Inhibition of rat prostate smooth muscle contractility by extracts of Costus speciosus(crepe ginger)
Nguok Ngie Su,Jamie S.Simpson,Philip E.Thompson,Sabatino Ventura
1Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, Monash University, VIC 3052, Australia.2Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences, Monash University, VIC 3052, Australia.3Sarawak Biodiversity Centre, KM20, Jalan Borneo Heights, Semengoh, Kuching 93250,Malaysia.
Abstract Background: This study examined the chemistry and biological effects of Costus speciosus (J.Koenig) Sm.(C.speciosus) which has been traditionally used by Sarawak natives to treat urological disorders for centuries. Methods: This study assesses the efficacy of C.speciosus in treating urological disorders by investigating its effects on the contractility of isolated prostate glands since this is the most effective way to relieve lower urinary tract symptoms that accompany benign prostatic hyperplasia.Plants were collected and extracts of C.speciosus rhizome, root, leaf and stem were made using water at different temperatures. Results: C.speciosus rhizome and root decoction (boiling, about 100 °C) combination extract (2 mg/mL)inhibited electrical field stimulation-induced neurogenic contractions of isolated rat prostatic gland by 44 ± 8% (p = 0.01, n = 4); whereas room temperature (about 20 °C) rhizome and root combination extract inhibited electrical field stimulation contractions by 62 ± 8% (p =0.003, n=4). C.speciosus rhizome(p=0.0004, n=6), root(p <0.0001, n=6) and stem (p= 0.0057, n = 6) room temperature extracts inhibited electrical field stimulation-induced contractions of rat prostatic smooth muscle but leaf extract did not (p = 0.09, n = 6).Contractions mediated by exogenous administration of noradrenaline, acetylcholine, adenosine 5’-triphosphate or tyramine were only weakly inhibited by rhizome extract.Fractions of C.speciosus rhizome room temperature extract separated by preparative high-performance liquid chromatography and evaluated by isolated organ bath bioassay revealed that inhibitory activity of the extract was due to highly polar soluble components present in the extract.Conclusion:C.speciosus extracts exert a direct inhibitory effect on prostate tissue which is likely to be therapeutically beneficial in treating lower urinary tract symptoms associated with benign prostatic hyperplasia.
Keywords: benign prostatic hyperplasia; Costaceae; lower urinary tract symptoms; smooth muscle contractility; traditional medicines
Background
Benign prostatic hyperplasia (BPH) is a progressive condition characterised by the enlargement of the periurethral and transition zones of the prostate.It is the non-cancerous aberrant proliferation of connective tissue, smooth muscle and glandular epithelium and is accompanied by lower urinary tract symptoms (LUTS), which can be bothersome and detrimental to the quality of life in ageing men [1–3].Despite several mechanisms appearing to be involved in the pathogenesis of BPH, aging and androgens represent the core mechanisms involved in its development [4].
Urinary obstruction in men with BPH is caused by both mechanical/static and dynamic components [5].The mechanical/static component is due to the anatomical obstruction caused by enlargement of the prostate that constricts the prostatic urethra and bladder outlet.Prostatic enlargement is stimulated by dihydrotestosterone which is converted from the less potent testosterone by the 5-α-reductase enzyme[6].Dynamic obstruction of the urethra is due to an increase in the force of prostatic smooth muscle, generated by an age-related increase in sympathetic nervous system activity leading to increased stimulation of α1-adrenoceptors by endogenously released noradrenaline [5, 7, 8].The conventional pharmacotherapies used to treat BPH by targeting these two components are α-adrenoceptor antagonists (prazosin, tamsulosin,alfuzosin, terazosin and doxazosin) and 5-α-reductase inhibitors(hormone therapy) (dutasteride and finasteride) [7, 9].Several other drugs such as antimuscarinic/anticholinergic drugs, β-adrenoceptor agonists, estrogen suppressors, and phosphodiesterase 5 inhibitors have also been used to ameliorate LUTS associated with BPH [7, 10].
Phytotherapy also plays a pivotal role in the management of BPH[11, 12].Several studies have shown that herbal supplements may have beneficial effects in treating BPH,such asPhellodendron amurense,Cucurbita peponis,Hypoxis rooperi,Pygeum africanum, rye, stinging nettle, pumpkin seeds, red clover, and saw palmetto [13–19].
Pharmacotherapies and prescribed medications are usually only effective in treating mild to moderate BPH symptoms.For severe or complicated BPH,invasive surgery remains the gold standard and BPH is the second most common cause of surgery in men older than 65 in the United States [20], with transurethral resection of the prostate being the most common procedure.Therefore, the generation of safer and more effective medicines to treat BPH is of great interest and economic value, as it may ameliorate the need for invasive and costly surgery in many cases.
Sarawak is one of the top 25 global biodiversity hot spots [21].Costus speciosus(J.Koenig) Sm.(C.speciosus), is a tropical herbaceous plant native to Sarawak that belongs to the Costaceae family in the order Zingiberales.C.speciosusis commonly known as crepe ginger or spiral ginger and contains a rich phytoconstituent reservoir with high concentrations of steroidal saponins that exhibit a variety of biological effects [22–40].The plant has been traditionally used by Sarawak natives to treat urological disorders in both men and women [41–43]indicated by symptoms such as haematuria, difficulty in urinating,painful urination as well as diabetes.However, to date, there is no scientific evidence justifying the use ofC.speciosusextracts in treating urological disorders.A schematic diagram of theC.speciosusplant with the different parts used in this study labelled, is shown in Figure 1.
