Fetal programming of obesity and type 2 diabetes
lNTRODUCTlON
Obesity and type 2 diabetes mellitus rates are rising globally. Obesity is the commonest form of malnutrition in the developed world, and is rapidly increasing in developing countries[1-3]. Obesity is strongly associated with insulin resistance and the development of type 2 diabetes. By 2050, it is predicted that half a billion men, women, and children will have type 2 diabetes, of whom three quarters will be from low and middle income countries (LMIC)[4]. Diabetes and its complications including kidney disease, heart disease, stroke, retinopathy and neuropathy, lead to premature mortality, morbidity, disability and reduced quality of life in affected individuals. At present, someone dies due to diabetes-related complications every 7 s[5]. Furthermore, it also leads to decreased workforce productivity, increased healthcare utilization and escalating healthcare costs[6]. Ten percent of the global health expenditure is spent on diabetes-related care[4].
Obesity is the main driver of type 2 diabetes. Obesity refers to the excess accumulation of body fat to an extent that it is harmful to an individual’s health. The fundamental cause of obesity is an imbalance between energy intake and expenditure, with excess energy being stored as fat in adipose tissue. This predisposes adipocytes to secrete more pro-inflammatory adipocytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), causing a state of low-grade inflammation and insulin resistance. An increase in insulin resistance necessitates a compensatory increase in insulin secretion from pancreatic β cells, and failure to achieve this demand results in diabetes. This, however, is a gradual process, and it may take years for diabetes to manifest clinically. Thus, early identification and modification of risk factors at the onset of the above trajectory could help prevent type 2 diabetes[5].
The etiology of obesity and type 2 diabetes is multifactorial and involves complex interactions between genetic, environmental and behavioral factors[3,7]. The rapid rise in obesity is mostly attributed to the unhealthy lifestyle associated with urbanization and technical advancement over the last three to four decades[8]. The present generations live within an obesogenic environment, with energy imbalance arising from excessive energy intake due to high fat, high-sugar, energy-dense processed foods, and a reduction in occupational, household and leisure-time physical activity[3,7,9]. However, there is evidence of an additional factor leading to increases in obesity and type 2 diabetes. This is the impact of the prenatal and early-life environment on long-term health
fetal programming.
The Developmental Origins of Health and Disease (DOHaD) concept states that early-life environmental influences at sensitive periods of development lead to lifelong effects on health and chronic disease risk[10]. There is evidence that exposure to an abnormal
environment disturbs the metabolic programming of the growing fetus, increasing the lifelong risk of chronic diseases including type 2 diabetes[11-15] This process is described as fetal or developmental programming[16]. Fetal programming is now recognized as a key factor contributing to the rapid rise in obesity and type 2 diabetes mellitus rates worldwide. Research in humans and animals over the past two decades has provided considerable evidence supporting ‘developmental programming’ by the intrauterine environment[17].
Fetal programming helps explain certain aspects of the obesity epidemic that cannot be fully explained by genetic and environmental factors. The relatively short time over which obesity and type 2 rates have escalated precludes genetic change as a major attributor[15,18]. Furthermore, energy homeostasis and body weight are regulated by biological systems established in early life. Thus, it is difficult to explain how lifestyle changes alone, can override these biological homeostatic mechanisms to bring about obesity[15,18]. Fetal programming is the most plausible reason for this phenomenon. Dysregulation of biological mechanisms maintaining body weight by early life fetal programming also helps explain why reversal of obesity is difficult[19].
FETAL PROGRAMMlNG OF OBESlTY AND TYPE 2 DlABETES
Epidemiological, clinical, and basic sciences research suggest that the foundations of an individual’s lifelong health, including predisposition towards obesity and type 2 diabetes is largely established during the ‘first 1000 days of life’ from day of conception to completion of the 2
year of life. This is a highly sensitive period of growth and development in humans, where biological systems are formed and developed[10,20,21].
It is difficult to separate out effects of
exposure from genetic and nurturing influences in humans. However, studies in small mammals and other animal models have shown that prenatal exposure to an adverse
environment associated with maternal overnutrition results in developmental programming of obesity and other disorders in offspring[22-25]. For example, in geneticallymodified obesity-prone rats, greater postnatal adiposity was observed in offspring born to overnourished dams, compared to normally nourished dams[22]. Furthermore, offspring of over-nourished dams developed greater body weight and body fat compared to offspring of lean dams, even when both groups were fostered by lean dams after birth[23]. These studies indicate that
exposure to maternal obesity
increases susceptibility to obesity in later life, beyond genetics or nurturing practices.
I stood up to look around, when a gentle hand touched my shoulder. I turned around to find wrinkled, little old lady beaming up at me with smile that lit up her entire being. She said, Hi handsome. My name is Rose. I m 87 years old. Can I give you a hug? I laughed and enthusiastically responded, Of course you may! , and she gave me a giant squeeze. Why are you in college at such a young, innocent age? I asked. She jokingly replied, I m here to meet a rich husband, get married, have a couple of kids... No seriously, I asked. I was curious what may have motivated her to be taking on this challenge at her age.
If the mother is obese during pregnancy, there is excess transfer of nutrients to the fetus, stimulating increased fetal insulin secretion, fetal overgrowth and increased adiposity. It is hypothesized that this tendency for fat accrual then tends to persist during childhood and adulthood. Furthermore, the metabolic milieu of overweight/obese mothers differs from normal weight mothers, with obese pregnancy being associated with higher insulin resistance, pro-inflammatory adipokines (leptin, IL-6, TNF-α) and lipid abnormalities.
exposure to this abnormal metabolic milieu is also implicated in fetal programming[31,35,44]. Factors associated with developmental programming of obesity in offspring of mothers who have obesity/diabetes in pregnancy include high glucose levels, triglycerides, free fatty acids, adiponectin, leptin, hypoxia, oxidative stress, inflammation, and the microbiome[25]. It is proposed that fetal exposure to this abnormal metabolic milieu leads to dysregulation of the offspring adipo-insular axis (leptin and insulin) causing alterations in the central nervous system regulation of appetite, activity level, energy balance and in adipocyte metabolism[14,34,44,45].
FACTORS PREDlSPOSlNG TO DEVELOPMENTAL PROGRAMMlNG OF OBESlTY/TYPE 2 DlABETES AND POTENTlAL MECHANlSTlC PATHWAYS
The field of developmental programming has begun to move beyond associations to potential causal mechanisms for developmental programming. Studies in humans and animal models are helping unravel underlying biological mechanisms underpinning fetal programming, including epigenetic, cellular, physiological, and metabolic processes[26]. We have however, still not gained a complete understanding of the complex ways in which the maternal genome, metabolome, and microbiome relate throughout pregnancy and lactation to increase the offspring’s disease risk across the life span[25]. Determining mechanisms of fetal programming has been complicated by rapid changes in the social environment and human behavior. Thus, more studies are needed to help better delineate the pathophysiological mechanisms underpinning fetal programming[25].
Epigenetics, and mechanisms of epigenetic modification have led to increased understanding of developmental programming, and how environmental, genetic and epigenetic factors inter-relate to cause lasting effects on offspring size, adiposity and future metabolic outcomes. Neonatal methylation markers associated with birth weight from several gene loci, have shown significant associations with the prenatal environment, as well as longitudinal associations with offspring size and/or adiposity in early childhood, providing evidence that developmental pathways to adiposity begin before birth and are influenced by environmental, genetic and epigenetic factors[16]. Disruption of the gut microbiome observed in maternal obesity, antibiotic use in pregnancy, delivery and early infancy, and cesarean section have also been implicated in increased childhood obesity risk. Disruption of microbiome colonization during critical periods of early development can predispose offspring to obesity, asthma, allergy and diabetes. This may occur due to cesarean delivery, and the use of prophylactic antibiotics during cesarean section, as well as maternal exposure to antibiotics in the second and third trimesters of pregnancy, and use of antibiotics in the offspring in early infancy. Furthermore, increased maternal body mass index (BMI)
is associated with altered intestinal microbial community structure of infants’ stool up to 2 years of age[25]. Future research in epigenetics and the gut microbiome could yield greater insights into the mechanistic pathways as well as potential methods of modulating fetal programming.
