Maternal low protein diet and fetal programming of lean type 2 diabetes
lNTRODUCTlON
Type 2 diabetes(T2D)is a metabolic disease,which is rapidly increasing among the human population both in developed as well as in developing countries.Diabetes is divided into four major categories:type1,type2,gestational,and other specific diabetes mellitus[1].As per the national diabetes statistics reports in 2020,10.5 %(34.2 million)of the United States population are diagnosed with some form of diabetes and 34.5%(88 million)of adults have pre-diabetes.T2D constitutes 90%-95% of all diabetes cases in the United States[2].The hallmarks of T2D are insulin resistance and insulin deficiency.In most cases of T2D,the etiology of insulin resistance and insulin deficiency can be traced back to obesity and lifestyle aspects.
Scientists from all over the world have spent a great deal of time and effort to understand the causes and consequences of obesity-induced T2D.However,the number of non-obese/lean T2D cases has also dramatically increased globally and especially in Asia and other developing countries.Recent estimates show that 10%-20% of T2D patients are not obese[3].Although the etiology is not clearly understood,lean T2D is clustered under the umbrella of T2D for patient care.Interestingly,clues from various studies indicate that lean T2D is often observed in the populations where the fetus is exposed to malnutrition during the intrauterine period or early childhood[3-7].Adequate birth weight and size of the newborn are often considered as indicators of appropriate fetal growth rate and optimal
environment[8-10].In contrast,deprivation of nutrition during fetal development is often marked by low birth weight and it is linked with adult-onset of metabolic diseases such as T2D[11].
He lay down where he was and fell asleep, and did not awake before it was bright daylight, and he heard steps outside, and the noise of the key being put into the lock
Historically,observational studies from the different parts of the world firmly indicate that the individuals exposed to
malnutrition due to famine were more prone to the hyperglycemic condition in adult life compared to those who are not born during a famine[12].For instance,the cohort exposed to Dutch famine(1944-45)
were more prone to glucose intolerance than the nonexposure cohort[13].Similarly,data associated with the Ukrainian famine of 1932-33 have exhibited a higher incidence of T2D among the people born in the famine-affected region than in the regions where no famine was reported[14].The link between Australian famines and T2D was studied and analysis showed a positive correlation with three years of famine and an increased number of T2D among those who were born during the famine years[12].Further,Li
[15]reported that people who were exposed to the Chinese famine in the fetal stage during 1959-1961 were more prone to hyperglycemia and T2D in their adult life,compared to those who were born after this period.
Recent studies indicate that adverse
nutrition could cause lean T2D later in life among certain ethnic and socio-economical groups,and people with certain lifestyles.Studies from India[16](up to 26%)and Caribbean islands[17,18](5%)report the predominance of lean T2D population.A study on American minorities showed that 13% of T2D patients are lean[4,16]with a fivefold higher incidence in Asians[4].These observations clearly show that not all diabetics are obese and obesity does not necessarily cause T2D[3,16,19].With > 42 million Americans experiencing food insecurity,it is a major problem even in the United States especially among the economically disadvantaged[20].WHO estimates that 1.1 million children had ≤ 2 standard deviations for weight for height ratio(an index of protein-energy malnutrition)in the United States[21].A recent German study shows that 38% of pregnant women did not consume enough protein[22].With vegetarian and vegan diets gaining popularity worldwide,low protein intake is more prevalent as these diets are often low in protein[23,24].Vegetarian mothers consume low protein diet[25,26]and give birth to children with lower birth weights,thus making them susceptible to T2D[26-28].
This atypical diabetic phenotype is known by various names such as Jamaica type diabetes,metabolically obese normal weight(MONW)diabetes,malnutrition-related diabetes mellitus,phasic insulindependent diabetes,tropical diabetes,mixed onset type diabetes,J,Z,M or type 3 diabetes,and ketosis resistant growth onset type diabetes[3,17,18,29-33].This concept of MONW individuals was first proposed in 1981[29]with subsequent validations in animal and human studies[34-37]and the existence of lean T2D has been observed for decades but the etiology and pathophysiology of lean T2D are poorly understood.
The
environment is crucial in the development of skeletal muscles,and the muscles growth is determined by the number,size,and type of muscle fibers formed during fetal development[130,131].Maternal undernutrition affects the quality and quantity of skeletal muscles and stem cell activity[132-134].A maternal low protein diet during gestation affects the normal proliferation and differentiation of bone marrow stem cells and satellite cell function[134,135].Therefore,imperfections of skeletal muscles development during fetal development are often deleterious to normal muscle functions in adulthood[133].Studies using maternal low protein diet-based animal models have reported lower expression of GLUT4 and mitochondrial dysfunction in skeletal muscles of low protein offspring[66,67,136-138].As skeletal muscle functions as one of the main sites for peripheral glucose disposal,functional or structural changes of the myofibrils leads to insulin resistance and glucose intolerance[139].
The most accepted and validated hypothesis that explains the link between early nutrition and metabolic diseases in adulthood was proposed by David Barker is called,‘thrifty phenotype hypothesis’[11,38].This hypothesis explains how impaired
nutrition availability results in compromised fetal growth and programs subcellular and metabolic effects in the developing fetus.Further,the hypothesis suggests that the metabolic fate of an individual predisposed to T2D is decided at the early developmental stage and thus attempts to explain why a sub-population of individuals born with low birth weight are more prone to lean T2D compare to normal birth weight individuals[11].Various subsequent studies have confirmed the reproducibility and epidemiological evidence for the 'thrifty phenotype hypothesis'[39].
43. A keen sense of smell: The giant in Jack and the Beanstalk is another popular fairy tale villain with a keen sense of smell.Return to place in story.
“You have already rewarded me,” said the nightingale. “I shall never forget that I drew tears from your eyes the first time I sang to you. These are the jewels that rejoice a singer’s heart. But now sleep, and grow strong and well again. I will sing to you again.”
In addition,studies based on this hypothesis showed the importance of a sufficient amount of protein in the maternal diet for the development of the fetus and the risk of diseases in adulthood[40].Although overall well-balanced nutrition is essential for a developing fetus and a healthy offspring,the role of protein is vital in the developmental programming paradigm[41].A low protein diet is well known to cause various programming effects leading to metabolic disorders in adulthood.Low protein or vegetarian diets are consumed due to various reasons such as poverty,famine,lack of availability,cultural,religious,or moral reasons,personal preference,
Although these are common in the developing world,the recent popularity of vegetarian and vegan diets in the developed world is also an important paradigm to be considered.The emerging popularity of vegetarian and vegan diets among the maternal population might compromise growing fetuses,as the amount and bio-availability of proteins are found to be inadequate from plant sources[23,42].A low protein diet during gestation is often connected with compromised renal function and impaired glucose metabolism[7].However,the mechanistic basis and the exact patient phenotype of the maternal low protein associated with lean T2D are not well understood.Therefore,a clear understanding of the epidemiological and clinical features of lean T2D is essential to the prevention or treatment strategies.
PATHOPHYSlOLOGY OF LEAN T2D
T2D is a complex metabolic disease with a spectrum of presentations.It is therefore essential to understand the pathophysiology of the disease to offer appropriate prevention and treatment strategies.The pathophysiology of lean T2D is not well defined,although we and others have considered them as a separate subset of T2D[3,43,44]body mass index(BMI)is widely used as a tool to classify T2D patients.Patients with a BMI greater than 25 are considered to have obese T2D[45].In contrast,the majority of lean T2D patients have a BMI of less than 25 but they have several metabolic characteristics associated with obesity[3].Observational studies in humans and experiments in rodents suggested that the various environmental and genetic factors could contribute towards the lean T2D phenotype[3,46].Poor
nutrition during fetal development is considered to be the main driver of lean T2D onset[44,46].We have shown using a novel rat model that the maternal low protein diet is one of the critical causes of the lean T2D phenotype[43].
Although the genetic factors may vary among the different populations,genetic predisposition to fragile beta cells was found to be common in lean T2D patients.The rapid beta-cell apoptosis is the major pathophysiological characteristic of lean T2D compared to elevated insulin resistance in obese T2D[47].Another interesting aspect that is noticed in this population is the prevalence of truncal obesity.Insulin sensitivity and insulin response are varied among the different ethnic groups(African,Caucasian,and East Asian),and East Asians have more vulnerable beta cells which make them more prone to T2D[48].Several studies on the South East Asian population have shown that lean T2D patients have central obesity or elevated visceral fat deposits[49].Even though lean T2D patients have lesser hyperglycemic values,their hemoglobin A1c levels are significantly higher than their obese counterparts[5,44].Further,the onset of lean T2D is reported at an early age than the obesity-associated T2D[3].Lean T2D patients showed a significantly lower incidence of hypertension and cardiovascular diseases compare to obese T2D patients but are more susceptible to peripheral neuropathy[3,5].Apart from environmental and hereditary factors,socioeconomic background is found to be an important aspect of lean T2D prevalence[10,50].Several studies have reported an inverse relationship between T2D and socioeconomic status[51].The National Health and Nutrition Examination Survey data indicated this relationship of poverty and higher incidence of T2D among African and Mexican origins in the United States[52].The Chicago cohort study further showed the prevalence of lean T2D among this minority community[4].
