Sensitivity of MCF-7 mammosphere CSCs to neutron radiation
Valentina G. Shuvatova, Yuliya P. Semochkina, Alexander N. Strepetov, Elizaveta Yu. Moskaleva
Kurchatov Complex of NBICS Technologies, National Research Centre “Kurchatov Institute”, Moscow 123098, Russia.
AbstractAim: Cancer stem cells (CSCs) are highly resistant to chemotherapy and γ-irradiation. Neutrons have a high linear energy transfer, which can lead to extensive damage to the DNA of tumor cells and CSCs. The aim of this work was to compare the sensitivity of MCF-7 human breast adenocarcinoma cells and CSCs to γ- and γ,n-irradiation.Methods: To increase the number of CSCs, MCF-7 cells were cultured as mammospheres. γ-irradiation was carried out in a GUT-200M device (60Co source) in the dose range of 1-8 Gy at a dose rate of 0.75 Gy/min. γ,n-irradiation was carried out in an IR-8 reactor in the dose range of 0.05-2 Gy at a dose rate of 0.06 Gy/min. DNA DSB formation was assessed by the level of γH2AX foci using fluorescence microscopy and flow cytometry. CSCs were identified by flow cytometry as CD44+/CD24-/low cells.Results: We showed that γ,n-irradiation induced the formation of γH2AX foci of a larger size than did γ-irradiation and led to more severe DNA damage per 1 Gy. Moreover, γ,n-radiation was found to have a high relative biological effectiveness (RBE) as assessed by the cell survival rate, the number of CSCs in culture, and the ability of CSCs to repopulate. The highest RBE of neutron radiation was observed at low doses, when cell survival rate decreased by only 5%-10%. With an increase in the radiation dose, the RBE value decreased for all studied parameters, but it remained as high as 5.Conclusion: γ,n-radiation is highly effective against CSCs. Our results explain the efficacy of neutron therapy for resistant forms of breast cancer.
Keywords: Cancer stem cells, radioresistance, neutron radiation, gamma radiation, relative biological effectiveness,γH2AX foci, mammospheres, MCF-7 cells, human breast adenocarcinoma
INTRODUCTION
Cancer incidence and mortality are rapidly increasing worldwide. Breast cancer is one of the most commonly diagnosed types of cancer in women. In 2018, the number of new cases amounted to 2,088,849(11.6% of the total number of cases), and the number of deaths was 626,679 cases (6.6% of deaths due to cancers of all localizations)[1]. The main problem that often makes existing therapy methods inefficient for various types of cancer is the presence of a small population of cells in the cancer tissue that are now called cancer stem cells (CSCs) or tumor-initiating cells[2,3].
The existence of CSCs was first evidenced by Bonnet and Dick in human acute myeloid leukemia[4]. These cells had the CD34+CD38-phenotype and could differentiate into leukemic cells, similar to differentiation of normal hematopoietic stem cells (SCs) into mature hematopoietic cells.
Breast cancer is the first type of human solid tumor in which the presence of CSCs has been detected.Al-Hajjet al. showed that only a small population of human tumor cells with the CD44+CD24-/lowphenotype can induce tumor development when transplanted into immunodeficient mice[5]. As few as 100 cells with this phenotype were capable of tumor induction in mice, while tens of thousands of cells with alternative phenotypes failed to induce tumors.
CSCs have been shown to have such properties of SCs as the ability to repopulate (to proliferate without differentiation for an unlimited time) and the ability for asymmetric division, in which one daughter cell is committed to differentiation, i.e., becomes more specialized, while the other remains an SC[6]. According to the model of carcinogenesis based on the concept of the existence of CSCs, malignant tumors have a hierarchical organization similar to normal tissues, in which only SCs are capable of self-renewal and formation of more differentiated progenitor cells in accordance with the tissue needs. Thus, tumor formation, growth, and development are determined by a small population of CSCs[7]. It is considered that CSCs most likely arise in the body as a result of accumulation of mutations in SCs of different tissues,although their formation from progenitor and differentiated cells may not be ruled out[6,8,9].
Overlapping sets of molecules and pathways have recently been identified to regulate both SCs migration and cancer metastasis. They constitute a complex network of cellular interactions that facilitate the initiation of the pre-metastasis niche by the primary tumor and the formation of a nurturing microenvironment for migrating CSCs. Therefore, it is CSCs that play a key role not only in the initiation and development of a tumor but also in the formation of metastases[10].
New, rapidly accumulating results of experimental studies on the properties of CSCs, their role in carcinogenesis, the specific markers of CSCs in different types of tumors, and the mechanisms of resistance of CSCs to various therapies are regularly summarized in detailed reviews[11,12]. CSCs are highly resistant to both chemotherapy[13,14]and the action of X-ray and gamma radiation[6,8,15-19].
Radioresistance of CSCs is one of the key problems of modern radiation oncology. The resistance of CSCs to X-ray and gamma radiation exposure is determined by such metabolic features as highly efficient repair of radiation-induced DNA damage[20-24]; state of proliferative dormancy[25,26]; lower reactive oxygen species levels, which are associated with increased expression of genes that control the synthesis of glutathione,superoxide dismutase, and catalase[27]; and activation of anti-apoptotic signaling pathways (STAT3, Wnt,Notch, Hedgehog, NF-κB, and PI3K/Akt/mTOR) in response to damage[28]. In addition to these biochemical features, the radioresistance of CSCs is also determined by their localization in the hypoxic regions of the tumor, which ensures their higher survival rate during radiation therapy[29].
It is known that γ-irradiation causes an increase in the proportion of CSCs in various types of tumors,including breast cancer[30]. One of the mechanisms for increasing the proportion of CSCs along with the higher radioresistance of CSCs may be γ-irradiation-induced activation of the intracellular STAT3 and Notch signaling pathways, which leads to accelerated repopulation of CSCs during and after radiation therapy[31,32].
