Non-alcoholic fatty liver disease and the impact of genetic, epigenetic and environmental factors in the offspring
Natalia Balassiano Wajsbrot,Nathalie Carvalho Leite, Gil F Salles,Cristiane A Villela-Nogueira
Abstract Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide and is strongly associated with metabolic deregulation. More recently, a significant impact of parental NAFLD in the offspring was demonstrated and has been widely discussed. However, pathogenetic pathways implicated in the inheritance by the offspring and relatives are still under debate.Probably, multiple mechanisms are involved as well as in NAFLD pathogenesis itself. Among the multifactorial involved mechanisms, genetic, epigenetic and environmental backgrounds are strongly related to NAFLD development in the offspring. Thus, based on recent evidence from the available literature concerning genetic, epigenetic and environmental disease modifiers, this review aimed to discuss the relationship between parental NAFLD and its impact on the offspring.
Key Words: Steatosis; Genetic; Epigenetic; Environmental; Offspring; Non-alcoholic fatty liver disease
INTRODUCTION
Although non-alcoholic fatty liver disease (NAFLD) is being replaced by metabolic dysfunctionassociated fatty liver disease[1], studies concerning genetic and epigenetic factors in this new scenario are still scarce. This way, we will still adopt the nomenclature NAFLD when discussing the studies in this review.
NAFLD affects about 25% to 45% of the world's western population[2]. The spectrum of the disease includes simple steatosis, steatohepatitis with or without fibrosis, leading to cirrhosis, hepatic decompensation and hepatocellular carcinoma (HCC)[3]. NAFLD is currently the third indication for liver transplantation worldwide, and it will potentially be the leading indication in 2030[4].
Many cofactors have been recognized and related to NAFLD's high prevalence and severity.Metabolic syndrome, obesity and type 2 diabetes mellitus (T2DM) are the most relevant factors associated with progression from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis(NASH) and fibrosis. Patients with T2DM have a higher prevalence of NAFLD, with a high prevalence of NASH and advanced fibrosis[5]. In a bidirectional relation, NAFLD also increases up to 5.5 times the risk of future development of T2DM and could be considered an early predictor of the disease[6].Ethnicity also influences NAFLD prevalence, with Hispanics presenting a higher prevalence than Caucasians and African Americans, independently of metabolic factors. The genetic and environmental basis could be responsible for these findings in diverse ethnic groups[7]. Accordingly, the observation of NASH and cirrhosis familial clusters suggests a substantial hereditary influence on NAFLD progression[8]. Data from diverse epidemiological, familial aggregation and twin-cohorts studies, with a welldesigned methodology, suggest that hepatic steatosis is highly heritable[9-12]. Some of these studies used magnetic resonance elastography to assess liver fibrosis or serum aminotransferase levels to infer hepatic steatosis. They demonstrated a high prevalence of NAFLD in family members of children with NAFLD, monozygotic and dizygotic twins, and first-degree family members of T2DM patients[11,13].So far, the risk of hepatic steatosis and more severe disease in family members and children of patients with NAFLD is not fully understood, as well as the pathogenetic pathways involved in this process.
Genome-wide association studies have demonstrated the association of single nucleotide polymorphisms (SNP) with NAFLD. Patatin-like phospholipase-domain-containing 3 (rs738409 C>G encoding for PNPLA3 I148M), also known as adiponutrin gene, is located at chromosome 22 and was the first SNP described[14]. Although this is the most robust variant linked to NAFLD, additional genetic variants have been identified subsequently: Transmembrane 6 superfamily member 2 (TM6SF2)[15], glucokinase regulator (GCKR)[16], membrane-bound O-acyltransferase domain-containing 7(MBOAT7)[17] and hydroxysteroid 17 β-dehydrogenase (HSD17B13)[18], among others. These variants have been associated with multiple pleiotropic effects, including a protective effect for NAFLD as seems to occur with the HSD17B13 polymorphism[18]. The different phenotypes resulting from these genes might partially explain the heritable component and metabolic profile of NAFLD patients and their offspring[9].
