Shenqihuatan formula (參七化痰方) reduces inflammation by inhibiting transforming growth factor-beta-stimulated signaling pathway in airway smooth muscle cells
CHEN Jingjing,WANG Yuanyuan,ZHANG Nianzhi,XUE Xiaoming
CHEN Jingjing,XUE Xiaoming,Shanxi Provincial Traditional Chinese Medicine Hospital,Taiyuan 030012,China
WANG Yuanyuan,Anhui University of Traditional Chinese Medicine,Hefei 230038,China
ZHANG Nianzhi,Department of Respiratory,the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine,Hefei 230031,China
Abstract OBJECTIVE:To study the effects and mechanism of Shenqihuatan formula (參七化痰方,SQHT) of the transforming growth factor-beta (TGF-β)-stimulated cell processes in airway remodeling.METHODS:The current study examined cell viability using a Cell Counting Kit-8 assay.Furthermore,a Transwell assay was conducted to detect the ability of cell migration,and apoptosis was detected via flowcytometry.Western Blot and quantitative real-time polymerase chain reaction (qRT-PCR) were used to determine the expression levels of apoptosis or inflammation-related factors,such as TGF-β,Interleukin-1β (IL-1β),B cell lymphoma 2 (Bcl-2),Bcl-2-Associated X (Bax),Ras homolog gene family,member A (RhoA),recombinant rho associated coiled coil containing protein kinase 1/2(ROCK1/2),extracellular regulated protein kinases 1/2(ERK1/2),Snail,and Slug.Finally,the expression levels of matrix metalloproteinase-9 (MMP-9) and Tissue inhibitor of metalloproteinase (TIMP-1) were admeasured by enzyme-linked immuno sorbent assay.RESULTS:The results demonstrated that SQHT inhibited the viability and migration,as well as the the F-actin formation and cytoskeletal reorganization of airway smooth muscle cells (ASMCs) stimulated by TGF-β.By monitoring the changes of critical regulators in the presence of the formula,it was observed that the expression levels of TGF-β,IL-1β,Bcl-2,RhoA,ROCK1/2,ERK1/2,Snail,and Slug were markedly suppressed,whereas Bax expression exhibited the opposite effect.Compared with a well-characterized RhoA pathway inhibitor,Fasudil,SQHT generated equivalent or even higher inhibitory effects on these processes in ASMCs.CONCLUSIONS:Collectively,these suggested that SQHT can reduce airway inflammation by inhibiting TGFβ-stimulated signaling pathways in ASMCs.These findings may provide a novel remedy for treating ASMC inflammation,which causes thickening and obstruction of the airway in chronic obstructive pulmonary disease.
Keywords:pulmonary disease,chronic obstructive;transforming growth factor beta;airway inflammation;fasudil;Shenqihuatan formula
1.INTRODUCTION
Chronic obstructive pulmonary disease (COPD),identified as a progressive obstructive lung disease,is usually associated with chronic airway inflammation induced by cell infiltration,which causes airflow obstruction and difficulties in breathing.1,2COPD has developed into one of the top-ranking diseases mortality,and thus,is a severe threat to humans,especially older generations3and cigarette smokers.4One of the most important features of COPD is airway smooth muscle thickening,5which is closely associated with airway smooth muscle cells (ASMCs).Besides,progressive airway remodeling in COPD,evident by hypertrophy,proliferation,and migration of ASMCs,6can finally lead to irreversible airflow obstruction,5especially with a long-term exposure to the growth factors,such as transforming growth factor-beta (TGF-β).7
TGF-β serves as a key regulator in airway remodeling and contributes to the hyperproliferation,excessive mass accumulation and migration of ASMCs in the airway wall.8ASMCs from patients with COPD exhibit enhanced proliferation in response to TGF-β compared with healthy individuals.Furthermore,TGF-β functions as a potent cytokine that mediates a wide range of cellular processes,including cell proliferation,migration,and apoptosis,by inducing the TGF-β signaling pathway.9In typical respiratory diseases,such as COPD,parenchymal lung diseases and asthma,elevated TGF-β promotes airway diseasesviaASMCs and induces inflammation and remodeling10through the activation of Smad2/3.11
TGF-β also serves an important role in the activation of the ras homolog family member A (RhoA)/Rho associated coiled-coil containing protein kinase (ROCK)and mitogen-activated protein kinase (MAPK)/extracellular regulated protein kinases (ERK) pathways.For instance,the activation of the RhoA pathway can be driven by TGF-β stimulation,12leading to enhanced RhoA translocation,ROCK1/2-dependent phosphorylation and contraction in ASMCs,which is involved in regulating sophisticated cellular processes,such as cell proliferation,migration,and invasion.13-15In ASMCs,RhoA and ROCKs are indispensable regulators during cell proliferation16and migration,8which indicates that these may be potential therapeutic targets for airway hyperresponsiveness treatment.17In addition,it was reported that the TGF-β receptor could activate the non-Smad signaling cascades,such as MAPK/ERK,to produce the full spectrum of TGF-β responses.18The MAPK/ERK pathway features a cascade of effector activations via phosphorylation to regulate gene transcription and protein expression.19This pathway also functions in the cellular proliferation,migration,and apoptosis of ASMCs,which further causes airway remodeling.8,20
The Traditional Chinese Medicine Shenqihuatan formula(SQHT),combining with the Shenqichongchao capsule and the Huatanjiangqi capsule,has been used for treating chronic obstructive lung diseases as it improve inflammation factor-involved airway remodeling and relieves inflammatory symptoms in the patients with Grade Ⅰ and Ⅱ COPD.21,22This study was aimed to investigate the effects and the underlying mechanism of SQHT on the TGF-β-stimulated cell processes in ASMCs.
