Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
WANG Chun-Chieh, CHEN Ruifang, YANG Xin, WEN Yulong,KUANG Yang, ZHANG Ce, ZHU Junhua, and KWAN Kit Yue, *
Asian Horseshoe Crab Bycatch in Intertidal Zones of the Northern Beibu Gulf: Suggestions for Conservation Management
WANG Chun-Chieh1), #, CHEN Ruifang1), #, YANG Xin2), WEN Yulong2),KUANG Yang2), ZHANG Ce2), ZHU Junhua2), and KWAN Kit Yue2), *
1),,,530007,2),,,535011,
Bycatch poses a severe challenge to global fishery management. Although studies have focused on marine megafauna bycatch, research on relevant issues regarding invertebrates is limited, particularly for the threatened horseshoe crabs. In this study, the distribution of fishing gears and the bycatch intensity of Asian horseshoe crabs in the intertidal zones of the northern Beibu Gulf that harbors the most abundant juvenile Asian horseshoe crabs populations in China were evaluated. Seven intertidal nursery habitats forandwere surveyed from May to July in 2021. A transect that crossed the tidal creeks and tidal flats between the high tide embankment/vegetation and low tide line of a given habitat was surveyed during the ebb tides. The type, number, and GPS of fishing gears were recorded when sighted, and the number and prosomal width of each horseshoe crab species were measured. Bycatch intensities differed among habitats, ranging from 0.3 to 18.4 and 1.2 to 22.7 individuals per kilometer of transect forand, respectively. Among the three identified fishing gear types, ground cages and stick net sets caused a stronger bycatch pressure on these two species. Mostbycatch andbycatch were large individuals in late juvenile and adult stages. Therefore, the ground cages and stick net sets along/near the tidal creeks, mangrove fringe, and low tide line should be regulated and removed to ensure the functionality of the intertidal zone as the spawning corridor and nursery habitat of Asian horseshoe crabs.
;; ground cage; stick net; tidal creek; mangrove; low tide line; spawning corridor
1 Introduction
Bycatch is a severe challenge to effective marine fish- ery management globally (Lewison, 2004; Davies, 2009; Komoroske and Lewison, 2015; Sims andQueiroz, 2016). In contrast to commercially fished species,whose landing and effort information are commonly avail- able, bycatch individuals are discarded during operation in most cases. Without long-term monitoring and data collec-tion, quantitative, reliable, and standardized assessmentsare difficult, thereby impeding strategic intervention on specific fishing gears and activities (Crowder and Mu- rawski, 1998; Temple, 2018; Savoca, 2020). Although bycatch is not an easily addressed issue, it has become one of the main research topics, especially non- target vertebrates, including marine megafaunas, such as mammals, sea turtles, and seabirds (Moore, 2009; Lewison, 2014). However, invertebrate bycatch has been rarely explored.
As characteristic marine invertebrates, horseshoe crabs are commonly known to be bycatch by trawling in coastal waters (Smith, 2017; Meilana and Fang, 2020). How- ever, their bycatch intensity across environmental gradi- ents has yet to be systematically evaluated. Steele(2002) assessed the efficiency of two bycatch reduction devices in otter trawling in Florida, United States. They found that the Atlantic horseshoe crabis the most abundant among the bycatch of inverte- brates. Supadminingsih(2019) observed that horse- shoe crab bycatch in bottom gillnetting, which is used to harvest the blue swimming crabin Java, Indonesia, mostly occurs at water depths within 5m. Zauki(2019) also reported the bycatch occurrence of Asian horseshoe crabs in artisanal fishing in Balok, Ma-laysia. However, the insufficient assessment on bycatch in- tensity and distributional hotspots throughout environmen-tal gradients hinders the implementation of species-spe- cific conservation management.
Three species of Asian horseshoe crabs,, tri-spine horseshoe crab, the coastal horse- shoe crab, and the mangrove horseshoe crab, are distributed along the Indo-Pacific coas- tal water (Vestbo, 2018). A comparison of these spe- cies with their Atlantic counterparthas sug- gested that the population baseline information about Asian horseshoe crabs is relatively limited, particularly in China, where most ecology- and conservation-related studies have focused on juvenile populations in intertidal nursery ha- bitats (Wang, 2020). These species are mainly threat-ened by exploitation foramebocyte lysate (TAL)production, food consumption, and habitat loss through land reclamation (Laurie, 2019; John, 2020). The threatened level ofhas been raised to endangered (EN) in the IUCN (International Union for Conservation of Nature) Red List of Threatened Species (Laurie, 2019), which is the highest grade amongthe four extant horseshoe crab species. In China,andare listed as Class II National Protected Animals issued by the Ministry of Agriculture and Rural Affairs, National Forestry and Grassland Admi- nistration of China in 2021 even thoughis maintained as data deficient (DD) in the IUCN Red List.Despite their increasing importance in conservation, the systematic evaluation of the perceived threats is widely lacking throughout the Indo-Pacific region, including the Chinese coastline.
Horseshoe crabs are mainly caught in not only subtidal coastal waters but also intertidal zones. Bycatch in inter- tidal zones remains relatively unexplored compared with that in coastal waters. With widespread tidal flats and the most abundant local populations of juvenile Asian horse- shoe crabs in China (Xie, 2020), the northern Beibu
Gulf offers a good opportunity to study horseshoe crab by- catch in intertidal zones. In this research, the bycatch of two species of Asian horseshoe crabs, namely,and, by fishing gears available in the intertidal zones of the northern Beibu Gulf in Guangxi, China, was investigated. Across the environmental gradi- ents in the intertidal zone, the density of fishing gears and the bycatch intensity of horseshoe crabs were evaluated. The spatial patterns of fishing gears and bycaught indi- viduals were also recorded. Recommendations for priori- tizing management to regulate particular fishing gears in specific places were provided to mitigate the adverse ef- fect of bycatch on the threatened horseshoe crab popula- tions.
2 Materials and Methods
2.1 Study Site
Beibu Gulf is a semi-closed bay at the northwestern South China Sea and is surrounded by Guangxi, Guang- dong, and Hainan regions/provinces of China and northern- most Vietnam. It has been viewed as the last wilderness for Asian horseshoe crabs (Brockmann and Smith, 2009; Weng, 2012). Seven intertidal nursery habitats along the coastline of northern Beibu Gulf, Guangxi, namely, Rong- genshan (RGS), Tieshangang (TSG), Xibeiling (XBL), Zhongsandun (ZSD), Shaluoliao (SLL), Yuzhouping (YZP),and Jiaodong (JD), which were previously demonstrated toharbor the most abundant juvenile Asian horseshoe crab populations in China (Xie, 2020), were chosen as the study sites (Fig.1). Each study site is located in a specific local bay or estuarine system, with different characteristic vegetations and dominant Asian horseshoe crab species (Table 1). Overall,dominates the eastern ha- bitats of northern Beibu Gulf, whereasis more abundant in the western shoreline (Xie, 2020).