Although our understanding of genetic influence has exponentially increased in the past few years, it cannot thoroughly explain the high prevalence of NAFLD in family members of patients with the disease. Experimental studies have investigated different pathways related to NAFLD development in the offspring[9,19,20]. In this context, environmental and epigenetic mechanisms play an essential role in the occurrence and progression of NAFLD. Epigenetic factors involve mechanisms that affect and regulate gene expression without changes in DNA sequences[21]. Therefore, gene expression and cell phenotype related to NAFLD might depend on the genetic information encoded by DNA sequences and epigenetic factors[22]. Figure 1 shows the multifactorial mechanisms implicated in the offspring's NAFLD development. This review aims to discuss the impact of genetic, epigenetic and environmentrelated variables associated with NAFLD in the offspring.
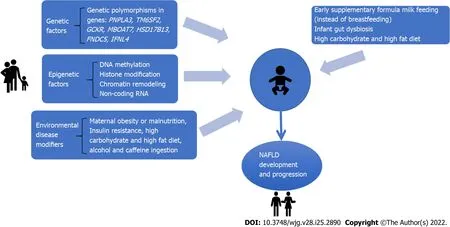
Figure 1 The interplay among genetic, epigenetic and environmental factors in pre and postnatal periods that impact the development of non-alcoholic fatty liver disease in the offspring. NAFLD: Non-alcoholic fatty liver disease.
HERITAGE AND GENETIC FACTORS
Studies in familial clusters and twin cohorts
Several studies have shown a solid familial clustering of NAFLD, particularly in coexisting metabolic traits[23-25]. Familial combined hyperlipidemia is the most frequent genetic dyslipidemia with a high risk of premature atherothrombotic cardiovascular disease. To assess whether liver steatosis is involved in the pathogenetic pathway of familial combined hyperlipidemia, Brouwerset al[23] studied family members with steatosis and twenty spouses. Fatty liver diagnosed by ultrasound was significantly more prevalent in familial combined hyperlipidemia probands (40%) and relatives (35%) compared with their spouses[23]. Moreover, the authors evaluated the correlations between indicators of fatty liver with plasma lipid levels. Liver steatosis and alanine aminotransferase levels correlated with triglyceride levels in all familial combined hyperlipidemia family members[23].
In the multigenerational Framingham Heart Study, a community-based study, individuals with at least one parent presenting hepatic steatosis had two-fold increased odds of having liver steatosis themselves than those without a parental history. More participants without metabolic diseases had liver steatosis if they had at least one parent with liver steatosis than those without any parent with steatosis. On the other hand, there was no difference in the prevalence of steatosis in patients with high cardiometabolic risk among participants with or without a parental history of liver steatosis. Based on these findings, this study suggested that a family history of liver steatosis was a significant risk factor for liver steatosis, but only in metabolically healthy participants[26]. This study goes against the previous one, which showed a higher prevalence of steatosis in those with familial hyperlipidemia.There was no investigation if the genetic aspects of those patients with familial hyperlipidemia could have influenced the higher prevalence of steatosis.
Schwimmeret al[11] evaluated 33 overweight children with biopsy-proven NAFLD and 11 overweight children without; NAFLD was significantly more observed in siblings and parents of the NAFLD children group. The correlation of liver fat fraction to body mass index (BMI) was more substantial in overweight children with NAFLD than without NAFLD, showing that there is likely an interaction between BMI and genetic factors on steatosis severity in families of children with NAFLD[11].
Similar to steatosis, hepatic fibrosis in NAFLD is also a heritable trait. Familial aggregation studies revealed a marked coexistence of advanced fibrosis or NAFLD cirrhosis among index patients and their first-degree relatives[24,25]. A cross-sectional analysis demonstrated that first-degree relatives of probands with NAFLD cirrhosis present a 12 times higher risk of advanced fibrosis compared with the relatives of non-NAFLD controls[25]. Interestingly, in another recent cross-sectional study of a prospective cohort comprising 156 twins and their families, the same authors identified a metabolite (3-4-hydroxyphenyl lactate) related to the abundance of several gut microbiota species in individuals with advanced fibrosis. Then, in their conclusions, they propose a link between genetics and microbiota composition concerning NAFLD heritability[27].