The results of somein vitroandin vivostudies indicate that CSCs are also resistant to fractionated irradiation due to an increased ability to repair sublethal damage in the intervals between sessions[16,33].
A quantitative analysis of the number of CSCs in tumors can have an important prognostic value. It was shown that CD44+/CD24-cells constituted an average of 6.12% (range, 0.11%-21.23%) of the primary human breast carcinomas, and a strong correlation was found between the percentage of these cells in primary tumors and distant metastasis development[34]. A particularly promising approach in this area of research was the combination of determining the number of CSCs in tumor tissue and tumor budding. Tumor budding, defined as the formation of cell clusters at the invasive tumor front, is an emerging prognostic biomarker in solid cancers. Xiang Z and co-authors showed that tumor budding score is an independent prognostic factor, high-grade tumor budding was correlated with worse disease-free survival, and CSC scores were correlated with tumor progression and tumor budding. A novel nomogram based on tumor budding and CSC score was shown to improve the prognostic evaluation of breast cancer[35].
The proportion of CSCs in uterine cervical cancer patients with the complete tumor regression decreased after irradiation, while in patients with partial regression, this indicator increased. However, the proportion of CSCs in cervical scrapings before the treatment did not have prognostic significance[36,37].
Due to the active development of hadron therapy, primarily proton, neutron, and heavy particle therapy,the sensitivity of CSCs to the fluxes of densely ionizing particles currently attracts considerable interest.According to the available data, in contrast to γ-irradiation, γ,n-radiation does not lead to an increase in the proportion of CSCs among surviving cells, which has thus far only been shown for melanoma cell culture[38]. This suggests that neutron therapy will have a number of advantages over the use of γ-radiation against CSCs. Data on the effects of neutrons and other types of densely ionizing radiation on CSCs of breast cancer remain unknown.
Dontuet al. developed a method for culturing cells under low-adhesion conditions in a serum-free medium with the addition of the necessary growth factors and special supplements to increase the number of SCs in normal human mammary gland cell cultures[39,40]. These conditions promoted the formation of spheroids,called mammospheres by the authors, and thus the obtained suspension culture was enriched in poorly differentiated cells and SCs. Cultivation of tumor cells in accordance with this method made it possible to obtain cultures with a higher content of CSCs, which facilitates their study.
The aim of this work was to characterize the sensitivity of CSCs and MCF-7 human adenocarcinoma cells cultured in mammospheres to γ,n-radiation in comparison with γ-radiation.
METHODS
Cell culture
The mammosphere culture was derived from MCF-7 human breast adenocarcinoma cells, which were cultured in DMEM medium (Gibco, USA) containing 10% fetal bovine serum (HyClone, USA) and 50 μg/mL gentamicin (Life Technologies, USA) at 37 °C and 5% CO2in a humidified atmosphere. Cells were removed from the substrate using a Versen’s solution with 0.05% trypsin, washed in phosphate-buffered saline (PBS, Sigma-Aldrich, Germany), and seeded in low-adhesion culture dishes (Corning Costar, USA) at a density of 20 cells/μL in a complete growth medium of the following composition: DMEM/F12 without phenol red (Gibco, USA) supplemented with 20 ng/mL EGF (Calbiochem, Germany), 10 ng/mL bFGF(PeproTech, USA), 2% B27 (Gibco, USA), 10 μg/mL insulin (Calbiochem, Germany), 4 μg/mL heparin(Serva, Germany), and 50 μg/mL gentamicin (Life Technologies, USA). After 5-6 days, spherical colonies with a diameter of 50-150 μm were formed (primary mammospheres), which were collected by centrifugation, dissociated into single cells using a solution of TrypLe Express proteases (Gibco, Denmark)in accordance with the manufacturer’s recommendations, and passaged under low-adhesion conditions in a complete growth medium. After five days of cultivation, the secondary mammospheres used in experiments were obtained.
Irradiation
After dissociation of mammospheres, a suspension of single cells was prepared in DMEM/F12 medium without growth factors and supplements. Mammosphere cells were exposed to γ-radiation using a GUT-200M device (60Co source) in the dose range of 1-8 Gy at a dose rate of 0.75 Gy/min. γ,n-irradiation was carried out in the dose range of 0.05-2 Gy at a dose rate of 0.07 Gy/min in the horizontal experimental channel of the IR-8 reactor at the National Research Center “Kurchatov Institute”. A beam consisting of neutrons and γ-quanta was formed using a collimator made of steel and borated polyethylene. Irradiation conditions and calculation of absorbed doses were described in detail previously[41]. The neutron flux density corresponding to 1 MW of reactor power was 0.71 ± 0.07 cm-2·s-1. The neutron energy varied from 0.5 eV to 10 MeV. The absorbed dose rate recalculated per 1 MW of reactor power (using the Geant4 software package) was 0.6 ± 0.1 Gy/h. Of this, 0.2 Gy/h was due to neutron radiation, and 0.4 Gy/h was due to γ-quanta. Plastic tubes with cell suspension were placed directly at the outlet of the collimator. After irradiation, the cells were cultured in low-adhesion six-well plates in a complete growth medium at an initial density of 50,000 cells/well in 2.5 mL of medium (20 cells/μL).
Cell counting
Five days after irradiation, we determined the total number of cells in each sample. Mammospheres were collected by centrifugation, dissociated into single cells as described above, and the cells were counted using a hemocytometer.
