The potential and the impact factors for phosphorus recovery from wastewater via struvite precipitation*
Erick K. MUTAI, WANG Xuan, MA Lin
(1. Key Laboratory of Agricultural Water Resources, Chinese Academy of Sciences / Hebei Key Laboratory of Soil Ecology / Center for Agricultural Resources Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Shijiazhuang 050022, China;2. University of Chinese Academy of Sciences, Beijing 100049, China; 3. Xiong’an Innovation Institute, Chinese Academy of Sciences, Xiong’an 071700, China)
Abstract:Phosphorous (P) is an essential nutrient for living organisms, and there is a concern regarding the challenges of both supply uncertainty and the linked aquatic eutrophication. Phosphorus recovery through struvite precipitation technology has attracted much attention in research as it prevents eutrophication and forms a slow-release fertilizer that serves as an alternative source of P in both synthetic wastewater (SW) and real waste (RW). However, only inorganic soluble P is involved in the reaction. Therefore, the ratio of organic P, which is up to 30%–40% of total P in real wastewater systems, leads to a variation of P recovery rates in practice.There is a lack of knowledge on the underlying differences and the factors causing the disparity in the P recovery, and few studies have attempted to quantify the gap in the P recovery rates of SW and RW. Data mining was conducted using 103 studies with 1186 observations to quantify the P recovery rate in SW and RW, establish the underlying factors affecting the P recovery, and derive solutions. Results showed that P recovery rate in SW (83.6%) was higher than that in RW (76.9%). A large variation in the P recovery rate(20.4% to 99.9%) in RW was detected since it was more sensitive to pH and foreign ions, such as calcium. Magnesium and calcium were found to impact struvite crystallization; magnesium had a positive impact at a specific Mg∶P ratio between 1 and 2, with SW being more sensitive, while calcium inhibited struvite formation. P precipitation is an abiotic process, and organic P concentration plays a crucial role in the total P recovery rate. Aeration played an important role in the mineralization of organic P. Therefore, supplying aeration at a flow rate of 6 L?min?1 was beneficial for both total and inorganic P recovery. Increasing the Mg∶P ratio was not a crucial factor for P recovery in practice, but it significantly impacts the components of the precipitate.
Keywords:Phosphorus; Recovery; Struvite; Synthetic wastewater; Real wastewater
Phosphorous (P) is an essential nutrient for living organisms, and it is, therefore, essential to ensure adequate supply and address its connection to aquatic eutrophication in the environment (Liu et al., 2016). The increasing consumption, demand, and preferential use of“clean” P rather than “dirty” P have aggravated the oneway journey of P from mineral rock to waste, such as manure, sewage, etc. (Withers, 2019). P recovery from wastewater is essential for preventing eutrophication in waterways (Schindler et al., 2016). Struvite crystallization has attracted much attention in literatures regarding resource recovery from wastewater; it provides an alternative source of P and closes the P cycle through struvite production for its reuse as a slow-release fertilizer to relieve the extensive use of mineral P (Shaddel et al., 2019).
Struvite crystals occur spontaneously in various biological media and were initially discovered inside pipes and pumps transporting waste as early as 1939 (Rawn et al., 1939). Over the last two decades, numerous studies have focused on P precipitation from solution for the subsequent recovery and reuse of P in wastewater (Doyle& Parsons, 2002; Le Corre et al., 2009; Sena et al., 2021). Most struvite crystallization studies have focused on the effects of process variables, such as temperature, foreign ions, and pH, in different wastewater streams. An ideal P recovery rate (almost 100%)can be achieved in a synthetic wastewater system under optimized conditions (pH 7–11) (Matynia et al., 2006) because only insignificant amounts of foreign impurities, such as calcium (Ca), are present (Capdevielle et al., 2013).
Several studies have investigated the recovery of P from dairy (Tao et al., 2016), swine (Vanotti et al., 2018), sewage (Kataki et al., 2016), and other sources of real wastewater systems. However, there are currently few real-world industrial applications. As only inorganic soluble P is involved in the reaction, a wide variation in the P recovery rates, ranging from 69% to 100%, were observed in different real wastewater systems. The fraction of dissolved organic P (DOP) varies from 10% to 80% of the total P in some wastewaters, especially wastewater from livestock farms (Santiviago Petzoldt et al., 2020), and this also affects the P recovery rates.