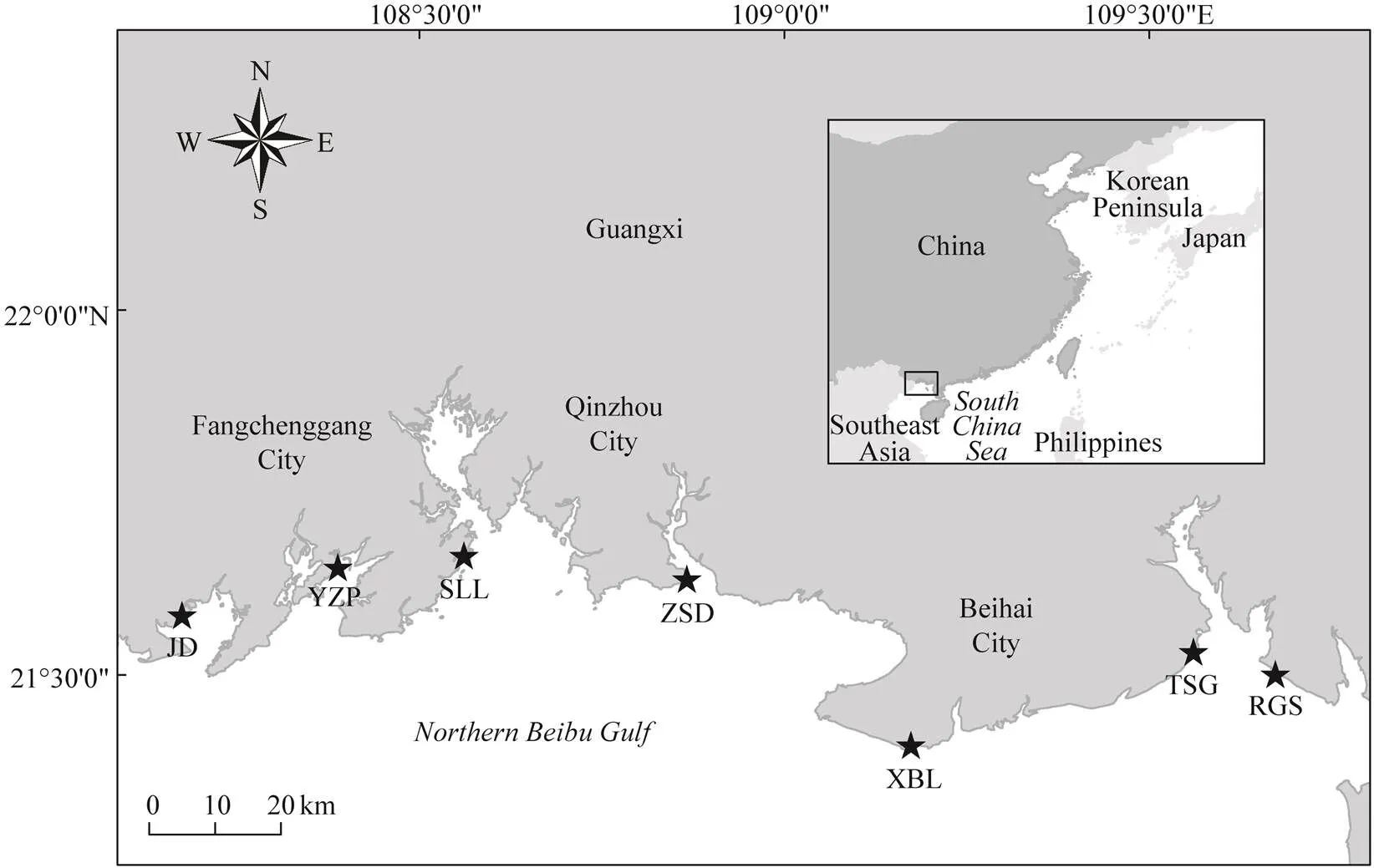
Fig.1 Seven study sites along the coastline of the northern Beibu Gulf, Guangxi, China. RGS, Ronggenshan; TSG, Tie- shangang; XBL, Xibeiling; ZSD, Zhongsandun; SLL, Shaluoliao; YZP, Yuzhouping; JD, Jiaodong.
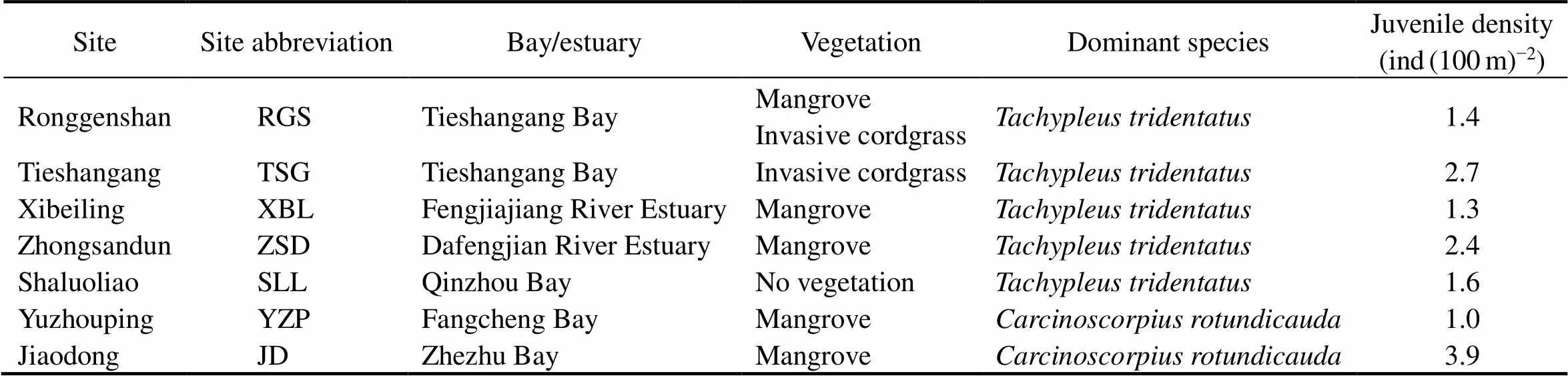
Table 1 Different characteristic vegetations and dominant Asian horseshoe crab species in the seven study sites
Note: Data are from Xie(2020).
2.2 Survey Methods
In each study site, a predefined transect line that cross- ed environmental gradients within the intertidal zone of ti- dal creeks and the tidal flat between an embankment/do- minant vegetation and a low tide line or river mouth was walked through during an ebb tide to search for fishing gears and examine the bycatch of Asian horseshoe crabs. The transect lines were designed in accordance with the method described in a recent population study on juvenile horseshoe crabs in the northern Beibu Gulf (Xie., 2020). This design was applied to include different land- scape elements, which are important for horseshoe crab spawning, including tidal creeks in mangrove forests and intertidal flats extending to the low tide line. ‘Stationary’ fishing gears rather than dynamic fishing activities in the intertidal zones were mainly considered, so small fishing gears commonly operated by local fishermen for beach- combing were excluded. When a fishing gear was sighted, the type, number, and GPS coordinate (Garmin Dakota 20) were recorded. A single coordinate was assigned for point- distributed fishing gears, whereas multiple coordinates of both ends and middle turning points were noted for fish- ing gears with a linear distribution. If any Asian horseshoe crab bycatch was found in the fishing gear, their species was identified in terms of the cross-section shape of the telson and morphological characteristics of the prosoma. For example,has a triangular telson, whereashas a round one. The prosoma ofis more convex and has more spines. Alive indivi- duals and dead/decayed bodies were included. Then, the number of ensnared horseshoe crabs was counted, and the prosomal width (PW, mm) of each individual of these two species was measured using calipers. The prosoma remainsintact even when a horseshoe crab is dead for a certain pe- riod, and the rest parts of the body (partially) have decay- ed or disappeared; as such, it serves as a good indicator to estimate the life history stage of the individual bycatch. Table 2 shows the range of PW and the corresponding life stages of(Sekiguchi, 1988; Kwan, 2021) and(Sekiguchi, 1988; Hu,2015; Fan, 2017). Once the measurement was finish- ed, the inspected individual was removed from the fishing gear to prevent repeat counts. The field surveys were im- plemented in the summer of 2021 (May–July).

Table 2 Prosomal width (PW, mm) and the corresponding life history stages of Tachypleus tridentatus (TT) and Carcinoscorpius rotundicauda (CR)
Notes: Data are from Sekiguchi(1988), Hu(2015), Fan(2017), and Kwan(2021). The distribution of bycatch fraction (%) of the three fishing gears in each life stage is shown.