Good maternal nutrition prior to and during pregnancy is important for optimizing offspring longterm health. Fetal programming, initially described in relation to fetal undernutrition, was associated with a higher risk of central obesity, diabetes, hypertension, coronary heart disease and stroke in adult life[12]. Fetal growth is influenced by the
environment, and there is trouble at both ends of the birthweight spectrum, with a ‘J’ shaped relationship between birth weight and future obesity risk[27]. It is proposed that the fetus ‘senses’ its future nutritional status
signals from the mother, and responds in ways which establish lasting influences on weight and appetite control[28]. Many umbilical cord blood metabolites and hormones are associated with birth weight and adiposity in human infants[25]. Paradoxically, both a nutritionally limited or nutritionally excessive
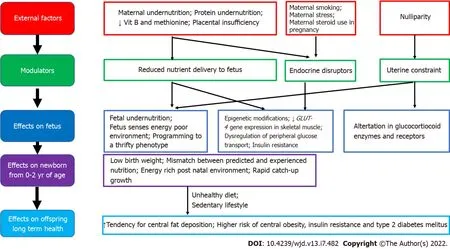
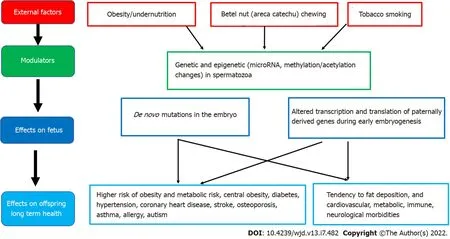
environment can lead to later obesity and associated co-morbidities[29]. More recent evidence emphasizes the adverse developmental programming effects of fetal overnutrition, and its association with increased risk of obesity in childhood and adulthood[29,30]. In addition, paternal factors are also now being recognized to play a role in fetal programming. The effects of maternal overnutrition, maternal undernutrition/ stress and paternal factors on fetal programming of obesity/type 2 diabetes including potential modulatory pathways and effects on the offspring are shown in Figures 1-3.
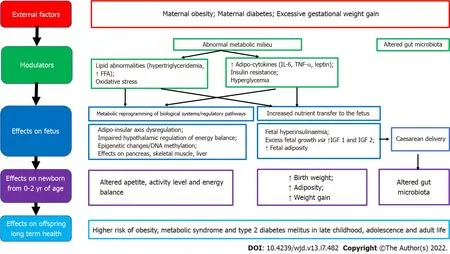
Maternal overnutrition
Concurrent with the global epidemic of obesity, the prevalence of overweight and obesity in women of reproductive age has risen rapidly over the last three decades[7,31,32]. There is now compelling evidence from human as well as animal studies that maternal obesity, diabetes and increased gestational weight gain all increase offspring birth weight and lead to fetal programming of obesity in the offspring[33]. It is thought that offspring obesity is programmed by the ‘obesogenic’ maternal metabolic environment the fetus is exposed to
during development, setting in an ‘obesity cycle’, where maternal obesity leads to neonatal obesity which continues to childhood and adulthood, propagating obesity in the next generation[34,35]. Thus, an increase in overweight and obesity among women of reproductive age should be considered an important modulator of the global obesity epidemic, which is further propagating obesity in future generations.
Are you crazy his eyes got funny and he said something like. The boat I want is the Supremo Numero-Uno blah-blah. Soon as I finish saving up 6,000 bucks2 for that baby I m going to order right from the manufacturer. Custom. In silver. Yesiree. This loser store wouldn t carry something like THAT. And I m sure not going near those sucker crowds.
Beyond infancy, promising interventional approaches for pre-school age children include age appropriate health and nutrition education for preschoolers, combined with teaching parents behavioral change strategies and increasing parenting skills[105]. For school children, school-based interventions have been shown to be effective in reducing excessive weight gain in children[106]. Programs involving both school and family and lasting ≤ 1 year were the most efficacious for primary school children aged between 6 and 12 years; while family-based interventions have been effective in children < 6 years old[107].
The prenatal environment in humans appears to be influenced by maternal body composition, metabolism, stress and diet from conception and throughout pregnancy. Paternal influences are also being recognized. Thus, parental lifestyle appears to influence the health of the offspring prior to birth,
fetal programming. From the maturation of gametes through to early embryonic development, parental lifestyle can adversely influence long-term risks of offspring metabolic, cardiovascular, immune, and neurological morbidities[26].
Maternal diabetes
Maternal diabetes during pregnancy is also strongly associated with fetal programming of obesity in the offspring. At present, approximately 20 million live births are affected by hyperglycemia in pregnancy, globally[4]. There is evidence that offspring of mothers with gestational diabetes mellitus have an increased risk of developing obesity, insulin resistance, type 2 diabetes, hypertension and cardiovascular complications at a relatively young age[46]. In the follow-up of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, 4160 children aged between 10-14 years, whose mothers had a 75-g oral glucose tolerance test at 28 wk of gestation demonstrated that exposure to higher maternal glucose spectrum levels
was significantly associated with childhood glucose levels and insulin resistance, independent of maternal and childhood BMI and family history of diabetes[47]. Studies have also shown associations between the sex of the fetus and maternal blood glucose concentrations during pregnancy, suggesting that fetal programming could be influenced by offspring gender as well[48,49].
Maternal undernutrition
Maternal undernutrition during critical periods of fetal development has been linked with fetal programming of central obesity, insulin resistance, metabolic syndrome and type 2 diabetes in later life, especially when exposed to an energy-rich diet postnatally. Adipogenesis, which begins
and accelerates in neonatal life, is a major candidate for developmental programming. According to the thrifty phenotype hypothesis, maternal undernutrition during critical periods leads to compensatory changes in the fetus, including tendency to store fat, which causes central obesity in later life, when there is a mismatch between the predicted and experienced postnatal nutritional environment[50,51].
Marta placed her fingers over his lips. Shhh, she hushed him. I do love you, Edward. I always have. And because of that, all I really want is your happiness. Go to her. As she gazed up at him, he saw for the first time how very beautiful she was. He recalled their walks in the meadows, their quiet evenings before the fire, her working beside him with the sandbags. It was then he realized what he had known for months. No, Marta. It is you I want. Sweeping17 her into his arms, he kissed her with all the love bursting inside him. Their families gathered around them chorusing(), We are here for the wedding!
Epigenetic pathways in fetal programming from
undernutrition described include histone modifications in skeletal muscle that directly decrease
gene expression, which leads to metabolic dysregulation of peripheral glucose transport and insulin resistance, which can contribute to the development of type 2 diabetes in later life. These fetal programming changes, combined with the effects of obesity, ageing and physical inactivity, are the most important factors in determining type 2 diabetes in those born with low birthweight[51]. Furthermore, specific maternal nutrient deficiencies during pregnancy, including low maternal protein consumption, and poor vitamin B and methionine status are also associated with an increased risk of metabolic derangements and type 2 diabetes in later life. Evidence from animal studies show that a protein-restricted diet
programs susceptibility to obesity, when exposed to overnutrition in postnatal life[50,52].
Prenatal stress could also be a modulating factor for fetal programming of obesity in severe maternal malnutrition[53]. During fetal development, the hypothalamic-pituitary-adrenal axis is extremely susceptible to programming, and alterations in the expression and function of glucocorticoid receptors and major glucocorticoid regulatory enzymes are observed in those exposed to undernourishment in early life[54]. Other factors associated with fetal programming include maternal exposure to endocrine disruptors, maternal infection and smoking and nulliparity[17,55]. Nulliparity is potentially associated with subtle adverse metabolic outcomes in overweight/obese mothers and their offspring, through uterine constraint effects[55].
Paternal factors
Epidemiological and animal studies suggest that many factors, including paternal under- and overnutrition, exposure to environmental toxins, father's health conditions such as diabetes, and even grandfather's nutritional status can program diseases in the offspring
germ cell-mediated transmission[56]. High paternal BMI has been linked with newborn adiposity[57]. Furthermore, paternal overweight/obesity appears to induces paternal programming of offspring phenotypes, through genetic and epigenetic changes in spermatozoa. Both human and rodent models have established that paternal obesity impairs sex hormones, basic sperm function, and molecular composition, which can result in perturbed embryo development and increase subsequent offspring disease burden[57]. Theories for the origin of male obesity-induced paternal programming include the accumulation of sperm DNA damage resulting in de novo mutations in the embryo and changes in sperm epigenetic marks (microRNA, methylation, or acetylation) altering the access, transcription, and translation of paternally derived genes during early embryogenesis[57].