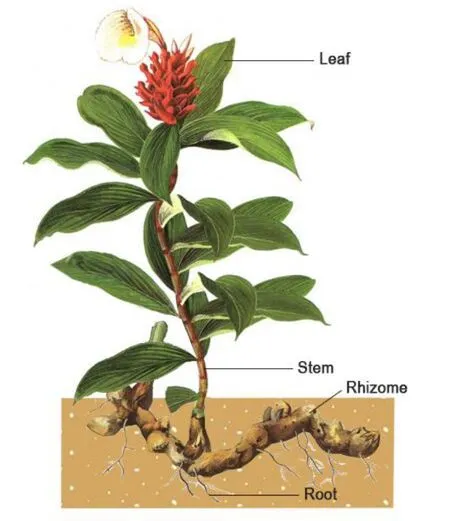
Figure 1 Schematic diagram of entire Costus speciosus plant with parts used in this study to assess bioactivity labelled
This study aimed to evaluate the effect ofC.speciosusplant extracts on the contractile responses of isolated rat prostatic smooth muscle using in vitro isometric tension studies.This is a commonly used bioassay to identify drugs that relieve LUTS in men.
Methods
Plant material preparation
C.speciosuswas identified, collected and authenticated by Sarawak Biodiversity Centre botanists in the villages of the northern region of Sarawak, Malaysia.Plants were checked against http://www.theplantlist.org.The plant voucher specimen (catalog number: SABC4300) was collected and deposited in the herbarium of the Sarawak Biodiversity Centre.Fresh rhizomes, roots, leaves, and stems were carefully separated and washed thoroughly under running tap water to clean the plant materials of soil, epiphytes and microbial contamination.The plant materials were cut into smaller pieces and shade-dried with good aeration and occasional shifting.After several days plant materials were transferred to a drying oven set at 45oC.The drying process took up to two weeks depending on different plant parts.The dry plant materials were ground into a coarse powder using a cutting mill and vacuum packed before shipping to Monash University, Melbourne, Australia.
Animals and tissues
Male Sprague-Dawley rats (8 weeks old) were housed at 22 °C with Fibrecycle or Aspen Chips bedding and exposed to a photoperiod cycle of 12 h light/12 h dark.Rats were allowed access to food and water ad libitum.Rats were placed in a CO2chamber and euthanased by inhalation of CO2gas.An incision was made along the midline of the abdomen, exposing the male urogenital tract.The penile muscles,excess fat and connective tissue were cut away to reveal the prostates lobes.The left and right lobes of the prostate were carefully dissected out providing two prostate preparations from each rat.The prostates were separately placed in specimen jars containing Krebs-Henseleit solution, pH 7.4 (mM: NaCl 118.1, KCl 4.69, KH2PO41.2, NaHCO325.0,D(+)glucose 11.7,MgSO4·7H2O 1.1,CaCl22.5).Ethical approval for use of animals in research was obtained from the Monash Institute of Pharmaceutical Sciences Animal Ethics Committees(ethics numbers:MIPS.2013.15, MIPS.2016.36).All studies abided by and conformed to the requirements from theAustralian Code for the Care and Use of Animals for Scientific Purposes.
Isolated organ bath studies
The dissected prostate lobes were placed on gauze pre-soaked with Krebs-Henseleit solution.The prostatic capsule, excess fat and connective tissue were removed.The separate prostate lobes were mounted on perspex tissue holders incorporating two parallel platinum electrodes used to conduct electricity during stimulation.The tissue holder with the isolated prostate was then mounted in a 10 mL water-jacketed organ bath containing Krebs-Henseleit solution bubbled with 95% O2/5% CO2, and maintained at 37oC.One end of the prostate tissue was then attached to an isometric Grass FT03 force-displacement transducer (Grass Instruments, MA, USA) which was connected to a PowerLab 4/SP data acquisition system(ADInstruments Pty.Ltd., New South Wales, Australia).LabChart software (version 5, ADInstruments Pty.Ltd., New South Wales,Australia) run on a personal computer was used for the measurement and recording of isometric contractions.The isolated prostates were equilibrated for a period of 1 h under a resting force of approximately 1 g prior to experimentation.During the 1 h equilibrium period, the isolated prostates were stimulated with electrical pulses of 0.5 ms duration and 60 V at 0.01 Hz, to ensure that tissues were viable.
Electrical field stimulation (EFS)
Frequency-response curves to EFS were constructed using frequencies of 0.1, 0.2, 0.5, 1, 2, 5, 10, and 20 Hz (0.5 ms pulse duration, 60 V).EFS was delivered at 10 min intervals in trains of pulses lasting 10 pulses(0.1–1 Hz)or 10 s(1–20 Hz).The tissue was allowed to recover for 10 min in between trains of EFS.An initial control frequency-response curve was constructed to determine the contractile response of the tissue at each frequency.A second frequency response curve was then constructed after the tissue had been exposed to the test extract or vehicle for 30 min.
Agonist concentration-response curves
Effects of extracts ofC.speciosuson direct smooth muscle stimulation by exogenously administered agonists were assessed by constructing discrete concentration-response curves to the agonists acetylcholine,noradrenaline, adenosine 5’-triphosphate(ATP)or tyramine.After the 1 hour equilibration period but before the construction of agonist concentration-response curves to acetylcholine or noradrenaline,tissues were exposed to a priming dose of 10μM noradrenaline or 100μM acetylcholine, respectively, to ensure reproducible responses.Following administration of each agonist concentration, the prostate was allowed to reach maximum contraction or plateau before being washed and allowing 10 min to recover before administration of the next concentration of agonist.If required, the tension was re-adjusted to approximately 1 g prior to the addition of agonists.
