrepancy might be due to the vertical alignment of rams’ testes compared to the horizontal orientation of stallion ones.Of the five parameters that were recorded in this study,there were three directly exhibiting the arterial blood flow velocity during the cardiac cycle (PSV,EDV,and TAmax);however,their values are known to be markedly irregular and not persistent between measurements of the same organ[36].On the other hand,spectral Doppler indices (RI and PI) are inherently accurate indicators of arterial blood flow,as they reflected information on the testicular vascular support,not only velocity.
The present study showed that RI and PI significantly decreased at 60 min after treatment in the oxytocin group compared to the control group.Fortunately,this reduction is useful for testicular functions,since the decrease in RI and PI values is followed by an increase in testicular vascular perfusion due to the absence of resistance of the arterial wall against the blood flow[37,38].Several studies have investigated that the reduction in RI and PI values is followed by an amelioration in testicular activities (spermatogenesis and steroidogenesis)[10,39].Oxytocin is a cardiovascular-reproductive hormone,where its receptors were found in the heart,brain,pituitary gland,and reproductive tract[29].In addition,oxytocin controls the blood vessels,since it has a modulatory effect on cardiac function and vascular tone.It is well-known that oxytocin exerts a vasodilatory effect on large and small peripheral arteries in humans[40].
In the present study,the oxytocin group exhibited a significant increase in testicular pixel intensity compared to the control group at 30 min post-injection.Besides,several reports have investigated that the changes in testicular pixel intensity are directly correlated with remarkable changes in semen characteristics[24,41].These changes could be due to further histomorphological alterations in the testicular tissues after treatment.In addition,pixel intensity is exhibited as a shade of gray in a range of 1 to 255,where 1 represents anechoic black and 255 resembles extremely white[24].Anjumet al[42] reported that oxytocin injections affected the testicular tissues of mice,where the testicular mass was significantly increased,and the seminiferous tubules exhibited more advanced stages of spermatogenic cellular development.This might explain the increased white range (grey pixel intensity) of the grey-scale images of testicular parenchyma in Baladi goats.
In the oxytocin group,there was a strong positive association between RI and PI values on one hand and a strong positive association between plasma concentrations of testosterone and estradiol-17β on the other hand.These clear correlations might be reflected by the vasodilatory role of oxytocin as well as the potent inhibitory effect of oxytocin on testosterone synthesis in male Baladi goats.
Overall,this study narrowed down the influence of oxytocin on testicular vascular irrigation and plasma steroid concentration in male goats.Our study generated insights into the reproductive impact of vasodilatory hormone (oxytocin) in goat bucks and provided a solid basis for further checking of its effect on semen quality and quantity characteristics.Moreover,the associations between the blood flow indices and plasma testosterone concentrations show the beneficial application of Doppler ultrasonography in the breeding soundness examination of male goats.
The lack of semen evaluation is a limitation in this study.The expected influence of the high frequency of semen collection (5 collections within 2 h) on testicular blood flow did not support the evaluation of semen.Only healthy bucks were used in this study,but further studies are important to validate the same findings in bucks with pathological affections deteriorating the testicular hemodynamics.
In conclusion,the administration of a single dose of oxytocin(0.7 IU/kg) has a favorable effect on testicular blood flow in male Baladi goats.This profitable effect may improve male reproductive performance through beneficial impacts on testicular hemodynamics.In the future,we recommend doing more studies to detect the possible effects of oxytocin injection on semen quantity and quality characteristics,and their association with testicular blood flow in male Baladi goats.We suggested further studies with a larger sample size to comprehensively evaluate the impact of oxytocin administration on the reproductive potential of male goats.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
The study received no extramural funding.
Authors’contributions
Mohamed Hedia was involved in conceptualization,writing,validation,review,and formal analysis.Amr El-Shalofy was involved in conceptualization,validation,investigation,and methodology.
 Asian Pacific Journal of Reproduction2022年5期
Asian Pacific Journal of Reproduction2022年5期
- Asian Pacific Journal of Reproduction的其它文章
- Impact of gamete health on fertilization and embryo development: An overview
- An incidental presentation of Herlyn-Werner-Wunderlich syndrome with secondary infertility: A case report
- L-carnitine improves developmental competence of buffalo oocytes in vitro
- Evaluation of intratesticular chlorhexidine gluconate for chemical contraception in dogs
- Association of the microbial culture of follicular fluid,vaginal swab and catheter tip with β-hCG IVF positive and negative
- Anti-Müllerian hormone and antral follicle count predict ovarian response in women less than 45 years following GnRH antagonist multiple-dose protocol
