Effects of delayed repair of peripheral nerve injury on the spatial distribution of motor endplates in target muscle
Dong-Dong Li,Jin Deng,Bo JinShuai HanXin-Yi GuXue-Feng Zhou,Xiao-Feng Yin
Abstract Motor endplates (MEPs) are important sites of information exchange between motor neurons and skeletal muscle,and are distributed in an organized pattern of lamellae in the muscle. Delayed repair of peripheral nerve injury typically results in unsatisfactory functional recovery because of MEP degeneration. In this study,the mouse tibial nerve was transected and repaired with a biodegradable chitin conduit,immediately following or 1 or 3 months after the injury. Fluorescent α-bungarotoxin was injected to label MEPs. Tissue optical clearing combined with light-sheet microscopy revealed that MEPs were distributed in an organized pattern of lamellae in skeletal muscle after delayed repair for 1 and 3 months. However,the total number of MEPs,the number of MEPs per lamellar cluster,and the maturation of single MEPs in gastrocnemius muscle gradually decreased with increasing denervation time. These findings suggest that delayed repair can restore the spatial distribution of MEPs,but it has an adverse effect on the homogeneity of MEPs in the lamellar clusters and the total number of MEPs in the target muscle. The study procedures were approved by the Animal Ethics Committee of the Peking University People’s Hospital (approval No. 2019PHC015) on April 8,2019.
Key Words: degeneration; delayed repair; lamellar cluster; light-sheet microscopy; motor endplates; peripheral nerve injury; threedimensional distribution; tissue optical clearing
Introduction
Peripheral nerve injury (PNI) is a common clinical condition. Because of the long distance between the target muscle and lesion site,delay in nerve repair,and the slow regeneration rate of the injured axons,it is difficult to effectively reconnect motor nerves with their target muscles in the clinical setting (Pisciotta et al.,2020; Zhang et al.,2021). With prolongation of denervation time,motor endplates (MEPs) progressively degenerate (Kobayashi et al.,1997; H?ke,2006),losing the capacity for information exchange between motor neurons and muscle fibers.
MEPs are important sites where motor neurons transform electrophysiological signals into chemical signals to control skeletal muscle activity. As neuromuscular junctions mature,acetylcholine receptor (AChR) clusters become perforated and complex,resembling pretzels with arrays or branches that are innervated by one axon per neuromuscular junction (Li et al.,2018). MEPs consist of AChRs possessing a high metabolic stability (half-life ≈ 10 days),and are induced and maintained by innervations (Bezakova et al.,2001). Muscle activity and lipid rafts also play important roles in maintaining the metabolic stability of AChRs (Rotzler et al.,1991; Bryndina et al.,2018). However,the AChRs become unstable (half-life ≈ 1 day) in denervated muscles after nerve injury because of denervation,loss of muscle activity,and perturbed lipid metabolism (Chao et al.,2013). The unstable AChRs impair the structural integrity of MEPs. AChR clusters gradually fragment and spread over a larger area without innervation after PNI (Magill et al.,2007; Slater,2020). Changes in the morphology of MEPs adversely affect the recovery of motor function (Sleigh et al.,2014a,b). Previous studies have investigated morphological and functional changes in single MEPs after PNI (Sleigh et al.,2014a; Slater,2020).
Using tissue optical clearing methods,our recent studies (Chen et al.,2016; Yin et al.,2019) focused on the spatial distribution of MEPs in intact muscle. We showed that MEPs in skeletal muscles are distributed in an organized pattern of lamellar clusters. The MEPs are concentrated around thinlayer areas (i.e.,lamellar clusters) in skeletal muscles. Each skeletal muscle has one or more distinctive MEP lamellar cluster. Based on the lamellar cluster,the intact muscle can be divided into one or more independent contractile units. We showed that the spatial distribution of MEPs is a key factor for the contraction of skeletal muscle. However,because of the limitations of the labeling and imaging methods for MEPs,the change in the spatial distribution of MEPs in intact skeletal muscle after delayed repair remained unknown. Therefore,in this study,we investigate the effects of delayed repair on the three-dimensional distribution of MEPs after PNI.