Concentration-response curves to the exogenously administered agonists noradrenaline(1 nM–100μM),acetylcholine(1 nM–100μM),ATP (300 nM–1 mM), or the indirectly acting agonist tyramine (10 nM–100μM) were constructed, with a concentration progression ratio of half a log molar unit.If no response was observed after 20 sec, the tissue was washed and allowed 10 min for recovery.The peak height of the contractile response observed after the addition of each concentration was used for data calculations.
Plant extract preparation
Water extraction.The water extraction protocol was optimized from preliminary studies.5 g of dried and groundC.speciosusplant materials (rhizome and root) were added to 500 mL of water and extracted at different temperatures, i.e.decoction (100oC), hot water(70oC) and room temperature (RT, 20oC).The extract was separated from the marc(plant material residue) with filter paper (Whatman 4).The extract collected was lyophilized to obtain the dry extract.The extract was weighed and dissolved and diluted in Milli-Q water(Merck, Darmstadt, Germany) to the required concentration to produce a final in vitro concentration of 2 mg/mL in the bath.2 mg/mL was used as preliminary experiments determined this concentration to be the optimum effective concentration ofC.speciosuswater extract needed to attenuate EFS-induced contractile responses of isolated rat prostates.A further maceration extract method was then developed and optimized (NS Method?) [44] to prepare a larger scale ofC.speciosusrhizome, stem, leaf and root extracts for subsequent experiments and chemical profiling.
Methanol extraction.Methanolic extracts ofC.speciosusrhizome have previously been reported to exhibit anticancer activity in cell-based assays, in lung carcinoma (NCI-H460) and breast cancer(MCF-7) cell lines [45].Therefore, a methanol extract was also prepared for comparison.Methanol (250 mL) (Merck Healthcare Pty.Ltd., New South Wales, Australia) was added to 10 g ofC.speciosusrhizome.The rhizome methanol mixtures were sonicated for 30 min and filtered using filter paper (Whatman 4).A further 150 mL of methanol was then added to the marc and sonicated for another 30 min followed by filtration.This step was repeated with addition of another 100 mL methanol.Lastly, the marc was submerged in a further 100 mL methanol for two days to ensure all chemical components in the rhizome had been retrieved and the extract appeared to be clear.The extract was then filtered and the total extract was collected and concentrated using a rotary evaporator.C.speciosusrhizome dried methanolic extract was weighed and dissolved in dimethyl sulfoxide to produce a final in vitro concentration of 2 mg/mL in the bath.2 mg/mL was used as the test concentration ofC.speciosusmethanol extract to align with the concentration of water extract used, and therefore allow a direct comparison of bioactivity between the two extracts.
Drugs and vehicle solutions
(-) Arterenol (noradrenaline) bitartrate salt, acetylcholine chloride,ATP magnesium salt, tyramine hydrochloride, and prazosin hydrochloride were purchased from Sigma (St Louis, MO, USA).Noradrenaline was dissolved in catecholamine diluent (mM: NaCl 154.0, NaH2PO41.2, ascorbic acid 0.2).All other drugs were dissolved in Milli-Q water.Milli-Q water was used for dilution of all dissolved compounds to the required working concentrations.
Centrifugation separation
A centrifugation separation method was used to separate insoluble particles present in the RT extract of theC.speciosusrhizome.1 mL of Milli-Q water was added to 200 mg of RT extract of theC.speciosusrhizome.The mixture was vortexed and sonicated to ensure the dried extract was fully dissolved.The dissolved extract was then centrifuged at 11,000 rpm for 15 minutes.Centrifugation of the extract successfully separated undissolved particles (namely, solid phase) of the extract from the extracted liquid (namely, liquid phase).The liquid phase extract was carefully transferred to a pre-weighed 1.5 mL microcentrifuge tube via pipetting.Both solid phase and liquid phase were frozen using liquid nitrogen and lyophilised to obtain the dried extract mass.
Fractionation by preparative high-performance liquid chromatography (HPLC)
The use of plants as a source of bioactive compounds is often challenging due to the ability in obtaining sufficient compound quantities needed for the thorough characterisation of pharmacological activity.Only the rhizome of theC.speciosuswas chosen for HPLC fractionation in this study, because even though the root had greater bioactivity, the potency and action were similar.The effective concentration (i.e 2 mg/mL) was subsequently used to determine the mechanism of action ofC.speciosusrhizome RT extract on isolated rat prostatic smooth muscle contractility.
Preparative-scale HPLC was used to fractionate chemical components of theC.speciosusrhizome liquid phase sample obtained from the centrifugation separation.Fractionation of the samples was performed on a WatersTMPrep LC preparative chromatography system(Waters, Milford, MA, USA) equipped with a WatersTM486 tunable absorbance detector (Waters, Part #WAT080690, Milford, MA, USA)and a WatersTMPrep LC controller (Waters, #WAT073660, Milford,MA, USA).Liquid phase extract (121 mg) was dissolved in 1 mL of Milli-Q H2O and manually injected into the system using a syringe through a sample injector for chemical component separation.An isocratic elution was employed to maintain 100% buffer A (100%Milli-Q H2O)for 10 min.This was followed by 15 min in 80%buffer B(100% acetonitrile) and 20% buffer A (100% Milli-Q H2O).This was maintained for 6 min before reversion back to 100% buffer A.The run was terminated at 40 min.The flow rate was 6 mL/min through a column Luna?10 μm C8(2) 100 ?, A X 1A P (250 × 21.2 mm)(Phenomenex, 00G-4250-P0-AX, Lane Cove West, Australia).The elution for each peak was collected every 30 mL.Data were managed and analysed using EmpowerTMsoftware (version 2) (Waters, Milford,MA, USA).Isolated fractions were frozen using liquid nitrogen and lyophilised to obtain the dry mass.Liquid chromatography-mass spectrometry and routine proton (1H) nuclear magnetic resonance(NMR) analyses were performed on all fractions obtained.