But the little grey dwarf began now to demand his beard again from the youth, for in his wicked heart he was determined to make an end of all their happiness; he knew that if only his beard were once more on his chin, he would be able to do what he liked with them all
The two crucial characteristic features of developing countries,which make them more vulnerable to lean T2D,are a rapid shift in lifestyle and impaired nutrition.Studies from India have reported the escalating number of lean T2D cases across the country,especially,the urban population of India[53-55].Similarly,Alemu and the group reported the increased number of lean T2D like cases in the urban population of sub-Saharan Africa[56].
GESTATlONAL LOW PROTElN PROGRAMMlNG AND SEX DlFFERENCES lN LEAN T2D
Sex differences in fetal development can be observed as early as the pre-implantation phase[57].There are major
differences between the sexes in growth and metabolic parameters leading to a faster fetal growth in males when compared to females.These differences are attributed to the genes expressed by sex-chromosomes and the actions of sex hormones[57-59].In addition,differences in the incidence of T2D can also be attributed to the differences in the leptin and insulin sensitivity between sexes[59,60].These metabolic hormones are influenced by the
nutritional environment[61].Many studies have found a link between T2D and maternal low protein diet[43,59,62,63].Further,as the nutritional environment often regulates the epigenetic machinery,any change
nutritional status may cause permanent alterations in the fetal gene expressions[64].
And in his heart were hidden chords, which might havesounded far out into the world if he had been placed anywhere elsethan in the fisherman s hut by the North Sea
Our research using a lean T2D rat model indicates a clear sex difference in glucose homeostasis with females developing glucose intolerance earlier in life with faster disease progression than males[43,65].These animals also showed differential regulation of gluconeogenesis and glycogenolysis as a result of gene expression changes in key genes involved in glucose metabolism[65-68].Sex difference in hepatic genes associated with glucose homeostasis such as phosphoenolpyruvate carboxykinase(PEPCK)and 11β-hydroxysteroid dehydrogenase type 1 were observed even in low protein programmed fetuses[69].Similarly,low protein programmed mice offspring were found to have lower birth weight with more glucose intolerant than the controls[70].In this study,maternal low protein diet activated the visceral adipose tissue neuropeptide Y-Y2 receptor system in female offspring but not in male offspring,which increased abdominal adiposity and insulin resistance in female offspring[70].This study indicates the importance of neuropeptide Y-Y2 receptor as a potential sex-specific marker and mediator of metabolic programming[70].
In the last decade,various studies have shown the importance of mitochondrial health and its relationship with glucose homeostasis in low protein programming.Zambrano and group have found maternal low protein diet-induced insulin resistance in male Wistar rats;however,females were responsive to glucose[71].This study,further,suggested that elevated mitochondrial dysfunction in the pancreatic islets of adult male rats might be the mechanism that leads to insulin resistance[71].Likewise,male offspring of low protein diet-fed mothers showed higher ROS production and impaired electron transport chain function in the mitochondria of the pancreatic islets when compared to female offspring indicating mitochondrial incompetence in males could predispose them to T2D[72].Similarly,the sex dependent fetal programming in glucose metabolism was also reported
low protein programmed piglets.In this study,hepatic gluconeogenesis in newborn male piglets was negatively affected by the maternal low protein diet during pregnancy[73].Epigenetic changes in the promoter region of the glucose-6-phosphatase gene were sex-specific and resulted in T2D in adult male pigs[73].In addition,maternal low protein diet diminished liver mtDNA copy number in males and altered the OXPHOS protein expression by the combined binding action of glucocorticoid receptor and methylation of on the hepatic mtDNA promoter,which effect the mtDNA replication and gene expression levels[73,74].
Studies in humans suggest greater prevalence and impact of lean T2D in males than females.Many studies have indicated that women are physiologically inclined to have better insulin sensitivity than men[75-77].Estrogen has a protective role in insulin sensitivity and glucose homeostasis by the inhibition of Foxo1 though activation of ERα-PI3K-Akt signaling[78].Another crucial way estrogen protects women from insulin resistance is through mitochondrial biogenesis,as testosterone reduce mitochondrial proliferation[79].The male preponderance of lean T2D was evident from the studies conducted in India,where more than 60% of lean T2D patients were men.Although the exact causes of sex differences are not clearly understood,it is suggested that the differences observed in this study may be due to predominant male exposure to oxidative stressors such as smoking and alcoholism[3,80].
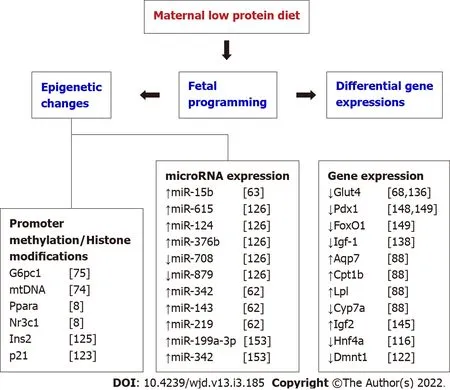
Several studies have reported various key genes that are epigenetically modified as a result of developmental programming.For instance,the transcription factor Hnf4a was found to be epigenetically regulated during gestation,and the maternal diet-induced changes in the expression of this gene can cause T2D in adulthood[116].Similarly,glucose transporter 4(GLUT4)expression in skeletal is epigenetically controlled by maternal diet during early development and the impaired gene expression often resulted in peripheral insulin resistance[117].Even though different biological mechanisms might contribute to fetal programming of lean T2D,many recent studies are indicating epigenetic changes as a potential single important driver of the fetal programming effects[118].Low protein diet exposure during pregnancy in animals exhibited changes in methylation in promoter regions of genes involved in the glucose homeostasis pathway thereby,affecting the gene expression either directly or indirectly[119].In recent years,many experimental studies in animals and observational studies in humans show that the epigenetic changes associated with gestational low protein are the main regulatory forces mediating the T2D phenotype[118,120].Changes in the fetal epigenome often mirror the unique
environment of the fetus.Epigenetic changes due to gestational low protein arise through the methylation of cytosine in CpG Island present in the promoter region of particular genes,histone protein modification by acetylation,and regulation microRNAs by post-transcriptional modification.The chromatin structure and expression of a specific gene are regulated through DNA methylation in association with histone modifications[121].
ANlMAL MODELS
Even though little is known about the mechanism of programming,the secondary effects of fetal programming and their mechanisms are well studied.For example,various organ systems that play vital roles in the metabolism,and how they are affected by the developmental programming of T2D are well characterized.
low protein exposure causes long-lasting structural and functional changes in metabolically active organs includes skeletal muscle,liver,pancreas,gonads,and brain.
Here came the suitors numbered according to their arrival, and they were ranged in rows, six in each row, and they were so tightly packed that they could not move their arms
To achieve a low protein diet model,the majority of studies followed a diet that has around a 50%reduction of total protein in the diet formulation[43,63,85-87].However,most of the investigations conserved the isocaloric nature of the diet by manipulating macronutrient proportions by various lipid and carbohydrate ratios[88-93].Although preferred protein,carbohydrate,and lipid ratio are varied among different research groups;a single research group often stick to one specific diet regimen[67,91,94-97].The other central deviation apparent among the different low protein models is the timing and duration of the maternal diet management.Majority of the studies have started giving low protein diet from the first day of the pregnancy and continued throughout pregnancy or lactation,although some studies initiated the low protein diet before pregnancy or in some cases in a specific period of
growth[65,86,98-102].The main aim of these refined diet manipulations is to develop a metabolically compromised adult offspring[103].Moreover,many studies have succeeded in mirroring low birthweight and catch-up growth pattern,which is considered by many as a hallmark of the developmental origin of metabolic disease[104-108].Pups from maternal low protein mothers weigh less compared to those from control diet-fed mothers.The differences in birth weight disappeared once the mothers were fed with a normal diet or pups were cross-fostered with control mothers.However,the weight differences were permanent,when the maternal low protein diet was continued throughout the weaning[43,100,109].In addition,due to the variation in macronutrients ratio and the time regime of the diet,the adult metabolic phenotypes reported by various groups are also varied.Insulin resistance,obesity,cardiovascular diseases,and dyslipidemia are the major clinical disorders observed in these models[104,110-113].A comprehensive list of different low protein programming animal models used are summarized in Table 1.