The potential genetic link of NAFLD regarding steatosis and fibrosis inheritance triggered the development of studies in twins to evaluate if both steatosis and fibrosis had a significant shared gene.The first study on twins regarding NAFLD inheritance included 60 monozygotic and dizygotic twins[13]. Both liver steatosis and fibrosis were non-invasively quantified by magnetic resonance imaging.The presence of hepatic steatosis by proton-density fat fraction magnetic resonance imaging (MRI) and fibrosis by magnetic resonance elastography correlated between monozygotic twins but not between dizygotic twins, providing evidence that both hepatic steatosis and fibrosis might be heritable traits as well[13].
Following the same rationale, Cuiet al[10] investigated a prospective cohort of community-dwelling monozygotic and dizygotic twin pairs living in Southern California, using non-invasive proton-density fat fraction MRI and magnetic resonance elastography to assess steatosis and fibrosis. They investigated if individuals prone to genetic susceptibilities to steatosis and fibrosis also had genetic susceptibilities to metabolic variables such as arterial hypertension, dyslipidemia, insulin resistance and diabetes mellitus.The authors have shown that hepatic steatosis and fibrosis have statistically and clinically significant shared genetic determination and metabolic traits such as high-density lipoprotein, triglycerides, insulin resistance, and glycosylated hemoglobin[10]. In another study, the same cohort of twins was evaluated regarding the metabolites of the gut microbiome and its effect on steatosis and liver fibrosis compared to a biopsy-proven NAFLD cohort. This proof of concept study provided a link between the gutmicrobiome and 3-lactate that shared gene-effect with hepatic steatosis and fibrosis[27]. Hence, the heritage of NAFLD might relate to multiple factors like a genetic inheritance that could directly affect steatosis and fibrosis and heritable traits of the gut microbiome inherited, or even be influenced by a shared lifestyle in the probands and its parents.
Genetic polymorphisms
Genetic polymorphisms are involved in NAFLD expression regarding its relation with liver steatosis,advanced stages of fibrosis, and even a possible protective effect for disease progression[9,18,28].However, studies evaluating their impact on the offspring of patients with NAFLD are scarce.
As previously described, PNPLA3 rs738409 C>G variant is associated with hepatic steatosis and severity of NAFLD, progression to cirrhosis and HCC, resulting in a worse prognosis[14]. PNPLA3 encodes a triacylglycerol lipase, and this variant promotes hepatic triglyceride accumulation by restricting substrate access to the catalytic dyad, thus inhibiting triglyceride hydrolysis in the cell[14].
TM6SF2 function is related to regulating cholesterol synthesis and secretion of lipoproteins.Individuals who carry the SNP rs58542926 C>T, which encodes the E167K amino acidic substitution,have a higher risk of NAFLD and histological disease severity. However, there is still a strong debate if it has a protective effect on coronary artery disease. A large study with 60801 patients with coronary artery disease compared to 123504 healthy individuals described a protective effect of the T variant of TM6SF2 on this disease and found an equivalent, although modest effect for the G variant of PNPLA3,that was more intense in the recessive model (genotype GG). At last, an exome study including more than 300000 individuals showed that both TM6SF2 and PNPLA3 polymorphisms induce a protective effect on coronary artery disease and an increased risk of NAFLD[29]. So far, there is no study regarding the evaluation of the impact of TM6SF2 in the offspring of NAFLD patients.
In young adolescents, the rs1260326 C>T variant in GCKR was significantly associated with de novo lipogenesis in those with TT genotype. Another variant in GCKR, the rs780094 A>G, was also associated with NAFLD in a meta-analysis involving 2091 cases and 3003 controls[30].
MBOAT7 was first studied in alcohol abusers and was related to a higher risk of cirrhosis. It encodes a protein involved in the re-acylation of phospholipids as a component of the phospholipid-remodeling pathway, known as the land cycle. Subsequently, the rs641738 C>T variant in this gene was associated with increased hepatic fat, more severe liver damage and fibrosis in NAFLD individuals of European descent; moreover, it has been demonstrated that the T allele may predispose to HCC in patients without cirrhosis[31].