2.3 Types of Fishing Gears
Fishing gears are categorized into three types, namely, ground cage, stick net set, and ghost net, after exploratory field surveys on the intertidal zones of the northern Beibu Gulf. Ground cage is a linearly distributed net with a meshsize of 10–20mm and a length of several meters. It has mul- tiple rectangle metal grids (length of each side: 30–60cm) inside that hold its shape as a cage (Figs.2A–2B). Both endsof a ground cage or a set of ground cages are tied on sta- tionary objects, such as sticks, anchors, and stones (Fig.2B). A ground cage has several openings at the lateral sides for animals to enter. Each section of a ground cage, where both ends are held by a pair of rectangular metal grids, has an internal concave net that only allows one directional entrance and prevents the animals from escaping. Eventually, most captured animals crowd in one end of the ground cage (Fig.2B). Fishermen usually deploy the ground cage along or near tidal creeks that still have water during the ebb tide (Fig.2A) to capture nonspecific species, such as fishes, crabs,and shrimps. In this study, most of the sighted ground cages were abandoned with irregular shapes, compressed metal grids, and point distribution (Fig.2C).
A stick net set is a set of long nets (mesh sizes: 10–30mm) that hang on multiple sticks. The height differs signi- ficantly among sets, ranging from several meters (Fig.2D) to centimeters. The net does not always hang on sticks, but it is laid down on the ground in some instances (Fig.2E). Stick net sets are generally deposited near the low tide line (Fig.2D) or along tidal creeks (Fig.2E), where some water remains even during the lowest tide period. Similar to ground cages, stick net sets do not target specific species; instead, they capture any organisms stuck inside during the ebb tide. Both ground cages and stick net sets are listed as illegal fishing gears by the Fisheries Bureau, Ministry of Agricul- ture and Rural Affairs of China.

Fig.2 (A) Case of a linearly distributed ground cage near a tidal creek, (B) an extended ground cage anchoring at a stationary end with several Carcinoscorpius rotundicauda bycatch along the mangrove fringe, and (C) a discarded irregularly shaped and point-distributed ground cage with compressed metal grids. (D) A stick net set with the net hung at a height of 2–3m along the low tide line and (E) a stick net set along a tidal creek that crosses the mangrove, where the net is laying down on the ground (indicated by the white arrows). (F) A case of point-distributed ghost net with a Tachypleus tridentatus bycatch.
A ghost net is not a specific kind of fishing gear, but fish- ing gears that cannot be categorized into either a ground cage or stick net set. It is a fragmented net that may come from a gill net, stick net, and other unknown sources. The nets of a discarded ground cage, where the net and metal grids are tightly entangled, are not considered a ghost net. Apart from a few exceptional cases, the encountered ghost nets generally have a point distribution (Fig.2F).
The length of the transect line ranged from 2.39km to 4.58km among the study sites (Table 3). Most ground cages and ghost nets were distributed in points except for the four linearly distributed cases of ground cages in RGS and SLL and ghost nets in SLL and JD (Table 3). The distribution of the stick net set was linear, and the length ranged from 7.5m to 671.9m per set.
2.4 Spatial Patterns, Density of Fishing Gears, and Bycatch Intensity
The distribution and number of fishing gears and Asian horseshoe crabs bycatch were mapped on Landsat images (Path/Row: 124045 and 125045; image date: 26th and 17th June 2021 for 124045 and 125045, respectively) by using ArcMap 9.3 (Esri) to clarify their spatial patterns. Level-1 data of Landsat 8 OLI were downloaded from the Earth- Explorer platform, USGS (https://earthexplorer.usgs.gov/, access date: 2nd July 2021). The band composite of RGB 753 was applied because this false-colored image could en-hance the contract of different landscape elements,., be-tween land and water, among vegetated and urbanized areas(Kerr and Ostrovsky, 2003), to display the characters of the studied intertidal zones clearly.
The total number (of ground cages and ghost nets) or to- tal set number (of stick net sets) was divided by the tran- sect line length (km) conducted during the survey to assessthe density of these three fishing gear types in a given studysite. The difference in the length of the linearly distributed stick net sets (m) among study sites was examined with theKruskal-Wallis test because the assumption of data norma- lity was not met. The lengths of a given transect line and a stick net set were measured with the Calculate Geome- try function of ArcMap 9.3 after they were mapped on the projected coordinate system of WGS 1984 UTM Zone 49N.
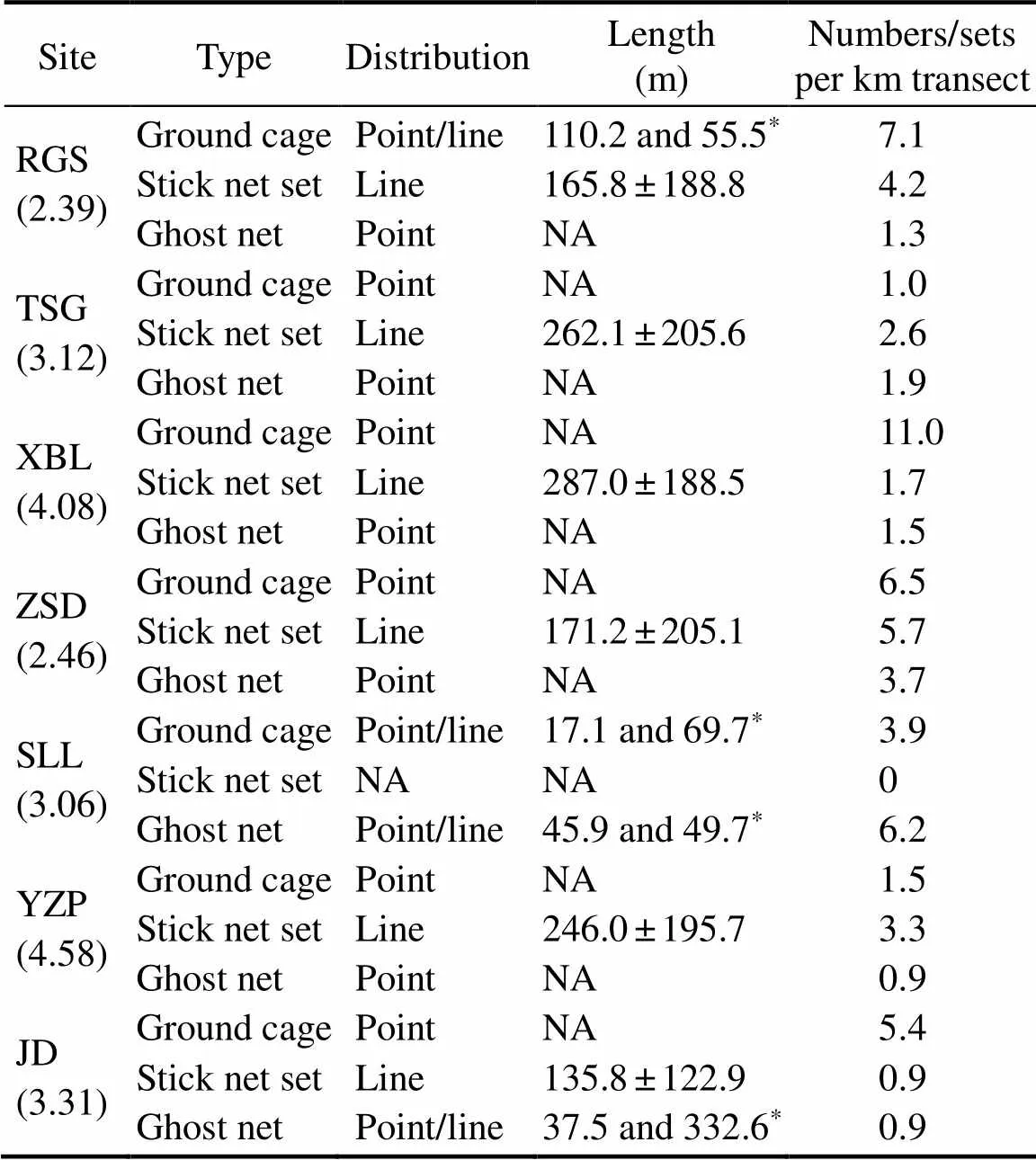
Table 3 General information about fishing gears on the intertidal zones during field surveys
Notes: Site abbreviations are mentioned in Table 1. Transect length (km) in each site is provided in the parentheses.*, only four specific cases of linearly distributed ground cages and ghost nets were en- countered, so the length is displayed individually rather than on aver- age. NA, not available.