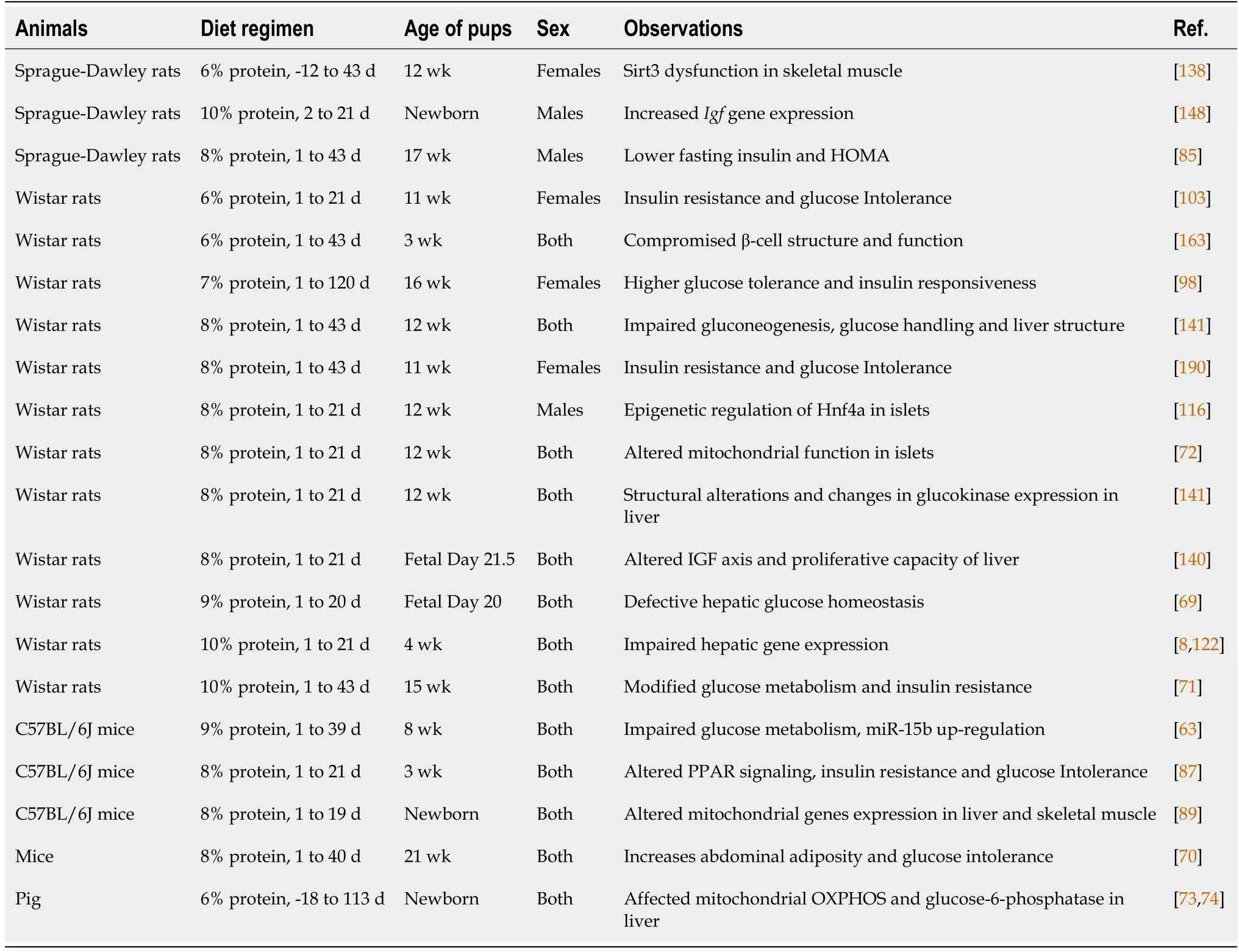
PHYSlOLOGlCAL EFFECTS AND MECHANlSMS
Many animal models based on a low protein diet have been successful in capturing the phenotypic characteristics of fetal programming of adult metabolic diseases.However,the exact mechanism that leads to these metabolic diseases is not well studied.The dominant hypothesis in the field of developmental programming of adult diseases attribute that the fetal epigenome play a central role.This hypothesis postulates that epigenome is reprogrammed as an adaptation in response to a low protein diet,the associated low birth weight,and the catch-up growth.A recent study in Japanese adults indicates that the reduced beta cell mass in low-birth-weight individuals is directly associated with the future development of T2D[114].Although the epigenome is prone to modification throughout the lifetime,
developmental period was found to be the most vulnerable time to be dysregulated by stressors[115].
Another interesting aspect to consider is the role of folate.Folate is routinely given to pregnant women throughout the world to prevent neural tube defect.Recent studies show that excessive folate can also have negative consequences at least in certain populations,ages and ethnicity/genetic background[81-83].One study in Indian population shows that it can lead to insulin resistance[7].The authors primarily attribute this to the deficiency of vitamin B12 which is primarily present in animal protein.Rat studies from our group showed that folate offered some protection in low protein programmed offspring by compensatory hyperinsulinemia but make insulin resistance worse in males[84].Although the mechanism of this sex dependent folate action in insulin resistance is not known and warrant further research,it is important to note how folate may have sex dependent effects and this may also hold a clue in the sex differences that are observed in the human population.
A study in pigs found a significant decrease in glucocorticoid receptor binding to the glucose-6-phosphatase(G6PC)promoter which was accompanied by hypomethylation of the G6PC promoter in association with gestational low protein diet[74].As G6PC is one of the crucial enzymes in glucose homeostasis that catalyzes gluconeogenesis and glycogenolysis,epigenetic changes in the promoter region might contribute to the onset of hyperglycemia[74].Further,this impaired maternal diet-induced reduction of mtDNA copy number and methylation of mtDNA promoter often leads to changes in OXPHOS gene expression.This may predispose to insulin resistance in adult offspring considering the importance of hepatic mitochondrial OXPHOS activity in glucose homeostasis[73].
Similarly,using maternal low protein programmed rats,Lillycrop
[8]established that the hepatic PPARα promoter and glucocorticoid receptors were hypomethylated
and these epigeneticchanges were persistent in adulthood.Further studies demonstrated reduced Dnmt-1 expression and its role in epigenetic changes of glucocorticoid receptors[73,95,96,122].Moreover,epigenetic changes in the promoter region of PEPCK were found to be the drawing force for impaired glucose homeostasis in animals[123,124].Anandwardhan and colleagues reported a decreased number of(pro)insulin 2 gene transcripts in the pancreas of low protein
programmed rats,due to the histone modification in the promoter region of the insulin 2 gene[125].Moreover,these epigenetic changes are potentially engaged in the trans-generational transmission of the induced phenotype[122,124,125].
Recently,Goyal and group demonstrated that the epigenetic modifications by miRNA,small noncoding RNAs consists of 20-22 nucleotides,is one of the molecular mechanisms of maternal low protein-induced T2D[119].Results from maternal low protein programmed mice found reduced betacell mass and insulin levels in the pancreatic islets of the programmed offspring due to the increased expression of miR-15b.As the activities of cyclins are negatively regulated by the presence of miR-15b,the up-regulation of this miRNA may inhibit pancreatic beta-cell proliferation,consequently,stem to T2D phenotype[63].A microarray study also demonstrated elevated expression of miR-615,miR-124,miR-376b,and decreased expression of miR-708 and miR-879 in maternal low protein programmed mice,which were associated with degenerated metabolic health of the offspring from the weaning age[126].
The ability of the pancreatic β-cell to secret insulin is dependent on its structural and functional integrity along with the nutritional availability[150].Consequently,protein deficiency in the maternal diet is a definite contributor to reduced insulin secretion and decreased β-cell proliferation in low protein programmed animals[151].The reduced islets area and β cell number are mainly due to the downregulation of genes
and
genes,or altered expression of Reg1 pathway genes[151-155].Epigenetic regulation of Hnf4a expression and expression of microRNAs such as miR-15b,miR-199a-3p,and miR 342,and signaling of mTOR in islets of the progeny also found to be associated with low protein-induced beta-cell dysfunction[62,63,156].Further,a maternal low protein diet demonstrated greater β-cell apoptosis rates and deviates the equilibrium of islet’s apoptosis and replication in the offspring[157-159].The pancreatic islet cells of these offspring exhibited greater oxidative stress and mitochondrial dysfunction[72,160].Consequently,lower β-cell reserve,β-cell dysfunction and impaired mitochondrial function in islets may drive towards T2D later in life[62,72,86,155].With the multiple pathways controlling β-cell functions are modulated by maternal low protein,it is reasonable to hypothesize that the low protein exposure predisposed the offspring to lean T2D[127].A list of key genes involved in low protein programming is compiled in Figure 1.
Considering the ethical and technical limitations in conducting impaired maternal nutrition and developmental programming studies in humans,various animal models that mimics several aspects of developmental programming have been developed.Due to the shorter lifetime and availability of genetic tools,a substantial amount of research is presently focused on developing clinically reliable rodent models of developmental programming.
In spite of his weariness the howling of the wolves kept him awake, and even when at last the day broke he was not much better off, for the falling snow had covered up every path, and he did not know which way to turn
Similarly,low protein-induced developmental programming caused functional and structural changes in the liver[140,141].The expression of genes associated with oxidative phosphorylation and glucose metabolism were altered in the liver.Further,
low protein exposed rat fetuses showed the altered structure of the liver with decreased proliferation of hepatocytes[142-146].These animals also had altered hepatic lipid metabolism and hepatic desaturase activities,which may account for fetal growth retardation and insulin resistance[94,147].In addition,a maternal low protein diet also induces epigenetic changes in methyltransferase machinery resulting in altered epigenetic regulation in the liver[148].Although further studies are warranted,it is clear from the existing studies that developmental programming induced by a low protein diet affects hepatic structure and function and this may,in turn,make them susceptible to impaired glucose metabolism[141,145,149].
Now the Princess happened to walk that way; and when she heard the tune13, shestood quite still, and seemed pleased; for she could play Lieber Augustine ;it was the only piece she knew; and she played it with one finger.
Apart from the epigenetic changes,maternal malnutrition is the major reason for low birth weight in newborns.Children who are small for gestation age and showed catch-up growth during the early age of development appeared to be more insulin resistant compared with normal-weight children[127].Moreover,several studies have shown epigenetic changes due to gestational diet-induced fetal programming adult diseases in these offspring[108,128,129].