NMR spectroscopy
1H NMR spectra were recorded on a Bruker Avance III Nanobay 400 MHz NMR spectrometer (Bruker Biospin AG, Billerica, MA, USA)coupled to a Bruker automated control system 60 automatic sample changer (Bruker Biospin AG, Billerica, MA, USA).The spectrometer is equipped with a 5 mm PABBO BB–1H/D Z–GRD probe(Bruker Biospin AG, Billerica, MA, USA).The NMR experiment was run in Bruker’s TOP-SPIN interface which consisted of an ICON-NMR component.1H NMR was performed by dissolving the sample in D2O (Cambridge Isotope Laboratories, Inc., DLM-4-100, Andover, MA, USA).The sample was fully dissolved and 300–500 μL of the solution was then transferred into a clean NMR tube.The number of scans performed was between 64 and 128.NMR data was analysed using Mestrelab MNova software (version 6.0.2-5475).For1H spectra, solvent peak reference for D2O was 4.79 parts per million.The chemical shifts were expressed in parts per million as δ values and the coupling constants in Hz.
Data analysis
The peak contractile force generated by isolated prostates in response to EFS or exogenously administered agonists were measured at each frequency or concentration.Baseline tone was subtracted from the peak contractile response of each frequency or concentration to minimize variability (maximum - minimum).Mean curves were constructed by pooling data from n experiments.Results are expressed as the mean ± standard error of the mean (SEM).Mean frequency or concentration in the presence ofC.speciosusextracts were compared with the mean frequency or concentration of the control using GraphPad Prism software (version 7.0) for Windows (La Jolla, CA,USA) and analysed using two-way repeated-measures analysis of variance (ANOVA).The value of n represents the number of animals used.Thep-values stated were used to evaluate the statistical significance of any difference between frequency/concentration and treatment,p≤0.05 was considered significant in all cases.
Results
Effects of different C.speciosus extracts on nerve-mediated rat smooth muscle contractility
EFS (0.5 ms, 60 V, trains of 10 pulses (0.1–1 Hz) or trains of 10 s(1–20 Hz)) evoked frequency dependent contractions that were reproducible over the time course of the experimental protocol and were unaffected by methanol(p=0.93,n=6)or water (p=0.46,n=86)alone.Nerve-mediated contractions of the isolated rat prostates treated withC.speciosusrhizome methanolic extract (2 mg/mL) were not different from control (Figure 2).However, incubation of isolated rat prostates withC.speciosusRT (about 20 °C) water extracts consistently attenuated electrically evoked contractions, whileC.speciosusboiling water (100 °C) extract (2 mg/mL) attenuated EFS-induced contractile responses to a lesser extent.Hot water extract(about 70 °C) did not attenuate EFS-induced contractions at all.Of note,C.speciosusrhizome (Figure 3A;p= 0.0004, n = 6), stem(Figure 3B;p=0.0057,n=6),and root(Figure 3C;p<0.0001,n=6)RT extract at a concentration of 2 mg/mL caused a similar degree of inhibition of EFS-induced contractile responses of the isolated rat prostate when compared to control.In contrast, no inhibitory effect was observed to the leaf RT extract(Figure 3D;p= 0.09, n = 6).
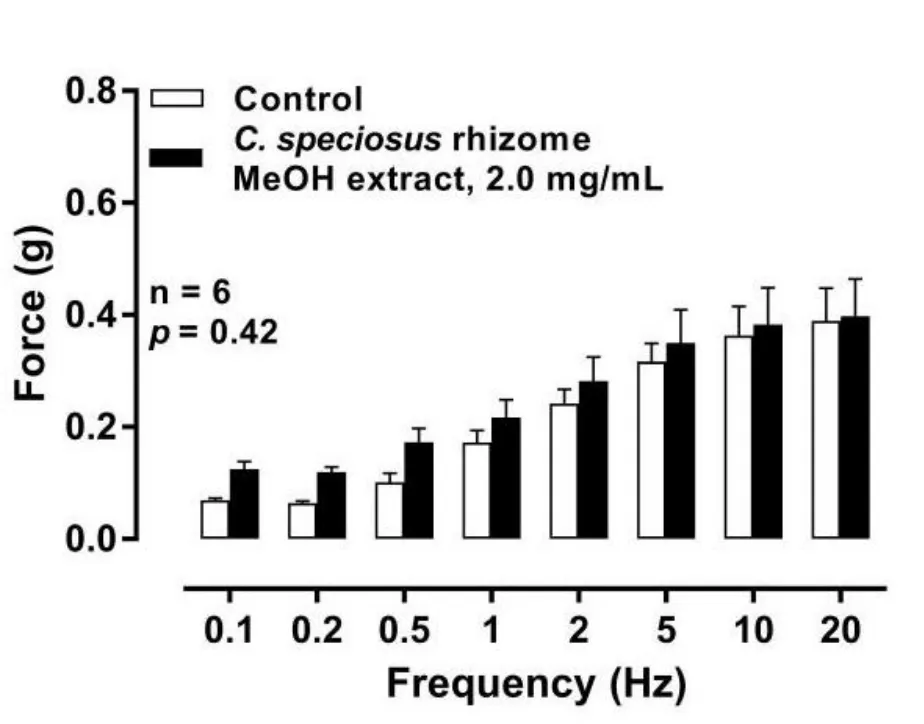
Figure 2 Mean contractile responses to electrical field stimulation(0.5 ms,60 V,1-20 Hz,10 s pulses)in isolated rat prostate before and after administration of C.speciosus rhizome methanolic extract at 2 mg/mL (p = 0.42, n = 6).Columns represent mean force ± SEM (y-axis (g)); the x-axis represents the frequency of electrical field stimulation(Hz).The p-value represents the probability of the change in the contractile responses being due to chance(two-way repeated-measures of ANOVA). C.speciosus,Costus speciosus;SEM,standard error of the mean;ANOVA,analysis of variance;MeOH,methanol.