The total number of bycatch individuals for each spe- cies in a given fishing gear was divided by the total num-ber of the given fishing gear in a study site to calculate the bycatch intensity of each type of fishing gear onand. The total number of each horse- shoe crab species was also divided by the length of the transect line in a given study site to evaluate the bycatch intensity in each habitat. The difference in the bycatch in- tensity among the three types of fishing gears was examined with the Kruskal-Wallis test. Pearson correlation was applied to explore the relationship between the density of fishing gears and the bycatch intensity of horseshoe crabs. The difference in the PW of(excluding in TSG where only one bycatch was found) andwas compared among the study sites through one- way analysis of variance (ANOVA) because data had nor- mal distribution and equal variance and among the three fishing gears via the Kruskal-Wallis test. Additionally, the difference in the status of the horseshoe crabs (., the fraction of alive individuals in the total bycatch for each species) among the fishing gears was compared using the Kruskal-Wallis test. The Dwass-Steel-Critchlow-Fligner testwas applied as the post hoc pairwise comparison for the Kruskal-Wallis test. Data were statistically analyzed withSystat 13.
3 Results
3.1 Density and Spatial Pattern of Fishing Gears
Fishing gears differed in their densities within any gi- ven site, and sites differed in the density of each type of fishing gear (Table 3). Although the three fishing gears had similar density throughout the study sites (1.0–7.1 cages, 0–5.7 sets, and 0.9–6.2 nets per kilometer of the transect line for the ground cage, stick net set, and ghost net, re- spectively), the density of ground cages was generally high- er than two other fishing gears in four of the seven habi- tats (., RGS, XBL, ZSD, and JD). The stick net set in SLL was not sighted during the field survey. The length of the stick net sets was not significantly different among ha- bitats (Kruskal-Wallis,=6.35,=0.27).
Both ground cages and stick net sets (green rectangles and light blue lines, respectively, in Fig.3) had a similar dis- tribution along the fringe of mangrove forests, along or near the tidal creeks, and along the low tide line close to estu- aries. No obvious spatial pattern was observed in ghost nets (purple triangles in Fig.3).
3.2 Bycatch Intensity, Spatial Distribution, Size, and Life History Stage
The bycatch intensity of each species of Asian horse- shoe crabs differed among the study sites and the three types of fishing gears (Table 4).was more intensive in RGS (18.4 individuals per km transect) and ZSD (14.2 individuals per km transect), butwas to a greater extent in YZP and JD (22.7 and 13.9 individuals per kilometer of the transect line in YZP and JD, respectively). In most cases, the bycatch intensity of each site matched the dominant species of juvenile horse- shoe crab (Table 1); however, in XBL and SLL, wheredominated the juvenile population, the inten- sity ofwas higher than that of(Table 4). The linear correlation between the density of fishing gears and the bycatch intensity of horseshoe crabs was not significant among all types of fishing gears (Pear- son=0.34, 0.39, and 0.25; and=0.31, 0.3, and 0.46 for ground cages, stick net sets, and ghost nets, respectively).
The ground cage and stick net set caught moreandthan the ghost net through- out their life stages (Table 2), although the difference in the bycatch intensity among the fishing gears was not sig- nificant (Kruskal-Wallis,=1.96,=0.16). Although the overall trend showed that higher proportions of individualswere caught in stick net sets during the four life stages, the fraction of bycatch in ground cages increased throughout the development of(Table 2). During the life stages of the 9th–10th instars and late instars, morewas caught in stick net sets, whereas the pro- portion of adults caught in ground cages was higher than that in stick net sets (Table 2).
The main threat differed among habitats (Table 4). In RGS, ground cages (1.8 individuals per ground cage) caught moreper unit than stick net sets (1.3 indi- viduals per stick net set). By comparison, stick net sets were the major threat forin ZSD (2.3 indi- viduals per stick net set). In the bycatch hotspots of, ground cages had more bycatches in YZP (8.6 individuals per ground cage). Conversely, stick net sets were the main threat in JD (9.3 individuals per stick net set). Overall, the intensity ofwas slightly higher (1.2–22.7 individuals per kilometer of the transect line) than that of(0.3–18.4 individuals per kilometer of the transect line) among habitats.

Fig.3 Spatial distribution of ground cages (green rectangles and greed lines), stick net sets (light blue lines), ghost nets (purple triangles and purple lines), Tachypleus tridentatus bycatch (TT, red circles), and Carcinoscorpius rotundicauda bycatch (CR, yellow circles) projected on 2021 Landsat 8 OLI false color images (RGB 753; Path/Row: 124045 and 125045; image date: 26th and 17th June for 124045 and 125045, respectively) in the intertidal zones of (A) Ronggenshan, (B) Tieshangang, (C) Xibeiling, (D) Zhongsandun, (E) Shaluoliao, (F) Yuzhouping, and (G) Jiaodong. Prosomal width (PW, mean±standard error) of each horseshoe crab species in a given study site is provided. Black lines represent the survey transects. The white arrows indicate tidal creeks or rivers. The dominant vegetation of mangrove forests or invasive cordgrass is marked. Note that the scaling is different among the panels.
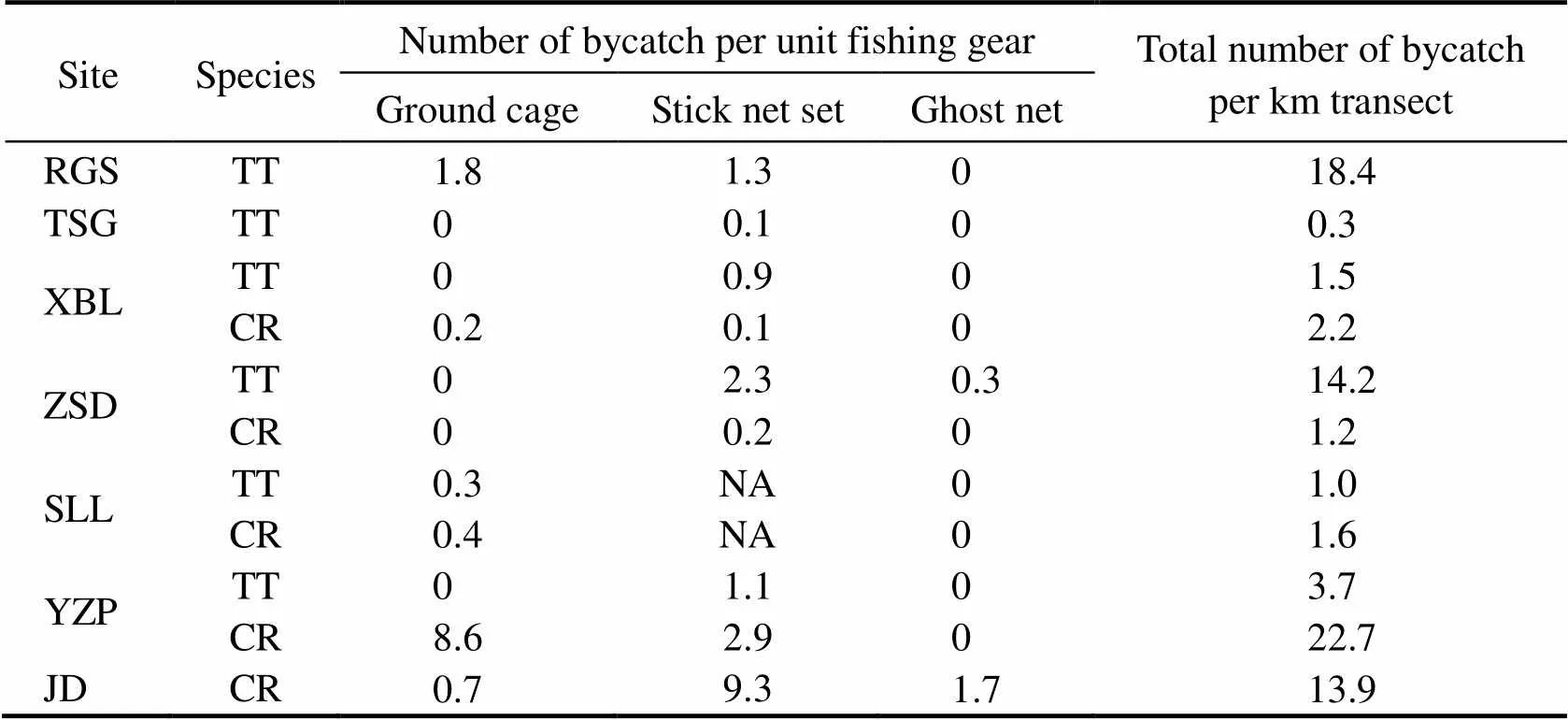
Table 4 Number of bycaught Asian horseshoe crabs per unit of each fishing gear type and per unit transect length (km)
Notes: Site abbreviations are mentioned in Table 1. TT,; CR,; NA, not available.