A gap exists in P removal and recovery efficiencies through struvite precipitation from actual and synthetic wastewater, which is attributed to the differences in their composition. Recent studies have observed that high organic matter content in agro-industrial wastewater is a major barrier hampering the application of P recovery via struvite precipitation (Muhmood et al., 2021). Real wastewater is characterized by high suspended matter and organic matter content (Huang et al., 2016; Zangarini et al., 2020). The organic matter content and its molecular weight influence P recovery from wastewater, and previous studies have reported that high molecular weight organic substances led to lower P recovery rates compared to those with low molecular weight (Wang et al., 2016). The dissolved organic matter in wastewater is composed of an array of recalcitrant organic compounds that apply unique inhibitory effects on struvite formation and growth (Michael-Kordatou et al., 2015; Muhmood et al., 2021; Zangarini et al., 2020); therefore, P recovery is greatly influenced by the mechanism of treatment and reaction parameters (Michael-Kordatou et al., 2015). Optimum reaction parameters for struvite crystallization, including the presence of magnesium (Mg), ammonium, and P at sufficient concentrations, optimum pH, and temperature (Edahwati et al., 2018), are also influenced by organic constituents in wastewater. Dissolved constituents in wastewater are mostly negatively charged and have a high affinity for positively charged ions such as Mgand NH, which play a major role in struvite formation by blocking struvite crystallization and reducing P recovery rate (Capdevielle et al., 2016; Edahwati et al., 2018). The kinetics of struvite crystallization in natural wastewater are negatively influenced by organic matter, even though the size of struvite crystals has a positive relationship with organic matter (Capdevielle et al., 2016).
Although struvite crystallization technology dates back many years and is a promising technique for removing and recovering nutrients from wastewater in the last two decades, there are few instances of real applications.This has created a disconnect between the laboratory studies that use synthetic wastewater and actual implementations in real wastewater systems. Notably, low recovery rates are among the uncertainties that contribute to its limited application. Therefore, data mining was conducted in this study: (i) to assess the P recovery potential from wastewater through struvite formation technology, (ii) investigate the factors affecting the P recovery rate in synthetic and actual wastewater, and (iii) establish possible solutions for improving P recovery and recovery rate of struvite crystallization to increase the potential of implementing this strategy in real nutrient recovery scenarios.
1 Methodology
1.1 Literature search
A systematic search of literatures from peer-reviewed articles, theses, and conference proceedings was performed using the following electronic search engines:PubMed, Google Scholar, Scopus, and Web of Science.The main keywords used for the search include“wastewater treatments” “phosphorus recovery” “phosphorus removal” “struvite crystallization” “l(fā)ivestock wastewater treatment” “nutrient recovery” and “magnesium ammonium phosphate hexahydrate (MAP)” from 1994 to 2021. Studies without sufficient information regarding the P recovery rate from wastewater were excluded, and data cited in the other articles were used to collect objective data relevant to the study.
1.2 Criteria for study selection
For data collection, studies were selected based on the following criteria: (i) synthetic or real wastewater(from livestock, industrial, or sewage plants) should be included in the reaction system; (ii) mechanical or aeration stirring should be applied in the study; (iii) the study should include P recovery rate from wastewater or have initial and final P concentrations of wastewater; (iv) real wastewater should include parameters such as total P and inorganic P; (v) at least one of the other parameters should be included, such as pH, temperature, Mg∶P, and Ca∶P ratios; (vi) the precipitation product should contain mainly struvite/MAP. If data were only presented in figures, they were digitized (using GetData version 2.22) for data extraction. Both inorganic P and total P concentrations were collected, and organic P was calculated based on the difference between inorganic P from total P.