Analysis of γH2AX foci by confocal microscopy
After γ-irradiation at a dose of 2 Gy and γ,n-irradiation at a dose of 1 Gy, control and irradiated cells were incubated in the growth medium for 1 h at 37 °C. After that, the medium was removed, and the cells were washed with PBS and fixed in absolute methanol for 60 min at +4 °C. Samples were stored in absolute methanol at -20 °C until testing. Samples for microscopy were incubated for 10 min at room temperature in a blocking solution (PBS containing 3% fetal bovine serum (FBS) and 0.05% Triton X-100), and then in a blocking solution with primary monoclonal mouse antibodies to histone γH2AX (Merck Millipore,Germany) at a concentration of 1 μg/mL (1:1000 dilution) for 1 h at room temperature. After incubation with primary antibodies, the samples were washed with PBS containing 3% FBS and incubated with secondary polyclonal goat antibodies conjugated to Alexa Fluor 488 (Biolegend, USA) at a concentration of 0.25 μg/mL (1:200 dilution) for 1 h at room temperature. The samples were washed with PBS and incubated in 0.3 μM 4′,6-diamidino-2-phenylindole (DAPI) solution for 10 min at room temperature to stain the nuclei. Then, the samples were washed with PBS, and the cells were suspended in 20 μL of PBS and applied to coverslips in drops of about 5 μL. After drying, the coverslips were mounted on glass slides using Mowiol.Visualization and image processing were performed using a CBIS-LSM-900 fluorescence microscope (Carl Zeiss, Germany).
Flow cytometry analysis of γH2AX
One hour after γ-irradiation at a dose of 2 Gy and γ,n-irradiation at a dose of 1 Gy, we determined the level of γH2AX histone in mammosphere cells. The cells were incubated in the culture medium for 1 h at 37 °C,and then washed with PBS and fixed in absolute methanol for 20 min at +4 °C. The samples were stored in methanol at -20 °C until measurements were taken. Before the study, cells were washed from methanol with PBS containing 3% FBS and 0.05% Triton X-100, and then incubated in this solution with mouse monoclonal antibodies to γH2AX histone conjugated to with Alexa Fluor 488 (BD Pharmingen, USA;dilution 1:100). For the measurement, we prepared a cell suspension in PBS with at least 100,000 cells per sample. The analysis was performed on a FACSCalibur flow cytometer (BD Biosciences, USA).
Identification of CSCs by flow cytometry
Cells with the CD44+/CD24-/lowphenotype were defined as CSCs and their percentage was assessed by flow cytometry. Cells were centrifuged in a cold buffer consisting of PBS, 0.1% sodium azide (Merck, Germany),and 0.1% bovine serum albumin (Diam, Russia), and then incubated in the same buffer supplemented with fluorescein isothiocyanate (FITC)-labeled mouse monoclonal antibodies to human CD44 (clone BJ18,Biolegend, USA) and phycoerythrin (PE)-labeled mouse monoclonal antibodies to human CD24 (clone ML5, Biolegend, USA) at a 1:20 dilution in the dark for 30 min at +4 °C. Non-specific binding was assessed by incubating cells of the control mammosphere culture with isotype control antibodies conjugated to the corresponding fluorochromes: FITC-labeled mouse IgG1, κ (Biolegend, USA) and PE-labeled mouse IgG2a,κ (Biolegend, USA). The relative content of CSCs was determined among the cells of the main population with the exception of aggregates. To identify the region for cells with the CSC phenotype (CD44+/CD24-/low),the x-axis cursor (CD44-FITC) was set to account for non-specific binding as determined by the isotype control. The position of the cursor along the y-axis (CD24-PE) to identify cells with a low level of CD24 antigen was set at 25% of the median fluorescence intensity of PE in the control mammosphere cells. The analysis was performed on a FACSCalibur instrument (BD Biosciences, USA) at the Resource Center for Molecular and Cellular Biology.
Calculating the number of CSCs
Using the obtained data on the total number of cells and the proportion of CSCs in the culture, the number of CSCs in the samples was calculated according to the formula: number of CSCs = (total number of cells in the sample) × (proportion of CSCs).
Determination of clonogenic activity
To assess the influence of γ- and γ,n-radiation at the applied doses on the ability of cells to form mammospheres, the cells after irradiation were seeded into 96-well low-adhesion plates (Corning, USA) at 100-1000 cells per well (the higher the radiation dose, the higher the amount of cells) in 200 μL of a complete growth medium and cultured for seven days. Seven days after irradiation, the number of mammospheres with a diameter of at least 50 μm was counted using a hemocytometer. The results are presented as clonogenic activity (CA) that was calculated by the formula: CA = PEirr/PEctrl× 100, where PEirris the plating efficiency of the irradiated cells (the number of mammospheres formed from the irradiated cells divided by the number of the irradiated cells seeded) and PEctrlis the plating efficiency of the control cells (the number of mammospheres formed from the control cells divided by the number of the control cells seeded).
Determination of relative biological effectiveness of γ,n-radiation
Relative biological effectiveness (RBE) is the ratio of the dose of reference radiation to the dose of test radiation to produce a similar effect. γ-radiation of60Co was used as reference radiation. To find the dose of γ,n-radiation which produces the same endpoint as γ-radiation, the dose-response dependence of MCF-7 mammosphere cells’ survival rate, CA, and the number of CSCs for γ- and γ,n-radiation were investigated,and the RBE of γ,n-radiation for different doses was determined[42].
Statistical analysis
The results were processed using the Student’st-test and Origin 8.1 software. Data are presented as mean ± standard error of the mean. Differences were considered statistically significant atP< 0.05.Regression analysis was also performed using Origin 8.1 software. All experiments were repeated five times.
RESULTS
To enrich the culture of MCF-7 human breast adenocarcinoma cells with CSCs, cells were cultured under low adhesion conditions in a serum-free medium supplemented with the necessary growth factors and such special additives as B27, insulin, and heparin. After 5-7 days under these conditions, floating colonies were formed - mammospheres consisting of CSCs and early progenitor cells [Figure 1A and B].
The relative content of CD44+/CD24-/lowCSCs in these cultures was determined by flow cytometry. We found that the cultivation of MCF-7 cells in mammospheres led to an increase in the proportion of CSCs in the culture by 17 times [Figure 1C and D].
