Clopidogrel delays and can reverse diabetic nephropathy pathogenesis in type 2 diabetic db/db mice
lNTRODUCTlON
Diabetes is a major public health issue, affecting more than 400 million people worldwide[1]. Type 2 diabetes is a highly prevalent condition that is associated with major vascular, renal, and neurologic complications. Diabetic nephropathy (DN) is the most common microvascular inflammatory disease among diabetes complications. Proteinuria is a hallmark of early DN, and the associated morphological abnormalities include glomerular hypertrophy, thickening of the glomerular basement membrane,expansion of the mesangial matrix, and renal tubular injury. Renal changes in the later phases include glomerulosclerosis and tubulointerstitial fibrosis[2-4].
Long ago there lived a rich merchant who, besides possessing more treasures than any king in the world, had in his great hall three chairs, one of silver, one of gold, and one of diamonds
The mechanism of DN is complex. A persistently high glucose level is considered the principal risk factor for DN; however, other factors, such as abnormal renal hemodynamics, may also be involved in the development of DN[5]. Hyperglycemia leads to the expression of proinflammatory mediators(chemokines and cytokines) in injured glomerular and tubular cells, which contributes to renal damage through various mechanisms, including mesangial proliferation, podocyte/tubular damage, and leukocyte infiltration[6,7]. Leukocytic interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α promote the release of vascular endothelial factors, stimulate the proliferation of glomerular mesangial cells,increase the permeability of the endothelium, and promote the synthesis and release of superoxide and proteolytic enzymes, which eventually cause renal structural remodeling and dysfunction[8,9]. An increasing amount of evidence indicates that diabetes-associated vascular and renal inflammation is likely to be associated with high platelet reactivity, which would contribute to high atherothrombotic risk[10,11].
Clopidogrel is an anti-platelet aggregation drug that with a pyridine-based structure. It can specifically and irreversibly bind to platelet P2RY12 purinergic receptors, which inhibits ADP-mediated platelet activation and aggregation. Clopidogrel not only inhibits platelet aggregation but also inflammatory responses in a platelet activation-dependent or independent manner[12,13]. Previous studies have shown that clopidogrel administration is an effective means of suppressing inflammation in conditions such as acute myocardial infarction, diabetes, and acute ischemic cerebral infarction[14,15].Lower expression levels of IL-6, TNF-α, and transforming growth factor-β1; lower matrix metalloproteinase (MMP)-2 and MMP-9 activity; and stabilization of the extracellular matrix (ECM) in clopidogreltreated mice with hyperlipidemia and acute myocardial infarction reflected the protective effect of the drug[16]. We have previously shown that clopidogrel significantly reduced renal collagen and fibronectin (FN) expression and thus ameliorated diabetes-induced renal fibrosis in a streptozotocininduced murine model of type 1 diabetes[17]. However, because 80%-90% of the population with diabetes has type 2 disease[1], in the present study, we aimed to determine whether clopidogrel treatment has a preventive or therapeutic effect in the kidneys of obese type 2 diabetic
/
mice, a widely used mouse model of type 2 diabetes for DN investigations[18].
MATERlALS AND METHODS
Experimental animals, groups, and drug administration
mice used in the present study were leptin receptor (Lepr) knockout mice with the C57BLKS background developed by GemPharmatech Co., Ltd. by using the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technique, (https://www.gempharmatech.us/en/strain/detail/?strain_id=3913). This mouse line has features similar to that from the Jackson Laboratory (https://www.jax.org/strain/000642): Significant increases in the body weight starting at 4 wk,hyperglycemia (6-h fasting blood glucose and hemoglobin A1c) at 8 wk, insulinemia (> 3-fold) at 8 wk,and hyperlipidemia (cholesterol, triglyceride, and low-density lipoprotein) along with the early onset of renal dysfunction (indicated by significantly increased microalbumin level in 24-h urine analysis) at 12 wk of age. Therefore, this mouse line has been widely used as a model for studies on type 2 diabetes[19,20], including for the studies on DN of type 2 diabetes[18,21].
Clopidogrel treatment reduced urinary albumin/creatinine ratio. Immunohistochemical staining revealed an amelioration of renal fibrosis, significantly less deposition of FN and collagen I. Lower expression of the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β and lower levels of urinary TNF-α, monocyte chemoattractant protein-1 and significantly reduced macrophage infiltration of the
mice.
Eight-week-old, specific pathogen-free male C57BLKS
/
diabetic mice (
= 24) and age-matched C57BLKS [wild-type (WT)] mice (
= 6) were purchased from the Model Animal Research Center of Nanjing University (Cat. No. T002407, GemPharmatech Co., Ltd., Nanjing, China). All animal experiments were approved by the ethics committee of the First Hospital of Jilin University and conformed to the internationally accepted principles for the care and use of laboratory animals. All the mice were adoptively fed until 12 wk of age when these
mice should show typical DN, defined as the baseline. Then the
/
mice were randomly assigned to vehicle or low-dose (5 mg/kg), mediumdose (10 mg/kg), or high-dose (20 mg/kg) clopidogrel groups. The mice were administered clopidogrel or vehicle daily by gavage for 3 mo. At the end of the experiment, the animals were intraperitoneally anesthetized with tribromoethanol (350 mg/kg) and sacrificed to collect blood and kidneys for subsequent experiments.
The dove then flew up on his shoulder and sat there and the prince thanked it, and stroked and caressed12 its white feathers, and kissed its little red beak
Sample collection
Blood glucose levels were measured at regular intervals by using samples collected from a tail vein.Twenty-four-hour urine collections were performed before and after 3 mo of treatment with the animals in metabolic cages, and the 24-h urine albumin output was measured using a mouse microalbuminuria enzyme-linked immunosorbent assay (ELISA) kit (Beijing XinQuan Tech Company, XQ-EN20536). The urine creatinine output was measured using a creatinine test kit (Nanjing Jiancheng Bioengineering Institute, C011-1-1).
Blood clotting time
Blood clotting time was measured using the mouse tail-vein bleeding assay. Briefly, the mouse’s tail was cut 1-2 mm from the tip, where its diameter was approximately 1 mm, and then, it was immediately dipped into a 50-mL tube filled with saline at 37 °C. The bleeding time was recorded over a period of up to 5 min, as in our previous study[17].