2.MATERIALS AND METHODS
2.1.Isolation of ASMCs and cell culture
Rat ASMCs were isolated from the lungs of newborn Sprague-Dawley (SD) rats based on a previous report.23The rats were sacrificedviaan intraperitoneal injection of pentobarbital sodium (150 mg/kg).The left lung was transferred to the Hank’s balanced salt solution followed by the isolation of bronchi tissues with the removal of endothelial cells.The isolated ASMCs were cut into 1 mm × 1 mm pieces in the presence of penicillin and treated with 1 mg/mL of Collagenase Type I at 37 ?C in a 5% CO2-atmospheric incubator for 1-h digestion.
Digested ASMCs were washed,dispersed and filtered.Cells then were pelleted by centrifugation at 1000 ×gfor 6 min and transferred to DMEM containing 20% FBS(Gibco,USA),penicillin (100 U/mL) and streptomycin(100 U/mL) for resuspension.All cultures were incubated in DMEM (Gibco,USA) with 10% FBS,penicillin (100 U/mL) and streptomycin (100 U/mL) at 37 ℃.Cells from passages 3-7 were used for all experiments.
2.2.Preparation of the formula of SQHT
The SQHT formula was composed of Xiyangshen (Radix Panacis Quinquefolii,10 g),Sanqi (Radix Notoginseng,3 g),Dongchongxiacao (Cordyceps,5 g),Zisuzi (Fructus Perillae Argutae,10 g),Baijiezi (Semen Sinapis,10 g),Xuanfuhua (Flos Inulae Japonicae,8 g) and Mahuang(Herba Ephedra Sinica,6 g).The formula was provided by the Preparation Center of The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine.Distilled water was used to prepare the corresponding concentration suspension.The crude drug content of the concentrated decoction was 2.5 g/mL.The extract was dried and stored at 4 ℃ for further usage.
2.3.Preparation of SQHT-containing serum and treatment of ASMCs
Formula-containing serum was isolated from SD rats[weight,(220 ± 20) g] after intragastric (ig)administration of the crude drug (25.2 g/kg) twice a day for 3 consecutive days.Then,8-10 mL blood was collected from the abdominal aorta of each rat.Blood samples were collected under local anesthesia with pentobarbital sodium injection (50 mg/kg) and centrifuged at 3000 ×g/min.The rats were sacrificed by pentobarbital sodium injection intraperitoneally (150 mg/kg).Serum samples were isolated and underwent a 30-min water bath at 56 ?C for serum complement inactivation.The post-inactivation serums were filtered using 0.22-μm filters,freeze-dried at-57 ?C for 48 h,and stored at-20 ?C for later use.
ASMCs were divided into seven groups,a model group,three treatment groups and a inhibition group.The control group was treated with 20% blank serum.The model group was stimulated with TGF-β (cat.No. 763102;BioLegend,San Diego,CA,USA).The three treatment groups were treated with 5,10 or 20% SQHTcontaining serum,and the inhibition groups were treated with 3 mmol/L Fasudil (cat.No.HY-10341A;MedChemExpress,Monmouth Junction,NJ,USA) with and without 10% SQHT-containing serum.Each group was performed in triplicate.With a 24-h incubation,TGF-β (10 ng/mL) was pipetted to all the groups except the control group and cells were cultured for an additional 24 h at 37 ℃.
2.4.Cell counting kit (CCK)-8 assay
ASMCs were suspended and inoculated (100 μL/well) in a 96-well plate that was pre-incubated (37 ?C;5% CO2).