Materials and Methods
Animals
Forty-two female wild-type C57BL/6J mice (weighing 22-25 g,aged 6-8 weeks),bred in the Laboratory Animal Centre of Peking University People’s Hospital (animal use license No. SYXK (Jing) 2016-0009),were used in the study. The study procedures were approved by the Animal ethics Committee of the Peking University People’s Hospital (approval No. 2019PHC015) on April 8,2019. All experiments complied with the ARRIVE guidelines,and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 85-23,revised 1996). The animals were maintained on standard mice chow and waterad libitum,under a 12/12-hour light/dark cycle and standardized housing conditions before and after operation. The mice were randomly divided into the following four groups: immediate repair group (n= 6; tibial nerve transection and immediate repair); 1-month delay group (n= 10; tibial nerve transection and repair after a 1-month delay); 3-month delay group (n= 20; tibial nerve transection and repair after a 3-month delay); and control group (n= 6; no damage to tibial nerve).
PNI modeling
Denervation of the target muscle
The animals were anesthetized using 1.5% isoflurane (RWD Life Science Co.,Shenzhen,China) inhalation. All surgeries were performed on the right tibial nerves,using standard microsurgical techniques under aseptic conditions. First,the tibial nerve,peroneal nerve and sural nerve were exposed and freed by gentle dissection. The right tibial nerves were transected approximately 3 mm distal to the bifurcation point of the sciatic nerve. Second,according to the previously described protocol (Wu et al.,2013),the proximal nerve stump was ligated and embedded into the nearby muscles by 7-0 nylon stitch. The distal nerve stump was straightened and sutured to neighboring muscles with 7-0 nylon to prevent retraction. At the end of the operation,the wound was closed in layers by 5-0 nylon sutures. Buprenorphine hydrochloride injection (Qinghai Pharmaceutical Factory Co. Ltd.,Qinghai,China),0.01 mg/kg,was given subcutaneously once immediately after the surgery and daily in the first 3 postoperative days. Diclofenac sodium (10 mg/L in drinking water; Novartis,Beijing,China) was given by oral administration for the first 7 postoperative days.
Reinnervation of the target muscle
The tibial nerve stumps were re-exposed for secondary anastomosis according to group assignment. The proximal and distal nerve stumps were freed,and the neuroma of the proximal stump and the scar of the distal stump were trimmed. Then,the proximal and distal stumps were inserted 1 mm into a biodegradable chitin conduit (made by our laboratory) using 11-0 nylon stitch. The gap between the proximal and distal stumps was approximately 2 mm. The biodegradable chitin conduit (tube length 4 mm,thickness 0.1 mm,inner diameter 0.6 mm) is a biocompatible and biodegradable artificial nerve graft,as described previously (Yu et al.,2016). No tension at the repair site and the ability to withstand maximal passive exercises with 11-0 sutures without tearing was considered successful nerve repair (Wu et al.,2013). The surgery site was then closed in layers. Buprenorphine hydrochloride injection and diclofenac sodium were given as described above for postoperative pain control.
Electrophysiological examination
After 3 months of reinnervation,a Medlec Synergy electrophysiological system (Oxford Instruments Inc.,Oxford,UK) was used to record compound muscle action potentials (CMAPs) from the gastrocnemius muscle in control (n= 6),immediate repair (n= 6),1-month delay (n= 6),and 3-month delay (n= 7) groups. The repaired nerve was exposed under anesthesia with sodium pentobarbital (Merck,Darmstadt,Germany; 30 mg/kg,intraperitoneal injection) at the experimental side,and then the stimulating bipolar electrode,recording electrode and ground electrode were placed in the proximal nerve trunk,gastrocnemius and thigh muscle,respectively. Rectangular pulses (duration 0.1 ms,intensity 0.06 mA,frequency 5 Hz) were applied to acquire CMAPs.