Recently, three polymorphisms have been identified as protective against advanced stages of NAFLD. Results from exome-sequence data from 46455 individuals have shown an association of rs72613567:TA in HSD17B13, a variant with an adenine insertion, with lower levels of aminotransferases and reduced risk of chronic liver disease, including NASH[18]. Pirolaet al[32] demonstrated the effect of this variant on a Hispanic population submitted to liver biopsy, investigating its association with histological parameters of NAFLD. They identified a lower risk of ballooning degeneration, lobular inflammation and liver fibrosis, mediated by reduced enzyme activity in converting retinol to retinoic acid, suggesting a protective effect in inflammation and fibrosis[32]. Di Sessaet al[33] evaluated 685 obese children (mean age 10.56 ± 2.94 years) and demonstrated that carriers of the HSD17B13 A allele had a lower percentage of liver steatosis on ultrasound imaging and lower serum aminotransferases levels[33].
Pettaet al[34] evaluated the role of irisin, a myokine encoded by the fibronectin type III domaincontaining protein 5 gene (FNDC5), in NAFLD patients. The variant rs3480 A>G was not associated with the severity of steatosis and NASH but was correlated with a lower prevalence of clinically significant fibrosis (F2-F4), showing a protective effect against fibrosis. They also found that irisin is expressed in human activated hepatic stellate cells, promoting profibrogenic actions and collagen synthesis. Thus, the FNDC5 genotype might affect hepatic fibrogenesis by modulating irisin secretion[34].
The genetic polymorphisms associated with NAFLD, their functions and effects are summarized in Table 1.
Concerning NAFLD and family inheritance, the PNPLA3 polymorphism was the only one studied.Overweight and obese children with NAFLD confirmed by histology were evaluated regarding the role of lifetime exposures in association with a genetic predisposition, parental obesity, economic income,programming during fetal life, being breastfed or not, and later biomarkers of dietary habits and lifestyle, correlating with fibrosis. In this study, 75% of the children had fibrosis, independently associated with PNPLA3-GG genotype, parental obesity, not being breastfed, vitamin D levels (< 20 mg/dL) and fructose consumption. Notably, a high socioeconomic maternal occupation was related to less severe fibrosis[35]. These findings reinforce the multifactorial impact of NAFLD inheritance.Recently, Jainet al[36] studied 51 patients with NAFLD and their parents compared to 50 individuals without NAFLD and their parents as a control group. They observed that parents of the NAFLD group had a higher frequency of GG genotype when compared to parents of those without NAFLD (15%vs5%)[36]. In this study, no other factors except for PNPLA3 polymorphism were evaluated.
ENVIRONMENTAL AND EPIGENETIC FACTORS: EVIDENCE IN EXPERIMENTAL AND CLINICAL STUDIES
In addition to the genetic information encoded by DNA sequences, epigenetic modifications increase or inhibit the expression of specific genes and affect chromatin structure without modifying nucleotide sequence. Epigenetics implies inheritable changes in the expression of genes, but they can also be acquired and may occur in response to environmental factors, such as nutrition, contributing to disease risk and severity[37]. These alterations can be transferred to the next generation and, in this way, may modify metabolic and NAFLD risk in the offspring. As epigenetic changes can be inheritable and modulated by environmental stimuli, they are considered reversible and could offer new individualized prevention and therapy[37]. So far, the impact of maternal and/or paternal risk factors on the clinical phenotypes of the offspring and the underlying epigenetic mechanisms has not been fully elucidated[37].
Epigenetic phenomena include four regulatory mechanisms: Modification in DNA methylation,covalent histone modification, chromatin remodeling, and RNA-based mechanisms, such as non-coding RNA. DNA methylation is the most studied[22,38].
Experimental studies
Some experimental studies, most of them in mice, tried to elucidate the mechanisms involved in the inheritance of NAFLD and the external factors that could modulate NAFLD development in the offspring through epigenetic factors. It has been shown that many factors during pregnancy may activate lipogenic and inflammatory pathways leading to NAFLD in the progeny[19]. Many authors have studied the impact of breastfeeding, maternal obesity and diet before or during pregnancy in animal models.
Obenet al[19] demonstrated that maternal obesity before and throughout pregnancy and lactation could be linked to dysmetabolism in the offspring of female mice. Offspring of obese dams showed a dysmetabolic pattern related to insulin resistance and NAFLD phenotype. Moreover, the offspring of lean dams fed by obese dams presented increased body weight and higher insulin levels and cytokines such as leptin, interleukin-6 and tumor necrosis factor-alpha. Raised levels of leptin were also observed in the breast milk of obese mice compared to lean ones. They proposed that a modified pathway over hypothalamic appetite nuclei signaling by maternal breast milk and neonatal adipose tissue-derived leptin in the early postnatal period was the mechanism behind these findings.