After overnight incubation,10 μL TGF-β-containing serum with or without the formula was added to each well for a further 48-h treatment at 37 ℃.According to the manufacturer’s instructions,following the addition of 10 μL CCK-8 (cat.no.BB-4202-01;Bestbio) solution to the cells,the plate was incubated for 4 h in the incubator at 37 ℃.The OD450nmwas measured using a microplate reader.
2.5.Transwell assay to examine the ability of cell migration
Serum starvation was performed for 12 h,followed by tryptic digestion to establish a cell suspension of 1 ×105/mL.The cell suspension was transferred to migration chambers for the 24-well plate and 500 μL different serums were added to specific wells.Serum-treated cells were then incubated for an additional 16 h at 37 ℃.The medium was removed from the chamber,and cells were washed twice in phosphate buffer saline (PBS).Formaldehyde (4% in PBS) was used to fix the cells for 30 min.After the disposal of formaldehyde and washing with PBS,cells were stained with crystal violet for 30 min.Stained cells were then visualized and imaged by light microscopy.
2.6.Immunofluorescence
The cells were washed and then fixed with 4%formaldehyde in PBS at room temperature for 20 min.After being rinsed with PBS,fixed cells were incubated with the F-actin and DAPI stains at room temperature for 30 min in the dark.Following washing with PBS three times,cells were mounted and imaged under the fluorescence microscope.
2.7.Flowcytometry
According to the instructions of flow cytometry kit (cat.No.BB-4101;Bestbio,Shanghai,China),the following experiments were conducted.In total,around 5 × 105cells were collected and washed.After removal of the supernatant,cells were resuspended in 100 μL 1 ×binding buffer.Fluorescein isothiocyanate (5 μL) and PI(5 μL) were added and gently swirled,followed by a 15-min incubation at room temperature in the dark.Cells were analyzed immediately via flow cytometry.
2.8.Western Blot
After collecting the cells,1 mL RIPA cell lysis solution was used for every 106cells.SDS-PAGE was run and transmembrane electrophoresis was performed using a constant current of 300 mA on ice.The transmembrane electrophoresis time for different proteins was as follows:45 min for β-actin (cat.No.TA-09;1:1000;OriGene Technologies,Rockville,MD,USA),20 min for B cell lymphoma 2 (Bcl-2,cat.No.ab59348;1:500;Abcam,Cambridge,UK),20 min for Bcl-2-Associated X (Bax,cat.No.ab32503;1:1000;Abcam,Cambridge,UK),20 min for RhoA (cat.No.ab 187027;1:5000;Abcam,Cambridge,UK),150 min for ROCK1 (cat.No.21850-1-AP;1:2000;ProteinTech,Manchester,UK),150 min for ROCK2 (cat.No.BS7216;1:2000;Bioworld Technology,Saint Louis,MN,USA),45 min for ERK1/2(cat.No.BS1112;1:500;Bioworld Technology,Saint Louis,MN,USA),45 min for phosphorylated (p)-ERK1/2 (cat.No.ab 201015;1:1000;Abcam,Cambridge,UK),30 min for Snail (cat.No.bs-1371R;1:500;BIOSS,Beijing,China) and 30 min for Slug (cat.No.bs-1382R;1:200;BIOSS,Beijing,China).The blots were washed in the wash solution and then blocked with 5% skim milk for 2 h.Primary antibodies (Abs) were prepared according to manufacturer’s instructions and blots were incubated with the primary Abs at 4 ?C overnight.Incubation of secondary Abs was conducted for 2 h at room temperature and blots were washed three times.Blots were then incubated with substrates and chemiluminescence was performed for sample evaluation.Quantity One software was used to determine the intensity of protein bands.Semi-quantification was performed by dividing the intensity of each target protein with the intensity of total β-actin on the blot.
2.9.Quantitative real-time polymerase chain reaction(qRT-PCR)
Groups of ASMCs were pelleted and lysed using TRIzol(cat.No.15596026,Thermo,Waltham,MA,USA).Samples were used to extractedRNAs firstly.Using the extracted mRNA as template,cDNA was reverse transcribed.Primer sequences are presented in Table S1.We followed the conventional method for RNA amplification.The 2ΔΔCt method was used to measure the RNA expression levels.
2.10.Enzyme-linked immunosorbent assay (ELISA)
The secreted Matrix metalloproteinase-9 (MMP-9),Tissue inhibitor of metalloproteinase (TIMP1),Interleukin-1β (IL-1β) and IL-6 were collected by pipetting the supernatant of the ASMCs culture with high-speed centrifugation at 10 000 ×gfor 10 min.The concentration of MMP-9,TIMP-1,IL-1β and IL-6 were carried out according to the protocol of the ELISA kits(JYMBio,Wuhan,China).The OD450 nm was recorded and the quantities of the two proteins were backcalculated based on the standard curve.