Osmium tetroxide staining and examination of the tibial nerve
The distal tibial nerves 2 mm distal to the repair site from control (n= 6),immediate repair (n= 6),1-month delay (n= 6),and 3-month delay (n= 7) groups were obtained after electrophysiological examination to assess nerve regeneration. The nerves were fixed in 1% w/v osmium tetroxide in double distilled water for 12 hours,sequentially dehydrated in 50%,70%,90% and 100% ethyl alcohol,and embedded in paraffin. Consecutive 2-μm-thick transverse sections were cut using a microtome (Leica RM2135,Wetzlar,Germany). The nerve sections were mounted onto slides and imaged under a microscope (Leica DM4B with Leica Application Suite X (LAS X) software,Wetzlar,Germany) with a 20× objective to count the number of myelinated axons.
Labeling of MEPs and tissue optical clearing
To observe the structure of regenerating MEPs,Alexa Fluor647-Bungarotoxin (Invitrogen,Carlsbad,NY,USA) was injected through the caudal vein to label MEPs after electrophysiological examination (Chen et al.,2016). First,1 hour after α-BTX injection (0.3 μg/g),the mice in control (n= 6),immediate repair (n= 6),1-month delay (n= 6),and 3-month delay (n= 7) groups were perfused transcardially with normal saline solution followed by 4% w/v paraformaldehyde in 0.01 M phosphate-buffered saline. Second,the intact gastrocnemius muscle was dissected and postfixed with 4% paraformaldehyde at 4°C overnight. Third,optical clearing was performed on the postfixed muscle using the 3DISCO technique (Ertürk et al.,2012; Wan et al.,2018). The postfixed muscle was dehydrated sequentially in 50%,70%,80% and 100% tetrahydrofuran (Sinopharm Chemical Reagent Co.,Ltd.,Beijing,China),and then treated with 100% dibenzyl ether (Sigma-Aldrich,St. Louis,MO,USA) to achieve complete transparency. The sample was shaken for 2.5 hours at 4°C during each clearing step.
Confocal microscopy observation
The transparent muscle in control (n= 6),immediate repair (n= 6),1-month delay (n= 6),and 3-month delay (n= 7) groups was imaged on a cover glass under an inverted confocal microscope (Zeiss LSM710,Oberkochen,Germany). The fluorescent signals of MEPs were observed with a 647-nm excitation wavelength. Three microscopic fields were randomly selected in every muscle to obtain 40-μmthick stacks in the Z-axis (1-μm steps) with a 10× objective. The two-dimensional images of MEPs were obtained by maximum-intensity projection (MIP) reconstruction using ImageJ software (National Institutes of Health,Bethesda,MD,USA). The MIP image was used to assess the maturation of MEPs by measuring their area and counting perforations. A perforation is defined as a region where there is no observable α-bungarotoxin staining within the MEP.
Spatial distribution of MEPs
To observe the spatial distribution of MEPs in gastrocnemius muscle,the intact muscles were imaged under a lightsheeting ultramicroscope (LaVisionBioTec,Bielefeld,Germany) equipped with MV PLAPO 2X/0.5 dry objective (W.D. 20 mm). The fluorescence image of MEPs (633 nm) in control (n= 6),immediate repair (n= 6),1-month delay (n= 6),and 3-month delay (n= 7) groups was captured by light-sheet microscopy. The Z-axis step size was 5 μm. The acquired images were analyzed using Imaris software (Bitplane,Zurich,Switzerland) to reconstruct the spatial distribution of MEPs and count their number. To obtain the cross-sectional images of the gastrocnemius and measure the width of the lamellar clusters,we resampled the image stacks,and the MIP of Z-stacks (thickness = 250 μm) were made with ImageJ. There are four lamellar clusters of MEPs (medial MEP lamella,and lateral MEP lamellae (LML) 1-3) on cross-sections of the gastrocnemius (Yin et al.,2019) (Figure 1). For each MEP lamella,to calculate the width,we selected one crosssectional MIP image of the proximal end,middle portion and distal end of the gastrocnemius. We took the average width of these three images as the MEP lamellar width.
Statistical analysis
SPSS 20.0 software (IBM,Armonk,NY,USA) was used for statistical analysis. One-way analysis of variance followed by the Student-Newman-Keuls test was used for two-group comparison and multi-group comparison,respectively. All data are expressed as mean ± standard deviation (SD),and statistical significance was defined asP< 0.05.