did not display a clear spatial pat- tern, although some of its bycatch occurred near the low tide line in RGS (Fig.3A) and ZSD (Fig.3D) or along the tidal creek in SLL (Fig.3E). For, most in- dividuals were found along the river (Fig.3C), tidal creeks (Figs.3D–3G), mangrove fringe (Fig.3F), and the bound- ary between the low tide line and estuary (Fig.3G).
The PWs of(ANOVA,=0.74,=0.6) and(ANOVA,=1.38,=0.25) were notsignificantly different among the study sites. Most of them were adults or at late instar stages (Fig.4). Althoughbycatch had more diverse life stages, which in-cluded the 10th–12th instars, late instars, and adults (Fig.4A), almost allspecimens were adults, except some 9th–10th instars and late instars in JD (Fig.4B). The PW ofdiffered significantly among the three fishing gears (Kruskal-Wallis,=38.7,<0.001). Inparticular, the largest individuals were found in ground cages (125.1mm±17.6mm), followed by those in stick net sets (105.6mm±22.1mm) and ghost nets (88.4mm±18.2mm; Dwass-Steel-Critchlow-Fligner test,<0.001 for all pairwise comparisons). Conversely, the PW ofdid not significantly differ among the fishing gears (Kru- skal-Wallis,=2.8,=0.25). As for the status of bycatch individuals, the percentage of alive individuals did not sig- nificantly vary among the three fishing gears (Kruskal-Wal- lis,=1.87,=0.39).
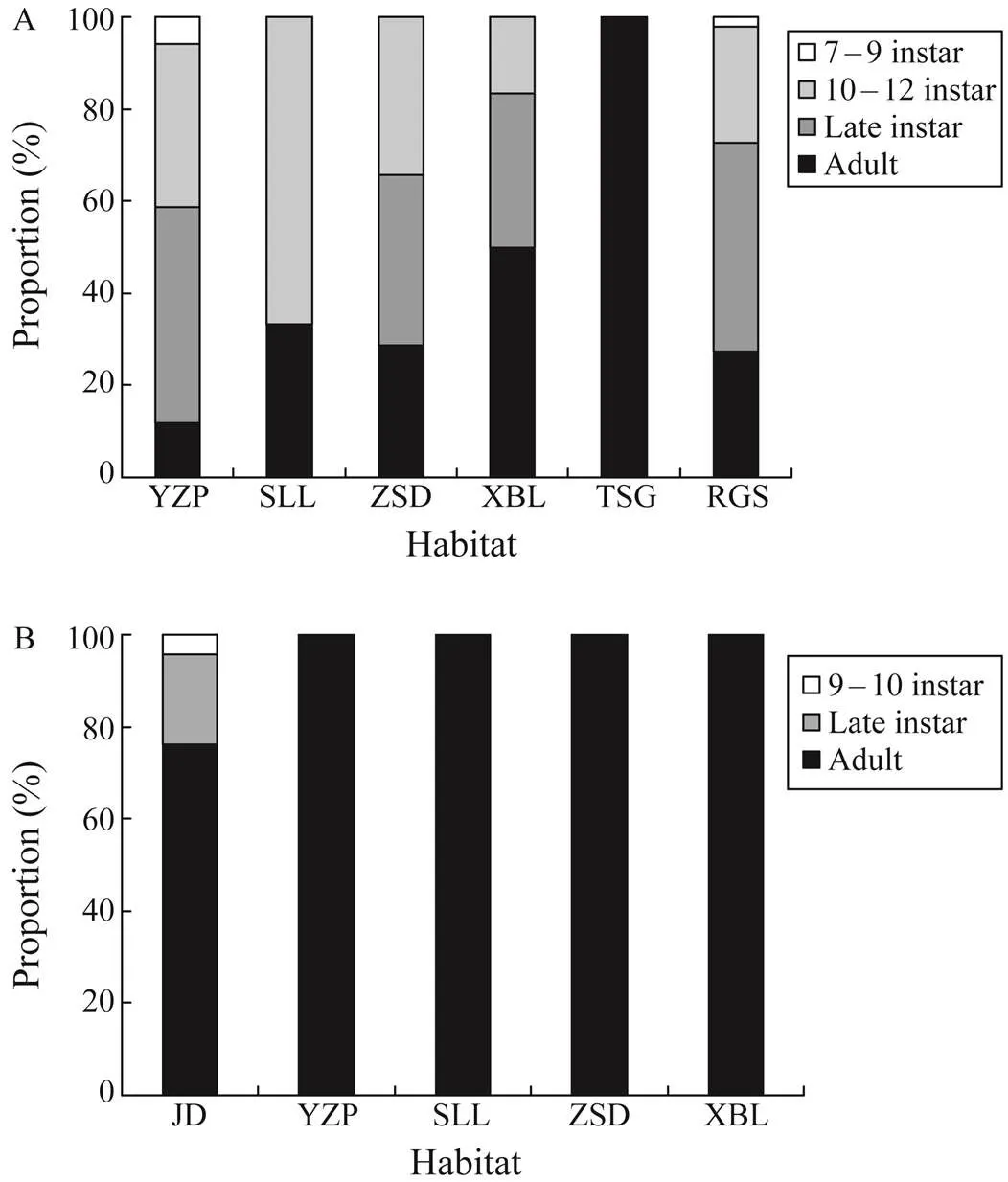
Fig.4 Proportion distribution of (A) Tachypleus tridenta- tus and (B) Carcinoscorpius rotundicauda at different life stages in the study sites. Site abbreviations are mentioned in Fig.1.
4 Discussion
In the seven studied intertidal zones along the coastline of northern Beibu Gulf, the density of the fishing gears (., ground cages, stick net sets, and ghost nets) had si- milar ranges, but the number of ground cages per kilome- ter of the transect line was more than that of the other two gears. Ground cages and stick net sets were mainly distri- buted along tidal creeks, mangrove fringe, and near the low tide line. They were identified as the major threat for the two Asian horseshoe crab species. Consistent with the spa- tial distribution pattern of these gears, the occurrence ofbycatch was mostly observed near tidal creeks, mangrove fringe, and low tide line, but thebycatch had no clear spatial pattern. The majority of the bycatch individuals of both species were late instars and adults.