Histology and immunohistochemical staining
The collected kidneys were fixed in 10% formalin, dehydrated in a graded series of alcohol, cleaned with xylene, embedded in paraffin, and sectioned at a thickness of 5 μm. Periodic acid-Schiff and Masson staining was performed to facilitate the examination of the glomerular basement membrane and mesangial matrix of the kidneys. Immunohistochemistry was used to assess the expression of TNF-α(Abcam, ab220210), IL-1β (Cell Signaling Technologies, #12242), F4/80 (Abcam, ab100790), FN (Santa Cruz Biotechnology, SC-8422), and collagen I (Abcam, ab253113) in the kidney sections. After 3,3’-diaminobenzidine staining, cells that were positive for target protein expression stained brown-yellow,whereas the cells without the said expression were unstained. The percentage of each area that was stained was quantified using the Image-Pro Plus software.
Measurement of TNF-α, MCP-1, and IL-6 levels in the urine
Urinary TNF-α, MCP-1, and IL-6 levels were measured using a mouse TNF-α precoated ELISA kit(DAKEWE, 1217202), mouse MCP-1 precoated ELISA kit (DAKEWE, 1217392), and mouse IL-6 precoated ELISA kit (DAKEWE, 1210602), respectively. These concentrations were normalized to the urinary creatinine concentrations.
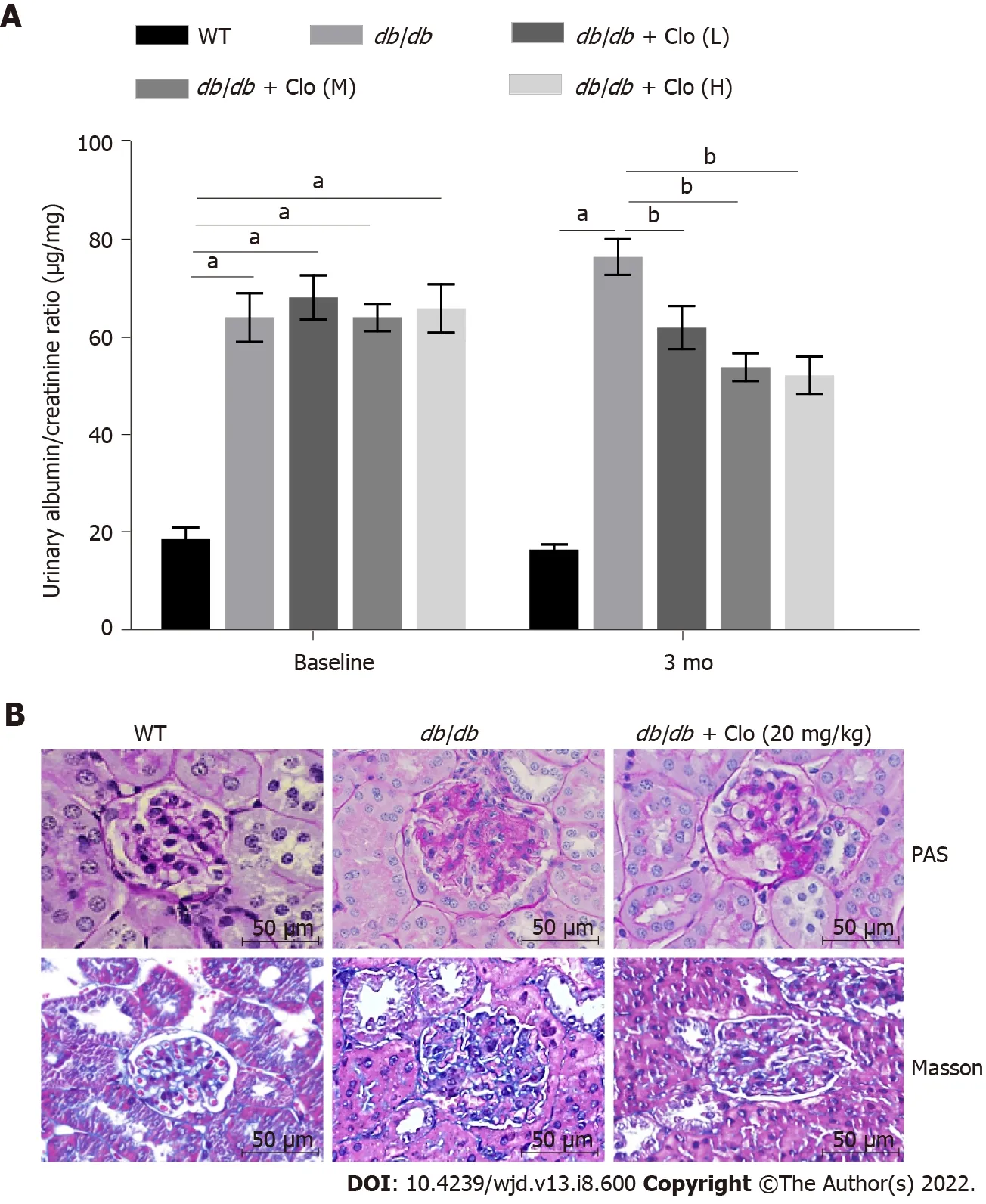
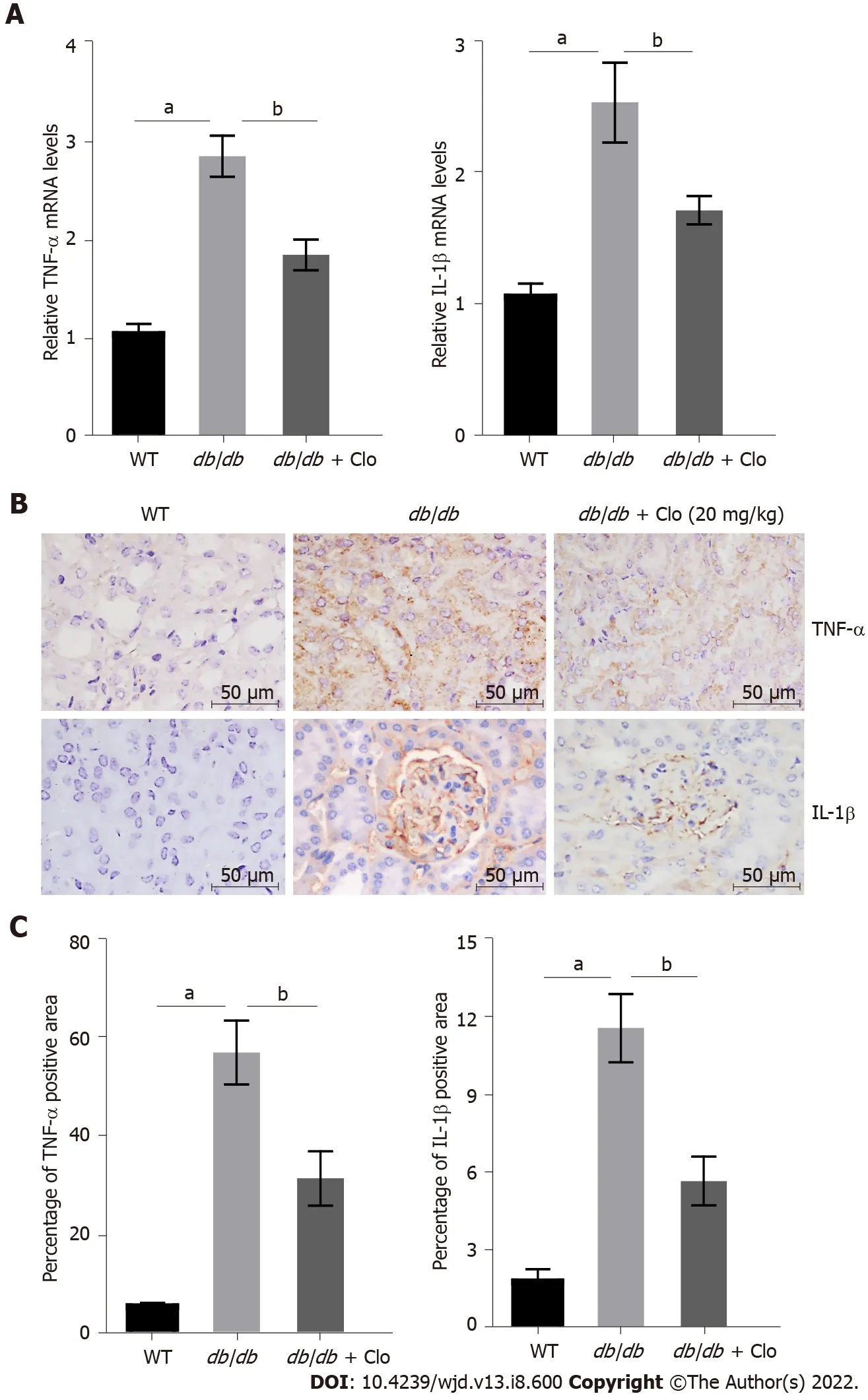
The urinary albumin concentration is closely related to the progression of glomerular lesions and kidney damage, and this was significantly reduced by clopidogrel administration in
/
mice to levels that were lower than those prior to treatment (Figure 2A). Inflammation plays an important role in the development and progression of DN, and this involves the production of chemokines and proinflammatory cytokines, infiltration of immune cells into the kidney, formation of immune complexes, and complement activation[29]. Chronic diabetic hyperglycemia causes an increase in the circulating concentration of advanced glycation end-products, which induce macrophage migration through advanced glycation end product receptor-mediated activation of the nuclear factor (NF)-κB inflammatory pathway[30,31]. Furthermore, the production of the proinflammatory cytokines TNF-α and IL-1β is induced by NF-κB activation. TNF-α promotes inflammatory cell aggregation and adhesion,microvascular dilation, vascular permeability, and exacerbation of the inflammatory