Figure 3 Mean contractile responses of isolated rat prostate before and after administration of C.speciosus. (A) Rhizome RT extract (2 mg/mL; p = 0.0004, n = 6); (B) stem RT extract (2 mg/mL; p = 0.0057, n = 6); (C) root RT extract (2 mg/mL; p <0.0001, n = 6); (D) leaf RT extract(2 mg/mL;p=0.09,n=6).Graph bars represent mean force±SEM(y-axis(g));x-axis represent frequencies of electrical field stimulation(Hz).(two-way repeated-measures of ANOVA). p-values represent probability of the treatment causing a significant change in the contractile responses. C.speciosus, Costus speciosus; RT, room temperature; SEM, standard error of the mean; ANOVA, analysis of variance.
Effects on agonist-induced contractile responses: noradrenaline,acetylcholine and ATP
Exogenously administered noradrenaline(1 nM–100μM) (p=0.88,n= 6), acetylcholine (1 nM–100 μM) (p= 0.17, n = 6) or ATP (300 nM–1 mM) (p< 0.001, n = 6) elicited reproducible concentration-dependent contractions of isolated rat prostate over the time course of the experiment.The RT extract ofC.speciosusrhizome(2 mg/mL) produced a slight reduction in the magnitude of the contractile responses to noradrenaline, acetylcholine and ATP of isolated rat prostate (Figure 4).
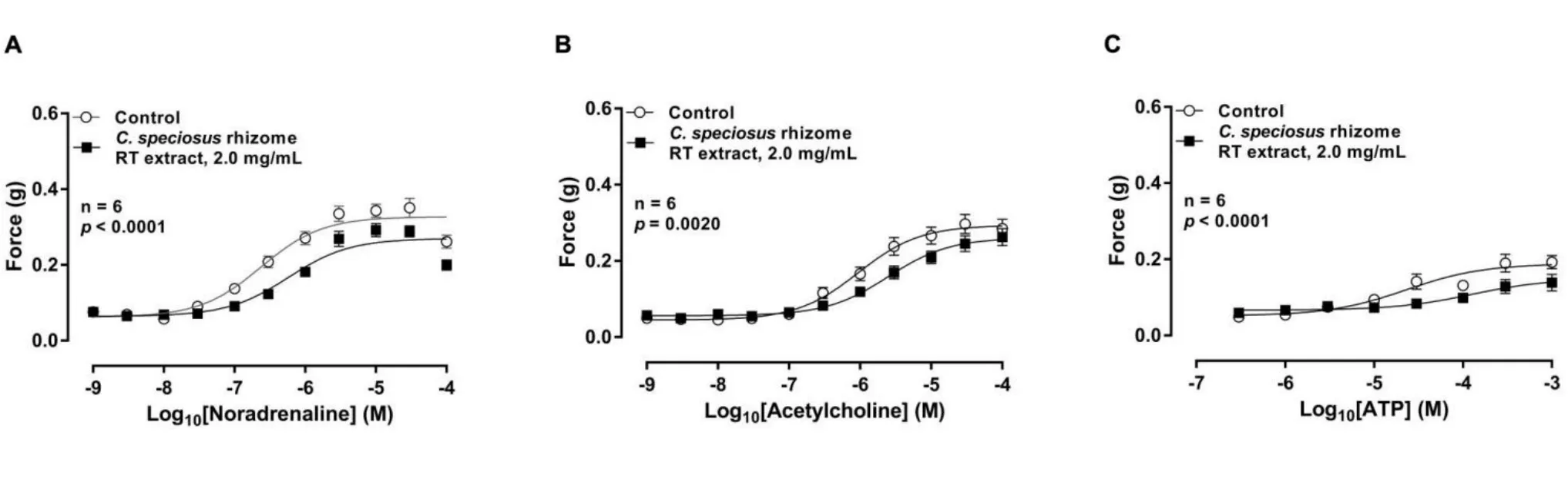
Figure 4 Mean log concentration-response curves to(A)noradrenaline(p <0.0001,n=6),(B)acetylcholine(p=0.0020,n=6)and(C)ATP (p <0.0001, n = 6) on isolated rat prostate in the presence (■) and absence (○) of C.speciosus rhizome RT extract (2 mg/mL).Symbols and error bars represent mean force ±SEM (y-axis(g)); x-axis represent concentrations of agonist(M). p-values represent the probability of a significant interaction between treatment and concentration being due to chance (two-way repeated-measures analysis of variance). C.speciosus,Costus speciosus; RT, room temperature; ATP, adenosine 5’-triphosphate; SEM, standard error of the mean; ANOVA, analysis of variance.
Effects on contractile response mediated by tyramine
Exogenous administration of the indirectly acting sympathomimetic tyramine (10 nM–100 μM) elicited reproducible concentrationdependent contractions of isolated rat prostate over the time course of the experiment.The concentration-response curve to tyramine was only very slightly shifted to the right in the presence of RT rhizome extract when compared to control (Figure 5; n = 4).