This study focused on bycatch by stationary fishing gears,., fishing gears remaining on the intertidal zone during ebb tides, so the information about bycatch from other fish-ing activities was not available. The status of fishing gears,such as ground cages with different extending levels, stick net sets hung on sticks or laid down on the ground, and di- verse gear/mesh sizes, may affect the results of bycatch. In our study, the potential effects of fishing gear status on horseshoe crab bycatch were not considered, but they shouldbe further explored. Additionally, the majority of the record-ed ground cages were not installed on any object, although some of them were partially buried in sediment or entan- gled in stick net sets. The position of these seemingly aban- doned ground cages and fragmented ghost nets might vary throughout the tidal cycles. The intertidal landscape that affects local tidal flow might further complicate the distri- bution of discarded fishing gears. These factors could in- teract simultaneously; as such, the general spatial pattern of horseshoe crabs bycatch could not be easily concluded. Although our focus and applied methods had limitations, the evaluated density of different fishing gears and the by-catch intensity of these two species of Asian horseshoe crabs helped reveal the main threats. They also provided a basis for developing management solutions to solve issues on horseshoe crab bycatch in the intertidal zones of the nor- thern Beibu Gulf.
Among the three types of fishing gears, ground cages and stick net sets showed clear spatial patterns along or near tidal creeks, mangrove fringe, and the low tide line at the boundary with estuaries. By contrast, ghost nets had nodistribution pattern. The spatial distribution of fishing gears reflects the general ways by which fishermen use them. For instance, our observations implied that ground cages and stick net sets are commonly used to capture nonspecific targets, such as fishes, crabs, and shrimps. Landscape ele- ments, such as tidal creeks and low tide lines, retain a thin water layer during ebb tides and may serve as the pathway for organisms to explore the increasingly immersed under- water area. Animals that move to the intertidal zone dur- ing flooding tides may get caught more easily by ground cages and stick net sets near tidal creeks and low tide lines. Therefore, nets of these fishing gears should stay at least partially immersed in estuarine water during ebb tides to ensure the accessibility and freshness of harvested orga- nisms.
Bycatch intensity differed among the study sites and va-rious types of fishing gears. The bycatch intensity of ground cages and stick net sets for these two Asian horseshoe crab species was stronger than that of ghost nets; that is, the proportions of bycatch individuals in ground cages and stick net sets throughout the life stages were higher than those in ghost nets. Generally,suffered from a more intensified bycatch pressure than(1.2– 22.7 and 0.3–18.4 individuals per kilometer of the tran- sect line forand, respective- ly). Although the bycatch hotspots ofwere unlikely found in this study, this result implied that the local population ofmight be in a poorer state with smaller population size and lower density than. In contrast to DD (data deficient)-listed,is upgraded to EN (endangered) in the IUCN Red List (Laurie, 2019). Experts from the Indo-Pacific region perceived an overall decline in the local population size of both species throughout their geo- graphical range (Wang, 2020). In the northern Beibu Gulf, most residents of coastal communities in Guangxi also recognized a decrease in the local population of(Fu, 2019; Liao, 2019). For the threaten- ed Asian horseshoe crab populations in the northern Bei- bu Gulf, especially the severely depleting, by- catch by stationary fishing gears in intertidal zones pose a remarkable challenge to an effective conservation.
The intensity ofbycatch in RGS and ZSD, which are the critical nursery habitats of(Xie, 2020), was higher than those in other sites. Similar- ly, more bycatch ofwas noted in YZP andJD, which was dominated by juvenile(Xie, 2020). In XBL and SLL, which harbored abundantjuveniles (Xie, 2020), the number ofbycatch was more than that of. For these two species, the majority of them were late instars and adults. The phenomenon that moreadults were found in juvenile-domi- nated nursery habitats indicated that the ‘spawning corridor’ function of intertidal nursery habitat for Asian horse- shoe crabs had been overlooked. For Asian horseshoe crabs, the baselines of juvenile populations and nursery habitats in intertidal zones are more abundant than those of spawn- ing habitats and adult populations, particularly in China (Wang, 2020). Most studies have focused on the po- pulation structure of juveniles and the characteristics of nursery habitats in intertidal zones (Chiu and Morton, 2003; Hsieh and Chen, 2009; Chen, 2015; Koyama, 2020; Meilana, 2021). Contrary to North American, whose spawning activities (Widener and Barlow, 1999; Smith, 2002; Cheng, 2015) and habitat characteristics have been well studied (Weber and Carter, 2009; Landi, 2015), Asian horseshoe crabs have been rarely described in terms of the function of in- tertidal zones in spawning (Shuster and Sekiguchi, 2009; Mohamad, 2019). Our results showed that most by- catch ofandwere adults, highlighting the importance of intertidal zones in their spawning activities.
Similar to the spatial pattern of major threats from ground cages and stick net sets, the distribution ofbycatch was mainly found along or near the tidal creeks, mangrove fringe, and low tide line. Conversely, the spatial distribution ofbycatch did not show a clear pattern, but it seemed to scatter across the area between the embankment/vegetation and low tide line. The density of the three fishing gears and bycatch intensity had no sig- nificant correlation, indicating that the spatial distribution of fishing gears could be more influential than their den- sity in determining the bycatch intensity of horseshoe crabs.Most bycatch individuals were adults, so spawning acti- vity might play an important role in determining the re- ported spatial distribution of bycatch. Therefore,andlikely had different spawning be-haviors and preferences. In the absence of systematic field surveys, adults ofwere widely observed alone or in amplexed mating pair in tidal creeks during theebb tides in summer. This result implied thatmight move to the high tide line for spawning even within the period of the ebb tide. The pathway to high tide spawning grounds is restricted in the low tide line and ti- dal creeks that cross mangroves and tidal flats; thuswas caught in fishing gears close to these land-scape elements. As for, we never observe any alive adults during the ebb tides in our long experience of field surveys, thus its spawning activity remains nearly un- known. They probably moved to spawning habitats dur- ing flood tides, where the intertidal zone was increasingly immersed under water. Mating pairs ofcould approach the high tide area through more diverse micro- habitats in the intertidal zone; as a result, the bycatch pat- terns were more scattered. Additionally, the proportion of these two species in ground cages increased from the ju- venile stages to the adult stage. Ground cages intended to catchwith a significantly larger PW than the other two fishing gears. This finding suggested that thedesign of ground cages met the instinctive and burying be- havior of horseshoe crabs. Therefore, ground cages were likely an ‘a(chǎn)ttractor’ for large individuals. However, future management endeavors should focus on the potential de- trimental effect of ground cages and clarify the behavior of adults.
The spatial pattern of fishing gears and the bycatch in- tensity of Asian horseshoe crabs in the intertidal zone in- dicated the need for managing ground cages and stick net sets, which are listed as illegal fishing gears (issued in 2013 by the Fisheries Bureau, Ministry of Agriculture and Rural Affairs of China). Under the limited manpower of law enforcement, we strongly recommend targeting bycatch hotspots, including tidal creeks, mangrove fringe, and low tide lines, to regulate and remove ground cages and stick net sets regularly. Given thatis severely en- dangered (Laurie, 2019) and the local population ofis potentially declining (Wang, 2020), the suggested management strategy can help mitigate the threat posed by stationary fishing gears and ensure the eco- logical function of intertidal zones as spawning corridors and nursery habitats for the threatened Asian horseshoe crabs.