2.11.Statistcal analysis
All statistical tests were conducted on SPSS 17.0 statistical software (IBM,Chicago,IL,USA).All measurement data were shown as the mean ± standard deviation from three independent experiments.The significance of the mean values between two groups was analyzed by Student’st-test and theP<0.05 was considered to be statistically significant.
3.RESULTS
3.1.SQHT inhibits the proliferation,migration and cytoskeletal reorganization of ASMCs
After TGF-β stimulation,ASMC proliferation is considered to be primarily responsible for the airway wall thickening that causes the airway remodeling.24,25To illustrate the influence of the SQHT formula on ASMC proliferation,the CCK-8 assay was performed(Figure 1A).It was found that SQHT inhibited the viability of ASMCs upon stimulation in a concentrationdependent manner compared with the TGF-β group(Figure 1A),as the inhibitory effect on cell proliferation was enhanced with the increasing SQHT concentrations.The cell viability in the TGF-β+10% SQHT and TGF-β+20% SQHT groups were not significantly different.It was identified that the 10% SQHT group exhibited higher inhibitory functions,and thus,10% SQHT was selected for the subsequent experiments.
The SQHT formula also showed activities in weakening cell migration (Figure 1B).It was revealed that after TGF-β stimulation,the migration of ASMCs was markedly enhanced compared with the the control group.Moreover,cell migration was increasingly hindered with the increments in the formula concentration.
When subjected to TGF-β stimulation,the cellular actin cytoskeleton can be reshaped and reorganizaed.26To gain insight into the influence of the SQHT formula on cytoskeletal development,the changes in F-actin assembling were examined using immunofluorescence(Figure 1C).Once subjected to the formula treatment,the formation and reorganization of the cytoskeleton were impeded even with TGF-β stimulation.Compared with the trackable prominent formation of the cytoskeleton by F-actin in the formula-free,TGF-β-stimulated ASMCs,the formula-containing groups had reduced stimulatory phenomena,in which the polymerization of F-actin filaments was efficiently limited without jeopardizing the intactness of nuclei.These results suggested that SQHT was capable of inhibiting the proliferation,migration and cytoskeletal reorganization of ASMCs.
3.2.SQHT drives ASMC apoptosis under TGF-β stimulation