Considering the hypothesis that diet during and after pregnancy might also be involved in NAFLD in the post-weaning period, Pruiset al[39] observed that a maternal western-type diet during pregnancy could stimulate metabolic programming or phenotype induction, leading to NAFLD development.
Another study[40] suggested that modifying the diet during pregnancy could benefit the offspring by preventing a disrupted liver lipid profile[61]. When pregnant mice were fed either with a high fat-slow digestive diet or a rapid digestive diet, the offspring of the high fat-rapid digestive diet showed an abnormal liver lipid profile. However, it was not observed in their counterparts born from high fat-slow digestive diet fed-mice.
The relationship between obesity in pregnancy and circadian cycle deregulation might affect metabolic pathways related to NAFLD in adults. Mouralidaraneet al[20] suggested that, in addition to an obesogenic post-weaning diet, obesity in the mother might lead to NAFLD by disrupting the liver's canonical metabolic rhythmicity gene expression. It implicates the role of abnormal circadian rhythm in the genesis of NAFLD, and alterations in this system during critical developmental periods might be responsible for the onset of the disease later in adulthood.
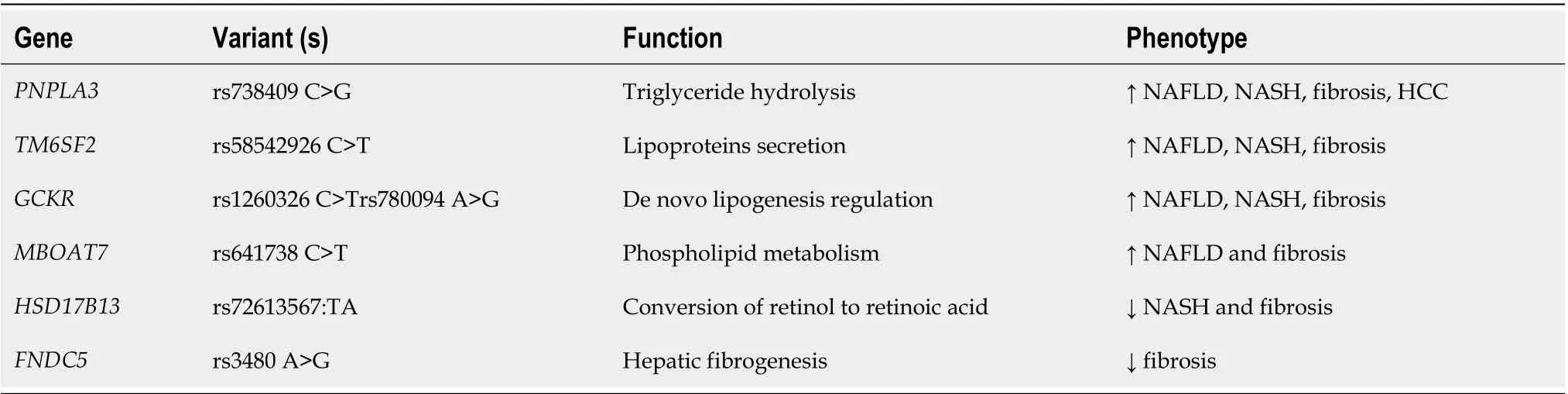
Table 1 Genetic polymorphisms involved in non-alcoholic fatty liver disease occurrence and related phenotypes
Another issue that might be considered regarding further development of NAFLD after birth is ethanol exposure[41]. Shenet al[41] developed a rat model of intrauterine growth retardation by prenatal ethanol exposure. These models were fed with normal and high-fat diets. Enhanced liver expression of the insulin growth factor-1 pathway, gluconeogenesis, lipid synthesis and diminished expression of lipid output were accompanied in prenatal ethanol exposure female offspring fed with a high-fat diet.