To compare the level of DNA damage in mammosphere cells as a result of γ- and γ,n-irradiation, we analyzed the number of DNA double-strand breaks, which was assessed by the number of γH2AX foci in cell nuclei using confocal microscopy after cell staining with fluorescently labeled antibodies. In addition, to assess the content of γH2AX in cells, we used flow cytometry. Previously, the RBE of fast neutrons with respect to X-ray radiation for the induction of cytogenetic damage assessed by micronuclei formation in peripheral blood reticulocytes has been shown to be 1.9 ± 0.3[43]. Therefore, we determined the γH2AX level 1 h after γ,n-irradiation (the time when the maximum number of foci can be detected) of mammosphere cells at a dose of 1 Gy and γ-irradiation at a dose of 2 Gy. Data are presented in Figures 2 and 3.
When analyzing micrographs of γH2AX foci, attention should be paid to the larger size of most foci formed after γ,n-irradiation of mammosphere cells compared to those after γ-irradiation [Figure 2B and C]. We showed that the numbers of γH2AX histone foci induced by γ-irradiation of mammosphere cells at a dose of 2 Gy [Figure 2B] and γ,n-irradiation at a dose of 1 Gy [Figure 2C] were practically the same and comprised 39 ± 15 and 46 ± 5 foci per nuclei, respectively.
The results of the assessment of the γH2AX histone level in mammosphere cells 1 h after γ-irradiation at a dose of 2 Gy and γ,n-irradiation at a dose of 1 Gy using flow cytometry are shown in Figure 3. We found that γ,n-irradiation of mammosphere cells at a dose of 1 Gy led to slightly higher accumulation of histone γ H2AX than γ-irradiation at a dose of 2 Gy, which corresponds to the results of confocal microscopy and suggests that the RBE for γ,n-radiation assessed by the formation of DNA double-strand breaks is close to 2.Thus, the results obtained by flow cytometry are in full agreement with the data obtained by confocal microscopy, except for the high level of fluorescence of control cells, which was not observed with confocal microscopy.

Figure 1. Micrographs of MCF-7 breast adenocarcinoma cells cultured as adherent cells (A) and as mammospheres (B), magnification 100×. (C,D) Examples of identification of CSCs with the CD44+/CD24-/low phenotype in the adherent culture and in the culture of mammospheres, respectively; the proportion of CSCs in the culture is shown. Isotype controls were performed (not shown). CSCs:Cancer stem cells.
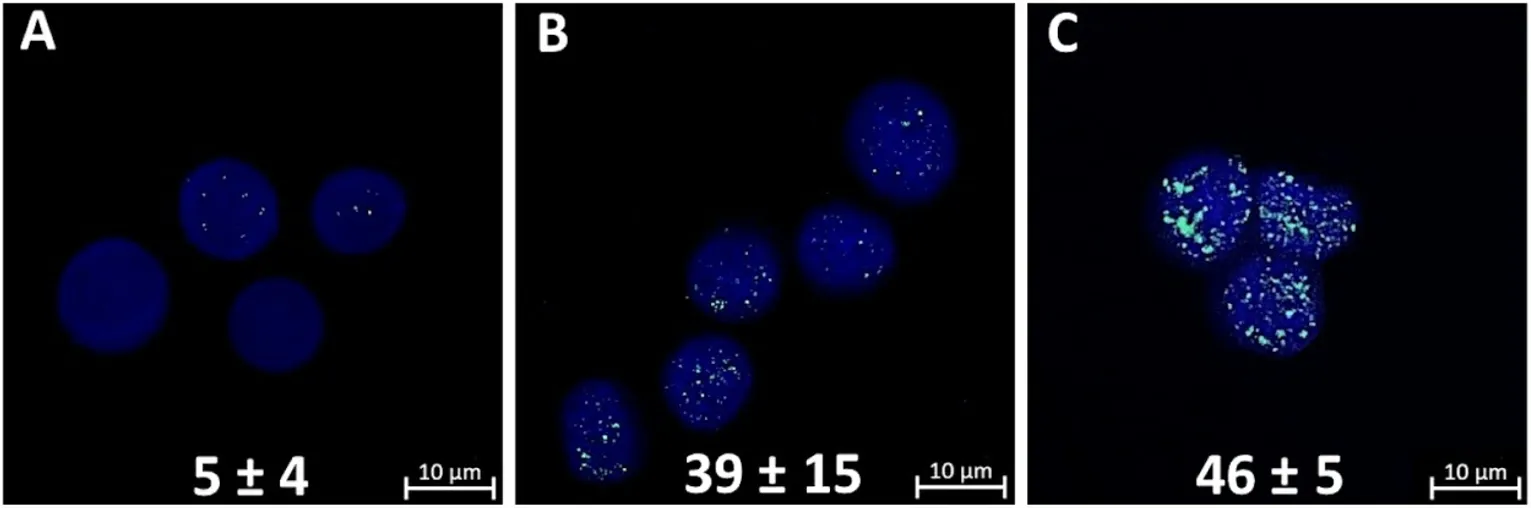
Figure 2. γH2AX foci in control mammosphere cells (A); radiation-induced γH2AX foci in mammosphere cells 1 h after γ-irradiation at a dose of 2 Gy (B); and radiation-induced γH2AX foci in mammosphere cells 1 h after γ,n-irradiation at a dose of 1 Gy (C). Confocal microscopy data are shown. Nuclei were stained with DAPI (blue) and γH2AX foci (green) were detected. The number of γH2AX foci per cell nucleus is indicated. Experiments were repeated five times.
To determine the RBE of γ,n-radiation for MCF-7 mammosphere cells and the corresponding CSCs, we examined the survival rate of these cells, the number of CSCs in culture, and their CA depending on the dose of γ- and γ,n-irradiation. The results obtained are presented in Figures 4-6.