A balanced
nutrition is essential for the normal development of the reproductive system.Epidemiological studies in humans and experimental in studies animals show the low protein/unbalanced diet
severely impacts the development of reproductive organs,sexual maturation,and reproductive function in the offspring[161-164],resulting in decreased testis weight,reduced Sertoli cell numbers,and late-onset of spermatogenesis in males[164-166].Moreover,the classical male fertility markers,sperm count,and serum testosterone were also diminished in the offspring of low protein exposed mothers[163,164,167].Similarly,the low protein programmed female offspring were found to be with compromised follicle development and follicle health[168,169].The numbers of primordial,primary,and secondary follicles were significantly reduced along with abnormal estrous cycle and redox homeostasis[170-172].The thyroid hormone production and hypothalamic-pituitary-gonadal axis are also found to be affected by maternal low protein diet[173,174].The impaired reproductive function of offspring may be due to the altered expression of genes associated with steroidogenesis,folliculogenesis,and steroid hormone receptors in gonads[175-178].In addition,changes in the hypothalamicpituitary-gonadal axis to low protein may have adverse effects on the normal development and function of gonads[168,179].
The hypothalamus of the brain plays a critical role in glucose homeostasis by controlling hepatic glucose production and peripheral glucose utilization.Therefore,functional,or structural alteration of hypothalamic neurons may often lead to the onset of T2D[180,181].The low protein programmed progenies have exhibited structural and functional changes in the neuronal centers,hypothalamic nuclei which regulate metabolism and body weight[102,181].In addition,maternal low protein can also differentially affect the hypothalamic-pituitary-gonadal axis depending on the timing of the impaired nutrition.Early gestational nutrition impairment has been shown to make the pituitary more sensitive to GnRH,resulting in reduced reproductive function[182].Further,it also alters hypothalamic-pituitaryadrenal axis function by deregulating corticosterone-inducible enzymes and associated enzyme receptors[183].Other reports also show that brain sparing may not be as effective during
low protein exposure leading to compromised brain development in the offspring along with long-lasting deterioration in cognitive and motor functions[184,185].
PREVENTlON AND TREATMENT
Insulin resistance and glucose intolerance are the cardinal signs of T2D,and if not prevented,they may lead to severe diabetes complications later in life.Hepatocytes,skeletal muscles,and adipocytes are the major insulin-dependent tissues that participate in the disposal of peripheral glucose.Thus,improving the muscle sensitivity towards insulin and enhancing hepatic glucose homeostasis,along with managing body weight are the central focus of T2D treatment strategies.Among the different drugs that have been prescribed for leanT2D management,metformin is a widely used drug for treating lean T2D along with nutritional and lifestyle modification[186].Over the past two decades,various randomized control trials conducted in many ethnic groups showed unambiguously that the prevention is feasible by drugs or lifestyle modification[187-190].
32.Faithful Falada was doomed to die: Falada, the dear horse, is doomed to die from the princess inability to assert herself or use her imagination. Her request to have Falada s head nailed above the gate shows little imagination. She uses her gold to keep the head nearby, not to spare the horse s life. Still, even this bribe86 and saving of Falada s head shows the most initiative she has had in the story so far. Return to place in story.
Most of the research on treatment or prevention of T2D has been done with obese individuals or animal models,even though 10%-16% of all T2D people have normal BMI.In addition,majority of the studies on the molecular mechanisms of prevention and reversal of T2D were performed in Caucasians.Consequently,it is essential to include other ethnic groups such as Southeast Asian and Chinese populations,which are more prone to diabetes at lower average BMIs or lean T2D compared with white Europeans[191].Regulating body weight is critical in the management of T2D associated with overweight or obese patients.However,in the case of leanT2D,it seems that leaner patients have severe beta-cell failure than normal-weight patients[4].Presently,it is not clear that the achievement of lower body weight will help to prevent or reverse the special variants of T2D such as lean T2D[3].
Although the underlying pathophysiology of lean T2D is not completely understood,many studies using the maternal low protein model have shown potential prevention approaches[157].The most promising approach among them is associated with one-carbon metabolism and the molecules involved in it.Some studies have reported the effectiveness of folic acid supplementation as a preventive treatment against the adverse effects of fetal programming[198-200].Similarly,Burdge and team reported that the folic acid supplementation reversed the maternal low protein-induced phenotype epigenetically in the offspring when treated during the juvenile-pubertal period[201].In contrast,our study reported a partial inhibition of gestational low protein-induced glucose intolerance only in female rats,when the maternal low protein diet was supplemented with folic acid from day 4 of the pregnancy until delivery[65].Similar to our data,Lillycrop and colleagues also reported the inefficiency of folic acid supplementation for the inhibition of gestational low protein-induced change in gene profile,although they found changes in the expression of genes associated with redox homeostasis[8].An observational study from Pune,India(Pune Maternal Nutrition Study),noticed that when the mother was vitamin B12 deficient,high amount of folic acid intake was not enough to prevent the insulin resistance in the offspring[7].However,the high protein to carbohydrate ratio in maternal diet was found to be effective in maintaining glucose homeostasis in the offspring[137].Thus ensuring sufficient protein in the maternal diet is essential to prevent lean T2D.
1872FAIRY TALES OF HANS CHRISTIAN1 ANDERSENDELAYING IS NOT FORGETTINGby Hans Christian AndersenTHERE was an old mansion2 surrounded by a marshy3 ditch with adrawbridge which was but seldom let down:- not all guests are goodpeople. Under the roof were loopholes to shoot through, and to pourdown boiling water or even molten lead on the enemy, should heapproach. Inside the house the rooms were very high and had ceilingsof beams, and that was very useful considering the great deal of smoke which rose up from the chimney fire where the large, damp logs of wood smouldered. On the walls hung pictures of knights4 in armour5 and proud ladies in gorgeous dresses; the most stately of all walked about alive. She was called Meta Mogen; she was the mistress of the house, to her belonged the castle.
The first trial associated with lifestyle modification and/drug therapy was started in China with a follow-up period of 23 years[192]and many other studies have followed since.Other studies include:the American diabetes prevention program outcome study[193];the Finnish diabetes prevention study[194];and the Indian short message service study[195]revealed the influence of lifestyle modification can persist long after the termination of the active phase of the trial.Although lifestyle modifications have been recognized to be very effective,safe,and ideal strategy for prevention,the effectiveness of relative risk reduction through these strategies exhibited some variations among different ethnic populations[192-196].A study conducted among the impaired insulin tolerant lean Indian population found that lifestyle modification alone prevented the diabetes onset,regardless of relatively low BMI and highly insulin-resistant characteristics of the population[197].
CONCLUSlON
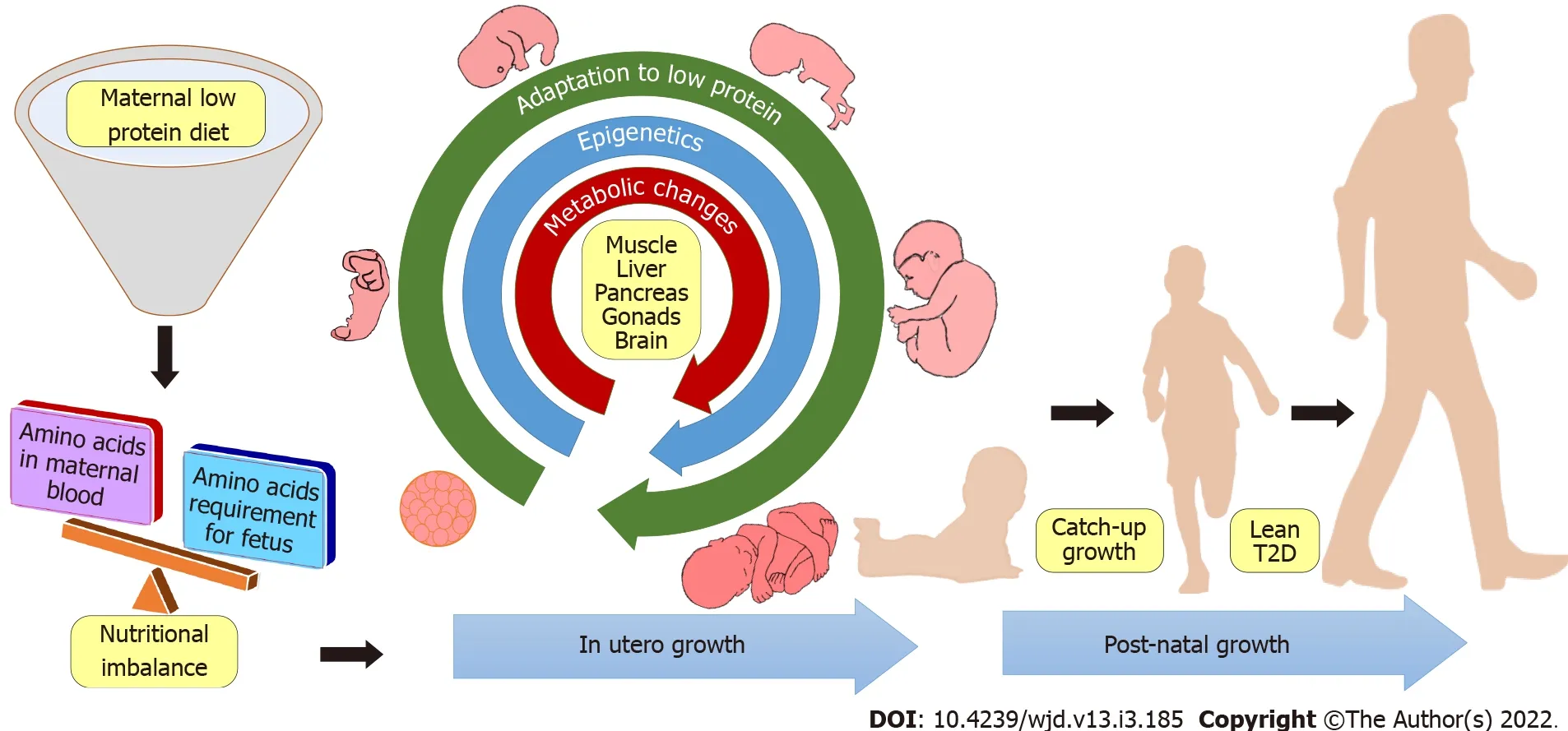
In summary,lean T2D is a discrete subgroup of T2D with a set of specific clinical profiles.Atypical characteristics of leanness associated with insulin resistance needed to be dissected further for a better understanding of the etiology of the disease.As the progression of T2D may take many years in humans,the assessment and prevention studies with human subjects may also warrant many years.Therefore,the development of a well-defined animal model,which mirrors not only the pathophysiology of lean T2D but also the etiology of the disease,might be the most important step in this area of research.Nevertheless,there is a lack of a single animal model that can constitute all pathophysiological and etiological changes similar to humans.In addition,the severity of lean T2D is different between sexes,due to sex hormones and sex dependent expression of genes.Among different molecular mechanisms involved in the onset of lean T2D,the epigenetic underpinning of metabolism appears to be the most promising lead.Although the mechanism of developmental programming is currently not well characterized.With the current literature,it may be summarized that maternal low protein diet leads to diminished essential amino acids levels in the maternal circulation and consequently to the fetus.In such low protein environment,fetus is acclimatized and revises its growth and metabolic set points.This adaptation is thought to be due to the overall alteration of epigenetic and metabolic attributes of fetal energy homeostasis.Although these adaptations may be beneficial for the fetus,a nutritional mismatch with protein abundance in the adulthood often leads to metabolic derangements leading to diseases such as lean T2D.This concept is summarized in Figure 2.A better understanding of the molecular mechanisms of the disease may pave the way for more effective preventive and treatment strategies.