Postnatal factors
In keeping with the concept of “the first 1000 days of life”, postnatal factors from the time of birth to the second birthday of a child, could also contribute towards adverse programming increasing the risk of obesity and type 2 diabetes in later life. Our present state of knowledge includes mainly early life nutritional practices, including breastfeeding duration, timing of introducing complementary feeding, and protein rich foods[58]. The underlying mechanisms are yet unclear, but there is emerging evidence that it is associated with altered neuro-endocrine programming, and modified by breastfeeding duration and maternal pre-pregnancy overweight[58,59]. Breastfeeding including longer duration of exclusive breastfeeding and longer duration of partial breastfeeding have been associated with a reduced risk of later life obesity and obesity-related complications. Breastfeeding for greater than 40 wk has been associated with lower weight gain by 1 year, and longer duration of breastfeeding with lower odds of developing hypercholesterolemia, hypertension, obesity and type 2 diabetes in later life[60]. Furthermore, mothers who are overweight and obese appear to breastfeed their babies for a shorter duration and introduce complementary foods earlier than mothers of normal weight, which could play a role in their offspring having increased weight and BMI from early childhood[59]. Exclusive breastfeeding for 6 mo or longer, and delaying the introduction of complementary feeding until 5
month of age, are also associated with lower risk of overweight at 5-6 years of age[61]. In addition, social factors including poor nurturing practices and role modeling by parents, early introduction of highly processed high fat, high sugar snacks/meals and exposure to unhealthy food advertising, are early life factors associated with increased offspring obesity.
The queen rode happy home to the hut, and happier still was the man, who had been sitting there in great anxiety, for now he was freed from all the power of the evil spirits
PREVENTlON OF FETAL PROGRAMMlNG
Primary and secondary prevention of obesity are at the foundation of diabetes prevention programs. While several medical and lifestyle strategies have shown promising effects in slowing progression to and minimizing complications of type 2 diabetes, implementing community measures to prevent obesity/type 2 diabetes are bound to be more cost-effective and beneficial to the community at large, compared to the cost of screening, treating and managing complications of established obesity/type 2 diabetes.
There is now increasing focus on primary prevention of obesity/type 2 diabetes targeting the first 1000-d of life[5]. The first 1000-d of life offers a unique and critical window of opportunity to shape long-term health at the population level, which can have a lasting effect on a country’s health and prosperity. Firstly, however, it is prudent to consider the important fundamental question of whether fetal programming of obesity can be minimized by interventions which improve the
environment of the fetus, in humans. The most promising research findings on preventing adverse fetal programming have come from animal models under experimental conditions. Whether these interventions could be applied in clinical practice, and their effectiveness remain uncertain. However, there are emerging data that improvement in fetal overnutrition and risk of obesity can be achieved
maternal interventions. Perhaps the best evidence available to date, is improvement in long-term health outcomes observed in offspring born to severely obese women, after maternal weight loss following bariatric surgery[62]. Studies comparing offspring pairs born to morbidly obese women conceived before and after substantial weight loss following bariatric surgery found that children conceived after surgery had a lower risk of macrosomia (birth weight > 4 kg) at birth, and continued to have better health outcomes in childhood and adolescence including a 50% lower risk of obesity, three-fold lower risk of severe obesity, and better insulin sensitivity and lipid profile, compared to their older siblings[62-64]. These findings confirm that pre-conception weight loss in severely obese mothers can lower fetal overnutrition and reduce the risk of obesity and metabolic complications in the offspring. However, weight reduction by bariatric surgery prior to conception is not an easily available or feasible option for most overweight and obese women of reproductive age, making it necessary to consider alternative interventions which could potentially improve offspring health outcomes.
I began to think I should like very well to stay in this pleasant country, and I said so to the stately lady, but she answered with the greatest disdain: Do you think I would keep you here? _You_! Why what do you suppose would be the good of you in this country, where everybody is wide-awake and busy? No, no, I have shown you all the hospitality you will get from me
The World Health Organization, having recognized and acknowledged the potential impact of fetal programming on the obesity epidemic, now advocates a life-course approach for the prevention and control of non-communicable diseases including obesity. This life cycle approach starts with maternal health including preconception, antenatal and postnatal care, and maternal nutrition[65,66]. Potential measures that can be taken at various stages of the life cycle to reduce adverse effects of fetal programming of obesity and type 2 diabetes in future generations are shown in Figure 4.
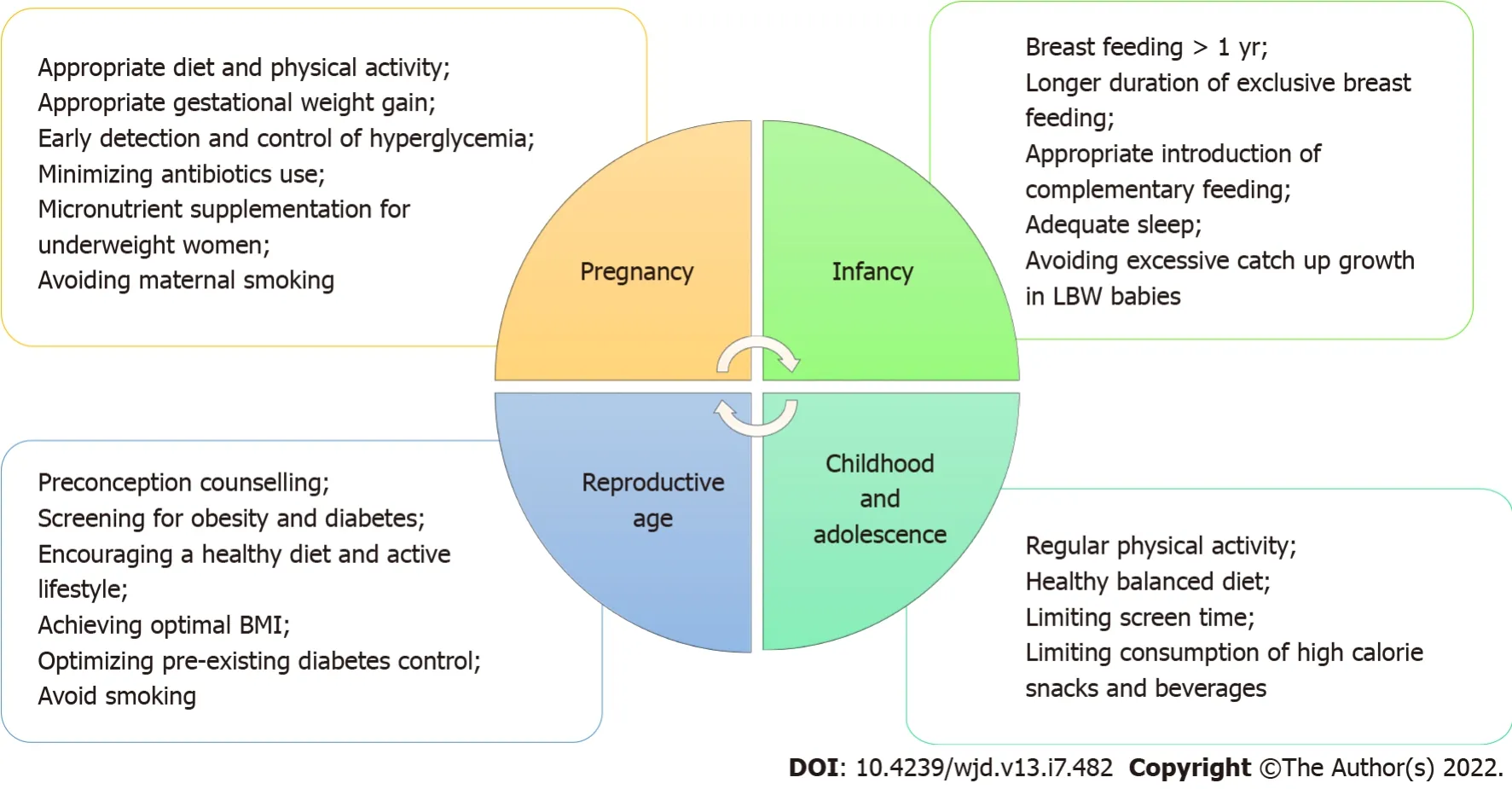
Lifestyle interventions during pregnancy
When considering the impact of intrauterine overnutrition and macrosomia on obesity risk in the next generation, public health measures for healthy maternal weight throughout the reproductive years is justified. Ideally body weight should be optimized to a healthy BMI in all women planning a pregnancy, but this is easier said than done. Pregnancy itself, however, can be an opportune period to commence healthy lifestyle changes, if heath care providers consider it as a "teachable moment" to educate pregnant women on the potential benefits to the baby as well as the mother, and utilize regular and frequent contact with heath care services during this time to provide encouragement and guidance to institute lifestyle interventions[67].
And they put Gerda to bed, and she folded her hands, thinking, as she fell asleep, How good people and animals are to me! The next day she was dressed from head to foot in silk and satin
Lifestyle interventions during pregnancy and postpartum appear to reduce gestational weight gain, pregnancy-induced hypertension, need for cesarean section and neonatal respiratory distress syndrome, without any risk of harm to the mother or neonate, across all maternal BMI categories[68]. Thus, a healthy diet and regular exercise for all healthy women during pregnancy and postpartum is a low-cost and feasible intervention which has been advocated as a global health policy[68,69].