TGF-β is known to prevent apoptotic processes,9therefore,flow cytometry was performed (Figure 2A).The results of the flow cytometry indicated that the formula had pro-apoptotic effects on ASMCs when stimulated by TGF-β,and these effects on apoptosis were in a concentration-dependent manner.
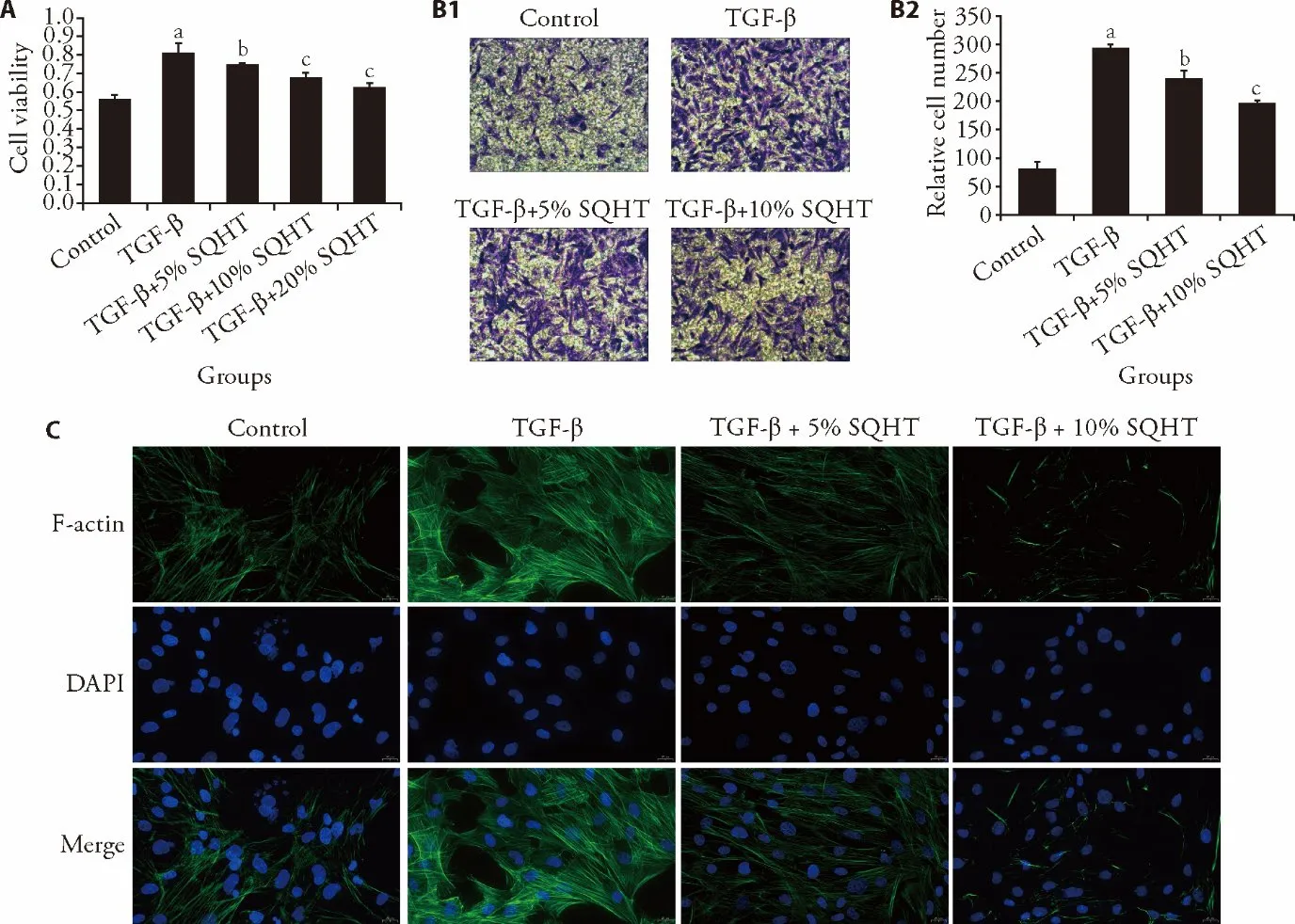
Figure 1 SQHT inhibits TGF-β-stimulated cell processes
Furthermore,the expression levels of two key proteins,Bax and Bcl-2,were measured in stimulated ASMCs(Figure 2B).Consistently,TGF-β upregulated the relative protein expression level of Bcl-2 and downregulated the relative protein expression level of Bax.However,the SQHT-treated groups had increased Bax expression and a simultaneous decrease in Bcl-2 expression.Thus,SQHT may have a notable suppressive effect on TGF-β-stimulated inflammation-associated cell processes of ASMCs,particularly at a 10% concentration.In addition,the expression levels of IL-1β and IL-6 in the TGF-β group were up-regulated significantly,in contrast to the control (Figure 2C).While,SQHT reversed the effects in a concentration-dependent manner.The results indicated that SQHT might play an anti-inflammatory effects.
3.3.SQHT exhibits comparable inhibition to Fasudil on the cell processes.
Furthermore,the current study quantified the abilities of SQHT formula and Fasudil to mitigate cell proliferation and migration (Figure 3).Both SQHT and Fasudil analogously inhibited the viability of TGF-β-stimulated ASMCs,in which the ASMC viability was significantly reduced compared with the TGF-β-stimulated group(Figure 3A).Similarly,SQHT elicited a comparable inhibition effect to Fasudil on cell migration (Figure 3B).The TGF-β activated migration could be abolished (>85%) in the groups treated with both SQHT and Fasudil.Furthermore,F-actin-involved cytoskeletal formation and reorganization could be weakened to similar degrees when ASMCs were treated by either SQHT or Fasudil,as evident by the attenuated formation of actin filaments(Figure 3C).To evaluate the pro-apoptotic activities of the SQHT formula and Fasudil,the cell status of ASMCs with different treatments was monitored using flow cytometry and the post-stimulation expression levels of Bax and Bcl-2 were determined (Figure 3D and 3E).Both of the treatments were able to promote the apoptotic process,as well as induced Bax expression and decreased Bcl-2 expression.