Based on these selection criteria, 103 papers with 1186 observations were obtained. The data included 1084 observations from 97 peer-reviewed journal publications, 61 observations from two Master’s theses, and 41 observations from three conference papers. The data were collected from 28 countries.
1.3 Data categorization
For a better understanding of the impacts of some factors and to compare the P recovery rate from real and synthetic wastewater, the results of the studies were categorized into factors influencing P recovery: pH, agitation type (mechanical and aeration), presence of foreign ions (Ca), molar ratio of Mg to P in wastewater, and organic matter content.
1.4 Calculation
When the rate of P recovery was not provided, it was calculated using the following formula (Wang et al., 2019):
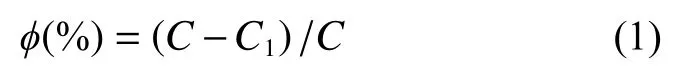
Whereis the rate of phosphate recovery;is the initial total phosphate mass concentration, mg?L;is the final total phosphate mass concentration, mg?L.
The total organic matter (TOM) concentration was calculated from the total organic carbon (TOC) based on the relationship: TOM=1.724 × TOC (Iglesias Jiménez &Pérez García, 1992) when not provided in the data.
2 Results and Discussion
2.1 Properties of wastewater
The increasing accumulation of livestock manure calls for effective handling, and the solid-liquid separation technique is widely employed to facilitate handling large volumes of slurry and ease subsequent treatments such as nutrient recovery through struvite precipitation.Nutrient concentration in various livestock wastes varies with species, as shown in Table1. After the separation of manure into solid and liquid fractions, a high concentration of inorganic P was recorded in the liquid manure, which makes the liquid portion suiTablefor P recovery via struvite crystallization. Organic P exists in the liquid separated from the slurry, and since it is not readily available for retrieval through crystallization, there is an impending need to transform organic P into inorganic P.The conversion of organic to inorganic P forms increases P recovery rate (Wang et al., 2016) and reduces its concentration in the treated water, thereby protecting the environment from P loading. The impacts of the organic P forms were evident in the hypothesized gap in the P recovery rate between synthetic wastewater and real wastewater. To improve P recovery rate in livestock wastewater, it is crucial to understand the underlying factors affecting the difference in the recovery rate between synthetic and real wastewater.
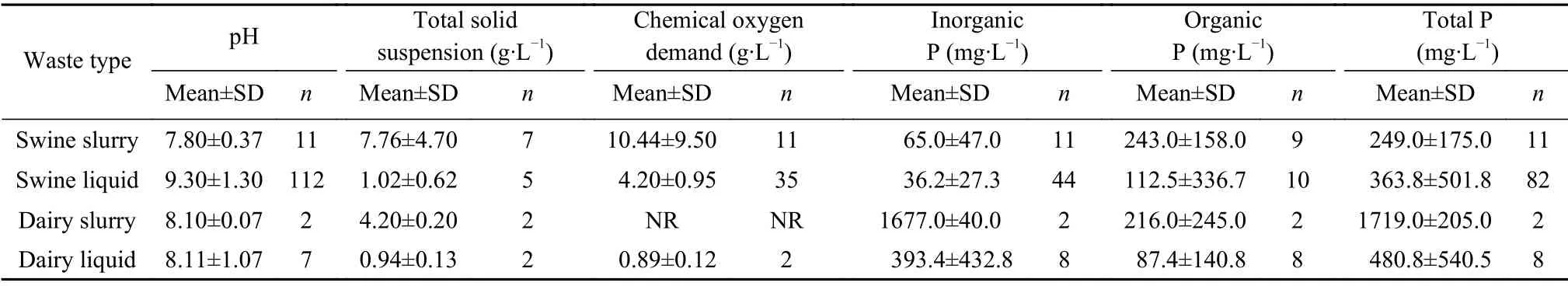
Table1 Distribution of P species and other constituents in livestock slurry and liquid waste
2.2 P recovery rates from synthetic and real wastewater
The study showed that the recovery rate of P was higher in synthetic wastewater than in actual wastewater, as shown in Fig.1. The total P recovery rate was slightly higher in synthetic wastewater. Real wastewater had a complex composition because it contains all P species as well as other nutrients and components, which vary depending on the source. The recovery rate of inorganic P was higher than that of organic P in the real wastewater.