Antenatal lifestyle intervention in maternal obesity
Given the rising rates of obesity in women of reproductive age, first in developed, and subsequently in developing countries over the past few decades, there is an urgent need for effective interventions to reduce adverse fetal programming due to maternal obesity[70]. There is expert consensus, that antenatal lifestyle interventions in overweight and obese pregnant women could alter adverse fetal programming and improve offspring health[71,72]. It is postulated that modifying the obesogenic
environment by lifestyle changes such as increased antenatal physical activity or improved dietary intake during pregnancy could reduce harmful programming effects in the offspring[72]. Antenatal nutritional/lifestyle interventions in overweight/obese pregnant women could potentially be effective by preventing excessive maternal gestational weight gain, and by reducing the risk of developing gestational diabetes, and improving the unhealthy maternal metabolic milieu (insulin resistance, hyperinsulinemia, hyperglycemia, hyperlipidemia, and increased inflammatory markers) which lead to adverse fetal programming[73,74].
Several studies have investigated if lifestyle interventions in overweight and obese mothers during pregnancy can attenuate offspring programming of obesity. In overweight/obese women, multicomponent interventions with both a diet and physical activity component have shown some promise, with reduction in gestational weight gain, pregnancy-induced hypertension, macrosomia and neonatal respiratory distress syndrome[68]. Diet was associated with greater reductions in the risk of gestational diabetes mellitus, pregnancy-induced hypertension and preterm birth, compared with any other intervention[68]. However, the effects of these interventions on long-term offspring health are unclear. Studies such as the LIMIT study in Australia reported that providing pregnant women who were overweight or obese with an antenatal dietary and lifestyle intervention improved maternal diet and physical activity during pregnancy, but did not alter 6-mo infant growth and adiposity, or childhood dietary intake, growth and adiposity at 3-5 years of age.
A recent review reported that several multicomponent trials promoting breastfeeding, responsive feeding, and a healthy diet (increased fruit and vegetables, and limiting sugar sweetened beverages and unhealthy snacks) through home visits or education at baby health clinics over 1-2 years duration, showed relative reductions in BMI in offspring at the end of the intervention, although early benefits were not maintained in the two trials reporting follow-up 1 year to 3 years later[102]. Thus, there is some evidence that nutrition or feeding interventions in the first two years of life can have a positive impact on a child's BMI, but maintaining this benefit may require continued intervention and sustainable environmental change[102].
One day, I hear frightening news: we are being shipped to another camp. This could mean the end for me. And it definitely means the end for me and my friend.
However, the effects of antenatal exercise during pregnancy on offspring health appear to vary depending on exercise intensity and frequency, as well as its timing in relation to the period of gestation[82-85]. Commencing exercise in early pregnancy appeared to stimulate feto-placental growth, and increase birth weight, while exercising in the second half of pregnancy appeared to reduce birth weight[83,86]. Furthermore, while it is postulated that regular antenatal exercise during the second half of pregnancy may lead to a reduction in birth weight and adiposity in the offspring, which may be protective against obesity in later life[87], it does not appear to be effective in practice, especially in overweight and obese women[88]. The results of clinical trials targeting antenatal exercise in overweight and obese women have led to varying/inconclusive findings on birthweight and other markers of fetal programming[89,90]. Many trials on supervised antenatal exercise interventions in overweight and obese women have reported a lack of effect on birth weight, or other markers of fetal programming[88,89,91,92]. One explanation for this could be that obese women, who are generally less physically active, tend to further reduce activity levels during pregnancy[70].
When these arrived at the palace with the beautiful young maiden everyone pitied her fate; but she herself was of good courage, and asked the queen for another bridal chamber11 than the one the lindorm had had before
Interventions for pregnancies complicated by gestational diabetes/pre-existing maternal diabetes
In woman with diabetes in pregnancy, tight glycemic control will help minimize adverse fetal programming of obesity and diabetes in the offspring. For women with both pre-existing and gestational diabetes, offspring outcomes can be optimized by ensuring appropriate gestational weight gain, and optimal glycemic control
close monitoring of blood glucose levels, and appropriate medical and nutritional therapy and exercise, throughout the pregnancy[93].
For women with pre-existing type 2 diabetes, insulin has long been considered the gold standard managing diabetes during pregnancy[93]. Careful blood glucose monitoring and titration of insulin doses are important as total daily insulin requirement increases linearly with advancing pregnancy[93]. For women with pre-existing diabetes, it is also important to provide preconception counseling, to achieve optimal pre-conceptional body weight and glycemic control, prior to pregnancy whenever possible. The onus is on health care providers to educate and counsel women with diabetes, particularly on the importance of these aspects not only for their own health status but also to protect their unborn baby from the risks of fetal programming.
For women with gestational diabetes, both insulin and metformin can be used to maintain blood glucose levels if lifestyle interventions are inadequate to achieve adequate glycemic control. Furthermore, for women with a previous history of gestational diabetes, post-partum weight reduction prior to pregnancy could potentially help reduce gestational diabetes mellitus and its associated complications in subsequent pregnancies[94]. The MiG TOFU study, a prospective longitudinal followup study in Australia and New Zealand which randomized pregnant women with gestational diabetes mellitus to either metformin or insulin therapy, found that mothers on metformin, had higher glycemia in pregnancy and higher rates of babies with birth weight > 90
percentile, compared to those on insulin therapy, while offspring had similar adiposity at 2 years of age, and similar total and abdominal percentage of body fat and metabolic measures at 7-9 years of age[95].
Interventions for maternal undernutrition
Due to a paucity of evidence from long-term follow-up studies, current recommendations to reduce adverse fetal programming effects of maternal undernutrition, presume that interventions helping to optimize pregnancy outcomes and promote healthy infant growth and development will also help improve the long-term risk of chronic diseases such as central obesity and type 2 diabetes. These recommendations include optimizing maternal nutrition prior to pregnancy, ensuring adequate micronutrient intake in the preconception period and throughout pregnancy before birth, and encouraging breastfeeding and high quality complementary foods to the offspring after birth[96]. Maternal multiple micronutrient supplementation including vitamin and mineral supplementation during the preconception period and early pregnancy have shown some benefit in reducing fetal undernutrition and other adverse fetal programming effects in undernourished mothers[21].
Long, long ago, in the days when fairies, witches, giants and ogres still visited the earth, there lived a king who reigned1 over a great and beautiful country
Balanced protein-energy supplementation also appears to be an effective intervention to reduce the prevalence of low birthweight and small-for-gestational-age births, especially in undernourished women[97]. Thus, ensuring appropriate and adequate intake of micronutrients, essential fats and protein supplementation in mothers with undernutrition during pregnancy, could improve the nutritional condition of the mother, and confer a protective benefit to the offspring by reducing fetal growth restriction and low birth weight in developing countries with high rates of maternal undernutrition[96].
Interventions in offspring in infancy and childhood
Maintaining a healthy maternal BMI and lifestyle from preconception and throughout pregnancy will help minimize the risk of future obesity in the offspring. A balanced diet with low glycemic load, and light-to-moderate intensity physical activity for 30-60 min daily, at least for 3-5 d per week is recommended[68]. Maternal pre-pregnancy and early pregnancy metabolic conditions often programs early placental function and gene expression in the first trimester of pregnancy, prior to when most intervention trials are initiated[110]. Interventions commenced during pregnancy have met with limited success in preventing adverse fetal programming effects. This could be because most interventions were instituted after the first trimester, where it may have been too late to have a positive impact on fetus programming.
30. Birds: Birds are predominant throughout this story. They keep the children trapped in the woods by eating the breadcrumbs. A bird leads the children to the witch s house. A bird also provides the final means of their escape by helping92 them cross the water (Tatar 2002).
Breastfeeding appears to protect against obesity in childhood, and could be a modifying factor to mitigate the adverse effects of fetal programming
. Promoting longer duration of full breastfeeding and partial breastfeeding, and delaying the introduction of complementary feeding could protect the offspring from obesity. Exclusive breastfeeding for 6 mo or longer, and delaying the introduction of complementary feeding until 5
month of age, has been associated with a lower risk of overweight at 5-6 years of age[61]. The protective effects of breastfeeding on the offspring of diabetic mothers in very early life appears somewhat conflicting, with one study suggesting a potential negative long-term influence on the risk of becoming overweight in offspring exposed to breast milk from mothers with diabetes (type 1 or gestational diabetes) during the first week of life[60]. However, overall, the benefits of breastfeeding appear to be beneficial, and protect infants from the adverse effects of fetal programming of obesity and type 2 diabetes. Women who were overweight or obese before pregnancy, appear to breastfeed their offspring for a shorter time and introduce complementary feeding earlier than normal weight women, which could contribute towards their children being heavier and having a higher BMI by end of infancy[59]. Thus, it is especially important to take measures to encourage and support longer duration of breastfeeding in women who are overweight or obese.