Obesity associated T2D is a serious public health problem throughout the developing and developed countries whereas nutritional deficiency especially protein deficiency is a major concern in under developed and developing countries.With studies showing a link between maternal protein consumption and T2D in offspring,it is essential to probe further and take action to avert a global crisis.Public health measures to alleviate poverty and access to nutritious and protein rich diet during pregnancy is essential to prevent lean T2D.Scientific understanding of the disease to prevent and treat T2D,along with effective health education and public policies can mitigate this growing global epidemic.
Last year a lady in the north of England was astonished to receive an extremely surprising Valentine s present from her husband of 28 years, a live donkey.
FOOTNOTES
Author Contribution:Vipin VA,Yallampalli C and Blesson CS had substantial contributions to conception,synthesis and survey of literature,and contributed to draft the manuscript.
the National Institutes of Health Grants,No.HL102866,HL58144 and DK114689.
Authors have no conflict of interest.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
The flakes8 of snow covered her long fair hair, which fell in beautiful curls around her neck; but of that, of course, she never once now thought. From all the windows the candles were gleaming, and it smelt9 so deliciously of roast goose, for you know it was New Year s Eve; yes, of that she thought.
United States
Vidyadharan Alukkal Vipin 0000-0002-1527-7753;Chandra Yallampalli 0000-0003-4873-8314;Chellakkan Selvanesan Blesson 0000-0003-0476-4457.
Zhang H
A
Zhang H
1 Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.
1997;20:1183-1197[PMID:9203460 DOI:10.2337/diacare.20.7.1183]
2 National diabetes statistics report,2020.[cited 28 Sep 2021]Available from:https://www.cdc.gov/diabetes/data/statisticsreport/index.html
3 George AM,Jacob AG,Fogelfeld L.Lean diabetes mellitus:An emerging entity in the era of obesity.
2015;6:613-620[PMID:25987958 DOI:10.4239/wjd.v6.i4.613]
4 Coleman NJ,Miernik J,Philipson L,Fogelfeld L.Lean versus obese diabetes mellitus patients in the United States minority population.
2014;28:500-505[PMID:24581791 DOI:10.1016/j.jdiacomp.2013.11.010]
5 Das S,Fonseca V.Low bodyweight type 2 diabetes in India:clinical characteristics and pathophysiology.
2009;3:60-66[DOI:10.1016/j.dsx.2009.01.001]
6 Kong X,Xing X,Hong J,Zhang X,Yang W.Genetic variants associated with lean and obese type 2 diabetes in a Han Chinese population:A case-control study.
2016;95:e3841[PMID:27281091 DOI:10.1097/MD.0000000000003841]
7 Yajnik CS,Deshpande SS,Jackson AA,Refsum H,Rao S,Fisher DJ,Bhat DS,Naik SS,Coyaji KJ,Joglekar CV,Joshi N,Lubree HG,Deshpande VU,Rege SS,Fall CH.Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring:the Pune Maternal Nutrition Study.
2008;51:29-38[PMID:17851649 DOI:10.1007/s00125-007-0793-y]
8 Lillycrop KA,Rodford J,Garratt ES,Slater-Jefferies JL,Godfrey KM,Gluckman PD,Hanson MA,Burdge GC.Maternal protein restriction with or without folic acid supplementation during pregnancy alters the hepatic transcriptome in adult male rats.
2010;103:1711-1719[PMID:20211039 DOI:10.1017/S0007114509993795]
9 Negrato CA,Gomes MB.Low birth weight:causes and consequences.
2013;5:49[PMID:24128325 DOI:10.1186/1758-5996-5-49]
10 Thomas N,Grunnet LG,Poulsen P,Christopher S,Spurgeon R,Inbakumari M,Livingstone R,Alex R,Mohan VR,Antonisamy B,Geethanjali FS,Karol R,Vaag A,Bygbjerg IC.Born with low birth weight in rural Southern India:what are the metabolic consequences 20 years later?
2012;166:647-655[PMID:22250073 DOI:10.1530/EJE-11-0870]
11 Hales CN,Barker DJ.Type 2(non-insulin-dependent)diabetes mellitus:the thrifty phenotype hypothesis.
1992;35:595-601[PMID:1644236 DOI:10.1007/BF00400248]
12 de Rooij SR,Roseboom TJ,Painter RC.Famines in the last 100 years:implications for diabetes.
2014;14:536[PMID:25173690 DOI:10.1007/s11892-014-0536-7]
13 Roseboom TJ,Van Der Meulen JH,Ravelli AC,Osmond C,Barker DJ,Bleker OP.Perceived health of adults after prenatal exposure to the Dutch famine.
2003;17:391-397[PMID:14629322 DOI:10.1046/j.1365-3016.2003.00516.x]
14 Lumey LH,Khalangot MD,Vaiserman AM.Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33:a retrospective cohort study.
2015;3:787-794[PMID:26342852 DOI:10.1016/S2213-8587(15)00279-X]
15 Li Y,He Y,Qi L,Jaddoe VW,Feskens EJ,Yang X,Ma G,Hu FB.Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood.
2010;59:2400-2406[PMID:20622161 DOI:10.2337/db10-0385]
16 Barma PD,Ranabir S,Prasad L,Singh TP.Clinical and biochemical profile of lean type 2 diabetes mellitus.
2011;15:S40-S43[PMID:21847453 DOI:10.4103/2230-8210.83061]
17 Hugh-Jones P.Diabetes in Jamaica.
1955;269:891-897[PMID:13264638 DOI:10.1016/s0140-6736(55)92530-7]
18 Diabetes mellitus.Report of a WHO Study Group.
1985;727:1-113[PMID:3934850]
19 Perry JR,Voight BF,Yengo L,Amin N,Dupuis J,Ganser M,Grallert H,Navarro P,Li M,Qi L,Steinthorsdottir V,Scott RA,Almgren P,Arking DE,Aulchenko Y,Balkau B,Benediktsson R,Bergman RN,Boerwinkle E,Bonnycastle L,Burtt NP,Campbell H,Charpentier G,Collins FS,Gieger C,Green T,Hadjadj S,Hattersley AT,Herder C,Hofman A,Johnson AD,Kottgen A,Kraft P,Labrune Y,Langenberg C,Manning AK,Mohlke KL,Morris AP,Oostra B,Pankow J,Petersen AK,Pramstaller PP,Prokopenko I,Rathmann W,Rayner W,Roden M,Rudan I,Rybin D,Scott LJ,Sigurdsson G,Sladek R,Thorleifsson G,Thorsteinsdottir U,Tuomilehto J,Uitterlinden AG,Vivequin S,Weedon MN,Wright AF;MAGIC;DIAGRAM Consortium;GIANT Consortium,Hu FB,Illig T,Kao L,Meigs JB,Wilson JF,Stefansson K,van Duijn C,Altschuler D,Morris AD,Boehnke M,McCarthy MI,Froguel P,Palmer CN,Wareham NJ,Groop L,Frayling TM,Cauchi S.Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases.