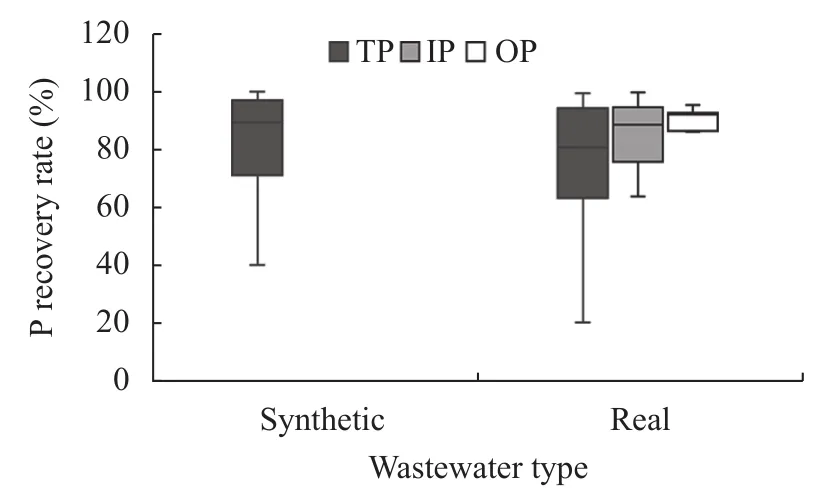
Fig.1 Recovery rates of different P species from both real and synthetic wastewater
Different P species exhibit different reaction dynamics in terms of recovery rate from wastewater. Inorganic P is readily removed by most of the recovery techniques, but organic P is not readily recoverable unless transformed (Venkiteshwaran et al., 2018). Approximately 54% of P in the waste was in the soluble phase and was available for struvite formation, while the remaining portion was in the insoluble or non-reactive form (Stratful et al., 2001).
One mole of struvite requires one mole of each of the participating ions (P, Mg, and ammonium). However, real wastewater is less likely to have this ideal ratio because it contains high concentrations of ammonium ions and relatively low Mg content (Doyle et al., 2000), which affects the crystallization process. The difference in the P recovery rate between synthetic and real wastewater was largely attributed to disparities between their composition and interactions with other factors that drive the crystallization process during struvite formation. Establishing the underlying reasons for this existing gap can provide a breakthrough in improving P recovery from real wastewater systems, boosting the recovery and reuse of P as struvite from livestock wastewater.
2.3 Impact of factors affecting P recovery rate
2.3.1 pH
Wastewater pH affects the crystallization process; struvite formation starts approximately at pH 7 and is optimal in the pH range of 9–11 (Fig.2), and then crystallization decreases at pH > 11. At a higher pH, ammonium ions (NH) can deprotonate into ammonia gas, resulting in ammonia vaporization, which inhibits struvite formation (Gong et al., 2018). The recovery of P at the same pH was higher in synthetic wastewater than in real wastewater. However, the real wastewater system was sensitive to pH change, as a noTableimprovement in P recovery rate was recorded with a slight change in pH. The P recovery rate was higher in the pH range of 9–11 in synthetic wastewater, while in real wastewater, it was higher at pH 8–10.Struvite crystallization and recovery rate from wastewater is reported to increase with increasing pH(Pastor et al., 2008).