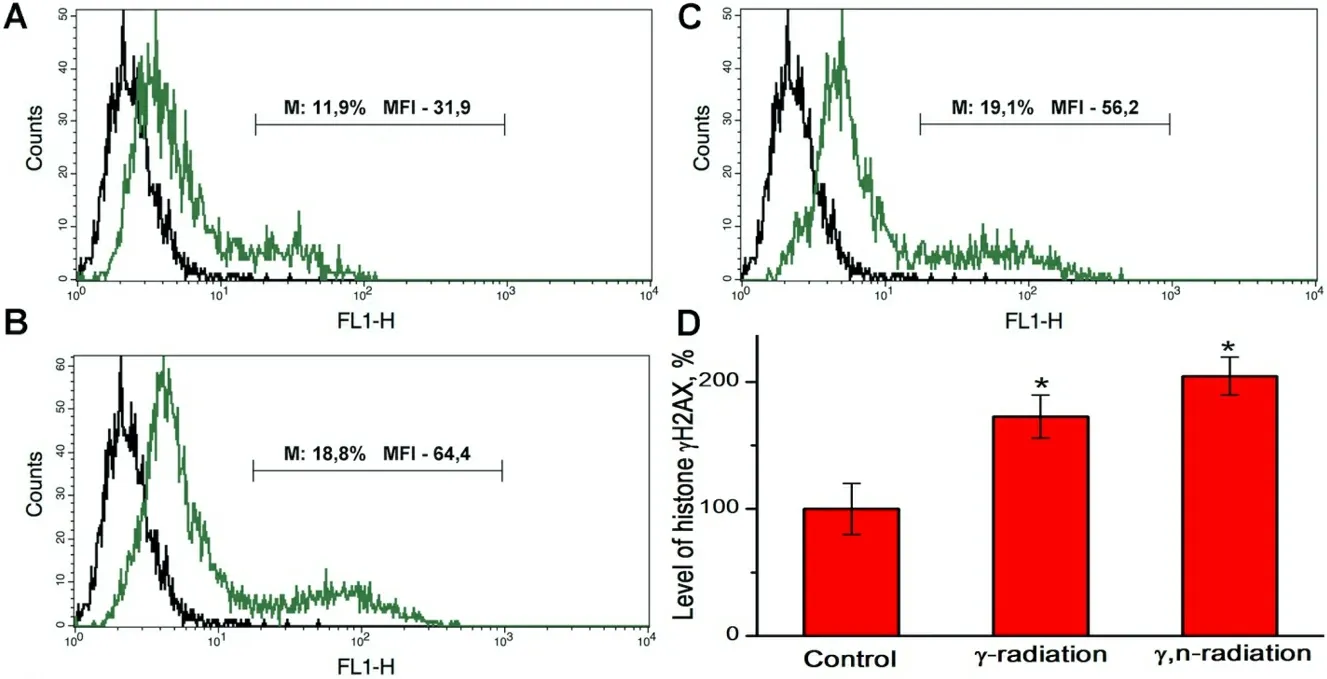
Figure 3. Typical overlaying histograms of autofluorescence (left peak) and fluorescence of MCF-7 mammosphere cells stained with fluorescently labeled antibodies to histone γH2AX (right peak): (A) control mammosphere cells; (B) mammosphere cells 1 h after γirradiation at a dose of 2 Gy; (C) mammosphere cells 1 h after γ,n-irradiation at a dose 1 Gy; and (D) the relative level of histone γH2AX calculated from the median fluorescence intensity (MFI) data for the M region which corresponds to γH2AX-positive mammosphere cells with high MFI values. The numbers on the histograms indicate the percentage of positive cells and MFI in the M region.★Differences from the control are statistically significant, P < 0.05. Experiments were repeated five times.

Figure 4. Dose dependence of the survival rate of MCF-7 mammosphere cells (A) and the RBE values of γ,n-irradiation determined from the data on the dose dependence of the mammosphere cells survival rate (B). Experiments were repeated five times. RBE: Relative biological effectiveness.
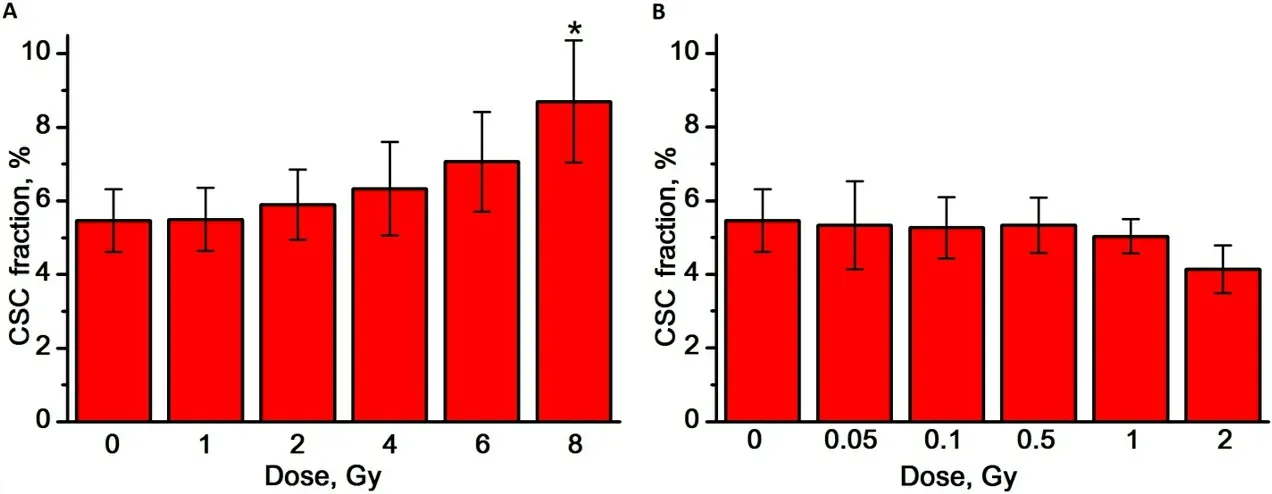
Figure 5. The effect of γ-radiation (A) and γ,n-radiation (B) on the proportion of CSCs in mammosphere cell cultures. Experiments were repeated five times. CSCs: Cancer stem cells. ★Differences from the control are statistically significant, P < 0.05.