response, thereby contributing to glomerular tissue damage. Therefore, the serum TNF-α concentration is considered a predictor of disease progression[9,32]. IL-1β stimulates the proliferation of glomerular cells in the kidneys, promotes the production of ECM, and accelerates the process of renal fibrosis[8,33]. Consistent with this, we found significantly higher expression of both TNF-α and IL-1β in the kidneys and urine of
/
mice. However, treatment with clopidogrel significantly reduced the expression of both cytokines,which implies that the drug has an anti-inflammatory effect in the kidneys of diabetic mice.
RNA isolation and real-time polymerase chain reaction
RNA was extracted from tissues by using Trizol and reverse-transcribed into cDNA. Real-time polymerase chain reaction (PCR) was performed using SYBR Green chemistry, with
as the reference gene. Gene expression was quantified using the 2
method. The primer sequences used were as follows:
(F): TATAAAGCGGCCGTCTGCAC,
(R): TCTTCTGCCAGTTCCACGTC;
(F): TTGACGGACCCCAAAAGATG,
(R): AGAAGGTGCTCATGTCCTCA; and
(F):CCCTGTATGCCTCTGGTCGT,
(R): CGTGGGGTGAAGCTGTAGCCACG.
Statistical analysis
Data are presented as mean ± SD values for
= 6 mice per group. Multiple comparisons of data were performed using one-way analysis of variance, with Tukey’s test for
pairwise comparisons. All statistical analyses were conducted using GraphPad Prism 5, and
< 0.05 was considered to represent statistical significance.
RESULTS
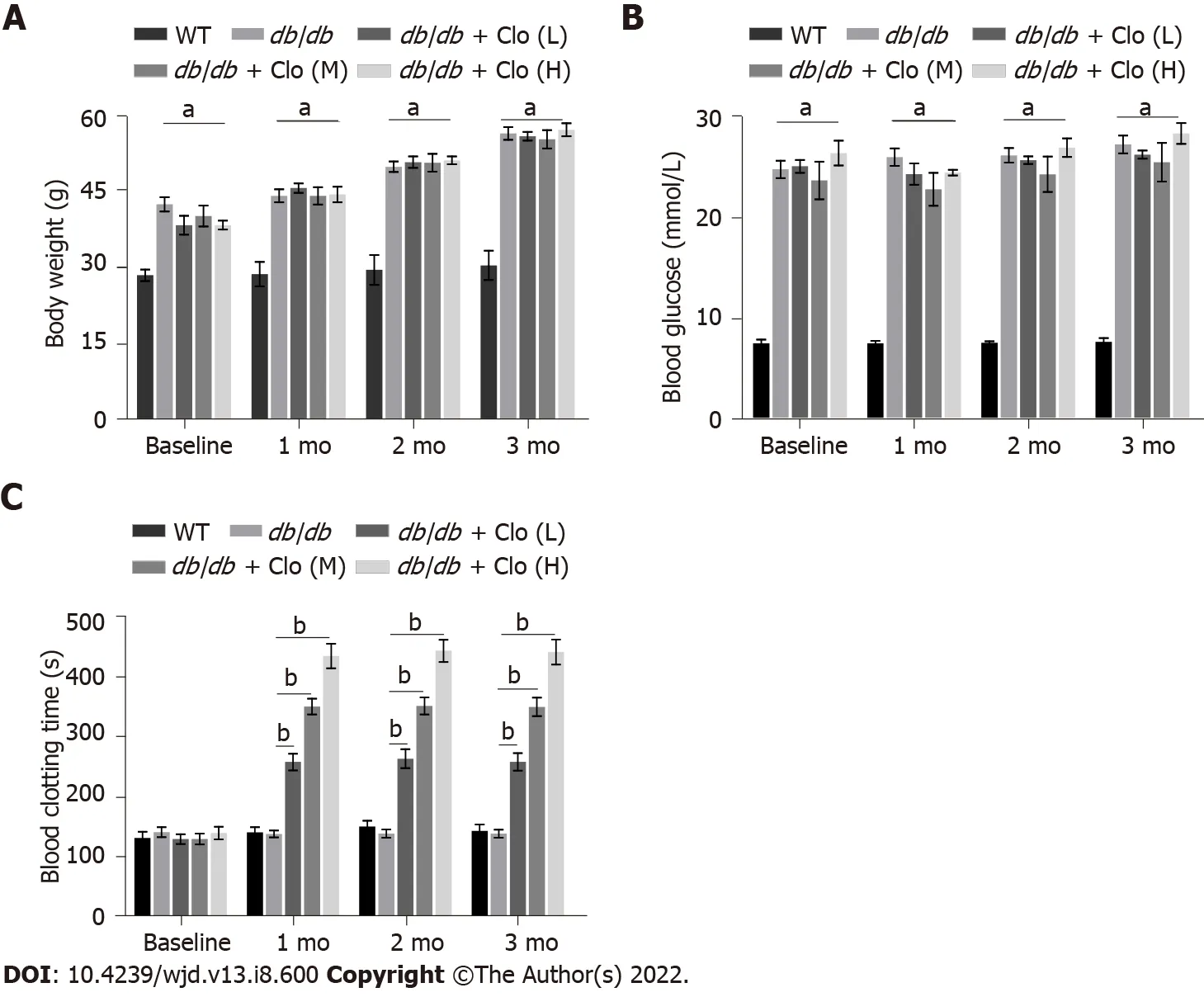
Effects of the db/db genotype and clopidogrel on the body mass, blood glucose level, and bleeding time of the mice
At the age of 12 wk, the
/
mice were significantly heavier and had higher fasting blood glucose levels compared to the WT mice (Figures 1A and B). Their animals’ body mass slightly increased with age thereafter, whereas their fasting blood glucose levels did not significantly change during the 3-mo treatment period. Because
/
mice start showing typical DN at 12 wk of age, we started to administer clopidogrel from this age with one of three doses (5, 10, or 20 mg/kg), to observe the drug’s therapeutic effects or delay DN progression. First, the bleeding times of the WT and diabetic mice that had or had not been administered clopidogrel were measured (Figure 1C). There was no difference between diabetic mice and WT nondiabetic mice at the baseline level, and treatment of the diabetic mice with clopidogrel increased the bleeding time in a dose-dependent manner during the 3-mo treatment.Furthermore, clopidogrel treatment did not affect the body mass or blood glucose levels of the diabetic mice during the experimental period (Figures 1A and B).