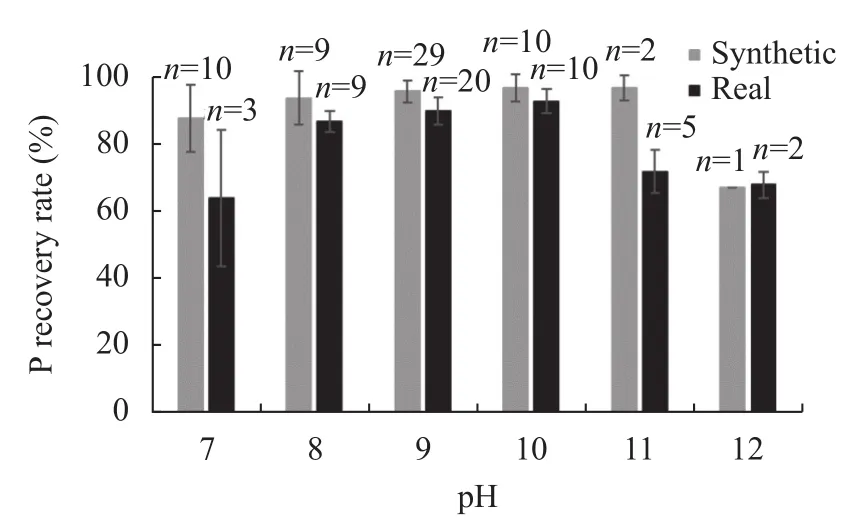
Fig.2 Impacts of pH on P recovery rates from both real and synthetic wastewater
The two most significant pH values crucial for controlling the struvite crystallization process are the pH at which struvite solubility is at its minimum and the pH level where struvite nucleation initiates (Doyle et al., 2000). Struvite precipitation in several studies reported that P recovery occurred in the pH range of 7.0–11.5, and optimum levels were attained at pH 8.0–9.5 (Kim et al., 2017). The optimum pH for struvite precipitation in real wastewater and synthetic wastewater is 9.0 (Cerrillo et al., 2015) and 10.0 (Gong et al., 2018), respectively, and struvite nucleation begins at pH 7.6 (Doyle et al., 2000).
Wastewater pH significantly affects magnesium ammonium phosphate (MAP) precipitation and influences the ratio of specific chemical constituents that participate in the reaction process (Cusick & Logan, 2012; Lee et al., 2015). The solution pH determines the equilibrium concentration and type of MAP forming ions, and the recovery rates of both nitrogen (N) and P increase rapidly with an increase in pH (Gong et al., 2018).
2.3.2 Mg/Ca∶ P ratio
Struvite crystallization is only possible in the presence of Mg ions, with a mole ratio equal to or higher than that of P in wastewater. The ions participating in the formation of struvite molecules included Mg, ammonium, and P. Fig.3a shows that the impact of the molar ratio of Mg to P was significant in driving the process of struvite precipitation. Low Mg∶P mole ratios result in less struvite formation, and as the concentration of Mg increases, the struvite formation rate increases (Liu et al., 2011). When the optimum level was reached, a further increase in Mg concentration did not significantly influence the P recovery rate. The optimum ratio of Mg∶P was observed to be 1.5 for actual wastewater and above 2.0 for synthetic wastewater.
Ca and Mg ions participate in the adsorption of P from wastewater through electrostatic interactions, leading to the formation of Ca–P or Mg–P complexes, which are critical in removing P (Fang et al., 2020).The presence of Ca ions in the wastewater significantly influences the P recovery rate (Lee et al., 2015). Under increased Ca concentrations in the synthetic wastewater system, P recovery exhibited a slow and steady decline, as shown in Fig.3b. In real wastewater systems, the same trend was recorded where a decline in P recovery as struvite was observed with an increase in Ca∶P ratio from 0 to 2, and a further increase above 2, showed a slight increase in P recovery rate, which can be attributed to the formation of Ca–P crystals. Ca in the wastewater inhibits the crystallization of struvite as it competes with Mg to bind P, leading to the formation of calcium phosphate.