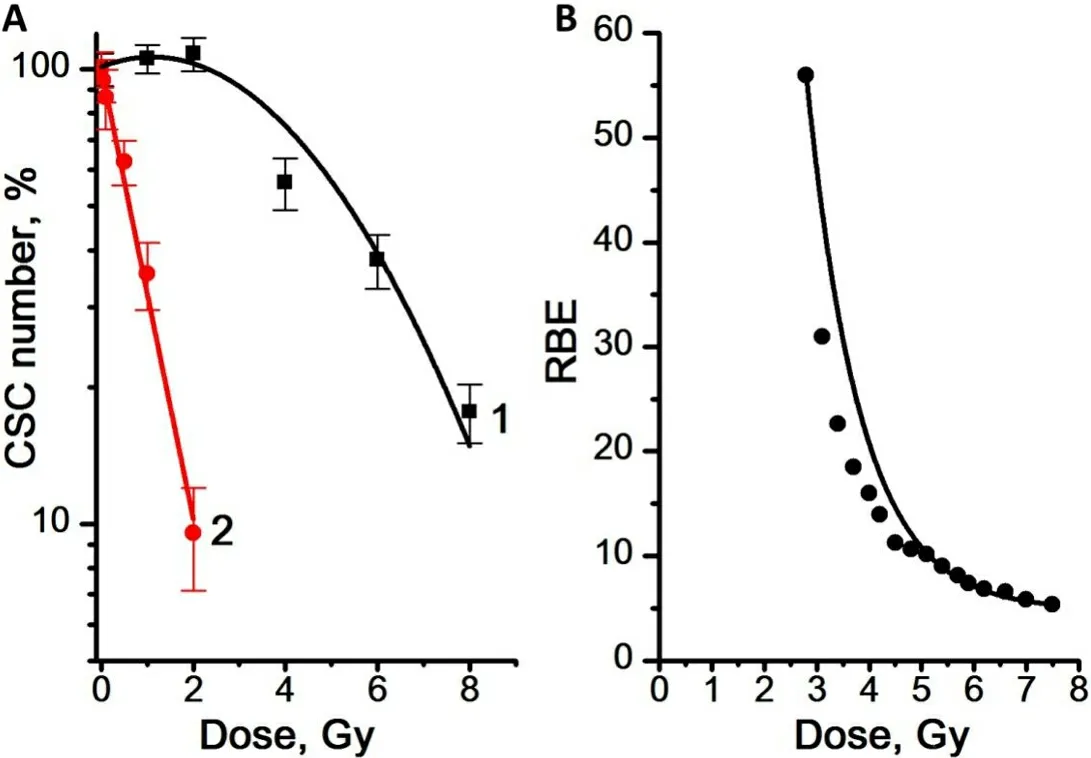
Figure 6. (A) Dose dependence of the number of CSCs in the mammosphere cell cultures for γ-radiation (Curve 1) and γ,n-radiation(Curve 2). (B) RBE values of γ,n-radiation determined from the data on the dose dependence of the number of CSCs in the mammosphere cultures. Experiments were repeated five times. CSCs: Cancer stem cells; RBE: relative biological effectiveness.
We showed that mammosphere cells are significantly more sensitive to γ,n-radiation than to γ-radiation[Figure 4]. The dose dependence of the survival rate of mammosphere cells was linear for γ,n-radiation(regression line equation for Curve 2: y = 2.0 - 0.44x, R2= 0.97) and linear-quadratic for γ-radiation(regression line equation for Curve 1: y = 2.0 - 0.02x - 0.02x2; R2= 0.95). The highest RBE values of γ,n-radiation were observed at low doses: with an increase in the radiation dose from 1 to 6 Gy, the RBE of γ,n-radiation assessed by the survival rate of mammosphere cells decreased from 18 to 4.
The proportion of CSCs in the mammosphere cell culture was determined by flow cytometry as a fraction of cells with the CD44+/CD24-/lowphenotype, as shown in Figure 1C and D. The proportion of CSCs in the control MCF-7 cells mammosphere cultures was 5.5 ± 0.9%. We found that, with an increase in the dose of γ-radiation, the proportion of CSCs tends to increase; on the contrary, with an increase in the dose of γ,n-radiation, the proportion of CSCs in the studied dose range does not change and at a dose of 2 Gy even tends to decrease [Figure 5A and B].
Using the data on the total number of cells and the proportion of CSCs in the cell culture, the number of CSCs per sample was calculated. The results obtained are shown in Figure 6.
After γ-irradiation at doses of 1 and 2 Gy, a slight increase in the number of CSCs was found, by 3% and 8%,respectively, compared to the control [Figure 6A]. At the same time, γ,n-irradiation in the studied dose range led to a decrease in the number of CSCs compared to the control. The dose dependence of the number of CSCs was linear for γ,n-radiation (regression line equation for Curve 2: y = 2.0 - 0.49x, R2= 0.99)and linear-quadratic for γ-radiation (regression line equation for Curve 1: y = 2.0 + 0.04x - 0.02x2, R2= 0.94).Regression Curves 2 in Figures 4A and 6A are similar. The obtained results indicate a very similar sensitivity of CSCs and the general population of mammosphere cells to γ,n-radiation. At the same time, CSCs were significantly more resistant to γ-radiation compared to mammosphere cells: 50% of CSCs died at a dose of 5.5 Gy, while 50% of mammosphere cells died at a dose of only 4 Gy. Higher values of the RBE of γ,n-radiation for CSCs were also observed at low doses: the RBE of γ,n-radiation assessed by the number of CSCs decreased from 56 to 4 with an increase in the radiation dose from 3 to 7.5 Gy.
The effect of γ- and γ,n-radiation on the CSC capability of self-renewal was assessed by determination of the dependence of the number of mammospheres formed seven days after irradiation on the radiation dose[Figure 7].