Oliveiraet al[42] studied Wistar rats fed with a standard diet and a high-fat diet. Rats born from mice fed with a standard diet were not affected by changes in liver morphology, as did the offspring of highfat-fed rats. Therefore, the study concluded that fructose intake during adolescence hastens NAFLD onset and reveals a differentiated hepatic response to metabolic insult, depending on the maternal diet.Notwithstanding, Nicolás-Toledoet al[43] showed that sucrose intake in adulthood increases fat content only in female rat offspring of dams fed with a low-protein diet during pregnancy, reinforcing the influence of maternal diet in the offspring[43]. Of note, regarding specific epigenetic mechanisms, Suteret al[44] have described that epigenetic changes to histones may act as a molecular memory of intrauterine exposure, rendering the risk of adult disease. The genome-wide epigenetic modifications in the fetal liver of susceptible offspring were analyzed, concluding that a maternal high-fat diet is associated with functional alterations to fetal hepatic histones, some of which may persist up to five weeks of age[44].
Another study by Weiet al[45] connected NAFLD with epigenetic methylation of specific genes in fathers that can be transmitted from gametes to embryos across generations. They have shown that even paternal diet patterns and prediabetes increase the risk of diabetes in the offspring through gametic epigenetic alterations such as different methylation of genes in the sperm of prediabetic fathers.
All these experimental studies in animal models have revealed that maternal obesity and parental diet during pregnancy or lactation may significantly influence NAFLD and lipid dysmetabolism in the offspring, either by environmental factors or through epigenetic factors, some yet to be better specified,mainly concerning environmental factors. Hence, cofactors as alcohol and fructose intake, among others not yet identified, may activate lipogenic and inflammatory pathways that can lead to NAFLD in the offspring.
Clinical studies
Studies in mothers and newborns: Animal studies confirmed that disruptions during early development could lead to increased susceptibility to metabolic dysfunctions later in life. Likewise, human data support that metabolic dysfunction and its contribution to NAFLD can be closely related to genetic and environmental predisposing factors. However, the precise mechanisms that link changes in pre and postnatal environments with NAFLD development risk in adolescence and adulthood remain poorly understood. These mechanisms involve shifts in lipid metabolism, mitochondrial dysfunction, altered gut microbiota, macrophage programming and activation of epigenetic changes.
In prior studies, it was demonstrated that low birth weight babies exhibit an altered postnatal metabolism after developing an adaptative response to a suboptimal fetal environment[46,47]. Although the mechanism is not entirely understood, exposure to excessive and deficient nutrition during the prenatal period may induce a nutritional mismatch between metabolic efficiency and energy expenditure, increasing the risk of future cardiometabolic diseases. If confirmed, an early and straightforward nutritional intervention might prevent the further development of metabolic diseases in adulthood.
Modiet al[48] evaluated 105 healthy mother-neonate pairs. They measured neonatal adipose tissue content by whole-body MRI and intrahepatic lipid content by a proton magnetic resonance spectroscopy. They have demonstrated that infant adiposity, particularly abdominal adipose tissue and intra hepatocellular lipid correlated with increased maternal BMI. Recently, Bedogniet al[49] studied the prevalence and risk factors associated with bright liver in 3911-year-old toddlers born from healthy mothers. The PNPLA3 I148M variant and maternal weight gain during pregnancy were related to the presence of bright liver in the ultrasonography[49]. Thus, interestingly, the authors suggested a potential gene-environment interchange between PNPLA3 and maternal environmental factors contributing to the risk of fatty liver disease at this earlier age, reinforcing the multifactorial inheritance of NAFLD.
In a large study, Ayonrindeet al[50] investigated the relation of maternal factors and infant nutrition with the future development of NAFLD in adolescents aged 17 years. They concluded that average pregestational BMI, breastfeeding for at least six months and avoiding early supplementary formula milk feeding reduce the risk of NAFLD diagnosis by liver ultrasound[50]. Additionally, more extended maintenance of breastfeeding resulted in multiple benefits on maternal metabolism and a lower risk of NAFLD in mid-life[51-53].
The Healthy Start study examined a cohort of 951 mothers from different ethnicities[54]. Similar to others, they found that maternal BMI was correlated to increased neonatal adiposity. It has also been demonstrated that increased maternal insulin resistance and fasting glucose levels contribute to this association. Excessive insulin resistance during pregnancy activates placental inflammatory pathways and affects the fetus indirectly by increasing placental nutrient transfer capacity[55].