And at the same time he changed himself into a mouse, and began to run about the floor. Puss no sooner perceived this but he fell upon him and ate him up.35
Clopidogrel ameliorated diabetes-associated renal dysfunction, glomerular sclerosis, and collagen fiber deposition in the mice
Clopidogrel administration for 3 mo reduced the urinary albumin/creatinine ratio (ACR) of the diabetic mice, especially in the high-dose group, which implies an improvement in the glomerular filtration rate(Figure 2A). The kidneys of the WT mice did not show any obvious pathology, whereas those of the
/
mice showed larger renal glomeruli, thickening of the substrate membranes, hyperplasia of mesangial cells, and an expansion of the ECM. These histopathological changes were markedly ameliorated by clopidogrel administration (Figure 2B). Masson’s trichrome staining showed greater collagen deposition in the
/
mice; however, this was less severe in the mice administered clopidogrel (Figure 2B).
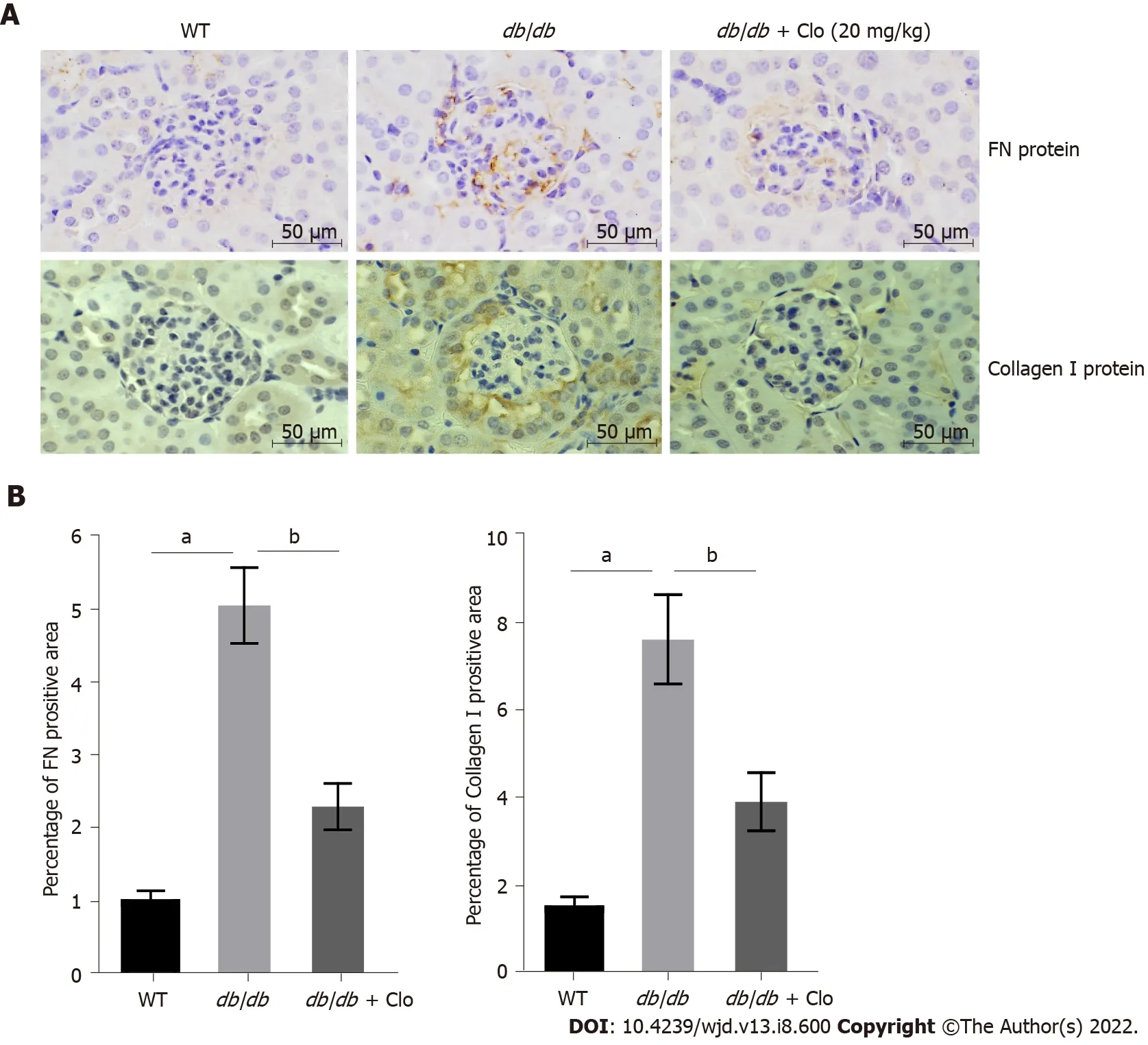
Potential mechanisms for the beneficial effects of clopidogrel: A reduction in the renal accumulation of ECM components responsible for fibrosis
It has been shown that in the early stages of DN, there is a significant increase in the deposition of collagen, FN, and laminin in the glomerular basement membrane and ECM in glomerular mesangial cells[22]. We assessed the protein expression of FN and collagen type I in the kidney cortex of the mice by immunohistochemical staining (Figure 3). No significant FN or collagen I protein expression was identified in the renal glomerulus of WT mice (Figure 3), and there was only a low level of expression around the blood vessels (data not shown). In contrast, there was an excessive deposition of both FN and collagen I in the glomerular membranes and mesangium of the
/
mice (Figure 3). However, the accumulation of both proteins was significantly lower in
/
mice that had been administered clopidogrel for 3 mo (Figure 3). These data imply that clopidogrel reduced collagen synthesis and ECM deposition and inhibited fibrosis in the kidneys of the
/
mice.