In addition to bycatch from these three types of fishing gears in intertidal zones, the intentional capture of Asian horseshoe crabs in subtidal coastal areas in Chinese watersremains largely unknown. For local communities in Guang-xi, horseshoe crabs are considered traditional seafood, par-ticularly the EN-listed(Fu, 2019; Liao, 2019). Another source of threat is the direct harvest of horseshoe crabs for TAL (amebocyte lysate) production. Although our observations suggested that the awareness of horseshoe crab conservation has increased sinceandwere upgraded to Class II National Protected Animals by the Chinese govern- ment in early 2021, potential needs for consumption and TAL production may enhance illegal exploitation through smuggling. Besides the explicit stress due to overharvest- ing, implicit factors such as habitat loss and degradation canalso affect the local populations of Asian horseshoe crabs(John, 2018; Laurie, 2019). Critical inter-tidal nurseries and spawning habitats have likely suffered from extensive land reclamation in the coastal waters of China (Tian, 2016). Therefore, future studies should target explicit and potential stresses to quantify and assess their impacts on Asian horseshoe crab populations syste- matically. An integrated method of field survey, question- naire inquiry, and satellite remote sensing should be deve- loped to explore the long-term changes in population size, distribution, and habitat status (Vihervaara, 2017), andhelp design, prioritize, and implement effective management interventions.
5 Conclusions
Among the three types of fishing gears, ground cages and stick net sets were the main threats for the two Asian horseshoe crab species, namely,and, in the intertidal zone of northern Beibu Gulf, Guangxi, China, during summer. The majority of horseshoe crabs bycatch were large individuals at the life stages of late instars and adults. The ground cages and stick net sets along/near the tidal creeks, mangrove fringe, and the bound- ary between the low tide line and nearby estuary imposed a strong bycatch intensity on. However, no clear spatial pattern was observed in. Our study suggested that regularly regulating and removing ground cages and stick net sets in the bycatch hotspots can help ensure the functionality of the intertidal zone as a spawning corridor and nursery habitat for horseshoe crabs. Combined with records on critical information about sta- tionary fishing gears and the bycatch of horseshoe crabs, our method of a transect line that crossed environmental gradients in the intertidal zone can serve as a standard for systematically evaluating the density of fishing gears and the bycatch intensity of endangered Asian horseshoe crabs and other non-targeted species.
Acknowledgements
This study was supported by the Basic Research Fund of Guangxi Academy of Sciences (No. 2020YBJ706), the National Natural Science Foundation of China (No. 3206 0129), the Guangxi Natural Science Foundation (No. 2018 GXNSFBA281071), the Guangxi Science and TechnologyBase and Talent Project (No. 2021AC19355), the Guangxi BaGui Youth Scholar Program, and the Guangxi Recruit- ment Program of 100 Global Experts. We would like to thank Dr. D. Christopher Rogers for his kind help in improving the English expression of the manuscript.
Brockmann, H. J., and Smith, M. D., 2009. Reproductive com- petition and sexual selection in horseshoe crabs. In:. Tanacredi, J. T.,., eds., Springer, Boston, MA, 199-221.
Chen, C. P., Yang, M. C., Fan, L. F., Qiu, G., Liao, Y. Y., and Hsieh, H. L., 2015. Co-occurrence of juvenile horseshoe crabsandin an estuarine bay, southwestern China., 24: 117- 126, https://doi.org/10.3354/ab00641.
Cheng, H., Chabot, C. C., and Watson III, W. H., 2015. The life history cycle ofin the Great Bay Estuary, New Hampshire U.S.A. In:. Car- michael, R. H.,., eds., Springer, Cham, 237-254.
Chiu, H. M. C., and Morton, B., 2003. The sediment and hydro- graphic characteristics of three horseshoe crab nursery beaches in Hong Kong., 2 (1): 35-43, https://doi.org/10.1007/s11802-003-0023-2.
Crowder, L. B., and Murawski, S. A., 1998. Fisheries bycatch: Implications for management., 23 (6): 8-17, https:// doi.org/10.1577/1548-8446(1998)023<0008:FBIFM>2.0.CO;2.
Davies, R. W. D., Cripps, S. J., Nickson, A., and Porter, G., 2009. Defining and estimating global marine fisheries bycatch., 33 (4): 661-672, https://doi.org/10.1016/j.marpol. 2009.01.003.
Fan, L. F., Chen, C. P., Yang, M. C., Qiu, G., Liao, Y. Y., and Hsieh,H. L., 2017. Ontogenetic changes in dietary carbon sources and trophic position of two co-occurring horseshoe crab species in southwestern China., 26: 15-26, https://doi. org/10.3354/ab00670.
Fisheries Bureau, Ministry of Agriculture and Rural Affairs, Peo- ple’s Republic of China, 2013. Catalogue of Prohibited Fish- ing Gears. Beijing, 30pp.
Fu, Y., Huang, S., Wu, Z., Wang, C. C., Su, M., Wang, X.,, 2019. Socio-demographic drivers and public perceptions of con- sumption and conservation of Asian horseshoe crabs in nor- thern Beibu Gulf, China., 29 (8): 1268-1277, https://doi.org/10. 1002/aqc.3125.
Hsieh, H. L., and Chen, C. P., 2009. Conservation program for the Asian horseshoe crabin Taiwan: Characterizing the microhabitat of nursery grounds and re- storing spawning grounds. In:. Tanacredi, J. T.,., eds., Springer, Bos- ton, MA, 417-438.
Hu, M., Kwan, B. K. Y., Wang, Y., Cheung, S. G., and Shin, P. K. S., 2015. Population structure and growth of juvenile horse- shoe crabsand(Xiphosura) in southern China. In:. Carmichael, R. H.,., eds., Springer, Cham, 167-180.
John, A., Shin, P. K. S., Botton, M. L., Gauvry, G., Cheung, S. G., and Laurie, K., 2020. Conservation of Asian horseshoe crabs on spotlight., 30: 253-256, https:// doi.org/10.1007/s10531-020-02078-3.
John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., War- diatno, Y., Dash, B. P.,, 2018. A review on fisheries and conservation status of Asian horseshoe crabs., 27: 3573-3598, https://doi.org/10.1007/s10531- 020-02078-3.
Kerr, J. T., and Ostrovsky, M., 2003. From space to species: Eco- logical applications for remote sensing., 18 (6): 299-305, https://doi.org/10.1016/S0169-5347 (03)00071-5.
Komoroske, L. M., and Lewison, R. L., 2015. Addressing fish- eries bycatch in a changing world., 2: 83, https://doi.org/10.3389/fmars.2015.00083.
Koyama, A., Hirata, T., Kawahara, Y., Iyooka, H., Kubozono, H., Onikura, N.,, 2020. Habitat suitability maps for juvenile tri-spine horseshoe crabs in Japanese intertidal zones: A mo- del approach using unmanned aerial vehicles and the struc-ture from motion technique., 15 (12): e0244494, https://doi.org/10.1371/journal.pone.0244494.
Kwan, K. Y., Bopp, J., Huang, S., Chen, Q., Wang, C. C., Wang, X.,, 2021. Ontogenetic resource use and trophic dyna- mics of endangered juvenileamong diversified nursery habitats in the northern Beibu Gulf, China., 16 (6): 908-928, https://doi.org/10.1111/ 1749-4877.12495.
Landi, A. A., Vokoun, J. C., Howell, P., and Auster, P., 2015. Pre- dicting use of habitat patches by spawning horseshoe crabs () along a complex coastline with field sur- veys and geospatial analyses., 25 (3): 380-395, https://doi.org/10. 1002/aqc.2440.
Laurie, K., Chen, C. P., Cheung, S. G., Do, V., Hsieh, H., John, A.,, 2019., tri-spine horseshoe crab. The IUCN Red List of Threatened Species 2019, Errata version, 58pp, https://dx.doi.org/10.2305/IUCN.UK.2019-1.RLTS. T21309A149768986.en.
Lewison, R. L., Crowder, L. B., Read, A. J., and Freeman, S. A., 2004. Understanding impacts of fisheries bycatch on marine megafauna., 19 (11): 598-604, https://doi.org/10.1016/j.tree.2004.09.004.