2012;8:e1002741[PMID:22693455 DOI:10.1371/journal.pgen.1002741]
20 Coleman-Jensen A,Rabbitt M,Gregory C,Singh A.Household food security in the United States,2015.
2016;44[DOI:10.2139/ssrn.2504067]
21 Nutrition Landscape Information System(NLiS).[cited 28 Sep 2021].Available from:https://www.who.int/teams/nutrition-and-food-safety/databases/nutrition-landscape-information-system
22 Diemert A,Lezius S,Pagenkemper M,Hansen G,Drozdowska A,Hecher K,Arck P,Zyriax BC.Maternal nutrition,inadequate gestational weight gain and birth weight:results from a prospective birth cohort.
2016;16:224[PMID:27528213 DOI:10.1186/s12884-016-1012-y]
23 McEvoy CT,Temple N,Woodside JV.Vegetarian diets,low-meat diets and health:a review.
2012;15:2287-2294[PMID:22717188 DOI:10.1017/S1368980012000936]
24 Clarys P,Deliens T,Huybrechts I,Deriemaeker P,Vanaelst B,De Keyzer W,Hebbelinck M,Mullie P.Comparison of nutritional quality of the vegan,vegetarian,semi-vegetarian,pesco-vegetarian and omnivorous diet.
2014;6:1318-1332[PMID:24667136 DOI:10.3390/nu6031318]
25 Ward RJ,Abraham R,McFadyen IR,Haines AD,North WR,Patel M,Bhatt RV.Assessment of trace metal intake and status in a Gujerati pregnant Asian population and their influence on the outcome of pregnancy.
1988;95:676-682[PMID:3415934 DOI:10.1111/j.1471-0528.1988.tb06529.x]
26 Piccoli GB,Clari R,Vigotti FN,Leone F,Attini R,Cabiddu G,Mauro G,Castelluccia N,Colombi N,Capizzi I,Pani A,Todros T,Avagnina P.Vegan-vegetarian diets in pregnancy:danger or panacea?
2015;122:623-633[PMID:25600902 DOI:10.1111/1471-0528.13280]
27 Reddy S,Sanders TA,Obeid O.The influence of maternal vegetarian diet on essential fatty acid status of the newborn.
1994;48:358-368[PMID:8055852]
28 Campbell-Brown M,Ward RJ,Haines AP,North WR,Abraham R,McFadyen IR,Turnlund JR,King JC.Zinc and copper in Asian pregnancies--is there evidence for a nutritional deficiency?
1985;92:875-885[PMID:3840032 DOI:10.1111/j.1471-0528.1985.tb03066.x]
29 Ruderman NB,Schneider SH,Berchtold P.The "metabolically-obese," normal-weight individual.
1981;34:1617-1621[PMID:7270486 DOI:10.1093/ajcn/34.8.1617]
30 Balasubramanyam A,Yajnik CS,Tandon N.Non-Traditional Forms of Diabetes Worldwide:Implications for Translational Investigation.In:Robertson RP.Translational Endocrinology &Metabolism:Type 2 Diabetes Update.Washington D.C.:The Endocrine Society;2011,43-68.
31 Morrison EY,Ragoobirsingh D,Thompson H,Fletcher C,Smith-Richardson S,McFarlane S,Pascoe K,DasGupta T,Fray JC.Phasic insulin dependent diabetes mellitus:manifestations and cellular mechanisms.
1995;80:1996-2001[PMID:7608247 DOI:10.1210/jcem.80.7.7608247]
32 Cuisinier-Raynal JC,Ducorps M,Grandpierre G.Le diabète sucré tropical,un nouvel indicateur nutritionnel?
1985;45:179-184[PMID:3927107]
33 Hoet JJ,Tripathy BB.Report of the International Workshop on types of Diabetes Peculiar to the Tropics.
1996;19:1014[PMID:8875102]
34 Ruderman N,Chisholm D,Pi-Sunyer X,Schneider S.The metabolically obese,normal-weight individual revisited.
1998;47:699-713[PMID:9588440 DOI:10.2337/diabetes.47.5.699]
35 St-Onge MP,Janssen I,Heymsfield SB.Metabolic syndrome in normal-weight Americans:new definition of the metabolically obese,normal-weight individual.
2004;27:2222-2228[PMID:15333488 DOI:10.2337/diacare.27.9.2222]
36 Carnethon MR,De Chavez PJ,Biggs ML,Lewis CE,Pankow JS,Bertoni AG,Golden SH,Liu K,Mukamal KJ,Campbell-Jenkins B,Dyer AR.Association of weight status with mortality in adults with incident diabetes.
2012;308:581-590[PMID:22871870 DOI:10.1001/jama.2012.9282]
37 Indulekha K,Surendar J,Anjana RM,Geetha L,Gokulakrishnan K,Pradeepa R,Mohan V.Metabolic obesity,adipocytokines,and inflammatory markers in Asian Indians--CURES-124.
2015;17:134-141[PMID:25478993 DOI:10.1089/dia.2014.0202]
38 Armitage JA,Khan IY,Taylor PD,Nathanielsz PW,Poston L.Developmental programming of the metabolic syndrome by maternal nutritional imbalance:how strong is the evidence from experimental models in mammals?
2004;561:355-377[PMID:15459241 DOI:10.1113/jphysiol.2004.072009]
39 Hales CN,Barker DJ.The thrifty phenotype hypothesis.
2001;60:5-20[PMID:11809615 DOI:10.1093/bmb/60.1.5]
40 Vaughan OR,Rosario FJ,Powell TL,Jansson T.Regulation of Placental Amino Acid Transport and Fetal Growth.
2017;145:217-251[PMID:28110752 DOI:10.1016/bs.pmbts.2016.12.008]
41 Chmurzynska A.Fetal programming:link between early nutrition,DNA methylation,and complex diseases.
2010;68:87-98[PMID:20137054 DOI:10.1111/j.1753-4887.2009.00265.x]
42 Shaikh MG,Anderson JM,Hall SK,Jackson MA.Transient neonatal hypothyroidism due to a maternal vegan diet.
2003;16:111-113[PMID:12585349 DOI:10.1515/jpem.2003.16.1.111]
43 Blesson CS,Schutt AK,Balakrishnan MP,Pautler RG,Pedersen SE,Sarkar P,Gonzales D,Zhu G,Marini JC,Chacko SK,Yallampalli U,Yallampalli C.Novel lean type 2 diabetic rat model using gestational low-protein programming.
2016;214:540.e1-540.e7[PMID:26874300 DOI:10.1016/j.ajog.2016.02.004]
44 Yajnik CS.Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries.
2004;134:205-210[PMID:14704320 DOI:10.1093/jn/134.1.205]
45 Gómez-Ambrosi J,Silva C,Galofré JC,Escalada J,Santos S,Gil MJ,Valentí V,Rotellar F,Ramírez B,Salvador J,Frühbeck G.Body adiposity and type 2 diabetes:increased risk with a high body fat percentage even having a normal BMI.
2011;19:1439-1444[PMID:21394093 DOI:10.1038/oby.2011.36]
46 Olaogun I,Farag M,Hamid P.The Pathophysiology of Type 2 Diabetes Mellitus in Non-obese Individuals:An Overview of the Current Understanding.
2020;12:e7614[PMID:32399348 DOI:10.7759/cureus.7614]
47 Cho YS,Chen CH,Hu C,Long J,Ong RT,Sim X,Takeuchi F,Wu Y,Go MJ,Yamauchi T,Chang YC,Kwak SH,Ma RC,Yamamoto K,Adair LS,Aung T,Cai Q,Chang LC,Chen YT,Gao Y,Hu FB,Kim HL,Kim S,Kim YJ,Lee JJ,Lee NR,Li Y,Liu JJ,Lu W,Nakamura J,Nakashima E,Ng DP,Tay WT,Tsai FJ,Wong TY,Yokota M,Zheng W,Zhang R,Wang C,So WY,Ohnaka K,Ikegami H,Hara K,Cho YM,Cho NH,Chang TJ,Bao Y,Hedman ?K,Morris AP,McCarthy MI;DIAGRAM Consortium;MuTHER Consortium,Takayanagi R,Park KS,Jia W,Chuang LM,Chan JC,Maeda S,Kadowaki T,Lee JY,Wu JY,Teo YY,Tai ES,Shu XO,Mohlke KL,Kato N,Han BG,Seielstad M.Metaanalysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians.
2011;44:67-72[PMID:22158537 DOI:10.1038/ng.1019]
48 Kodama K,Tojjar D,Yamada S,Toda K,Patel CJ,Butte AJ.Ethnic differences in the relationship between insulin sensitivity and insulin response:a systematic review and meta-analysis.
2013;36:1789-1796[PMID:23704681 DOI:10.2337/dc12-1235]
49 Mohan V,Vijayaprabha R,Rema M,Premalatha G,Poongothai S,Deepa R,Bhatia E,Mackay IR,Zimmet P.Clinical profile of lean NIDDM in South India.
1997;38:101-108[PMID:9483373 DOI:10.1016/s0168-8227(97)00088-0]
50 Chan JC,Malik V,Jia W,Kadowaki T,Yajnik CS,Yoon KH,Hu FB.Diabetes in Asia:epidemiology,risk factors,and pathophysiology.
2009;301:2129-2140[PMID:19470990 DOI:10.1001/jama.2009.726]
51 Everson SA,Maty SC,Lynch JW,Kaplan GA.Epidemiologic evidence for the relation between socioeconomic status and depression,obesity,and diabetes.