Potential mechanisms for the beneficial effects of clopidogrel: Inhibition of the diabetes-associated renal inflammatory response and macrophage infiltration
We next measured the mRNA expression of
and
by using real-time PCR, which showed that both mRNA expression levels were significantly lower in the clopidogrel treatment group(Figure 4A). Furthermore, immunohistochemical staining of kidney sections showed that TNF-α and IL-1β were expressed at low levels in the kidneys of the WT mice. However, there was higher TNF-α expression principally in the renal tubules and higher IL-1β expression in both the glomeruli and renal tubules of the
/
mice (Figure 4B). Semi-quantitative analysis showed significantly higher protein expression of TNF-α and IL-1β in the kidneys of diabetic mice. Nevertheless, clopidogrel significantly reduced the expression of these renal inflammatory cytokines (Figure 4C).
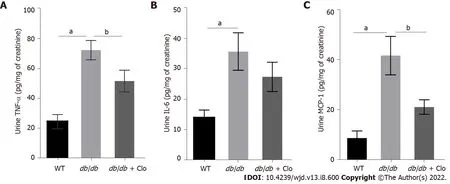
We also examined these inflammatory cytokine levels in the urine, which may be indirect indicators of systemic inflammation. Furthermore, considering that MCP-1 is a potent chemokine in the recruitment of macrophages[23], urinary TNF-α, IL-6, and MCP-1 levels were measured by ELISA(Figure 5). The results showed that the development of diabetes significantly increased urinary TNF-α,IL-6, and MCP-1 levels. However, clopidogrel reduced the levels of urinary TNF-α and MCP-1 (Figures 5A and C), and urinary IL-6 level in
mice had a slight decrease (Figure 5B). These results indicate that clopidogrel can inhibit the diabetes-associated renal inflammatory response and probably systemic inflammation.
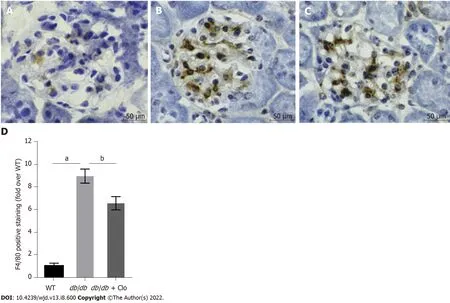
Macrophages, lymphocytes, and mast cells secrete large amounts of proinflammatory mediators and cytokines in the diabetic kidney, which directly or indirectly induce renal damage and accelerate local fibrosis[24,25]. F4/80 is a macrophage marker expressed on the surface of these cells in mice. Consistent with the urinary MCP-1 results, there was no obvious expression of F4/80 in normal mouse kidney tissue (Figure 6). On the other hand, macrophage infiltration was apparent around the glomeruli in diabetic mice. Furthermore, the number of macrophages at this location was significantly high in
mice; this number was significantly reduced by clopidogrel treatment (Figure 6). These results suggest that clopidogrel reduces macrophage infiltration and inhibits cytokine secretion, thereby reducing damage to the kidneys caused by the inflammatory response.
DlSCUSSlON
25.Fetch one of his best suits: Once again, clothes make the man. Puss resorts to more trickery to gain grand apparel for the youngest son. In other versions of the tale, such as Basile s Gagliuso, (also know as Caglioso), the cat arrives at the castle before a scheduled appointment announcing that the Marquis servants have stolen all of his clothing. The king then sends a wardrobe to the Marquis. Once the youngest son appears in the king s own majestic clothing, he cannot be recognized as a lowly commoner.Return to place in story.
Clopidogrel at doses of 5, 10, or 20 mg/kg was administered by gavage for 12 wk. The body mass, blood glucose, and urinary creatinine and albumin concentrations were measured. Immunohistochemistry,enzyme-linked immunosorbent assay and real-time quantitative polymerase chain reaction were used to evaluate the expression of cytokines. Fibronectin (FN), and collagen I was assessed using immunohistochemistry.
In addition, although clopidogrel is an anti-platelet aggregation drug, its preventive or therapeutic effect on the development of DN in
/
mice does not seem to be predominantly related to its effect on platelet aggregation. We believe this is because the
mice did not demonstrate shorter bleeding times than those noted in the WT mice before or after 3 mo of clopidogrel administration (Figure 1C).However, clopidogrel increased the bleeding times of the
mice in a dose-dependent manner(Figure 1C) during the 3-mo treatment period, parallel to the dose-dependent effects of improvement in renal function (Figure 2A).