Results
General condition of the mice with delayed repair after PNI
There were six mice in the immediate repair group (100% success rate),six in the 1-month delay group (60% success rate) and seven in the 3-month delay group (35% success rate) that had successful repair without tension. All of the mice survived and had a good nutritional status after the second surgery. However,there were varying degrees of muscle atrophy in the right hind limbs of mice in the repair nerve groups.
Electrophysiological examination of the gastrocnemius in mice with delayed repair after PNI
Three months after nerve repair,gastrocnemius CMAPs were recorded in each group. The waveforms in the repair groups (immediate repair,1-month delay,and 3-month delay) were the same as that in the control group (Figure 2). The amplitudes of the gastrocnemius CMAPs were 16.0 ± 1.6,10.4 ± 1.1,1.2 ± 0.5 and 28.5 ± 3.1 mV in the immediate repair,1-month delay,3-month delay and control groups,respectively. CMAP amplitudes in the four groups were significantly different (P< 0.05).
Pathology of the tibial nerve in mice with delayed repair after PNI
Osmium tetroxide staining revealed regenerated myelinated axons in the distal tibial nerve (Figure 3). The number of regenerated axons decreased with increasing denervation time. Except for the immediate repair and 1-month delay groups,there were significant differences (P< 0.05) in the number of regenerated axons among the groups.
Regeneration of MEPs in the gastrocnemius of mice with delayed repair after PNI
Confocal microscopic images revealed the structure of regenerating MEPs. The shape of MEPs in the gastrocnemius appeared uniformly pretzel-shaped in the immediate repair and control groups,whereas the shapes of the MEPs in the 1-month delay and 3-month delay groups were irregular and the fluorescent signal was heterogeneous (Figure 4A-D). The mean area of MEPs in the 3-month delay group was significantly smaller than those in the immediate repair and control groups (P< 0.05;Figure 4E). There were no differences between the immediate repair and control groups (P> 0.05). We also assessed maturation of MEPs by counting perforations in single MEPs,and found significant difference among the four groups (P< 0.05;Figure 4F). The maturity of MEPs gradually decreased with increasing denervation time.
Spatial distribution of MEPs in the gastrocnemius of mice with delayed repair after PNI
To assess whether delayed repair influences the spatial redistribution of MEPs in gastrocnemius muscle,light-sheet microscopy was used to observe structural changes. Threedimensional reconstruction (Additional Video 1) showed that the regenerated MEPs were also distributed within lamellar clusters after delayed repair (Figures 5and6A-D). However,the homogeneity of MEPs and lamellar cluster dimensions were changed in the 3-month delay group. The width of the lamellar clusters on cross-sections of the gastrocnemius in the 3-month delay group was larger than in the other three groups (P< 0.05). However,there was no difference in width of the lamellar clusters in cross-sections of the gastrocnemius among the immediate repair,1-month delay and control groups (P> 0.05;Figure 6E). Moreover,we counted the MEPs in each group. This revealed differences among the four groups (P< 0.05;Figure 6F). To examine MEP regeneration in the lamellar clusters,we further counted the MEPs per lamellar cluster. As with the number of total MEPs,the number of MEPs per lamellar cluster also gradually declined with increasing denervation time (P< 0.05;Figure 6G),and the rate of decline was different among the lamellar clusters in the 3-month delay group (Table 1).

Figure 1|Schematic diagram of the measurement of the width of MEP lamellar clusters on cross-sections of the gastrocnemius.
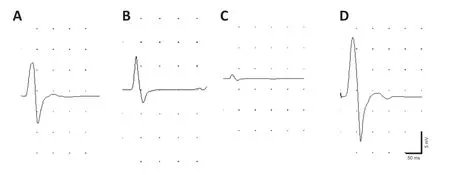
Figure 2|Electrophysiological examination of gastrocnemius muscles in mice with delayed repair after peripheral nerve injury.