2002;53:891-895[PMID:12377299 DOI:10.1016/s0022-3999(02)00303-3]
52 Robbins JM,Vaccarino V,Zhang H,Kasl SV.Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men:evidence from the Third National Health and Nutrition Examination Survey.
2001;91:76-83[PMID:11189829 DOI:10.2105/ajph.91.1.76]
53 Ramachandran A,Snehalatha C.Current scenario of diabetes in India.
2009;1:18-28[PMID:20923516 DOI:10.1111/j.1753-0407.2008.00004.x]
54 Ramachandran A,Snehalatha C,Kapur A,Vijay V,Mohan V,Das AK,Rao PV,Yajnik CS,Prasanna Kumar KM,Nair JD;Diabetes Epidemiology Study Group in India(DESI).High prevalence of diabetes and impaired glucose tolerance in India:National Urban Diabetes Survey.
2001;44:1094-1101[PMID:11596662 DOI:10.1007/s001250100627]
55 Ramachandran A,Snehalatha C,Viswanathan V,Viswanathan M,Haffner SM.Risk of noninsulin dependent diabetes mellitus conferred by obesity and central adiposity in different ethnic groups:a comparative analysis between Asian Indians,Mexican Americans and Whites.
1997;36:121-125[PMID:9229196 DOI:10.1016/s0168-8227(97)00040-5]
56 Alemu S,Dessie A,Seid E,Bard E,Lee PT,Trimble ER,Phillips DI,Parry EH.Insulin-requiring diabetes in rural Ethiopia:should we reopen the case for malnutrition-related diabetes?
2009;52:1842-1845[PMID:19565213 DOI:10.1007/s00125-009-1433-5]
57 Gutiérrez-Adán A,Perez-Crespo M,Fernandez-Gonzalez R,Ramirez MA,Moreira P,Pintado B,Lonergan P,Rizos D.Developmental consequences of sexual dimorphism during pre-implantation embryonic development.
2006;41 Suppl 2:54-62[PMID:16984469 DOI:10.1111/j.1439-0531.2006.00769.x]
58 Chen X,McClusky R,Itoh Y,Reue K,Arnold AP.X and Y chromosome complement influence adiposity and metabolism in mice.
2013;154:1092-1104[PMID:23397033 DOI:10.1210/en.2012-2098]
59 Dearden L,Bouret SG,Ozanne SE.Sex and gender differences in developmental programming of metabolism.
2018;15:8-19[PMID:29773464 DOI:10.1016/j.molmet.2018.04.007]
60 Sun B,Purcell RH,Terrillion CE,Yan J,Moran TH,Tamashiro KL.Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity.
2012;61:2833-2841[PMID:22751689 DOI:10.2337/db11-0957]
61 Fowden AL,Forhead AJ.Endocrine mechanisms of intrauterine programming.
2004;127:515-526[PMID:15129007 DOI:10.1530/rep.1.00033]
62 Alejandro EU,Jo S,Akhaphong B,Llacer PR,Gianchandani M,Gregg B,Parlee SD,MacDougald OA,Bernal-Mizrachi E.Maternal low-protein diet on the last week of pregnancy contributes to insulin resistance and β-cell dysfunction in the mouse offspring.
2020;319:R485-R496[PMID:32877242 DOI:10.1152/ajpregu.00284.2019]
63 Su Y,Jiang X,Li Y,Li F,Cheng Y,Peng Y,Song D,Hong J,Ning G,Cao Y,Wang W.Maternal Low Protein Isocaloric Diet Suppresses Pancreatic β-Cell Proliferation in Mouse Offspring
miR-15b.
2016;157:4782-4793[PMID:27754789 DOI:10.1210/en.2016-1167]
64 Edelmann MN,Auger AP.Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala.
2011;25:1299-1304[PMID:21352906 DOI:10.1016/j.bbi.2011.02.009]
65 Blesson CS,Schutt A,Chacko S,Marini JC,Mathew PR,Tanchico D,Balakrishnan M,Yallampalli C.Sex Dependent Dysregulation of Hepatic Glucose Production in Lean Type 2 Diabetic Rats.
2019;10:538[PMID:31447783 DOI:10.3389/fendo.2019.00538]
66 Blesson CS,Chinnathambi V,Kumar S,Yallampalli C.Gestational Protein Restriction Impairs Glucose Disposal in the Gastrocnemius Muscles of Female Rats.
2017;158:756-767[PMID:28324067 DOI:10.1210/en.2016-1675]
67 Blesson CS,Sathishkumar K,Chinnathambi V,Yallampalli C.Gestational protein restriction impairs insulin-regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring.
2014;155:3036-3046[PMID:24797633 DOI:10.1210/en.2014-1094]
68 Blesson CS,Schutt AK,Vipin VA,Tanchico DT,Mathew PR,Balakrishnan M,Betancourt A,Yallampalli C.In utero low-protein-diet-programmed type 2 diabetes in adult offspring is mediated by sex hormones in rats?.
2020;103:1110-1120[PMID:32766739 DOI:10.1093/biolre/ioaa133]
69 Kwong WY,Miller DJ,Wilkins AP,Dear MS,Wright JN,Osmond C,Zhang J,Fleming TP.Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses.
2007;74:48-56[PMID:16941667 DOI:10.1002/mrd.20606]
70 Han R,Li A,Li L,Kitlinska JB,Zukowska Z.Maternal low-protein diet up-regulates the neuropeptide Y system in visceral fat and leads to abdominal obesity and glucose intolerance in a sex- and time-specific manner.
2012;26:3528-3536[PMID:22539639 DOI:10.1096/fj.12-203943]
71 Zambrano E,Bautista CJ,Deás M,Martínez-Samayoa PM,González-Zamorano M,Ledesma H,Morales J,Larrea F,Nathanielsz PW.A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake,glucose metabolism and serum leptin in the rat.
2006;571:221-230[PMID:16339179 DOI:10.1113/jphysiol.2005.100313]
72 Theys N,Bouckenooghe T,Ahn MT,Remacle C,Reusens B.Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat.
2009;297:R1516-1525[PMID:19759337 DOI:10.1152/ajpregu.00280.2009]
73 Jia Y,Li R,Cong R,Yang X,Sun Q,Parvizi N,Zhao R.Maternal low-protein diet affects epigenetic regulation of hepatic mitochondrial DNA transcription in a sex-specific manner in newborn piglets associated with GR binding to its promoter.
2013;8:e63855[PMID:23691106 DOI:10.1371/journal.pone.0063855]
74 Jia Y,Cong R,Li R,Yang X,Sun Q,Parvizi N,Zhao R.Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver.
2012;142:1659-1665[PMID:22833655 DOI:10.3945/jn.112.160341]
75 Karakelides H,Irving BA,Short KR,O'Brien P,Nair KS.Age,obesity,and sex effects on insulin sensitivity and skeletal muscle mitochondrial function.
2010;59:89-97[PMID:19833885 DOI:10.2337/db09-0591]
76 H?eg L,Roepstorff C,Thiele M,Richter EA,Wojtaszewski JF,Kiens B.Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling.
2009;107:824-831[PMID:19574502 DOI:10.1152/japplphysiol.91382.2008]
77 Vistisen B,Hellgren LI,Vadset T,Scheede-Bergdahl C,Helge JW,Dela F,Stallknecht B.Effect of gender on lipidinduced insulin resistance in obese subjects.
2008;158:61-68[PMID:18166818 DOI:10.1530/EJE-07-0493]
78 Yan H,Yang W,Zhou F,Li X,Pan Q,Shen Z,Han G,Newell-Fugate A,Tian Y,Majeti R,Liu W,Xu Y,Wu C,Allred K,Allred C,Sun Y,Guo S.Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis
the Transcription Factor Foxo1.
2019;68:291-304[PMID:30487265 DOI:10.2337/db18-0638]
79 Capllonch-Amer G,Lladó I,Proenza AM,García-Palmer FJ,Gianotti M.Opposite effects of 17-β estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes.
2014;52:203-214[PMID:24604890 DOI:10.1530/JME-13-0201]
80 Shrivastav M,Kharkwal N,Tiwari A,Gupta KK.A Cross Sectional Study of Type 2 Diabetes Mellitus Comparing Different Factors between Lean Body Weight,Non Obese and Obese Patients in Western Uttar Pradesh.
2020;3:405-409[DOI:10.15520/jcmro.v3i01.248]
81 Selhub J,Rosenberg IH.Excessive folic acid intake and relation to adverse health outcome.
2016;126:71-78[PMID:27131640 DOI:10.1016/j.biochi.2016.04.010]
82 Li Z,Gueant-Rodriguez RM,Quilliot D,Sirveaux MA,Meyre D,Gueant JL,Brunaud L.Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity.
2018;37:1700-1706[PMID:28780990 DOI:10.1016/j.clnu.2017.07.008]
83 Maruvada P,Stover PJ,Mason JB,Bailey RL,Davis CD,Field MS,Finnell RH,Garza C,Green R,Gueant JL,Jacques PF,Klurfeld DM,Lamers Y,MacFarlane AJ,Miller JW,Molloy AM,O'Connor DL,Pfeiffer CM,Potischman NA,Rodricks JV,Rosenberg IH,Ross SA,Shane B,Selhub J,Stabler SP,Trasler J,Yamini S,Zappalà G.Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid:a summary,and perspectives,from an NIH workshop.