Though Placida s kingdom was a large one; his horse had carried him gallantly24 to the limit of it, but it could go no further, and the Prince was obliged to dismount and continue his journey on foot, though this slow mode of progress tired his patience severely
Macrophages are the principal type of immune cells that promote kidney damage in diabetes[34,35].F4/80 is a macrophage-specific antigen that participates in the maturation and activation of this cell type. Consistent with the increased urinary level of MCP-1, which is a potent chemokine in macrophages, we found a significant increase in macrophage infiltration, mirrored by F4/80-positive staining (Figure 6), in the kidneys of the
/
mice. However, the number of macrophages in the kidneys of the clopidogrel-treated mice was significantly lower. Chronic renal inflammation causes glomerular membrane cells to produce large amounts of type I and type IV collagen and FN, which leads to thickening of the glomerular basement membrane and ECM accumulation, ultimately resulting in glomerulosclerosis[36,37]. We also found higher expression of collagen I and FN in the kidneys of the
/
mice. However, this was much lower in the kidneys of mice that had been treated with clopidogrel for 3 mo.
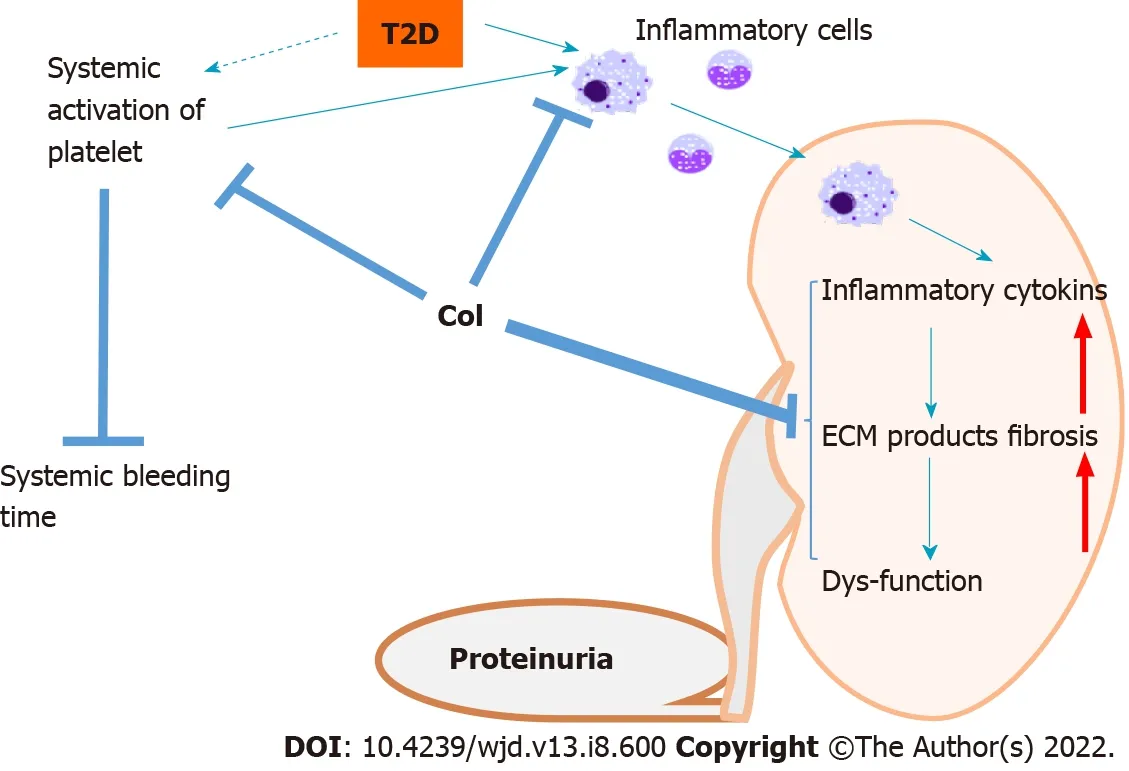
Thus, as illustrated in Figure 7, the results of the present study suggest that clopidogrel administration for 3 mo to
/
mice, which had significant renal dysfunction (high urinary ACR), delayed the progression of DN and possibly improved renal function. This effect is accompanied by an amelioration of features of the renal pathology of DN, including lower renal accumulation of ECM components, such as collagen and FN. The beneficial effects of clopidogrel on the renal deposition of collagen in the kidneys of
/
mice may be explained by its inhibition of diabetes-associated macrophage infiltration and proinflammatory cytokine release. However, further research is required to determine whether there is a genuine cause-and-effect link between these findings. In addition, the lower macrophage infiltration may explain the reduction in ECM accumulation and fibrosis. However, it may be that clopidogrel also directly inhibits renal inflammation and fibrosis. This possibility requires further investigation.
In the course of the beat his dogs disturbed a beautiful snow-white stag, and directly he saw it the king determined3 that he would have it at any cost
CONCLUSlON
We found that clopidogrel significantly reduced renal collagen deposition and fibrosis and prevented renal dysfunction in
/
mice. These findings suggest a promising alternative approach to the treatment of diabetes and prevention of DN because clopidogrel is in current use and could be coadministered with other antidiabetic drugs.
ARTlCLE HlGHLlGHTS
Research background
Diabetic nephropathy (DN) is the leading cause of end-stage kidney disease in the United States and most developed countries. New strategies are required to delay the development and the progression of DN.
Research motivation
Pei J and Li HQ contributed to conception and design of the study; Li HQ and Liu N performed the experiment; Zheng ZY organized the database; Teng HL performed the statistical analysis; Li HQ and Liu N wrote the draft of the manuscript; and all authors contributed to manuscript revision, read, and approved the submitted version.
Clopidogrel is an antagonist of the P2RY12 receptor, which is expressed on the surfaces of platelets. It not only inhibits platelet aggregation but also reduces ventricular inflammation and fibrosis in animal models[26-28]. Consistent with this, in this study, we found that clopidogrel administration for 3 mo delayed or prevented the progression of DN in
/
diabetic mice. These beneficial effects of clopidogrel in
/
mice appear to be mediated by inhibition of the renal deposition of collagen,probably through suppression of macrophage infiltration into the kidney and thus a reduction in proinflammatory cytokine secretion, as illustrated in Figure 7.
Research objectives
We aimed to determine whether treatment with clopidogrel has a preventive or therapeutic effect in the kidneys of obese type 2 diabetic
mice.
Losing a father whom you have no recollection of ever living with is difficult. Grieving is tricky13; I didn t have any obvious close father-daughter memories to cling to and mull and cry over. Most of my memories were of stilted14 meetings and uncomfortable times together. But I desperately15 missed him being alive.