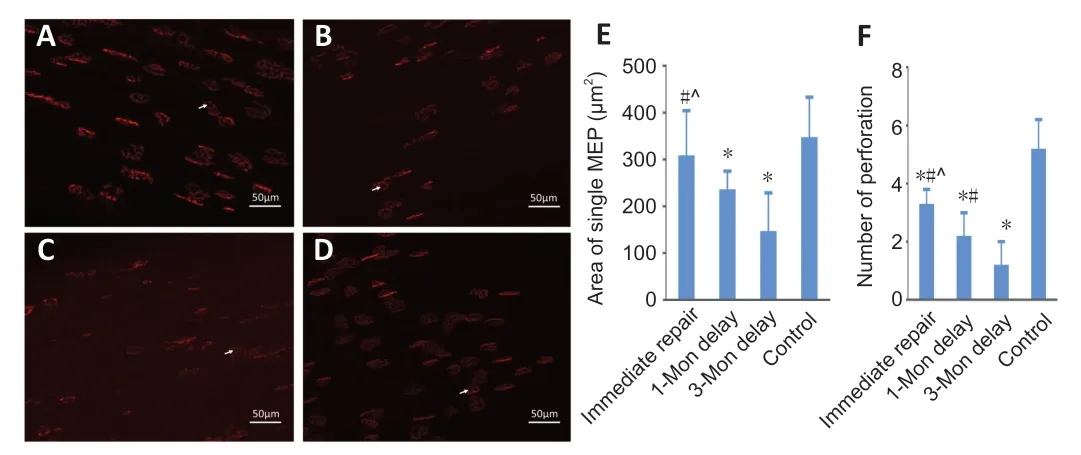
Figure 3|Osmium tetroxide staining of the regenerated tibial nerve in the gastrocnemius of mice with delayed repair after peripheral nerve injury.
Figure 4|Confocal microscopy of the MEPs in the gastrocnemius of mice with delayed repair after peripheral nerve injury.

Figure 5|Threedimensional reconstruction of MEPs in the gastrocnemius in the different groups of mice with delayed repair after peripheral nerve injury.
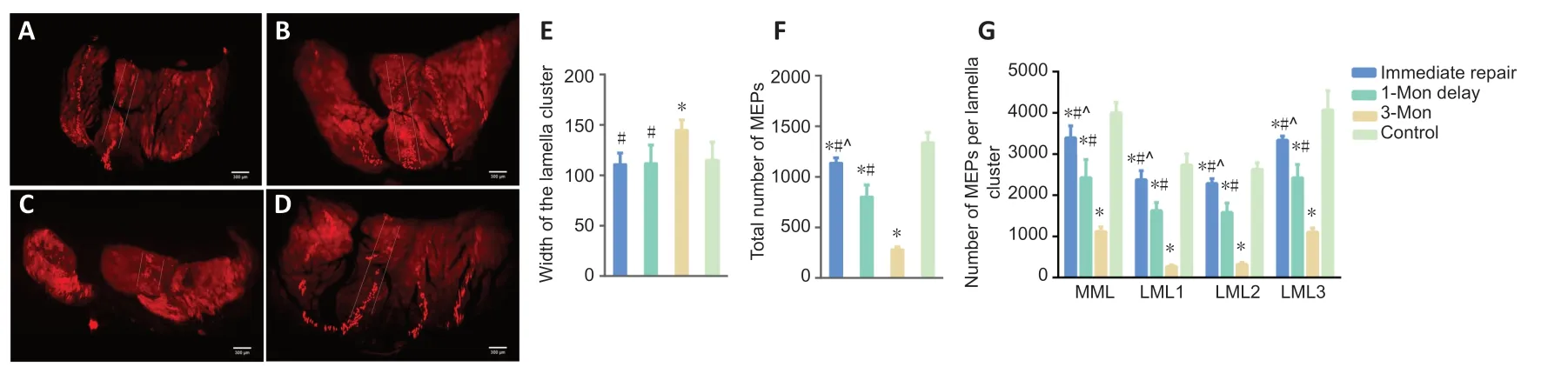
Figure 6|Cross-sectional images of MEPs in the gastrocnemius in the different groups of mice with delayed repair after peripheral nerve injury.