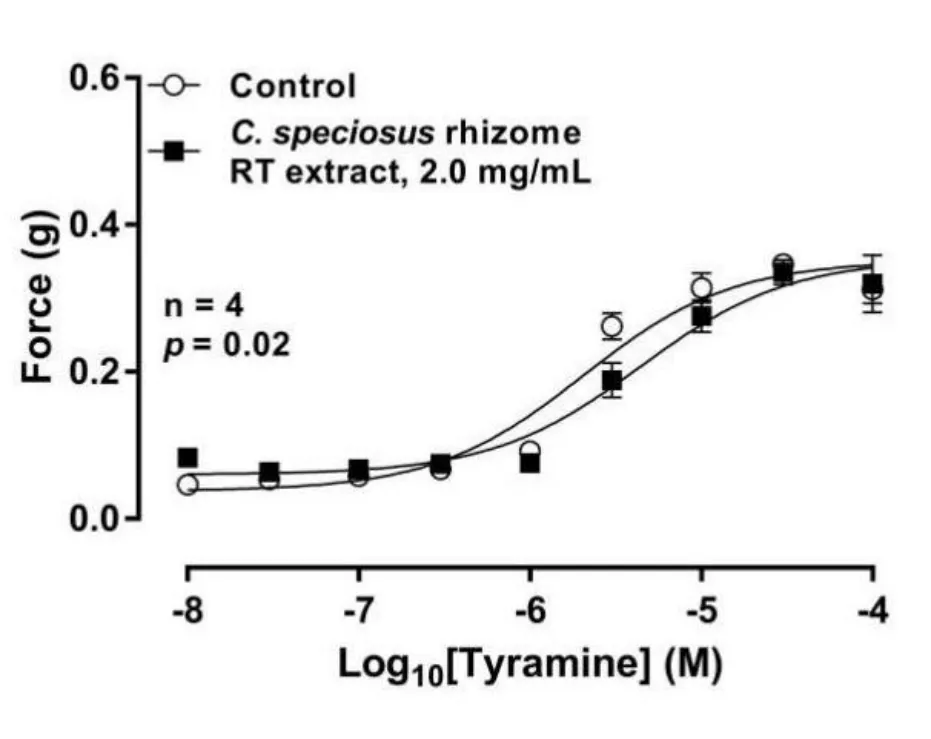
Figure 5 Mean log concentration-response curves to tyramine on isolated rat prostate in the presence (■) and absence (○) of C.speciosus rhizome RT extract (2 mg/mL) (P = 0.02, n = 4).Symbols and error bars represent mean force ± SEM (y-axis (g)); the x-axis represents the concentration of agonist (M).The p-value represents the probability of a significant interaction between treatment and concentration due to chance (two-way repeated-measures ANOVA). C.speciosus, Costus speciosus; RT, room temperature; SEM, standard error of the mean; ANOVA, analysis of variance.
Centrifugation separation
Centrifugation separation followed by preparative reverse phase –high performance liquid chromatography (RP-HPLC) removed some inactive constituents, leaving a simpler sample for analysis that still contained multiple components.The active components from 200 mg of RT water extract ofC.speciosusrhizome were concentrated into a 58 mg sample.Figure 6 shows the effects of the liquid and solid phases on EFS-induced contractions of the isolated rat prostate gland.The liquid phase extract (1.3 mg/mL) (Figure 6A;p= 0.05, n = 4)attenuated EFS-induced contractile responses of isolated rat prostates whereas the solid phase extract did not (0.5 mg/mL) (Figure 6B;p=0.40, n = 4).
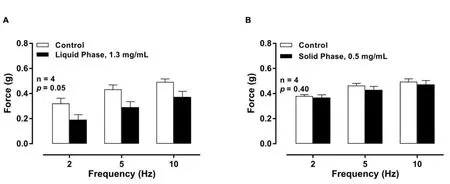
Figure 6 Mean contractile responses to EFS (0.5 ms, 60 V, 2 Hz, 5 Hz, and 10 Hz, 10 s trains) of isolated rat prostates before (open columns) and after (solid columns) administration of (A) liquid phase (1.30 mg/mL; p= 0.05,n = 4) and (B) solid phase (0.5 mg/mL; p= 0.40, n = 4) extracts obtained from the centrifugation separation of C.speciosus rhizome RT crude extract.Columns represent mean force ± SEM (y-axis (g)); x-axis represent frequencies of EFS (Hz) (*p <0.05; two-way repeated-measures of ANOVA). p-values represent probability of a significant change in the contractile responses being due to chance. C.speciosus, Costus speciosus; RT, room temperature; SEM,standard error of the mean; EFS, electrical field stimulation; ANOVA, analysis of variance.
Fractionation via preparative HPLC
To further fractionate the mixture, the soluble portion of the centrifuged extract was subject to preparative RP-HPLC separation yielding 10 fractions (i.e.Costus speciosus-preparative HPLC liquid phase fraction 1–10 (CS-PLP1–10)) (Figure 7).CS-PLP1 constituted the main recovered material (58.3 mg) harboured the bioactive constituent also.CS-PLP1 attenuated contractile responses of isolated rat prostates in response to EFS at 0.97 mg/mL.Other fractions did not inhibit EFS-induced contractile responses of isolated rat prostates.