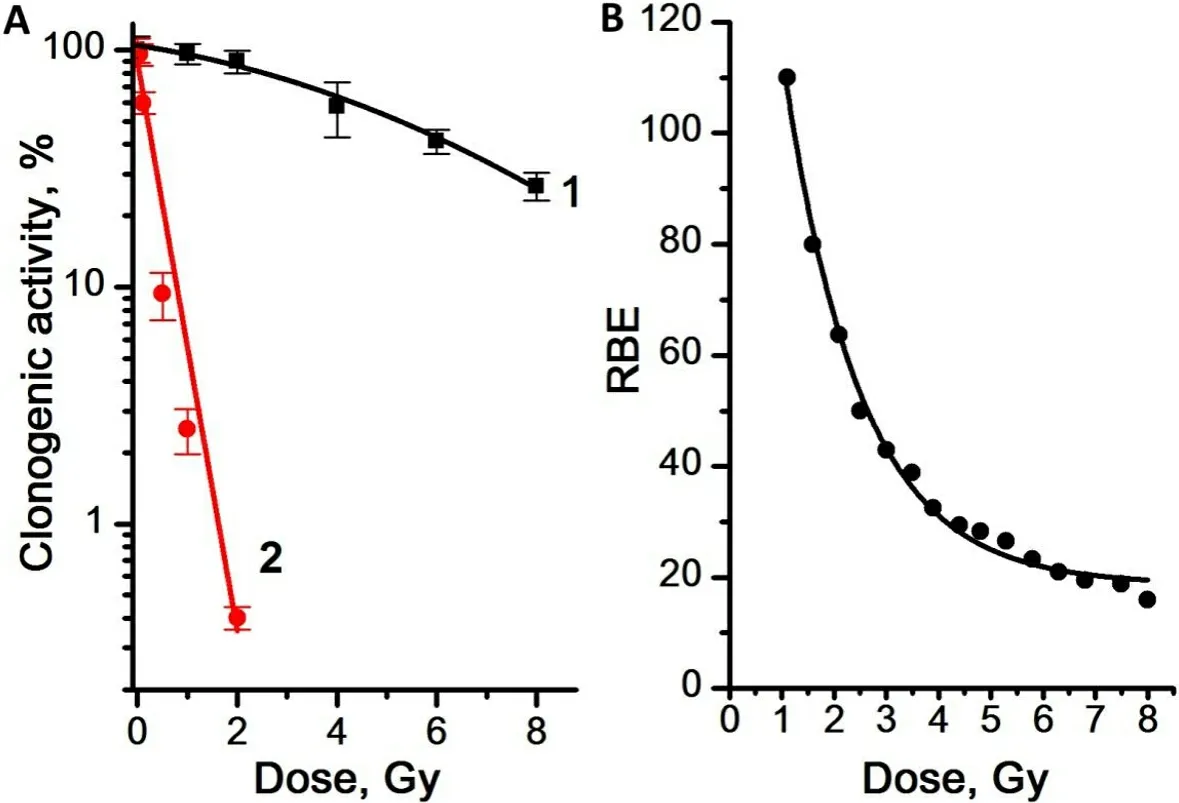
Figure 7. (A) Dose dependence of the CA of CSCs in mammosphere culture for γ-radiation (Curve 1) and γ,n-radiation (Curve 2). (B)Dose dependence of the RBE of γ,n-radiation assessed by the CA of the mammosphere cells. Experiments were repeated five times. CA:clonogenic activity; CSCs: Cancer stem cells; RBE: relative biological effectiveness.
We showed that, after γ-irradiation in all the studied dose range up to 8 Gy, a significant number of CSCs remained capable of self-renewal and formation of mammospheres, while γ,n-irradiation led to a decrease in the CSC capability of self-renewal, which confirms the much higher sensitivity of CSCs to γ,n-radiation compared to γ-radiation. The dose dependence of the CA of CSCs was linear for γ,n-radiation (regression line equation for Curve 2: y = 1.9 - 1.2x, R2= 0.98) and linear-quadratic for γ-radiation (regression line equation for Curve 1: y = 2.0 - 0.03x - 0.01x2, R2= 0.99). The highest values of the RBE of γ,n-radiation assessed by the change in CA were observed at low doses as well: with an increase in the radiation dose from 1 to 8 Gy, the RBE of γ,n-radiation assessed by the CA of mammosphere cells decreased from 110 to 18.
DISCUSSION
Radiation therapy is the most effective treatment for various types of cancer. The high resistance of CSCs to X-ray and gamma radiation exposure and the existence of radioresistant types of cancer determine the need for the development and implementation of new methods of radiation therapy for malignant tumors. At present, along with the use of stereotactic radiosurgery, hadron therapy using protons and accelerated ions is being actively developed. During particle radiation therapy, depth-dose curves of proton and carbon ion beams sharply increase when the particles come to rest in the tissue and form the Bragg peak[44]. This makes it possible to focus the beam of accelerated particles precisely on the tumor area and reduce the level of radiation exposure to normal tissues. Neutrons do not form the Bragg peak, but they have such radiobiological advantages as high linear energy transfer, which is about 200 times greater than that of photon beams, high RBE[21], and are effective against radioresistant breast cancers[45]. However, the mechanisms of high neutron efficiency are little studied.
We compared the effects of γ- and γ,n-radiation on MCF-7 human breast adenocarcinoma cells cultured as mammospheres and showed that irradiation with neutrons leads to greater DNA damage: the number of γH2AX foci comprised 19.5 ± 7.5 per 1 Gy 1 h after γ-irradiation and 46.0 ± 5.0 after neutron irradiation. It should be noted that similar data have been obtained for the effectiveness of accelerated carbon ions12C+6when learning 53BP1 foci[46]. The protein 53BP1 is rapidly recruited to DNA double-strand breaks, where it forms sub-nuclear foci that co-localize with γH2AX, a prominent marker of double-strand breaks.