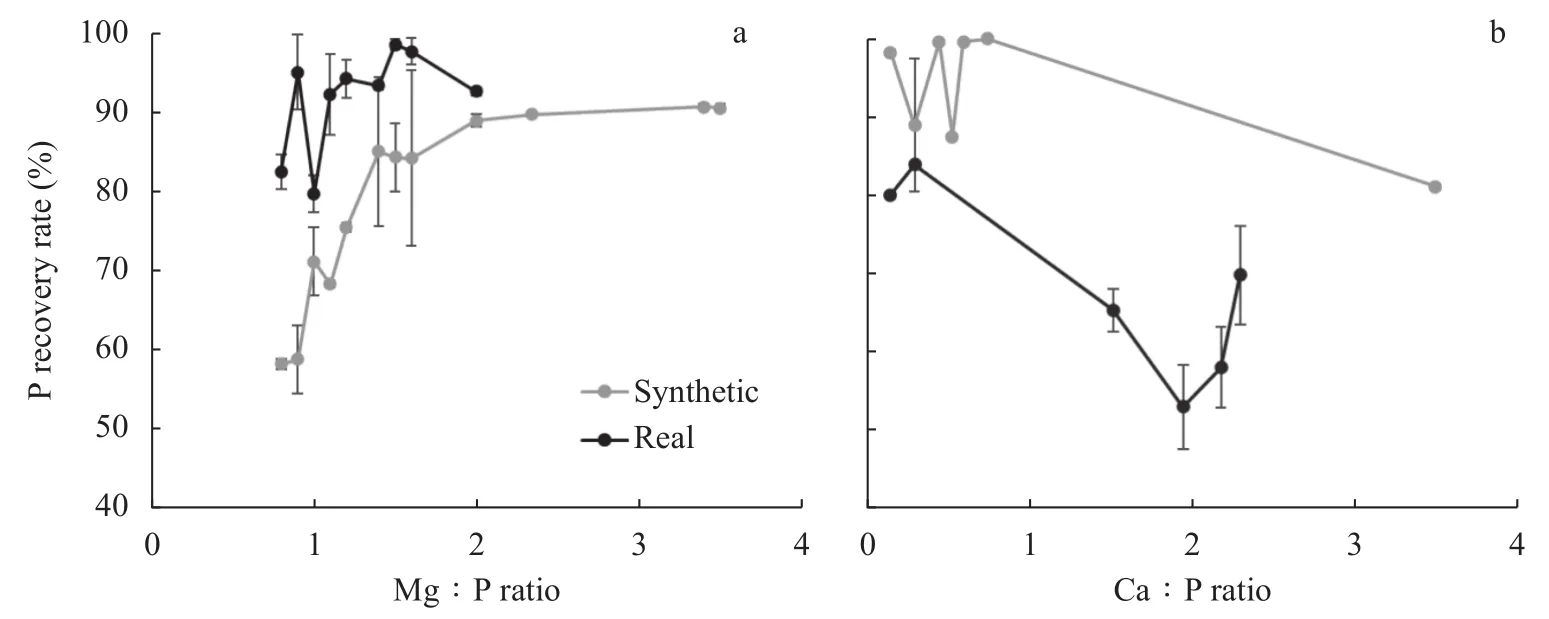
Fig.3 Effect of mole ratios of Mg∶P (a) and Ca∶P (b) on P recovery rate from both real and synthetic wastewater
Low Ca∶P mole ratios inhibit P recovery through induced hydroxyapatite (HAP). Although it enhances P recovery through struvite formation (Zou & Wang, 2017), interfering ions such as Cain wastewater affect the pH range for struvite precipitation, thereby affecting P recovery (Kim et al., 2017). They also contribute to the formation of fines, which are discharged with effluents, thereby negatively impacting the recovery rate of P (Pastor et al., 2008). Less struvite was realized when Ca participated in the reaction, lowering the recovery of P as struvite crystals.
2.3.3 Mechanical agitation
Agitation during struvite crystallization affected the recovery rate of P, as shown in Fig.4. There was a positive correlation between the stirring speed and P recovery rate; increasing the stirring speed from 0 rpm to 200 rpm increased the P recovery rate in synthetic wastewater, whereas in real wastewater, increase from 200 rpm to 300 rpm showed an increase in P recovery. A high mixing rate tends to lower the P recovery rate. Stirring creates a hydraulic shear force that causes the disintegration of struvite crystals, leading to the formation of fines(Morales et al., 2019) and lowering the P recovery rate.