Figure 2 SQHT drives apoptosis in stimulated ASMCs

Figure 3 SQHT exhibits comparable inhibition to Fasudil on the cell processes
3.4.SQHT inhibits the RhoA and ERK pathway under TGF-β stimulation
After induction with TGF-β,the RhoA signaling pathway contributes to the remodeling of actinomycin cytoskeleton and regulation of cellular processes.14To examine the functions of SQHT on the RhoA signaling pathway,the protein and mRNA expression levels of RhoA GTPase,ROCK1/2 were measured after treatment with the formula (Figure 4A and 4B).TGF-β induced the expression levels of these three important proteins and mRNA.The results of western blotting and RT-qPCR analysis indicated that the formula and Fasudil counteracted the upregulation of not only RhoA,but also the downstream factors ROCK1 and ROCK2,compared with TGF-β group.As SQHT attenuated the RhoApathway-involved cell processes,it was suggested that SQHT was cross-functional in both RhoA and ERK pathways. Thus,the expression level and phosphorylation of ERK1/2 was determined in ASMCs(Figure 4C).TGF-β induced the expression and phosphorylation of ERK1/2 in ASMCs.However,after treatment with SQHT formula,both the expression and phosphorylation of ERK1/2 were reduced in ASMCs,as was the p-ERK/ERK ratio.
3.5.SQHT inhibits the Snail/Slug signaling pathway
SQHT inhibits the Snail/Slug signaling pathway.As the SQHT formula inhibits both RhoA and ERK pathways,the present study determined the anti-inflammatory activities of the SQHT formula on the expression levels of Snail and Slug,and the secretion of MMP-9 and TIMP-1 (Figure 5).The mRNA and protein expression levels of the two transcription regulators were found to be inhibited after treatment with the formula (Figure 5A and 5B).The secretory levels of MMP-9 and TIMP-1 in different ASMC groups were then examined using ELISA (Figure 5C).The addition of SQHT to stimulated ASMCs decreased the secreted quantities of both proteins.
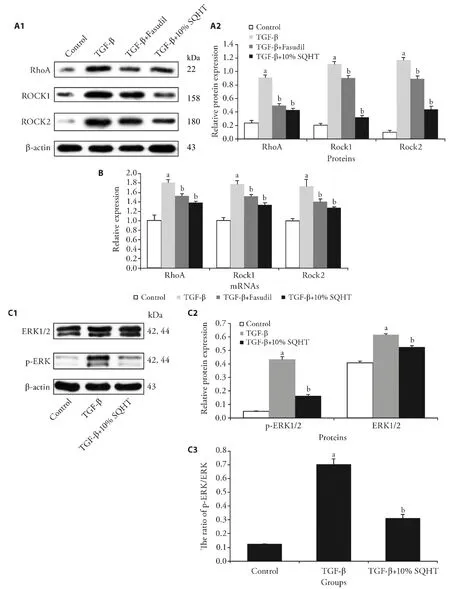
Figure 4 SQHT or Fasudil inhibit the RhoA and ERK1/2 pathway in TGF-β-stimulated ASMCs

Figure 5 SQHT inhibits the Snail/Slug signaling pathway
4.DISCUSSION
The inflammatory response in COPD is determined by the activation of epithelial cells and macrophages.27TGF-β,released by epithelial cells and macrophages,is able to drive fibroblast proliferation and tissue remodeling.9,10The present study demonstrated that the SQHT formula effectively counteracted the TGF-βstimulated elevation of cell viability,migration and antiapoptosis in ASMCs,thus indicating its ability to inhibit inflammatory phenomena.It was found that the regulation of these processes by SQHT was associated with its deactivation of TGF-β-mediated pathways.By counteracting the stimulatory function of TGF-β in several pathways,such as reducing the expression levels of critical effector proteins,SQHT inhibited TGF-βactivated cell proliferation,migration,apoptosis,inflammatory factors and protein secretion,which are usually associated with airway remodeling in COPD.Moreover,regulation of these processes involved complicated pathways,such as the RhoA/ROCK pathway and the MAPK/ERK pathway.
The RhoA/ROCK pathway and MAPK/ERK pathways contribute to the regulation of the elevated secretion of MMP-9 and TIMP-1,28,29which can further cause epithelial-mesenchymal transition,and thus are an indication of airway remodeling and inflammation in COPD.30,31The present study identified that SQHT markedly downregulated the expression levels of essential proteins in the RhoA pathway.The potency of SQHT was comparable to that of Fasudil,and was higher under certain regulatory circumstances,such as in inhibiting the RhoA pathway. In terms of pharmacodynamics,the formula worked more efficiently not only on protein expression levels but also on apoptosis,compared with Fasudil.