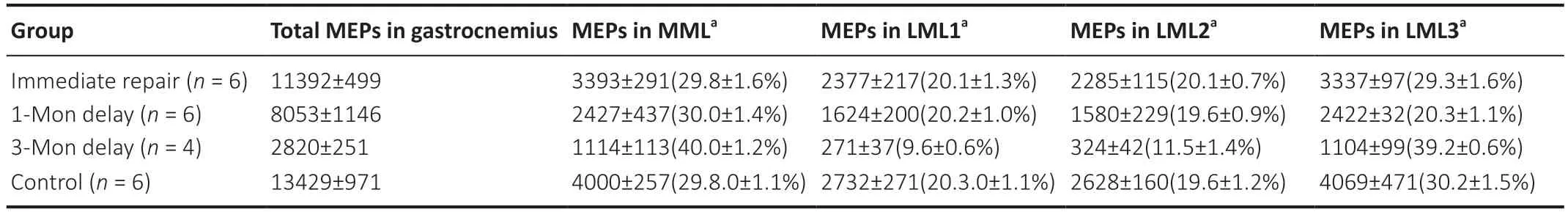
Table 1|The number and ratio of MEPs per lamellar cluster
Discussion
In this study,to further explore the regeneration of MEPs after PNI,a delayed tibial nerve repair model was successfully established with a biological conduit small gap sleeve bridging as secondary repair (Zhang et al.,2015). The 52% overall success rate of tension-free direct repair was lower than that reported by Wu et al. (2013). In their study,the Lewis rat sciatic nerve was transected and maintained for 0,1,4,6,8 or 12 weeks before being repaired through anastomosis. A successful repair was defined when the gap was shorter than 4.0 mm and the stumps could be reapproximated with 10-0 stitches without detachment. We postulated that the lower success rate in our study might be associated with the shorter lengths of the nerves in mouse compared with rat. In this study,we found that delayed repair partially restored motor function in the mice. However,the CMAPs and number of regenerated axons were significantly reduced by prolonged denervation. These data are in accordance with those reported previously (Kobayashi et al.,1997; Jiao et al.,2015).
In this study,a novel and convenient approach combining MEP labeling with the optical clearing technique (Chen et al.,2016) was used to explore the number and spatial distribution of MEPs after delayed repair. The technique is simple and efficiently obtains the 3D distribution of MEPs. However,the technique cannot display the 3D distributionin vivobecause it requires a series of chemical treatments on intact musclein vitro. A previous study reported a method of labeling and imaging MEPsin vivo(Martinez-Pena y Valenzuela et al.,2010). Their method allows observing changes in MEPsin vivo,but it only displays superficial MEPs in muscle.
In the present study,we found that regenerated MEPs were also distributed within lamellar clusters after delayed repair. However,the homogeneity of MEPs in the lamellar clusters changed in the 3-month delay group compared with the immediate repair,1-month delay and control groups. The reason may be that the remaining MEP fragments cannot guide axons accurately after long-term denervation. Previous studies have demonstrated that the arrangement of AChRs in skeletal muscles is important for guiding axons to innervate MEPs and promote the development of neuromuscular junctions in the developmental stage (Flanagan-Steet et al.,2005; Panzer et al.,2005; Kim and Burden,2008; Jing et al.,2009). However,long-term denervation typically contributes to the degeneration of MEPs (Shyng and Salpeter,1989; Bezakova et al.,2001),which may disrupt the arrangement of AChRs. In addition,atrophy makes the muscle unable to undergo reinnervation after long-term denervation. The degradation and fibrosis of the distal nerve stumps is another factor that impedes MEP regeneration. After nerve injury,distal myelin sheaths and nerve terminals undergo a series of cellular and molecular biological alterations and gradually cause degeneration (Benito et al.,2017; Gomez-Sanchez et al.,2017). The synthesis of fibrous tissue acts as a barrier to axonal growth and MEP reinnervation (Pellegrino and Spencer,1985; Virtanen et al.,1992). Distal nerve segments become less conducive to axonal regeneration and cannot provide pathways for regenerated axons to reconnect MEPs effectively after delayed repair (Aydin et al.,2004; Jonsson et al.,2013). Studies show that lipids,especially cholesterol,are important for maintaining the stability of MEPs. However,after denervation,skeletal muscle starts to atrophy,and the lipid content decreases (Bouteloup et al.,2009),which may also contribute to the fragmentation of MEPs. It has been reported that administering simvastatin after nerve injury reduces cholesterol to promote the recovery of neurological function (Xavier et al.,2012; Morishita et al.,2014). Therefore,the effect of cholesterol on MEPs in denervated skeletal muscle remains unclear and needs to be studied systematically. The combination of adverse factors affects the homogeneous distribution of regenerated MEPs. Therefore,to restore the spatial distribution of regenerated MEPs and their number,nerve terminals should be reconnected to the MEPs within 3 months after nerve injury.