Neutron irradiation-induced γH2AX foci had a larger size compared to foci formed after γ-irradiation. The same results have been obtained after irradiation of human tumor cells with accelerated carbon ions when learning 53BP1 foci[47]and after neutron irradiation of mouse neural SCs when learning γH2AX foci[48]. The larger γH2AX foci reflect the cluster character of DNA damage referred to as complex damage. Such damage may include closely spaced double-strand breaks, end-modified single-strand breaks, multiple modified bases, and multiple sites with loss of bases or nucleotides, which are especially challenging to repair[47,49]. The significantly higher level of mammosphere cell death observed in case of neutron irradiation fully correlates with the larger scale of DNA damage.
It is important to emphasize that the dose dependence of the survival rate of all mammosphere cells and the survival rate of mammosphere CSCs completely match each other. This indicates that the sensitivity of mammosphere CSC subpopulation and all mammosphere cells to γ,n-radiation was the same, in contrast to the significantly higher resistance of CSCs compared to other mammosphere cells to γ-radiation.
We found that with an increase in the dose of γ-radiation, the proportion of CSCs tends to increase; on the contrary, with an increase in the dose of γ,n-radiation, the proportion of CSCs in the studied dose range does not change and at a dose of 2 Gy even tends to decrease. Ghisolfi and co-authors showed that ionizing radiation induces stemness in cancer cells: irradiation of CSC-depleted heterogeneous cancer cell populations induced the emergence of sphere-forming cells, and, at the molecular level, analysis of the pluripotency gene expression following gamma irradiation showed upregulation of Sox2 and Oct3/4 mRNA and protein[30]. An increase in the CSCs number with an increase in the dose of gamma-irradiation can be also associated with the death of the main part of radiation-sensitive tumor cells and the preservation of CSCs due to their higher radioresistance. In addition, the effect of gamma radiation leads to the exit of the CSCs from the state of rest and to the stimulation of their proliferation, which can also increase the amount of CSCs.
The highest values of neutron RBE for CSCs, in terms of both survival rate and CA, that is, the ability of CSCs to repopulate, were observed at low doses. The higher is the irradiation dose, the lower is the RBE,which is typical for the biological effect of neutrons[49]; however, in the therapeutic dose range of 1.2-2.4 Gy for neutrons, the RBE of neutrons for CSCs remained very high and comprised at least 40, while for the general population of tumor cells it was at least 10.
The high resistance of some tumors to radiation is determined by the same mechanisms as the resistance of CSCs, namely highly efficient repair of radiation-induced DNA damage[17], slow proliferation, and activation of anti-apoptotic signaling pathways (STAT3, Wnt, Notch, Hedgehog, NF-κB, and PI3K/Akt/mTOR) in response to damage[28]. Therefore, our data on a higher level of tumor cells DNA damage under the action of neutrons than under the action of gamma irradiation allow us to explain the high efficiency of neutrons in regard to resistant tumors and tumor recurrences after conventional radiation therapy.
Tumor growth, in addition to the properties of the tumor cells and of CSCs, is largely determined by stromal cells, the tumor microenvironment, and the regulatory factors that these cells secrete[50,51]. Our data on a higher level of DNA damage under the action of neutrons allow us to suggest that neutron irradiation can also damage the cells of the tumor microenvironment and interrupt the tumor growth stimulation by the influence of microenvironment factors.
In clinical practice, neutron irradiation has shown good results in terms of local control of tumor growth and improvement in the quality of life for patients with localized forms of breast cancer and its relapses, as well as with resistant tumors of the salivary gland, thyroid gland, and larynx[52,53].
Therapeutic use of fast neutrons is limited due to a relatively small beam penetration depth and the lack of unified treatment protocols. Spechtet al. reported that, since there is a growing interest in the development of hadron therapy using heavy ions, clinical and experimental data obtained from fast neutron studies may be useful in planning therapy with heavy particles[53]. At the same time, the use of new neutron generators to obtain neutron beams directly in clinics will make it possible to overcome the inconveniences that physicians previously encountered when using reactors and accelerators to obtain neutrons for therapy[54],so that neutron therapy will take its place in the arsenal of nuclear medicine techniques. Neutron therapy for breast cancer and other resistant cancers management may be useful in multidisciplinary approaches combining surgical procedures and modern radiotherapy techniques have become established practice. The most recent breast cancer fast neutron trials have thus evaluated this method in a multimodality approach[45].
In Conclusion, The results obtained show that MCF-7 human adenocarcinoma CSCs have a significantly higher sensitivity to γ,n-radiation than to γ-radiation, which explains the higher efficacy of neutron therapy for resistant tumors known from clinical data. We also demonstrated that one of the possible mechanisms for the high sensitivity of tumor cells and CSCs to γ,n-radiation is the formation of a larger number of DNA double-strand breaks per radiation dose, which were measured as γH2AX foci.
DECLARATIONS
Authors’ contributions
Performed experiments: Shuvatova VG, Semochkina YP
Managed the irradiation process: Strepetov AN
Designed experiments: Moskaleva EY
Analyzed data: Shuvatova VG, Semochkina YP, Moskaleva EY
Wrote the manuscript: Shuvatova VG, Moskaleva EY
Translated the manuscript: Semochkina YP
Availability of data and materials
All data utilized in this study are publicly available. See Methods for data sources.
Financial support and sponsorship
The work was supported by the National Research Center “Kurchatov Institute”.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2022.
 Journal of Cancer Metastasis and Treatment2022年5期
Journal of Cancer Metastasis and Treatment2022年5期
- Journal of Cancer Metastasis and Treatment的其它文章
- GENERAL INFORMATION
- Follicular lymphoma: the diminishing role of chemotherapy
- AUTHOR INSTRUCTIONS
- Lymph node metastasis from non-melanoma skin cancer
- Tumors due to chronic exposure to benzene and biomarkers of exposure
- Molecular pathology of thymoma and thymic carcinoma