ERK serves as an indispensable mediator in the MAPK/ERK pathway and is dependent on its phosphorylation,which further activates downstream transcription and expression of effector proteins for cell proliferation and migration.32,33In the present study,the phosphorylation ERK was almost completely abolished with the treatment of SQHT,which could also sequentially cause a suppressed regulation of downstream effectors in the pathway to decrease cell proliferation,migration and invasion.Moreover,the expression levels of MMP-9 and TIMP-1 are upregulated by TGF-β stimulationviaSnail and Slug transcription factors,34,35which are responsible for inflammatory cell processes.36The present study found that SQHT might hinder the secretion of MMP-9 and TIMP-1 by inhibiting the transcription and expression of Snail and Slug transcription factors under TGF-β stimulation.Briefly,SQHT may abate the airway remodeling and inflammation in COPD by effectively inhibiting these pathways and processes.However,the regulatory mechanisms underlying these processes remain unknown,as these can be sophisticated and complicated.The present study demonstrated that SQHT functions in the regulation of multiple cell signaling pathways.
In conclusion,the current study demonstrated that this formula was potent and could be more widely used for therapeutic purposes.In addition,the revelation of the SQHT-mediated regulation of the signaling pathways not only furthers the understanding of traditional Chinese medicines,but also establishes a good example of interpreting inherited knowledge using modern technologies.
5.ACKNOWLEDGEMENTS
The Anhui University of Traditional Chinese Medicine and The First Affiliated Hospital of Anhui University of Traditional provided the help to support the running of the research.
6.REFERENCES
1.Wang Y,Xu J,Meng Y,Adcock IM,Yao X.Role of inflammatory cells in airway remodeling in COPD.Int J Chron Obstruct Pulmon Dis 2018;13:3341-8.
2.Higham A,Quinn AM,Cancado JED,Singh D.The pathology of small airways disease in COPD:historical aspects and future directions.Respir Res 2019;20:49.
3.Burney PG,Patel J,Newson R,Minelli C,Naghavi M.Global and regional trends in COPD mortality,1990-2010.Eur Respir J 2015;45:1239-47.
4.Laniado-Laborin R.Smoking and chronic obstructive pulmonary disease (COPD).Parallel epidemics of the 21 century.Int J Environ Res Public Health 2009;6:209-24.
5.Chung KF.The role of airway smooth muscle in the pathogenesis of airway wall remodeling in chronic obstructive pulmonary disease.Proc Am Thorac Soc 2005;2:347-54;discussion 371-2.
6.Goldsmith AM,Bentley JK,Zhou L,et al.Transforming growth factor-beta induces airway smooth muscle hypertrophy.Am J Respir Cell Mol Biol 2006;34:247-54.
7.Gawaziuk JP,Sheikh F,Cheng ZQ,Cattini PA,Stephens NL.Transforming growth factor-beta as a differentiating factor for cultured smooth muscle cells.Eur Respir J 2007;30:643-52.
8.Salter B,Pray C,Radford K,Martin JG,Nair P.Regulation of human airway smooth muscle cell migration and relevance to asthma.Respir Res 2017;18:156.
9.Zhang Y,Alexander PB,Wang XF.TGF-beta family signaling in the control of cell proliferation and survival.Cold Spring Harb Perspect Biol 2017;9:a022145.
10.Ojiaku CA,Yoo EJ,Panettieri RA,Jr.Transforming growth factor beta1 function in airway remodeling and hyperresponsiveness.The missing link? Am J Respir Cell Mol Biol 2017;56:432-42.
11.Pan Y,Liu L,Li S,et al.Activation of AMPK inhibits TGF-beta1-induced airway smooth muscle cells proliferation and its potential mechanisms.Sci Rep 2018;8:3624.
12.Chaudhury A,Howe PH.The tale of transforming growth factorbeta (TGFbeta) signaling:a soigne enigma.IUBMB Life 2009;61:929-39.
13.Kumper S,Mardakheh FK,McCarthy A,et al.Rho-associated kinase (ROCK) function is essential for cell cycle progression,senescence and tumorigenesis.Elife 2016;5:e12994.
14.Priya R,Liang X,Teo JL,Duszyc K,Yap AS,Gomez GA.ROCK1 but not ROCK2 contributes to RhoA signaling and NMIIA-mediated contractility at the epithelial zonula adherens.Mol Biol Cell 2017;28:12-20.
15.Shaifta Y,MacKay CE,Irechukwu N,et al.Transforming growth factor-beta enhances Rho-kinase activity and contraction in airway smooth muscleviathe nucleotide exchange factor ARHGEF1.J Physiol 2018;596:47-66.
16.Takeda N,Kondo M,Ito S,Ito Y,Shimokata K,Kume H.Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin.Am J Respir Cell Mol Biol 2006;35:722-9.
17.Chiba Y,Matsusue K,Misawa M.RhoA,a possible target for treatment of airway hyperresponsiveness in bronchial asthma.J Pharmacol Sci 2010;114:239-47.