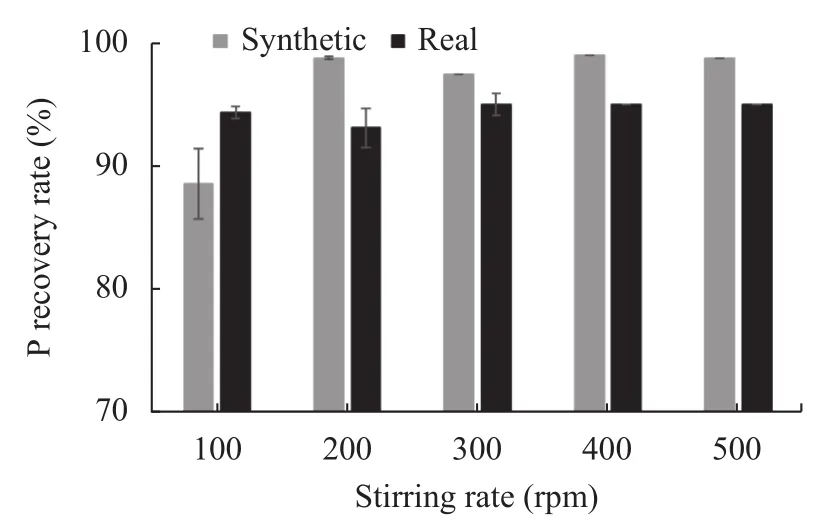
Fig.4 Impact of mechanical agitation speed on P recovery rate from both real and synthetic wastewater
The maximum particle size of the struvite precipitates decreased as the stirring speed increased from 100 rpm to 200 rpm (Cerrillo et al., 2015). There is a strong correlation between stirring speed and particle size distribution (Morales et al., 2019). The mechanical stirring rate had a distinct impact on the struvite particle size in both synthetic and real wastewater. Mixing at 100 rpm in real wastewater led to the development of more extended crystals than when mixing at the rate of 200 rpm; on the other hand, longer crystals were obtained in synthetic wastewater at 200 rpm when compared to those formed at 100 rpm (Cerrillo et al., 2015). Synthetic wastewater systems showed a significant improvement in P recovery with increasing stirring rate compared with real wastewater systems.
2.3.4 Aeration rate
Aeration facilitated the recovery of nutrients from the wastewater, as shown in Fig.5. An increase in the aeration flow rate increased the recovery of P up to certain optimum level. Further increase in aeration led to a decrease in the P recovery rate. An increase in aeration flow rate from 0–6 L?minresulted in an increase in total P recovery from 60% to 94%.However, further increasing the aeration rate resulted in a decline in total P recovery. An increase in aeration flow rate from 2–4 L?minled to an increase in inorganic P recovery rate from 56% to 74%, and a further increase resulted in a decline in the recovery rate.
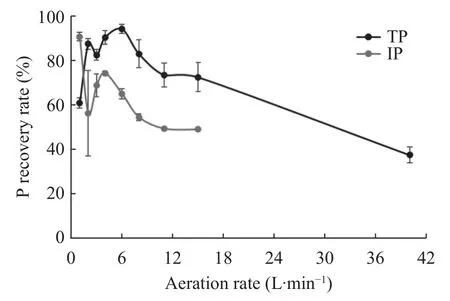
Fig.5 Impact of aeration rate on the recovery of both total P (TP) and inorganic P (IP).
Aeration facilitates carbon dioxide stripping, which buffers the pH of the wastewater significantly (Lee et al., 2015) and facilitates the fluidization of seed crystals, thereby promoting struvite formation (Zou & Wang, 2017). Increasing the airflow rate decreases the liquid density and enhances the circulation of small particles, which encourages crystallization and subsequent crystal growth, thereby improving P recovery (Tarragó et al., 2016). A high aeration rate inhibited struvite formation by inducing the dissolution of the crystals due to their interaction with a fresh solution, decreasing the P recovery rate (Casoni et al., 2018). High circulation rates lead to the formation of fines and break the crystals that are formed; these fines can be discharged with the effluent and lower the P recovery. However, other studies have circumvented this limitation by increasing the hydraulic retention time, thereby allowing time for the sedimentation of the crystals (Ueno & Fujii, 2001). The total P recovery rate enhancement with increased aeration rate can be attributed to the transformation of organic P to inorganic P forms, which are easily recoverable. Aeration facilitated organophosphorus transformation facilitating P recovery in real wastewater systems.