Further protective measures that could be helpful in optimizing long-term health during infancy include ensuring adequate sleep and minimizing antibiotic use. Early antibiotic use before 2 years of age has been associated with disruption of the gut microbiota, and a higher risk of childhood overweight and obesity[98]. Recent evidence on the associations with gut microbiota and infant weight gain or child weight status, suggest that dietary manipulation with human milk and pre/probiotic formulations holds promise for preventing obesity[99].
Furthermore, as short sleep duration increased the risk of childhood obesity, public health efforts that encourage children to have sufficient sleep time are also important in combating obesity[100]. Project Viva prospectively studied the cumulative number of modifiable early-life risk factors associated with programming of obesity/type 2 diabetes in mother-offspring pairs including:maternal smoking and consumption of high sugar-sweetened beverages during pregnancy, excessive gestational weight gain; breastfeeding for less than 1 year; complementary food introduction before 4 mo; and infant sleep duration less than 12 h daily. When reassessed in early adolescence, they found that offspring with 5-6 risk factors had a 2.5 higher rate of obesity and metabolic syndrome, compared to those with 0-1 risk factors[101]. Thus, it appears that promoting exclusive breastfeeding for at least the first 4 mo of life, and continuation of breastfeeding beyond the first year of life, as well as ensuring adequate sleep for infants, could potentially reduce the risk of further life obesity in infants who have already been exposed to risk factors for adverse fetal programming
.
Other potential strategies to reduce the adverse impact of fetal programming include identifying and targeting young children at higher risk of fetal programming of obesity/diabetes such as offspring of mothers with obesity/diabetes/undernutrition during pregnancy, especially those being reared in highly urbanized obesogenic environments, for healthy lifestyle interventions during early childhood to encourage them towards a healthier lifestyle, and prevent adverse metabolic health outcomes in later life[46]. Pairing breastfeeding with healthy weaning foods is likely to promote healthy weight trajectories.
Prenatal exercise has been considered a potential intervention to reduce adverse fetal programming, especially in pregnancies complicated by obesity and/or diabetes[70,75]. Previously, there were concerns regarding the safety of exercise during pregnancy, due to fears regarding teratogenicity from exercise-induced hyperthermia, and fetal hypoxia and intrauterine growth retardation from redistribution of blood flow and nutrients away from the utero-placental circulation during exercise[76]. However, studies on maternal antenatal exercise over the past 20 years have demonstrated that mild-tomoderate intensity antenatal exercise in healthy well-nourished women does not cause observable harm to the fetus[77-80]. There has since been a gradual change of opinion that moderate antenatal exercise is not only safe, but may also be beneficial to offspring health[71,78]. Detailed small scale studies have shown that offspring of physically active lean women who engaged in regular vigorous exercise during pregnancy had lower birthweight and subcutaneous fat at birth, and continued to have lower weight and subcutaneous fat in childhood[81].
Observational studies suggest that rapid weight gain in infancy also increases the long-term risk of obesity and type 2 diabetes in infants from both low-and high-income countries, among infants born preterm or at term, with normal or low birth weight for gestation[103]. Furthermore, it has been hypothesized that the increased risk of adverse long-term outcomes including central obesity and type 2 diabetes in low birth weight infants may be driven by accelerated postnatal catch-up growth. While some studies on health outcomes in babies with low birth weight have reported that increased catch up growth was associated with higher BMI or higher serum cholesterol levels in early adolescence, the quality and quantity of the evidence is limited[102]. Thus, it is prudent to recommend “striking a healthy balance”, especially for low and middle income countries, until more information on underlying mechanisms and suitable interventions on minimizing adverse effects of catch up growth in low birth weight infants become available[104].
Children born to overweight/obese women have increased birth weight and an increased risk of obesity and metabolic dysregulation throughout life[34,36-38]. At birth, these babies have increased birth weight and adiposity[39], and thus, an increased risk of assisted delivery as well[40,41]. Exposure to maternal obesity and diabetes accelerates adipogenesis and impairs energy sensing, affecting neurodevelopment, liver, pancreas, and skeletal muscle development in the offspring, creating a lifelong impact on multiple systems[25]. The influence of maternal obesity on the risk of offspring obesity starts manifesting from early life[36,42]. These children show increased weight for age and length, in comparison to offspring of normal weight women, as early as six months of age[42], and their risk of obesity is increased two-fold as preschoolers, even after controlling for birth weight and other confounding factors[36]. They also have an increased risk of metabolic syndrome by late childhood[43]. Furthermore, high maternal BMI in pregnancy is an independent predictor of obesity in the adult offspring, at 30 years of age[37].
Preconception care
Healthy lifestyle behaviors during the preconception period are important to optimize maternal and child outcomes. Community nurses and midwifery professions who are active across both preconception and pregnancy could play an important role in such interventions[108]. Many women of reproductive age do not appear to have optimal preconception lifestyle behaviors, and a recent systematic review identified the absence of knowledge on healthy behaviors as the most common barrier[109]. The need for further studies on how to best improve preconception women's capability, opportunity, and motivation to modify their lifestyle behaviors has been emphasized[109]. At present, there is a lack of international consensus guidelines on weight management preconception, and its impact on fertility, pregnancy and subsequent maternal and infant outcomes.
The reversibility of obesity-induced parental programming has only recently received attention. These programmed changes to offspring health may be partially restored
diet/exercise interventions in obese fathers, prior to conception,
improvement in sperm DNA integrity. Promising results in animal models utilizing diet and exercise interventions have shown improvements in sperm function and molecular composition, resulting in restorations of both embryo and fetal health and subsequent male offspring fertility[57]. However, it is noteworthy, that most data surrounding paternal obesity and offspring phenotypes have come from rodent models, and implications for clinical practice warrants further research[57].
Many balls would start in the late evening and last until the early morning hours. Cinderella s need to leave at midnight would be an early departure from most balls.Return to place in story.
CHALLENGES AND THE WAY FORWARD
Intervention strategies to reduce adverse effects of fetal programming of later life obesity may be more effective if they target multiple modifiable factors, focusing on the first 1000-d of life.
Of course they soon found out about the donkey eating Nur Mahomed s cabbages, and about the young man s hot words; but although the lad assured them that he had never said anything about murdering anyone, they replied they were ordered to arrest him, and bring him to take his trial before the king
Given the widespread and long-lasting impact of adverse fetal programming, a population-based lifecourse approach is warranted, until more focused and specific ways to prevent adverse fetal programming are discovered. As the evidence on the peri-conceptional environment on offspring longterm health is compelling, updated guidelines and guidance for parental preparation for pregnancy, prior to conception to protect the health of offspring is required[26,110]. This should be followed by proper guidance for parents regarding appropriate nutrition, physical activity, and screen time in early childhood. Furthermore, school based interventions with family involvement could be effective in improving dietary habits and lifestyle in primary school children[111]. Whole community interventions addressing both policy and behavior change are needed[112]. Wider dissemination of health messages advocating healthy lifestyle as a means of providing a better chance of a healthier life for future generations is recommended[112].
Thus, at present, lifestyle interventions during pregnancy in women with obesity/diabetes have not shown much effect on infant or childhood outcomes. However, many such clinical trials started later in pregnancy, and it is possible that developmental programming occurs much earlier and interventions focusing on healthy lifestyle interventions in pregnant humans are missing the crucial time period for effectiveness[25].
CONCLUSlON
Fetal programming is an important contributor to the global obesity epidemic. Risk factors for adverse fetal programming include maternal obesity, diabetes, undernutrition, smoking, stress, operative delivery and use of antibiotics in pregnancy, as well as paternal factors including over/undernutrition. These factors lead to fetal programming
multiple complex pathways including alterations in organ formation and homeostatic pathways, epigenetic changes, and changes in gut microbiota. Specific mechanistic pathways are still being unraveled. Using this knowledge to find effective and feasible methods of preventing adverse fetal programming is an issue of global importance. The current state of knowledge dictates that future research should be directed towards earlier interventions starting in the pre-conceptional period. Until such time, a multi-pronged life-course approach, focusing on maternal health, antenatal and postnatal care, as well as healthy lifestyle interventions for preschoolers, school children, and young adults of reproductive age is advocated.