18.Rojas A,Padidam M,Cress D,Grady WM.TGF-beta receptor levels regulate the specificity of signaling pathway activation and biological effects of TGF-beta.Biochim Biophys Acta 2009;1793:1165-73.
19.Wortzel I,Seger R.The ERK cascade:distinct functions within various subcellular organelles.Genes Cancer 2011;2:195-209.
20.Yap HM,Israf DA,Harith HH,Tham CL,Sulaiman MR.Crosstalk between signaling pathways involved in the regulation of airway smooth muscle cell hyperplasia.Front Pharmacol 2019;10:1148.
21.Zhang N,Chen W,Li G,et al.Clinical study on treatment of chronic obstructive pulmonary disease at stable stage with Yiqi Huoxue Huatan formula.Zhong Yi Yao Lin Chuang Za Zhi 2014;26:151-2.
22.Wang Y.The Distribution of TCM syndromes in COPD patients of grade Ⅰ and Ⅱ and the effect of SQHTF on the expression of inflammatory makers in COPD rats.Hefei:Anhui University of Chinese Medicine,2019:33-7.
23.An SS,Laudadio RE,Lai J,Rogers RA,Fredberg JJ.Stiffness changes in cultured airway smooth muscle cells.Am J Physiol Cell Physiol 2002;283:C792-801.
24.Stamatiou R,Paraskeva E,Gourgoulianis K,Molyvdas PA,Hatziefthimiou A.Cytokines and growth factors promote airway smooth muscle cell proliferation.ISRN Inflamm 2012;2012:731472.
25.Wang J,Wang HS,Su ZB.MicroRNA-142 inhibits proliferation and promotes apoptosis in airway smooth muscle cells during airway remodeling in asthmatic ratsviathe inhibition of TGFbeta-dependent EGFR signaling pathway.Cell Physiol Biochem 2018;47:1682-95.
26.Hubchak SC,Runyan CE,Kreisberg JI,Schnaper HW.Cytoskeletal rearrangement and signal transduction in TGF-beta1-stimulated mesangial cell collagen accumulation.J Am Soc Nephrol 2003;14:1969-80.
27.Aghasafari P,George U,Pidaparti R.A review of inflammatory mechanism in airway diseases.Inflamm Res 2019;68:59-74.
28.Wu YJ,Neoh CA,Tsao CY,Su JH,Li HH.Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways.Int J Mol Sci 2015;16:16469-82.
29.Qiu Q,Yang M,Tsang BK,Gruslin A.EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways.Reproduction 2004;128:355-63.
30.Li Y,Lu Y,Zhao Z,et al.Relationships of MMP-9 and TIMP-1 proteins with chronic obstructive pulmonary disease risk:a systematic review and Meta-analysis.J Res Med Sci 2016;21:12.
31.Churg A,Zhou S,Wright JL.Series "matrix metalloproteinases in lung health and disease":Matrix metalloproteinases in COPD.Eur Respir J 2012;39:197-209.
32.Eblen ST.Extracellular-regulated kinases:signaling from Ras to ERK substrates to control biological outcomes.Adv Cancer Res 2018;138:99-142.
33.Srinivasan R,Zabuawala T,Huang H,et al.Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis.PLoS One 2009;4:e8283.
34.Sun L,Diamond ME,Ottaviano AJ,Joseph MJ,Ananthanarayan V,Munshi HG.Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression.Mol Cancer Res 2008;6:10-20.
35.Welch-Reardon KM,Ehsan SM,Wang K,et al.Angiogenic sprouting is regulated by endothelial cell expression of Slug.J Cell Sci 2014;127:2017-28.
36.de Herreros AG,Peiro S,Nassour M,Savagner P.Snail family regulation and epithelial mesenchymal transitions in breast cancer progression.J Mammary Gland Biol Neoplasia 2010;15:135-47.
 Journal of Traditional Chinese Medicine2022年4期
Journal of Traditional Chinese Medicine2022年4期
- Journal of Traditional Chinese Medicine的其它文章
- Editorial Board Listing
- Mixed methods research in complementary and alternative medicine:a scoping review
- Herbal anthelmintic agents:a narrative review
- Factors influencing physician's behavioral intention to use Traditional Chinese Medicine to treat coronavirus disease 2019 based on the theory of planned behavior
- Identification of novel biomarkers and therapeutic target candidates for stasis-heat symptom pattern of acute intracerebral hemorrhage by quantitative plasma proteomics
- Effect of three tongue needles acupoints Lianquan (CV23) and Hegu(LI4) combined with swallowing training on the quality of life of laryngeal cancer patients with dysphagia after surgery