Still regarding insulin-glucose metabolism, elevated blood glucose and insulin concentrations exacerbate de novo lipogenesis, resulting in increased intrahepatic lipids. Additionally, reduced glucose and pyruvate consumption in parallel with increased triglyceride concentrations and excess fatty acids incompletely oxidized can impair mitochondrial function and gene expression, limiting mitochondrial biogenesis and leading to NAFLD[55].
Peroxisome proliferator-activated receptor γcoactivator 1 (PGC1) gene is a transcriptional coactivator that participates in mitochondrial biogenesis and function and hypermethylation PGC1 promoter was associated with decreased mitochondrial DNA content and insulin resistance in NAFLD patients[56]. In a cross-sectional analysis, Gemmaet al[57] noticed a positive correlation between maternal BMI and methylation of the PGC1 gene in the umbilical cord of their babies[57]. Based on their findings, the authors speculated that PGC1 might be a promising candidate gene involved in metabolic programming by epigenetic regulation[57]. DNA methylation in regulatory regions of different genes participates in NAFLD development and progression. Other epigenetic mechanisms affecting NAFLD pathogenesis include histone modification and microRNA (miRNA)-mediated processes. Notably, circulating miRNAs have been associated with the presence and heritability of NAFLD in a population study in 40 pairs of twins. Serum miR-331-3p and miR-30c were identified among the 21 miRs that differed between NAFLD and non-NAFLD individuals. These miRNAs are highly inheritable and correlate with each other suggesting a common pathway related to NAFLD[58].
Although shreds of evidence support that high pre-pregnancy BMI in the mothers may lead to significant modifications in the infant gut microbiome[59], few studies link maternal obesity and infant dysbiosis with NAFLD risk in later life. The neonatal gut microbiome can be essential for later homeostasis, and disruption of this early process may increase the risk of future metabolic diseases[60].Emerging data provides evidence that the gut-liver axis is a fundamental element in the onset and progression of NAFLD. Gut microbiota dysbiosis may contribute to NAFLD by increasing concentrations of bacteria-derived endotoxins, pro-inflammatory cytokines, amino-acid metabolites, shortchain fatty acids and bile acids, all of which might exert effects that promote macrophage programming and activation, favoring liver injury[61].
CONCLUSION
The interplay among multiple genetic, epigenetic and environmental factors determine an individual's susceptibility to NAFLD. Current evidence points to genetic polymorphisms as pleiotropic tools that lead to diverse traits and phenotypes, including typical metabolic profiles in parents and their offspring.Importantly, epigenetic markers can also be transferred to successors by transgenerational epigenetic inheritance. Current studies in mothers and their offspring, although still small, show a direct effect of these factors and their related outcome, NAFLD. Future studies may clarify what interventions are essential for preventing this complex disease in the perinatal or postnatal period to reach the better liver and metabolic-related outcomes in the upcoming adult population.
FOOTNOTES
Author contributions:Leite NC, Salles GF and Villela-Nogueira CA contributed to study design and critical revision of the manuscript; all authors contributed to data analysis, wrote the initial draft and approved the final version.
Conflict-of-interest statement:The authors have nothing to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORCID number:Natalia Balassiano Wajsbrot 0000-0001-5780-4390; Nathalie Carvalho Leite 0000-0001-9066-0405; Gil F Salles 0000-0001-6318-7077; Cristiane A Villela-Nogueira 0000-0003-1355-2368.
Corresponding Author's Membership in Professional Societies:American Association for the Study of Liver Diseases, No.114358; European Association for the study of Liver Diseases, No. 60371.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年25期
World Journal of Gastroenterology2022年25期
- World Journal of Gastroenterology的其它文章
- Correction to “Gut microbiota dysbiosis in Chinese children with type 1 diabetes mellitus: An observational study”
- Acupuncture and moxibustion for treatment of Crohn’s disease: A brief review
- Correlation of molecular alterations with pathological features in hepatocellular carcinoma: Literature review and experience of an Italian center
- Mapping the global research landscape on nutrition and the gut microbiota: Visualization and bibliometric analysis
- Upregulated adenosine 2A receptor accelerates post-infectious irritable bowel syndrome by promoting CD4+ T cells’ T helper 17 polarization
- Fecal gene detection based on next generation sequencing for colorectal cancer diagnosis