We wish to acknowledge Dr. Jayakody C and Dr. Hewakuruppu S for their assistance with development of the figures for this manuscript. We are grateful to Dr. Perera BJC, Joint Editor of The Sri Lanka Journal of Child Health, for editing the final manuscript.
Seneviratne SN and Rajindrajith S conceptualized and designed this manuscript; Seneviratne SN reviewed the literature and wrote the manuscript; both authors read and approved the final manuscript.
All the authors report no relevant conflicts of interest for this article.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See:https://creativecommons.org/Licenses/by-nc/4.0/
Sri Lanka
Sumudu Nimali Seneviratne 0000-0003-2960-9269; Shaman Rajindrajith 0000-0003-1379-5052.
The messenger awoke with such a start, and when he saw that the hour had almost run out he snatched up the answer and rushed back with such speed that the clock had not yet struck when he entered the palace
Gao CC
Webster JR
Gao CC
1 Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, Paciorek CJ, Farzadfar F, Riley L, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in adult overweight and obesity prevalences.
2012; 10:22 [PMID:23167948 DOI:10.1186/1478-7954-10-22]
2 Gupta N, Goel K, Shah P, Misra A. Childhood obesity in developing countries:epidemiology, determinants, and prevention.
2012; 33:48-70 [PMID:22240243 DOI:10.1210/er.2010-0028]
3 Maggi S, Busetto L, Noale M, Limongi F, Crepaldi G. Obesity:Definition and Epidemiology. In:Lenzi A, Migliaccio S, Donini LM. Multidisciplinary Approach to Obesity. Cham:Springer, 2015:31-39
4 International Diabetes Federation. IDF Diabetes Atlas. Brussels, Belgium 2019. [cited 6 May 2021]. Available from:https://www.diabetesatlas.org/
5 Estampador AC, Franks PW. Precision Medicine in Obesity and Type 2 Diabetes:The Relevance of Early-Life Exposures.
2018; 64:130-141 [PMID:29097511 DOI:10.1373/clinchem.2017.273540]
6 Lehnert T, Sonntag D, Konnopka A, Riedel-Heller S, K?nig HH. Economic costs of overweight and obesity.
2013; 27:105-115 [PMID:23731873 DOI:10.1016/j.beem.2013.01.002]
7 Obesity:preventing and managing the global epidemic. Report of a WHO consultation.
2000; 894:i-xii, 1 [PMID:11234459]
8 World Health Organization. Obesity and overweight 2012. [cited 28 April 2021]. Available from:http://www.who.int/mediacentre/factsheets/fs311/en/inwordex.html
9 Eby JG, Colditz GA. Obesity/Overweight:Prevention and Weight Management. In:Kris H. International Encyclopedia of Public Health. Oxford:Academic Press, 2008:602-609
10 Gillman MW. Developmental origins of health and disease.
2005; 353:1848-1850 [PMID:16251542 DOI:10.1056/NEJMe058187]
11 Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease.
2008; 359:61-73 [PMID:18596274 DOI:10.1056/NEJMra0708473]
12 Barker DJ. In utero programming of chronic disease.
1998; 95:115-128 [PMID:9680492]
13 Barker DJ. The fetal and infant origins of adult disease.
1990; 301:1111 [PMID:2252919 DOI:10.1136/bmj.301.6761.1111]
14 Oken E, Gillman MW. Fetal origins of obesity.
2003; 11:496-506 [PMID:12690076 DOI:10.1038/oby.2003.69]
15 Gluckman PD, Hanson M, Zimmet P, Forrester T. Losing the war against obesity:the need for a developmental perspective.
2011; 3:93cm19 [PMID:21795585 DOI:10.1126/scitranslmed.3002554]
16 Lin X, Lim IY, Wu Y, Teh AL, Chen L, Aris IM, Soh SE, Tint MT, MacIsaac JL, Morin AM, Yap F, Tan KH, Saw SM, Kobor MS, Meaney MJ, Godfrey KM, Chong YS, Holbrook JD, Lee YS, Gluckman PD, Karnani N; GUSTO study group. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome.
2017; 15:50 [PMID:28264723 DOI:10.1186/s12916-017-0800-1]
17 Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes.
2019; 62:1789-1801 [PMID:31451874 DOI:10.1007/s00125-019-4951-9]
18 Chung WK, Leibel RL. Considerations regarding the genetics of obesity.
2008; 16 Suppl 3:S33-S39 [PMID:19037210 DOI:10.1038/oby.2008.514]
19 Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight.
1995; 332:621-628 [PMID:7632212 DOI:10.1056/NEJM199503093321001]
20 Hanson MA, Gluckman PD, Ma RC, Matzen P, Biesma RG. Early life opportunities for prevention of diabetes in low and middle income countries.
2012; 12:1025 [PMID:23176627 DOI:10.1186/1471-2458-12-1025]
21 Kinshella MW, Moore SE, Elango R. The missing focus on women's health in the First 1,000 days approach to nutrition.
2021; 24:1526-1530 [PMID:33023698 DOI:10.1017/S1368980020003894]
22 Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny.
1998; 275:R1374-R1379 [PMID:9756571 DOI:10.1152/ajpregu.1998.275.4.R1374]
23 Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring.
2008; 294:R528-R538 [PMID:18032473 DOI:10.1152/ajpregu.00316.2007]
24 Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain.
2006; 20:1257-1259 [PMID:16684802 DOI:10.1096/fj.05-5241fje]
25 Friedman JE. Developmental Programming of Obesity and Diabetes in Mouse, Monkey, and Man in 2018:Where Are We Headed?
2018; 67:2137-2151 [PMID:30348820 DOI:10.2337/dbi17-0011]
26 Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ, Hanson MA, Forrester T, Gluckman PD, Godfrey KM. Origins of lifetime health around the time of conception:causes and consequences.
2018; 391:1842-1852 [PMID:29673874 DOI:10.1016/S0140-6736(18)30312-X]
27 Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort:longitudinal study.
2001; 323:1331-1335 [PMID:11739217 DOI:10.1136/bmj.323.7325.1331]
28 Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease:a life history and evolutionary perspective.
2007; 19:1-19 [PMID:17160980 DOI:10.1002/ajhb.20590]
29 Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes.
2011; 60:1849-1855 [PMID:21709280 DOI:10.2337/db11-0400]
30 Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, Godfrey K, Gluckman P, Hanson M, Kuh D, Nathanielsz P, Nestel P, Thornburg KL. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD).
2007; 61:625-629 [PMID:17413866 DOI:10.1203/pdr.0b013e3180459fcd]
31 Catalano PM. Obesity, insulin resistance, and pregnancy outcome.
2010; 140:365-371 [PMID:20457594 DOI:10.1530/REP-10-0088]
32 Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980:systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants.
2011; 377:557-567 [PMID:21295846 DOI:10.1016/S0140-6736(10)62037-5]
33 Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity.
1998; 132:768-776 [PMID:9602184 DOI:10.1016/s0022-3476(98)70302-6]
34 Oken E. Maternal and child obesity:the causal link.
2009; 36:361-377, ix [PMID:19501319 DOI:10.1016/j.ogc.2009.03.007]
35 Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity:modifiable determinants of pregnancy outcome.
2010; 16:255-275 [PMID:19966268 DOI:10.1093/humupd/dmp050]
36 Whitaker RC. Predicting preschooler obesity at birth:the role of maternal obesity in early pregnancy.
2004; 114:e29-e36 [PMID:15231970 DOI:10.1542/peds.114.1.e29]
37 Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain:influences on offspring adiposity in young adulthood.
2010; 95:5365-5369 [PMID:20702520 DOI:10.1210/jc.2010-0697]
38 Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk.
2010; 140:387-398 [PMID:20562299 DOI:10.1530/REP-10-0077]
39 Guihard-Costa AM, Papiernik E, Kolb S. Maternal predictors of subcutaneous fat in the term newborn.
2004; 93:346-349 [PMID:15124837 DOI:10.1080/08035250410023007]
40 Oral E, Ca?da? A, Gezer A, Kaleli S, Aydinli K, O?er F. Perinatal and maternal outcomes of fetal macrosomia.
2001; 99:167-171 [PMID:11788165 DOI:10.1016/s0301-2115(01)00416-x]
41 Wikstr?m I, Axelsson O, Bergstr?m R, Meirik O. Traumatic injury in large-for-date infants.
1988; 67:259-264 [PMID:3176946 DOI:10.3109/00016348809004216]
42 Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes.
2011; 158:221-226 [PMID:20863516 DOI:10.1016/j.jpeds.2010.08.008]
43 Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood:association with birth weight, maternal obesity, and gestational diabetes mellitus.