Lewison, R. L., Crowder, L. B., Wallace, B. P., Moore, J. E., Cox, T., Zydelis, R.,, 2014. Global patterns of marine mam- mal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots., 111 (14): 5271-5276, https://doi.org/10.1073/pnas.1318960111.
Liao, Y., Hsieh, H. L., Xu, S., Zhong, Q., Lei, J., Liang, M.,, 2019. Wisdom of Crowds reveals decline of Asian horseshoe crabs in Beibu Gulf, China., 53 (2): 222-229, https://doi. org/10.1017/S003060531700117X.
Meilana, L., and Fang, Q., 2020. Local knowledg-based study on the status of horseshoe crabs along the Indonesian coast., 36: 101252, https://doi.org/10.1016/j.rsma.2020.101252.
Meilana, L., Hakim, A. A., and Fang, Q., 2021. Nursery habitat of three species of juvenile Asian horseshoe crabs in Teritip Beach, East Kalimantan, Indonesia: Characterization and implication., 26: e01453, https://doi.org/ 10.1016/j.gecco.2021.e01453.
Mohamad, F., Sofa, M. F. A. M., Manca, A., Ismail, N., Cob, Z. C., and Ahmad, A. B., 2019. Nests placements and spawning in the endangered horseshoe crab(Leach, 1819) (Merostomata: Xiphosurida: Limulidae) in Sabah, Ma- laysia., 39 (6): 695-702, https:// doi.org/10.1093/jcbiol/ruz070.
Moore, J. E., Wallace, B. P., Lewison, R. L., ?ydelis, R., Cox, T. M., and Crowder, L. B., 2009. A review of marine mammal, sea turtle and seabird bycatch in USA fisheries and the role of policy in shaping management., 33 (3): 435- 451, https://doi.org/10.1016/j.marpol.2008.09.003.
Savoca, M. S., Brodie, S., Welch, H., Hoover, A., Benaka, L. R., Bograd, S. J.,, 2020. Comprehensive bycatch assessment in US fisheries for prioritizing management., 3: 472-480, https://doi.org/10.1038/s41893-020-0506- 9.
Sekiguchi, K., Seshimo, H., and Sugita, H., 1988. Post-embryo- nic development of the horseshoe crab., 174 (3): 337-345, https://doi.org/10.2307/1541959.
Shuster Jr., C. N., and Sekiguchi, K., 2009. Basic habitat require- ments of the extant species of horseshoe crabs (Limulacea). In:. Tanacredi, J. T.,., eds., Springer, Boston, MA, 115-130.
Sims, D. W., and Queiroz, N., 2016. Unlimited by-catch limits recovery., 531: 448, https://doi.org/10.1038/531448a.
Smith, D. R., Brockmann, H. J., Beekey, M. A., King, T. L., Mil- lard, M. J., and Zaldívar-Rae, J., 2017. Conservation status of the American horseshoe crab, (): A regional assessment., 27 (1): 135-175, https://doi.org/10.1007/s11160-016-9461-y.
Smith, D. R., Pooler, P. S., Swan, B. L., Michels, S. F., Hall, W. R., Himchak, P. J.,, 2002. Spatial and temporal distribu- tion of horseshoe crab () spawning in De- laware Bay: Implications for monitoring., 25 (1): 115-125, https://doi.org/10.1007/BF02696055.
Steele, P., Bert, T. M., Johnston, K. H., and Levett, S., 2002. Efficiency of bycatch reduction devices in small otter trawls used in the Florida shrimp fishery., 100 (2): 338-350.
Supadminingsih, F. N., Wahju, R. I., and Riyanto, M., 2019. Com- position of blue swimming craband horse- shoe crab Limulidae on the gillnet fishery in Mayangan waters, Subang, West Java., 12 (1): 14-24.
Temple, A. J., Kiszka, J. J., Stead, S. M., Wambiji, N., Brito, A., Poonian, C. N. S.,, 2018. Marine megafauna interactions with small-scale fisheries in the southwestern Indian Ocean: A review of status and challenges for research and management., 28: 89-115, https://doi. org/10.1007/s11160-017-9494-x.
Tian, B., Wu, W., Yang, Z., and Zhou, Y., 2016. Drivers, trends, and potential impacts of long-term coastal reclamation in Chi- na from 1985 to 2010., 170: 83-90, https://doi.org/10.1016/j.ecss.2016.01.006.
Vestbo, S., Obst, M., Fernandez, F. J. Q., Intanai, I., and Funch, P., 2018. Present and potential future distributions of Asian horseshoe crabs determine areas for conservation., 5: 164, https://doi.org/10.3389/fmars.2018. 00164.
Vihervaara, P., Auvinen, A. P., Mononen, L., T?rm?, M., Ahl-roth, P., Anttila, S.,, 2017. How essential biodiversityvariables and remote sensing can help national biodiversity mo- nitoring., 10: 43-59, https:// doi.org/10.1016/j.gecco.2017.01.007.
Wang, C. C., Kwan, K. Y., Shin, P. K. S., Cheung, S. G., Itaya, S., Iwasaki, Y.,, 2020. Future of Asian horseshoe crab conservation under explicit baseline gaps: A global perspective., 24: e01373, https://doi.org/ 10.1016/j.gecco.2020.e01373.
Weber, R. G., and Carter, D. B., 2009. Distribution and deve- lopment ofegg clusters on intertidal beaches in De- laware Bay. In:. Tanacredi, J. T.,., eds., Springer, Boston, MA, 249-266.
Weng, Z. H., Xie, Y. J., Xiao, Z. Q., Huang, L. M., Li, J., Wang, S. H.,., 2012. Distribution and resource of Chinese horse- shoe crab () in Fujian and other coastal water of China., 47 (3): 40-48,https://doi.org/10.13859/j.cjz.2012.03.004 (in Chinese withEnglish abstract).
Widener, J. W., and Barlow, R. B., 1999. Decline of a horseshoe crab population on Cape Cod., 197 (2): 300- 302, https://doi.org/10.2307/1542664.
Xie, X., Wu, Z., Wang, C. C., Fu, Y., Wang, X., Xu, P.,, 2020. Nursery habitat for Asian horseshoe crabs along the northern Beibu Gulf, China: Implications for conservation management under baseline gaps., 30 (2): 260-272, https://doi.org/10.1002/aqc. 3259.
Zauki, N. A. M., Satyanarayana, B., Fairuz-Fozi, N., Nelson, B. R., Martin, M. B., Akbar-John, B.,, 2019. Horseshoe crab bio-ecological data from Balok, East Coast Peninsular Malay- sia., 22: 458-463, https://doi.org/10.1016/j.dib. 2018.12.027.
J. Ocean Univ. China(Oceanic and Coastal Sea Research)
https://doi.org/10.1007/s11802-022-5214-9
ISSN 1672-5182, 2022 21 (3): 611-621
(September 29, 2021;
November 8, 2021;
December 14, 2021)
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
#The two authors contributed equally to this work.
Corresponding author. E-mail: kityuekwan@bbgu.edu.cn
(Edited by QiuYantao)
 Journal of Ocean University of China2022年3期
Journal of Ocean University of China2022年3期
- Journal of Ocean University of China的其它文章
- Roles of Equatorial Ocean Currents in Sustaining the Indian Ocean Dipole Peak
- Transcriptome Analysis of the Digestive Tract of Tachypleus tridentatus and Carcinoscorpius rotundicauda
- Application of Principal Component Analysis (PCA) to the Evaluation and Screening of Multiactivity Fungi
- Impact of Different Diets on Adult Tri-Spine Horseshoe Crab, Tachypleus tridentatus
- Indiscriminate Dietary Compositions of Two Asian Horseshoe Crabs, Tachypleus tridentatus and Carcinoscorpius rotundicauda:Evidence from Hemolymph Stable Isotopes
- Effect of Probiotics on Juvenile Tachypleus tridentatus Gut Microbiota