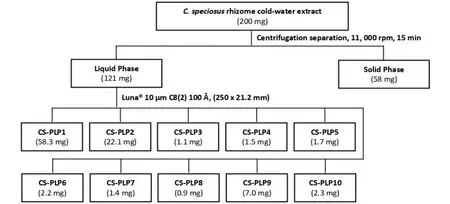
Figure 7 Isolation flowchart of the liquid phase and solid phase extract from centrifugation separation of C.speciosus rhizome room temperature crude extract.Preparative reverse phase – high performance liquid chromatography fractionation of liquid phase yielded 10 fractions (CS-PLP1–CS-PLP10). C.speciosus, Costus speciosus; CS-PLP1–10, Costus speciosus-preparative high-performance liquid chromatography liquid phase fraction 1–10.
1H NMR spectroscopic analysis of the water extract (Figure 8A) and CS-PLP1 (Figure 8B) shows them to have largely comparable constituents, although as yet no single component has been identified.The NMR spectrum shows multiple and strong resonances between 3.5 and 4 parts per million as well as the polar nature of the material that allows extraction into water is suggestive of carbohydrate structures[46].
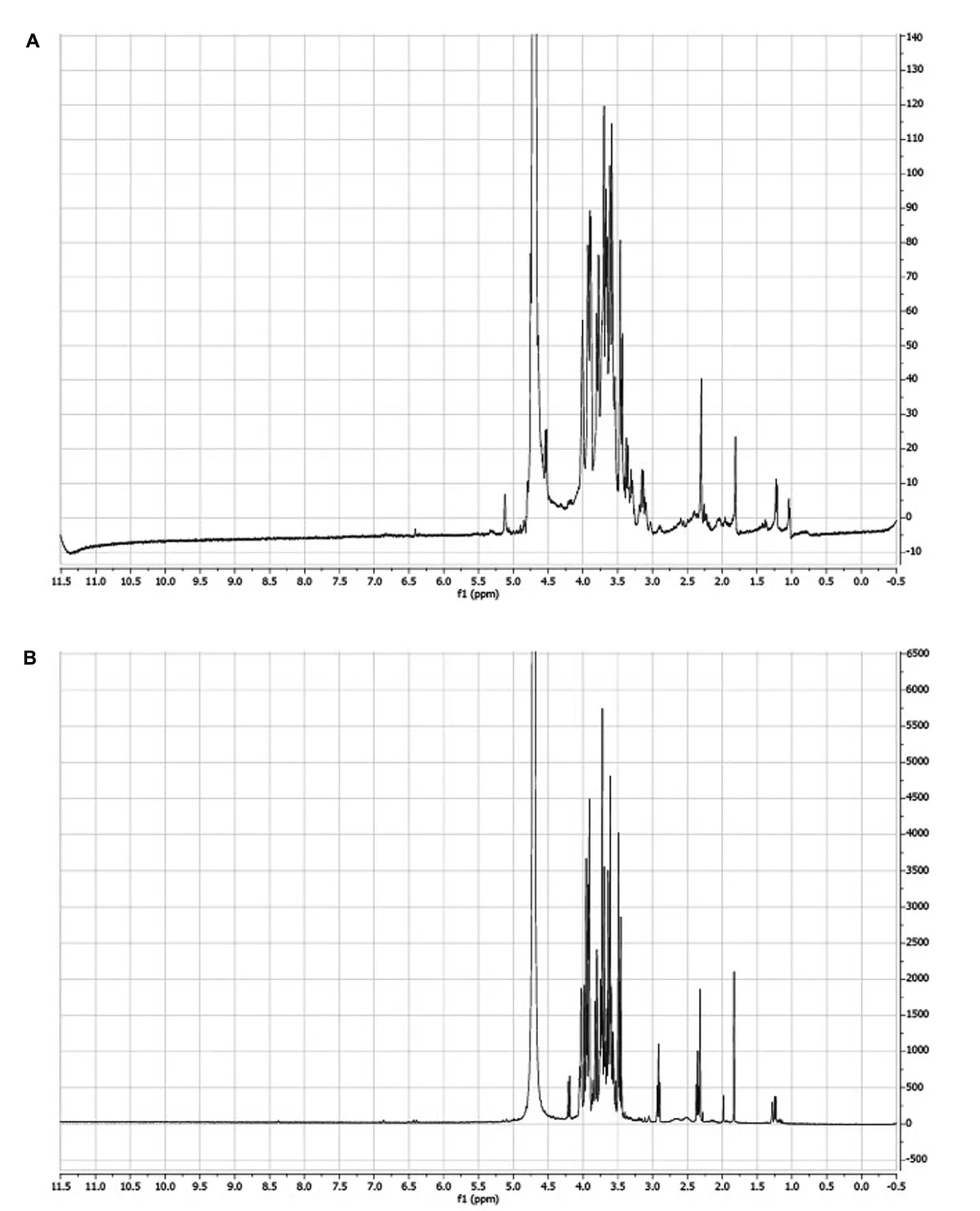
Figure 81H NMR spectrum.Horizontal axis represents the chemical shift (δ).The chemical shifts were expressed in parts per million as δ values(usually from 1–10 ppm) and the coupling constants in Hz.The vertical axis represents the intensity of the resonance signals.(A) C.speciosus rhizome RT water extract, and (B) CS-PLP1 isolated from liquid phase extract from centrifugation separation of C.speciosus rhizome RT water extract.The samples were dissolved in D2O. C.speciosus, Costus speciosus; RT, room temperature; CS-PLP1, Costus speciosus-preparative high-performance liquid chromatography liquid phase fraction 1;1H, routine proton; NMR, nuclear magnetic resonance; D2O, deuterium oxide.