2005; 115:e290-e296 [PMID:15741354 DOI:10.1542/peds.2004-1808]
44 Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero.
2009; 32:1076-1080 [PMID:19460915 DOI:10.2337/dc08-2077]
45 McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth:implications for the early programming of adult obesity.
2006; 131:415-427 [PMID:16514185 DOI:10.1530/rep.1.00303]
46 Bianco ME, Josefson JL. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes.
2019; 19:143 [PMID:31754898 DOI:10.1007/s11892-019-1267-6]
47 Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, Brickman WJ, Clayton P, Ma RC, McCance D, Tam WH, Catalano PM, Linder B, Dyer AR, Lowe WL Jr, Metzger BE; HAPO Follow-up Study Cooperative Research Group; HAPO Follow-Up Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome Followup Study (HAPO FUS):Maternal Glycemia and Childhood Glucose Metabolism.
2019; 42:381-392 [PMID:30617141 DOI:10.2337/dc18-2021]
48 Seneviratne SN, Derraik JGB, Jiang Y, McCowan LME, Gusso S, Cutfield WS, Hofman PL. The sex of the foetus affects maternal blood glucose concentrations in overweight and obese pregnant women.
2017; 37:667-669 [PMID:28019134 DOI:10.1080/01443615.2016.1256970]
49 Retnakaran R, Kramer CK, Ye C, Kew S, Hanley AJ, Connelly PW, Sermer M, Zinman B. Fetal sex and maternal risk of gestational diabetes mellitus:the impact of having a boy.
2015; 38:844-851 [PMID:25693837 DOI:10.2337/dc14-2551]
50 Taylor PD, Poston L. Developmental programming of obesity in mammals.
2007; 92:287-298 [PMID:17170060 DOI:10.1113/expphysiol.2005.032854]
51 Hales CN, Barker DJ. The thrifty phenotype hypothesis.
2001; 60:5-20 [PMID:11809615 DOI:10.1093/bmb/60.1.5]
52 Ozanne SE, Hales CN. Lifespan:catch-up growth and obesity in male mice.
2004; 427:411-412 [PMID:14749819 DOI:10.1038/427411b]
53 Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, Wadhwa PD. Fetal programming of body composition, obesity, and metabolic function:the role of intrauterine stress and stress biology.
2012; 2012:632548 [PMID:22655178 DOI:10.1155/2012/632548]
54 Correia-Branco A, Keating E, Martel F. Maternal undernutrition and fetal developmental programming of obesity:the glucocorticoid connection.
2015; 22:138-145 [PMID:25001018 DOI:10.1177/1933719114542012]
55 Seneviratne SN, Derraik JGB, Jiang Y, McCowan LME, Gusso S, Biggs JB, Parry GK, Chiavaroli V, Cutfield WS, Hofman PL. Nulliparity is associated with subtle adverse metabolic outcomes in overweight/obese mothers and their offspring.
2017; 87:545-551 [PMID:28727231 DOI:10.1111/cen.13426]
56 Li J, Tsuprykov O, Yang X, Hocher B. Paternal programming of offspring cardiometabolic diseases in later life.
2016; 34:2111-2126 [PMID:27457668 DOI:10.1097/HJH.0000000000001051]
57 McPherson NO, Fullston T, Aitken RJ, Lane M. Paternal obesity, interventions, and mechanistic pathways to impaired health in offspring.
2014; 64:231-238 [PMID:25300265 DOI:10.1159/000365026]
58 Marseglia L, Manti S, D'Angelo G, Cuppari C, Salpietro V, Filippelli M, Trovato A, Gitto E, Salpietro C, Arrigo T. Obesity and breastfeeding:The strength of association.
2015; 28:81-86 [PMID:25595034 DOI:10.1016/j.wombi.2014.12.007]
59 M?kel? J, Vaarno J, Kaljonen A, Niinikoski H, Lagstr?m H. Maternal overweight impacts infant feeding patterns--the STEPS Study.
2014; 68:43-49 [PMID:24219892 DOI:10.1038/ejcn.2013.229]
60 Plagemann A, Harder T. Breast feeding and the risk of obesity and related metabolic diseases in the child.
2005; 3:222-232 [PMID:18370791 DOI:10.1089/met.2005.3.222]
61 Sirkka O, Vrijkotte T, Halberstadt J, Abrahamse-Berkeveld M, Hoekstra T, Seidell J, Olthof M. Prospective associations of age at complementary feeding and exclusive breastfeeding duration with body mass index at 5-6 years within different risk groups.
2018; 13:522-529 [PMID:29695025 DOI:10.1111/ijpo.12289]
62 Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years.
2006; 118:e1644-e1649 [PMID:17142494 DOI:10.1542/peds.2006-1379]
63 Richards DS, Miller DK, Goodman GN. Pregnancy after gastric bypass for morbid obesity.
1987; 32:172-176 [PMID:3572896]
64 Ducarme G, Revaux A, Rodrigues A, Aissaoui F, Pharisien I, Uzan M. Obstetric outcome following laparoscopic adjustable gastric banding.
2007; 98:244-247 [PMID:17433814 DOI:10.1016/j.ijgo.2007.02.020]
65 World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. [cited 12 May 2021]. Available from:https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf?sequence=1
66 Hanson MA, Gluckman PD. Developmental origins of health and disease--global public health implications.
2015; 29:24-31 [PMID:25225058 DOI:10.1016/j.bpobgyn.2014.06.007]
67 Phelan S. Pregnancy:a "teachable moment" for weight control and obesity prevention.
2010; 202:135.e1-135.e8 [PMID:19683692 DOI:10.1016/j.ajog.2009.06.008]
68 Farpour-Lambert NJ, Ells LJ, Martinez de Tejada B, Scott C. Obesity and Weight Gain in Pregnancy and Postpartum:an Evidence Review of Lifestyle Interventions to Inform Maternal and Child Health Policies.
2018; 9:546 [PMID:30319539 DOI:10.3389/fendo.2018.00546]
69 Armengaud JB, Simeoni U. Offspring of Mothers with Hyperglycemia in Pregnancy:Short-Term Consequences for Newborns and Infants.
2020; 28:194-200 [DOI:10.1159/000480175]
70 Seneviratne SN, McCowan LM, Cutfield WS, Derraik JG, Hofman PL. Exercise in pregnancies complicated by obesity:achieving benefits and overcoming barriers.
2015; 212:442-449 [PMID:24909342 DOI:10.1016/j.ajog.2014.06.009]
71 Impact of physical activity during pregnancy and postpartum on chronic disease risk.
2006; 38:989-1006 [PMID:16672855 DOI:10.1249/01.mss.0000218147.51025.8a]
72 Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity?
2012; 9:1263-1307 [PMID:22690193 DOI:10.3390/ijerph9041263]
73 Weight Gain During Pregnancy:Reexamining the Guidelines. Washington (DC):National Academies Press (US); 2009- [PMID:20669500]
74 Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity.
2003; 111:e221-e226 [PMID:12612275 DOI:10.1542/peds.111.3.e221]
75 Davenport MH, Meah VL, Ruchat SM, Davies GA, Skow RJ, Barrowman N, Adamo KB, Poitras VJ, Gray CE, Jaramillo Garcia A, Sobierajski F, Riske L, James M, Kathol AJ, Nuspl M, Marchand AA, Nagpal TS, Slater LG, Weeks A, Barakat R, Mottola MF. Impact of prenatal exercise on neonatal and childhood outcomes:a systematic review and meta-analysis.
2018; 52:1386-1396 [PMID:30337465 DOI:10.1136/bjsports-2018-099836]
76 McMurray RG, Mottola MF, Wolfe LA, Artal R, Millar L, Pivarnik JM. Recent advances in understanding maternal and fetal responses to exercise.
1993; 25:1305-1321 [PMID:8107536]
77 Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy.
2006; CD000180 [PMID:16855953 DOI:10.1002/14651858.CD000180.pub2]
78 Gavard JA, Artal R. Effect of exercise on pregnancy outcome.
2008; 51:467-480 [PMID:18463475 DOI:10.1097/GRF.0b013e31816feb1d]
79 Barakat R, Ruiz JR, Rodríguez-Romo G, Montejo-Rodríguez R, Lucia A. Does exercise training during pregnancy influence fetal cardiovascular responses to an exercise stimulus?
2010; 44:762-764 [PMID:19752154 DOI:10.1136/bjsm.2009.062547]
80 Szymanski LM, Satin AJ. Exercise during pregnancy:fetal responses to current public health guidelines.
2012; 119:603-610 [PMID:22314872 DOI:10.1097/AOG.0b013e31824760b5]
81 Clapp JF 3rd. Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy.
1996; 129:856-863 [PMID:8969727 DOI:10.1016/s0022-3476(96)70029-x]
82 Clapp JF 3rd, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy:effect of exercise volume on fetoplacental growth.