Research methods
We have also shown that clopidogrel administration does not significantly affect the body mass or blood glucose level of
/
diabetic mice, which implies that the dose and duration of clopidogrel administration used in these mice were safe, without evidence of potential side-effects. Furthermore,these beneficial effects of the drug against DN development in these mice were independent of glycemic control. There have been few previous studies on the effects of clopidogrel on blood glucose. However,in one study, patients with type 2 diabetes were treated daily with one 70 mg clopidogrel tablet for 2 mo, which improved their fasting blood glucose level (from 9.7 ± 0.7 mmol/L to 7.5 ± 0.5 mmol/L).Therefore, the potential hypoglycemic effect of clopidogrel requires further investigation.
Research results
Why didn t you call me up and tell me about your dream? he asked. They say that the more you talk about bad dreams, the sooner you ll stop having them.
Research conclusions
Clopidogrel prevented renal dysfunction in
mice, most likely through inhibition of renal macrophage infiltration and the associated inflammation.
Research perspectives
The present findings suggest a promising alternative approach to the treatment of patients with diabetes and the prevention of DN because clopidogrel is in current use and could be co-administered with other antidiabetic drugs.
FOOTNOTES
Previous studies have shown that clopidogrel administration is an effective means of suppressing inflammation in diabetes. Moreover, clopidogrel can ameliorate diabetes-induced renal fibrosis in a streptozotocin-induced murine model of type 1 diabetes.
I might as well try. I think Stacy had a sponge toy like this and hers grew. Wendy smiled and removed the junk from her bed. Mom, can you get me two bowls of water?
All animal experiments conformed to the internationally accepted principles for the care and use of laboratory animals.
All the authors report no relevant conflicts of interest for this article.
No additional data are available.
But when it came, and the princess saw the horrible figure, she screamed out, What! marry this dirty beggar? Never, never! Ah, child, answered the king, how could I ever guess that the rich Don Giovanni would ever look like that? But I have passed my royal word, and I cannot break it, so there is no help for you
The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
China
Hong-Qin Li 0000-0002-3260-922X; Nian Liu 0000-0003-4518-4045; Zong-Yu Zheng 0000-0001-5523-1891; Hao-Lin Teng 0000-0001-8222-2536; Jin Pei 0000-0001-8735-1994.
Wang JJ
The incident bothered me for the rest of the week. I had money in my pocket and it wouldn t have killed me to hand over a buck2 or two even if he had been lying. On a frigid3() , cold night, no less, I assumed the worst of a fellow human being.
A
Wang JJ
1 Khursheed R, Singh SK, Wadhwa S, Kapoor B, Gulati M, Kumar R, Ramanunny AK, Awasthi A, Dua K. Treatment strategies against diabetes: Success so far and challenges ahead.
2019; 862: 172625 [PMID: 31449807 DOI: 10.1016/j.ejphar.2019.172625]
2 Soldatos G, Cooper ME. Diabetic nephropathy: important pathophysiologic mechanisms.
2008; 82 Suppl 1: S75-S79 [PMID: 18994672 DOI: 10.1016/j.diabres.2008.09.042]
3 Lehmann R, Schleicher ED. Molecular mechanism of diabetic nephropathy.
2000; 297: 135-144 [PMID:10841915 DOI: 10.1016/s0009-8981(00)00240-0]
4 Zeng LF, Xiao Y, Sun L. A Glimpse of the Mechanisms Related to Renal Fibrosis in Diabetic Nephropathy.
2019; 1165: 49-79 [PMID: 31399961 DOI: 10.1007/978-981-13-8871-2_4]
5 de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ,Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy.
2014; 25: 1083-1093[PMID: 24722445 DOI: 10.1681/ASN.2013080830]
6 Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy.
2016; 12: 13-26 [PMID:26568190 DOI: 10.1038/nrneph.2015.175]
7 Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P. Is podocyte injury relevant in diabetic nephropathy?
2003; 52: 1031-1035 [PMID: 12663476 DOI: 10.2337/diabetes.52.4.1031]
8 Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy.
1991; 40: 1007-1012 [PMID: 1762301 DOI:10.1038/ki.1991.308]
9 Yeo ES, Hwang JY, Park JE, Choi YJ, Huh KB, Kim WY. Tumor necrosis factor (TNF-alpha) and C-reactive protein(CRP) are positively associated with the risk of chronic kidney disease in patients with type 2 diabetes.
2010;51: 519-525 [PMID: 20499416 DOI: 10.3349/ymj.2010.51.4.519]
10 Chen G, Huang W, Hong S, Li X, Su B, Tang S. Effectiveness of antiplatelet drugs for the prevention of diabetic nephropathy: A meta-analysis of randomized controlled trials.
2018; 90: 419-426 [PMID: 30378535 DOI:10.5414/CN109588]
11 Westein E, Hoefer T, Calkin AC. Thrombosis in diabetes: a shear flow effect?
2017; 131: 1245-1260[PMID: 28592700 DOI: 10.1042/CS20160391]
12 Jansen MP, Emal D, Teske GJ, Dessing MC, Florquin S, Roelofs JJ. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps.
2017; 91: 352-364 [PMID: 27692564 DOI: 10.1016/j.kint.2016.08.006]
13 Cerda A, Pavez M, Manriquez V, Luchessi AD, Leal P, Benavente F, Fajardo CM, Salazar L, Hirata MH, Hirata RDC.Effects of clopidogrel on inflammatory cytokines and adhesion molecules in human endothelial cells: Role of nitric oxide mediating pleiotropic effects.
2017; 35 [PMID: 28371087 DOI: 10.1111/1755-5922.12261]
14 Woodward M, Lowe GD, Francis LM, Rumley A, Cobbe SM; CADET Study Investigators. A randomized comparison of the effects of aspirin and clopidogrel on thrombotic risk factors and C-reactive protein following myocardial infarction: the CADET trial.
2004; 2: 1934-1940 [PMID: 15550024 DOI: 10.1111/j.1538-7836.2004.01017.x]
15 Ramadan R, Dhawan SS, Syed H, Pohlel FK, Binongo JN, Ghazzal ZB, Quyyumi AA. Effects of clopidogrel therapy on oxidative stress, inflammation, vascular function, and progenitor cells in stable coronary artery disease.
2014; 63: 369-374 [PMID: 24336012 DOI: 10.1097/FJC.0000000000000057]
16 Korish AA. Clopidogrel Prophylaxis Abates Myocardial Ischemic Injury and Inhibits the Hyperlipidemia-Inflammation Loop in Hypercholestrolemic Mice.
2020; 51: 515-523 [PMID: 32487324 DOI:10.1016/j.arcmed.2020.05.003]
17 Zheng Z, Ma T, Lian X, Gao J, Wang W, Weng W, Lu X, Sun W, Cheng Y, Fu Y, Rane MJ, Gozal E, Cai L. Clopidogrel Reduces Fibronectin Accumulation and Improves Diabetes-Induced Renal Fibrosis.