In this study,we also found that long-term denervation negatively affects the maturation of regenerated MEPs. Efficient acetylcholine release and receptor binding depends on MEP maturation (Marques et al.,2000; Sleigh et al.,2014a,b),which is important to the functional recovery of target muscles after delayed repair (Miyamaru et al.,2008). The total number of MEPs gradually decreased as denervation time increased. The decline in the maturation and number of MEPs may be a critical causative factor in target muscle dysfunction after PNI.
There are some limitations to this study. For example,the relationship between the spatial distribution of MEPS and functional recovery was not specifically assessed. Additionally,we only investigated delayed repair of less than 3 months after nerve injury. Delayed repair of longer duration may be required to clarify the long-term effects of denervation on regenerated MEPs. Our previous study showed that the distribution of MEPs is constant in mice of different sexes (Buhtz et al.,1989; Yin et al.,2019). In this study,female mice were used because they have smaller muscles than male mice,and thus require less time for tissue optical clearing. Furthermore,the success rate of delayed repair was lower than immediate repair; the longer the time of delayed repair,the lower the success rate. Thus,the numbers of mice in the four groups were not the same; however,this should not have affected the results.
In summary,we investigated the changes in the number and spatial distribution of regenerated MEPs after delayed repair following nerve injury. Our findings show that delayed repair restores the spatial distribution of MEPs,but adversely affects the homogeneity of MEPs and lamellar MEP clusters in mice.
Acknowledgments:The authors wish to thank Dan Zhu,Ting-Ting Yu and Jian-Yi Xu of the Britton Chance Center for Biomedical Photonics,Wuhan National Laboratory for Optoelectronics,Huazhong University of Science and Technology for their technical support.
Author contributions:Study design: XFY and XFZ; experimental implementation: DDL and JD; data analysis: BJ,SH and XYG; paper writing: DDL. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support:This study was supported by the National Natural Science Foundation of China,Nos. 82072162 (to XFY),81971177; and the Natural Science Foundation of Beijing of China,No. 7192215 (to XFY). The funding sources had no role in study conception and design,data analysis or interpretation,paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by Animals thics Committee of the Peking University People’s Hospital (approval No. 2019PHC015) on April 8,2019.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Zoya Katarova,Institute of Experimental Medicine,Hungarian Academy of Sciences,Hungary.
Additional files:
Additional file 1:Open peer review report 1.
Additional Video 1:3D reconstruction of MEPs in gastrocnemius muscle after delayed reinnervation.
 中國(guó)神經(jīng)再生研究(英文版)2022年2期
中國(guó)神經(jīng)再生研究(英文版)2022年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- A Drosophila perspective on retina functions and dysfunctions
- Celeboxib-mediated neuroprotection in focal cerebral ischemia: an interplay between unfolded protein response and inflammation
- Pramipexole,a dopamine D3/D2 receptor-preferring agonist,attenuates reserpine-induced fibromyalgia-like model in mice
- Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: a single-arm study
- Gene and protein expression profiles of olfactory ensheathing cells from olfactory bulb versus olfactory mucosa
- Inhibition of microRNA-29b suppresses oxidative stress and reduces apoptosis in ischemic stroke