2002; 186:142-147 [PMID:11810100 DOI:10.1067/mob.2002.119109]
83 Hopkins SA, Cutfield WS. Exercise in pregnancy:weighing up the long-term impact on the next generation.
2011; 39:120-127 [PMID:21519301 DOI:10.1097/JES.0b013e31821a5527]
84 Bell RJ, Palma SM, Lumley JM. The effect of vigorous exercise during pregnancy on birth-weight.
1995; 35:46-51 [PMID:7771999 DOI:10.1111/j.1479-828x.1995.tb01829.x]
85 Leet T, Flick L. Effect of exercise on birthweight.
2003; 46:423-431 [PMID:12808392 DOI:10.1097/00003081-200306000-00021]
86 Atapattu N, Mohsin F, Zabeen B, Seneviratne SN. Pediatric diabetes care in Sri Lanka and Bangladesh:Reaching the community.
2021; 22:112-115 [PMID:33232549 DOI:10.1111/pedi.13102]
87 Seneviratne SN, Parry GK, McCowan LM, Ekeroma A, Jiang Y, Gusso S, Peres G, Rodrigues RO, Craigie S, Cutfield WS, Hofman PL. Antenatal exercise in overweight and obese women and its effects on offspring and maternal health:design and rationale of the IMPROVE (Improving Maternal and Progeny Obesity Via Exercise) randomised controlled trial.
2014; 14:148 [PMID:24767604 DOI:10.1186/1471-2393-14-148]
88 Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, Craigie S, Gusso S, Peres G, Rodrigues RO, Ekeroma A, Cutfield WS, Hofman PL. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes:a randomised controlled trial.
2016; 123:588-597 [PMID:26542419 DOI:10.1111/1471-0528.13738]
89 Sui Z, Grivell RM, Dodd JM. Antenatal exercise to improve outcomes in overweight or obese women:A systematic review.
2012; 91:538-545 [PMID:22229625 DOI:10.1111/j.1600-0412.2012.01357.x]
90 Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum:a systematic review and meta-analysis of randomized controlled trials.
2013; 56:351-364 [PMID:23480971 DOI:10.1016/j.ypmed.2013.02.021]
91 Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, Carballo MT, Schmidt MI. Aerobic exercise and submaximal functional capacity in overweight pregnant women:a randomized trial.
2005; 106:243-249 [PMID:16055571 DOI:10.1097/01.AOG.0000171113.36624.86]
92 Nascimento SL, Surita FG, Parpinelli M?, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women:a randomised clinical trial.
2011; 118:1455-1463 [PMID:21895947 DOI:10.1111/j.1471-0528.2011.03084.x]
93 Berry DC, Boggess K, Johnson QB. Management of Pregnant Women with Type 2 Diabetes Mellitus and the Consequences of Fetal Programming in Their Offspring.
2016; 16:36 [PMID:26983624 DOI:10.1007/s11892-016-0733-7]
94 Kim SY, Sharma AJ, Callaghan WM. Gestational diabetes and childhood obesity:what is the link?
2012; 24:376-381 [PMID:23000698 DOI:10.1097/GCO.0b013e328359f0f4]
95 Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, Hague WM. Metformin in gestational diabetes:the offspring follow-up (MiG TOFU):body composition and metabolic outcomes at 7-9 years of age.
2018; 6:e000456 [PMID:29682291 DOI:10.1136/bmjdrc-2017-000456]
96 Triunfo S, Lanzone A. Impact of maternal under nutrition on obstetric outcomes.
2015; 38:31-38 [PMID:25194427 DOI:10.1007/s40618-014-0168-4]
97 Imdad A, Bhutta ZA. Maternal nutrition and birth outcomes:effect of balanced protein-energy supplementation.
2012; 26 Suppl 1:178-190 [PMID:22742610 DOI:10.1111/j.1365-3016.2012.01308.x]
98 Miller SA, Wu RKS, Oremus M. The association between antibiotic use in infancy and childhood overweight or obesity:a systematic review and meta-analysis.
2018; 19:1463-1475 [PMID:30035851 DOI:10.1111/obr.12717]
99 Koleva PT, Bridgman SL, Kozyrskyj AL. The infant gut microbiome:evidence for obesity risk and dietary intervention.
2015; 7:2237-2260 [PMID:25835047 DOI:10.3390/nu7042237]
100 Li L, Zhang S, Huang Y, Chen K. Sleep duration and obesity in children:A systematic review and meta-analysis of prospective cohort studies.
2017; 53:378-385 [PMID:28073179 DOI:10.1111/jpc.13434]
101 Hu J, Aris IM, Lin PD, Rifas-Shiman SL, Perng W, Woo Baidal JA, Wen D, Oken E. Longitudinal associations of modifiable risk factors in the first 1000 days with weight status and metabolic risk in early adolescence.
2020 [PMID:33184628 DOI:10.1093/ajcn/nqaa297]
102 Koplin JJ, Kerr JA, Lodge C, Garner C, Dharmage SC, Wake M, Allen KJ. Infant and young child feeding interventions targeting overweight and obesity:A narrative review.
2019; 20 Suppl 1:31-44 [PMID:31419047 DOI:10.1111/obr.12798]
103 Singhal A. Long-Term Adverse Effects of Early Growth Acceleration or Catch-Up Growth.
2017; 70:236-240 [PMID:28301849 DOI:10.1159/000464302]
104 Jain V, Singhal A. Catch up growth in low birth weight infants:striking a healthy balance.
2012; 13:141-147 [PMID:22415299 DOI:10.1007/s11154-012-9216-6]
105 Ling J, Robbins LB, Wen F, Zhang N. Lifestyle Interventions in Preschool Children:A Meta-analysis of Effectiveness.
2017; 53:102-112 [PMID:28237633 DOI:10.1016/j.amepre.2017.01.018]
106 Liu Z, Xu HM, Wen LM, Peng YZ, Lin LZ, Zhou S, Li WH, Wang HJ. A systematic review and meta-analysis of the overall effects of school-based obesity prevention interventions and effect differences by intervention components.
2019; 16:95 [PMID:31665040 DOI:10.1186/s12966-019-0848-8]
107 Gori D, Guaraldi F, Cinocca S, Moser G, Rucci P, Fantini MP. Effectiveness of educational and lifestyle interventions to prevent paediatric obesity:systematic review and meta-analyses of randomized and non-randomized controlled trials.
2017; 3:235-248 [PMID:29071100 DOI:10.1002/osp4.111]
108 Walker R, Morris H, Lang S, Hampton K, Boyle J, Skouteris H. Co-designing preconception and pregnancy care for healthy maternal lifestyles and obesity prevention.
2020; 33:473-478 [PMID:31812498 DOI:10.1016/j.wombi.2019.11.005]
109 Kandel P, Lim S, Pirotta S, Skouteris H, Moran LJ, Hill B. Enablers and barriers to women's lifestyle behavior change during the preconception period:A systematic review.
2021; 22:e13235 [PMID:33754474 DOI:10.1111/obr.13235]
110 Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring:why lifestyle interventions may have not achieved the desired outcomes.
2015; 39:642-649 [PMID:25777180 DOI:10.1038/ijo.2015.15]
111 Seneviratne SN, Sachchithananthan S, Gamage PSA, Peiris R, Wickramasinghe VP, Somasundaram N. Effectiveness and acceptability of a novel school-based healthy eating program among primary school children in urban Sri Lanka.
2021; 21:2083 [PMID:34774025 DOI:10.1186/s12889-021-12041-8]
112 Nader PR, Huang TT, Gahagan S, Kumanyika S, Hammond RA, Christoffel KK. Next steps in obesity prevention:altering early life systems to support healthy parents, infants, and toddlers.
2012; 8:195-204 [PMID:22799545 DOI:10.1089/chi.2012.0004]
 World Journal of Diabetes2022年7期
World Journal of Diabetes2022年7期
- World Journal of Diabetes的其它文章
- More studies are necessary to establish the effectiveness of Jinhuang powder in the treatment of diabetic foot
- Epidemiology for public health practice:The application of spatial epidemiology
- Relationship between quality of life and adolescent glycolipid metabolism disorder:A cohort study
- Factors associated with trabecular bone score in postmenopausal women with type 2 diabetes and normal bone mineral density
- Elevated levels of fructosamine are independently associated with SARS-CoV-2 reinfection:A 12-mo follow-up study
- Efficacy and mechanism of anti-vascular endothelial growth factor drugs for diabetic macular edema patients