2019; 15: 239-252[PMID: 30662363 DOI: 10.7150/ijbs.29063]
18 Wang Y, Niu A, Pan Y, Cao S, Terker AS, Wang S, Fan X, Toth CL, Ramirez Solano MA, Michell DL, Contreras D, Allen RM, Zhu W, Sheng Q, Fogo AB, Vickers KC, Zhang MZ, Harris RC. Profile of Podocyte Translatome During Development of Type 2 and Type 1 Diabetic Nephropathy Using Podocyte-Specific TRAP mRNA RNA-seq.
2021; 70: 2377-2390 [PMID: 34233930 DOI: 10.2337/db21-0110]
19 Kuang S, He F, Liu G, Sun X, Dai J, Chi A, Tang Y, Li Z, Gao Y, Deng C, Lin Z, Xiao H, Zhang M. CCR2-engineered mesenchymal stromal cells accelerate diabetic wound healing by restoring immunological homeostasis.
2021;275: 120963 [PMID: 34153785 DOI: 10.1016/j.biomaterials.2021.120963]
20 Wu QR, Zheng DL, Liu PM, Yang H, Li LA, Kuang SJ, Lai YY, Rao F, Xue YM, Lin JJ, Liu SX, Chen CB, Deng CY.High glucose induces Drp1-mediated mitochondrial fission
the Orai1 calcium channel to participate in diabetic cardiomyocyte hypertrophy.
2021; 12: 216 [PMID: 33637715 DOI: 10.1038/s41419-021-03502-4]
21 Wang S, Yang Y, He X, Yang L, Wang J, Xia S, Liu D, Liu S, Liu W, Duan H. Cdk5-Mediated Phosphorylation of Sirt1 Contributes to Podocyte Mitochondrial Dysfunction in Diabetic Nephropathy.
2021; 34: 171-190[PMID: 32660255 DOI: 10.1089/ars.2020.8038]
22 Giralt-López A, Molina-Van den Bosch M, Vergara A, García-Carro C, Seron D, Jacobs-Cachá C, Soler MJ. Revisiting Experimental Models of Diabetic Nephropathy.
2020; 21 [PMID: 32438732 DOI: 10.3390/ijms21103587]
23 Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy.
2011; 7: 327-340 [PMID: 21537349 DOI:10.1038/nrneph.2011.51]
24 Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, IJpelaar DHT. Macrophages in diabetic nephropathy in patients with type 2 diabetes.
2017; 32: 1322-1329 [PMID: 27416772 DOI:10.1093/ndt/gfw260]
25 Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy:correlation with diabetic state and progressive renal injury.
2004; 65: 116-128 [PMID: 14675042 DOI:10.1111/j.1523-1755.2004.00367.x]
26 Jia LX, Qi GM, Liu O, Li TT, Yang M, Cui W, Zhang WM, Qi YF, Du J. Inhibition of platelet activation by clopidogrel prevents hypertension-induced cardiac inflammation and fibrosis.
2013; 27: 521-530 [PMID:23887740 DOI: 10.1007/s10557-013-6471-z]
27 Wu L, Zhao F, Dai M, Li H, Chen C, Nie J, Wang P, Wang DW. P2y12 Receptor Promotes Pressure Overload-Induced Cardiac Remodeling
Platelet-Driven Inflammation in Mice.
2017; 70: 759-769 [PMID: 28827474 DOI:10.1161/HYPERTENSIONAHA.117.09262]
28 An X, Jiang G, Cheng C, Lv Z, Liu Y, Wang F. Inhibition of Platelets by Clopidogrel Suppressed Ang II-Induced Vascular Inflammation, Oxidative Stress, and Remodeling.
2018; 7: e009600 [PMID: 30608200 DOI:10.1161/JAHA.118.009600]
29 Hanefeld M, Appelt D, Engelmann K, Sandner D, Bornstein SR, Ganz X, Henkel E, Haase R, Birkenfeld AL. Serum and Plasma Levels of Vascular Endothelial Growth Factors in Relation to Quality of Glucose Control, Biomarkers of Inflammation, and Diabetic Nephropathy.
2016; 48: 620 [PMID: 27756080 DOI:10.1055/s-0036-1585504]
30 Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway.
2004; 165: 2033-2043 [PMID:15579446 DOI: 10.1016/s0002-9440(10)63254-3]
31 Sanajou D, Ghorbani Haghjo A, Argani H, Aslani S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions.
2018; 833: 158-164 [PMID: 29883668 DOI: 10.1016/j.ejphar.2018.06.001]
32 Sartain SE, Turner NA, Moake JL. TNF Regulates Essential Alternative Complement Pathway Components and Impairs Activation of Protein C in Human Glomerular Endothelial Cells.
2016; 196: 832-845 [PMID: 26673143 DOI:10.4049/jimmunol.1500960]
33 Manchanda PK, Kumar A, Bid HK, Mittal RD. Interleukin-1beta and receptor antagonist (IL-1Ra) gene polymorphisms and the prediction of the risk of end-stage renal disease.
2006; 11: 164-173 [PMID: 16766392 DOI:10.1080/13547500500525383]
34 Li T, Shen K, Li J, Leung SWS, Zhu T, Shi Y. Glomerular Endothelial Cells Are the Coordinator in the Development of Diabetic Nephropathy.
2021; 8: 655639 [PMID: 34222276 DOI: 10.3389/fmed.2021.655639]
35 Wei TT, Yang LT, Guo F, Tao SB, Cheng L, Huang RS, Ma L, Fu P. Activation of GPR120 in podocytes ameliorates kidney fibrosis and inflammation in diabetic nephropathy.
2021; 42: 252-263 [PMID: 32948825 DOI:10.1038/s41401-020-00520-4]
36 Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy.
2003; 14: 1358-1373[PMID: 12707406 DOI: 10.1097/01.asn.0000065640.77499.d7]
37 Gruden G, Perin PC, Camussi G. Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology.
2005; 1: 27-40 [PMID: 18220580 DOI: 10.2174/1573399052952622]
 World Journal of Diabetes2022年8期
World Journal of Diabetes2022年8期
- World Journal of Diabetes的其它文章
- Loss of skeletal muscle mass is not specific to type 2 diabetes
- Association of rs1137101 with hypertension and type 2 diabetes mellitus of Mongolian and Han Chinese
- Metformin toxicity: A meta-summary of case reports
- In vivo evaluation and mechanism prediction of anti-diabetic foot ulcer based on component analysis of Ruyi Jinhuang powder
- lmproved systemic half-life of glucagon-like peptide-1-loaded carbonate apatite nanoparticles in rats
- Diabetic kidney disease in pediatric patients: A current review