2.3.5 Organic matter
The presence of organic matter in wastewater plays a critical role in nutrient recovery, as shown in Fig 6.There was a positive correlation between total organic matter and P recovery during wastewater treatment. In swine wastewater treatment, the total P recovery rate was correlated with the total organic matter recovery, with anvalue of 0.963. The recovery rates of P in wastewater having suspended solids was enhanced through sedimentation, and the bound P accumulated as sediments.However, the presence of suspended solids in wastewater had an inhibitory effect on struvite precipitation, and the recovery of the solid suspensions prior to struvite precipitation enhanced the process (Muhmood et al., 2019).Organic matter in wastewater exists as particulate(>50 nm), colloidal (2 –450 nm), and dissolved(<2 nm) form (Jin et al., 2017) and impacts P recovery from different perspectives either by inhibiting or promoting the process dynamics and also by influencing the morphological characteristics and quality of the resulting crystals (Wang et al., 2016).
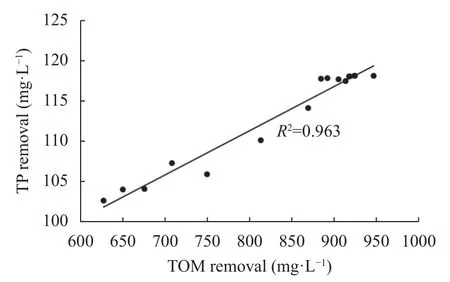
Fig.6 Relationship between total P (TP) removal and total organic matter (TOM) in swine wastewater
As demonstrated in previous studies, high molecular weight organic matter concentrations have shown an inhibitory effect on the struvite crystallization process. It can affect the size and shape of the crystals as they coprecipitate with struvite, and low molecular weight organics facilitate struvite formation through adsorption of Ca, thereby blocking its competitive P binding with magnesium ions (Wang et al., 2016). Organic matter (dissolved and particulate) slows down the increase in wastewater pH during treatment (Capdevielle et al., 2016). Previous studies have shown that in the absence of natural organic matter, some nutrient recovery systems are efficient for phosphate recovery over a wide pH range between 4–10. However, at pH 10, less P recovery rate was recorded compared to that in the presence of natural organic matter. Under higher pH, natural organic matter showed negligible inhibition of struvite crystallization (Lei et al., 2018).
3 Conclusion and Recommendation
Data mining was conducted by using 103 studies with 1186 observations to quantify the P recovery rate from wastewater. The results indicated that there was a gap in the total P recovery rate between real and synthetic wastewater. Synthetic wastewater systems exhibited a higher recovery rate (84%) compared to that in real wastewater systems (77%). P recovery in synthetic and real wastewater occurs at pH 7 to 12, with an optimum recovery rate at pH 10 for synthetic wastewater and pH 9 for real wastewater. In the SW system, P recovery rate increased from 58% to 89% when the Mg∶P mole ratio was between 1 and 2. In contrast, the dependency of P precipitation on Mgwas reduced in the RW system. Organophosphorus transformation is crucial for improving P recovery, particularly from real wastewater. Aeration accelerated the mineralization of organic P, improving the total P recovery rate from 61% to 94% when aeration flow rate increased from 0–6 L?min.
The impact factors of P recovery through struvite crystallization drive the process in both real and synthetic wastewater systems; however, the two systems showed varying degrees of response. The real wastewater system exhibited high sensitivity to pH change, Ca∶P ratio, and aeration rate. Aeration is necessary to improve the P recovery rate. A good understanding of the dynamics of the struvite crystallization process is required for successful implementation. Detailed experiments are needed to investigate the mechanism of aeration on the organic/inorganic P recovery efficiency through struvite crystallization in real wastewater. This study provides an important theoretical basis and practical guidance for P recovery from wastewater.