Discussion
BPH affects over two-thirds of men older than 50 years of age and prevalence increases proportionally with age to affect 80% of men over 70 [47].Epidemiological studies have revealed that 26.2% of all men are affected by BPH [48].In a global survey, the prevalence of severe LUTS in men reported from four research centres in Auxerre(France), Boxmeer (the Netherlands), Birmingham (United Kingdom),and Seoul (Republic of Korea), was seen to increase with age from 5.4% in men aged 60–69 to 7.5% in men between aged 70–79.Severity of LUTS had also increased with increasing age across all four centres indicating that there is no significant cultural variation [49].Due to the prevalence of BPH in ageing men, extensive studies have been performed, particularly in the field of phytotherapy, to treat or ease the LUTS secondary to BPH.Despite this,evidence for the clinical efficacy of different plant extracts in the management of LUTS associated with BPH in ageing men, is still lacking.
C.speciosushas been used by the Sarawak indigenous community as a traditional medicine to treat urological disorders for centuries.Our results showed that a methanolic extract ofC.speciosusrhizome did not affect the nerve-mediated smooth muscle contraction of the isolated rat prostates induced by EFS.Traditionally, medicinal plants are prepared either singly or in combination with other herbal extracts in an aqueous form.This is in agreement with our water extraction method that mimics traditional preparation.In addition, our results showed that RT water extraction (about 20 °C) showed greater potency when compared to a decoction extract(100°C).This indicates that potential bioactive constituents inC.speciosusmay be thermally labile with high extraction temperatures leading to degradation of heat-sensitive compounds.
Interestingly, different plant parts ofC.speciosusscreened in this study displayed different degrees of inhibition.In particular, bioactive constituents that attenuate contractile responses of isolated rat prostate appear to be present in lower concentrations in leaf than in root, rhizome or stem.This is consistent with traditional medicine practices by the Sarawak indigenous community.The inhibitory effect is indicative of a smooth muscle relaxant effect on the prostate gland.Relaxation of prostatic smooth muscle tone is a biological property that would be useful in a treatment targeting the dynamic component of BPH.In assessing the mechanism of action,C.speciosusrhizome extract did not affect direct smooth muscle stimulation mediated contractions mediated by noradrenaline, acetylcholine and ATP to the same degree as contractions mediated by EFS.Interestingly, the extract also had little or no effect on contractions elicited by tyramine,an indirectly acting sympathomimetic agonist that releases neurotransmitters through non-exocytotic means.This suggests that the extract affects the exocytotic release of neurotransmitters.
C.speciosusextracts displayed a comparable degree of inhibition to the therapeutically used antagonist, prazosin, in relaxing isolated preparations of rat prostatic smooth muscle [50].C.speciosusrhizome extract for example yielded an average inhibition of 50 ± 6% across all frequencies which compares favourably to prazosin causing a similar degree of inhibition using the same bioassay and under the same conditions in our laboratory [50].This implies that theC.speciosusrhizome extract may provide a similar degree of symptomatic relief from LUTS associated with BPH as the therapeutically used α1-adrenoceptor antagonist class of BPH medications.
Identifying the pure bioactive compounds from medicinal plants is crucial to validate their efficacy and safety as pharmacotherapeutics.Chemical separation ofC.speciosusrhizome RT extract showed that the inhibitory activity of the extract was due to the soluble components that were present in the highly polar fraction of the liquid phase but not the undissolved particles.The components of this active fraction are yet to be identified, but are consistent with carbohydrate-based structures.Several carbohydrate-based compounds have been isolated fromC.speciosusseed, rhizome, and root.These carbohydrate-based compounds are saponins and three of these (costucoside A, costucoside D or dioscin, and costucoside E or gracillin) that have been reported isolated from the rhizome are spirostanol saponins [24, 40].It is desirable to determine the active compound(s) responsible for the muscle relaxation activity ofC.speciosusrhizome RT extract and whether the crude extract might be preferable to the activity of the purified active compound(s) alone.Isolation and elucidation of active components fromC.speciosusrhizome RT extract and their mechanisms of action are important, as they will provide basic guidelines for the safety and efficacy of its use in the pharmacotherapy of BPH.
Conclusion
Knowledge of traditional medicines has re-emerged as a crucial contemporary resource that contributes to the augmentation of the drug discovery process via ethnopharmacology, which ultimately leverages the identification of novel therapeutic targets [51–54].C.speciosusrhizome RT extracts possess prostatic smooth muscle relaxant efficacy similar to that seen with prazosin or tamsulosin.ThereforeC.speciosusextracts are likely to provide similar therapeutic relief from LUTS associated with BPH.To our knowledge, this is the first study to report a relaxant effect ofC.speciosuson rat prostatic smooth muscle thus lending support to the traditional medical knowledge of the Sarawak indigenous community.In addition, asC.speciosushas been used to treat urinary diseases in both men and women, the smooth muscle relaxant effect observed in this study might not be specific to the prostate, as this would only be beneficial in men.The observed action might therefore have similar effects on other smooth muscles such as the bladder.
 Traditional Medicine Research2022年4期
Traditional Medicine Research2022年4期
- Traditional Medicine Research的其它文章
- Comment on “A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis”
- Non-inclusion of certified herbal medicines in the National Health Insurance Scheme affects patient utilization of the integrated herbal medicine services in Ghana
- Jujube (Ziziphus jujuba Mill.(Rhamnaceae)): a review on its pharmacological properties and phytochemistry
- Toxicological advances of traditional medicine in 2021
- Advances in traditional Chinese medicine for respiratory disease therapy in 2021
- Biologically active components for cosmeceutical use extracted from Chaetomorpha aerea