2020;112:1390-1403[PMID:33022704 DOI:10.1093/ajcn/nqaa259]
84 Blesson CS,Schutt A,Mathew PR,Tanchico D,Balakrishnan M,Yallampalli U,Yallampalli C.Folate treatment partially reverses gestational low-protein diet-induced glucose intolerance and the magnitude of reversal is age and sex dependent.
2018;49:81-89[PMID:29500969 DOI:10.1016/j.nut.2017.10.014]
85 Arentson-Lantz EJ,Zou M,Teegarden D,Buhman KK,Donkin SS.Maternal high fructose and low protein consumption during pregnancy and lactation share some but not all effects on early-life growth and metabolic programming of rat offspring.
2016;36:937-946[PMID:27632913 DOI:10.1016/j.nutres.2016.06.014]
86 Rodríguez-Trejo A,Ortiz-López MG,Zambrano E,Granados-Silvestre Mde L,Méndez C,Blondeau B,Bréant B,Nathanielsz PW,Menjivar M.Developmental programming of neonatal pancreatic β-cells by a maternal low-protein diet in rats involves a switch from proliferation to differentiation.
2012;302:E1431-E1439[PMID:22436693 DOI:10.1152/ajpendo.00619.2011]
87 Zheng J,Xiao X,Zhang Q,Yu M,Xu J,Wang Z.Maternal protein restriction induces early-onset glucose intolerance and alters hepatic genes expression in the peroxisome proliferator-activated receptor pathway in offspring.
2015;6:269-279[PMID:25969711 DOI:10.1111/jdi.12303]
88 Mesquita FF,Gontijo JA,Boer PA.Expression of renin-angiotensin system signalling compounds in maternal proteinrestricted rats:effect on renal sodium excretion and blood pressure.
2010;25:380-388[PMID:19793932 DOI:10.1093/ndt/gfp505]
89 Mortensen OH,Olsen HL,Frandsen L,Nielsen PE,Nielsen FC,Grunnet N,Quistorff B.A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle;protective effect of taurine.
2010;17 Suppl 1:S38[PMID:20804614 DOI:10.1186/1423-0127-17-S1-S38]
90 Ostreicher I,Almeida JR,Campean V,Rauh M,Plank C,Amann K,D?tsch J.Changes in 11beta-hydroxysteroid dehydrogenase type 2 expression in a low-protein rat model of intrauterine growth restriction.
2010;25:3195-3203[PMID:20566573 DOI:10.1093/ndt/gfq354]
91 Sathishkumar K,Elkins R,Yallampalli U,Yallampalli C.Protein restriction during pregnancy induces hypertension in adult female rat offspring--influence of oestradiol.
2012;107:665-673[PMID:21787449 DOI:10.1017/S0007114511003448]
92 Snoeck A,Remacle C,Reusens B,Hoet JJ.Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas.
1990;57:107-118[PMID:2178691 DOI:10.1159/000243170]
93 Zhou D,Pan YX.Gestational low protein diet selectively induces the amino acid response pathway target genes in the liver of offspring rats through transcription factor binding and histone modifications.
2011;1809:549-556[PMID:21777709 DOI:10.1016/j.bbagrm.2011.07.003]
94 Ozanne SE,Martensz ND,Petry CJ,Loizou CL,Hales CN.Maternal low protein diet in rats programmes fatty acid desaturase activities in the offspring.
1998;41:1337-1342[PMID:9833942 DOI:10.1007/s001250051074]
95 Lillycrop KA,Phillips ES,Torrens C,Hanson MA,Jackson AA,Burdge GC.Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring.
2008;100:278-282[PMID:18186951 DOI:10.1017/S0007114507894438]
96 Lillycrop KA,Phillips ES,Jackson AA,Hanson MA,Burdge GC.Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring.
2005;135:1382-1386[PMID:15930441 DOI:10.1093/jn/135.6.1382]
97 Petry CJ,Dorling MW,Pawlak DB,Ozanne SE,Hales CN.Diabetes in old male offspring of rat dams fed a reduced protein diet.
2001;2:139-143[PMID:12369717 DOI:10.1155/edr.2001.139]
98 Berleze KJ,Müller AP,Schweigert ID,Longoni A,Sordi F,de Assis AM,Rotta LN,de Souza DO,Perry ML.Gestational and postnatal low protein diet alters insulin sensitivity in female rats.
2009;234:1437-1444[PMID:19934364 DOI:10.3181/0903-RM-111]
99 Chen JH,Martin-Gronert MS,Tarry-Adkins J,Ozanne SE.Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice.
2009;4:e4950[PMID:19308256 DOI:10.1371/journal.pone.0004950]
100 Qasem RJ,Cherala G,D'mello AP.Maternal protein restriction during pregnancy and lactation in rats imprints long-term reduction in hepatic lipid content selectively in the male offspring.
2010;30:410-417[PMID:20650349 DOI:10.1016/j.nutres.2010.05.008]
101 Zheng S,Rollet M,Yang K,Pan YX.A gestational low-protein diet represses p21(WAF1/Cip1)expression in the mammary gland of offspring rats through promoter histone modifications.
2012;108:998-1007[PMID:22152918 DOI:10.1017/S0007114511006222]
102 Crossland RF,Balasa A,Ramakrishnan R,Mahadevan SK,Fiorotto ML,Van den Veyver IB.Correction:Chronic Maternal Low-Protein Diet in Mice Affects Anxiety,Night-Time Energy Expenditure and Sleep Patterns,but Not Circadian Rhythm in Male Offspring.
2018;13:e0201079[PMID:30016362 DOI:10.1371/journal.pone.0201079]
103 Dahri S,Snoeck A,Reusens-Billen B,Remacle C,Hoet JJ.Islet function in offspring of mothers on low-protein diet during gestation.
1991;40 Suppl 2:115-120[PMID:1748239 DOI:10.2337/diab.40.2.s115]
104 Aroutiounova N,Fandrich R,Kardami E,Tappia PS.Prenatal exposure to maternal low protein diet suppresses replicative potential of myocardial cells.
2009;19:707-712[PMID:19346111 DOI:10.1016/j.numecd.2008.12.014]
105 Alwasel SH,Kaleem I,Sahajpal V,Ashton N.Maternal protein restriction reduces angiotensin II AT(1)and AT(2)receptor expression in the fetal rat kidney.
2010;33:251-259[PMID:20606474 DOI:10.1159/000317739]
106 Coupé B,Grit I,Darmaun D,Parnet P.The timing of "catch-up growth" affects metabolism and appetite regulation in male rats born with intrauterine growth restriction.
2009;297:R813-R824[PMID:19605764 DOI:10.1152/ajpregu.00201.2009]
107 Cianfarani S,Germani D,Branca F.Low birthweight and adult insulin resistance:the "catch-up growth" hypothesis.
1999;81:F71-F73[PMID:10375369 DOI:10.1136/fn.81.1.f71]
108 Veening MA,Van Weissenbruch MM,Delemarre-Van De Waal HA.Glucose tolerance,insulin sensitivity,and insulin secretion in children born small for gestational age.
2002;87:4657-4661[PMID:12364453 DOI:10.1210/jc.2001-011940]
109 Desai M,Crowther NJ,Lucas A,Hales CN.Organ-selective growth in the offspring of protein-restricted mothers.
1996;76:591-603[PMID:8942365 DOI:10.1079/bjn19960065]
110 Gao H,Yallampalli U,Yallampalli C.Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries.
2012;86:68[PMID:22088913 DOI:10.1095/biolreprod.111.095844]
111 Augustyniak RA,Singh K,Zeldes D,Singh M,Rossi NF.Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring.
2010;298:R1375-R1382[PMID:20200128 DOI:10.1152/ajpregu.00848.2009]
112 Erhuma A,McMullen S,Langley-Evans SC,Bennett AJ.Feeding pregnant rats a low-protein diet alters the hepatic expression of SREBP-1c in their offspring
a glucocorticoid-related mechanism.
2009;36:333-338[PMID:19672729 DOI:10.1007/s12020-009-9225-8]
113 Sohi G,Marchand K,Revesz A,Arany E,Hardy DB.Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter.
2011;25:785-798[PMID:21372147 DOI:10.1210/me.2010-0395]
114 Sasaki H,Saisho Y,Inaishi J,Watanabe Y,Tsuchiya T,Makio M,Sato M,Kitago M,Yamada T,Itoh H.Associations of birthweight and history of childhood obesity with beta cell mass in Japanese adults.
2020;63:1199-1210[PMID:32239263 DOI:10.1007/s00125-020-05127-2]
115 Dolinoy DC,Weidman JR,Waterland RA,Jirtle RL.Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome.
2006;114:567-572[PMID:16581547 DOI:10.1289/ehp.8700]
116 Sandovici I,Smith NH,Nitert MD,Ackers-Johnson M,Uribe-Lewis S,Ito Y,Jones RH,Marquez VE,Cairns W,Tadayyon M,O'Neill LP,Murrell A,Ling C,Constancia M,Ozanne SE.Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets.
2011;108:5449-5454[PMID:21385945 DOI:10.1073/pnas.1019007108]
117 Raychaudhuri N,Raychaudhuri S,Thamotharan M,Devaskar SU.Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring.
2008;283:13611-13626[PMID:18326493 DOI:10.1074/jbc.M800128200]
118 Stevenson K,Lillycrop KA,Silver MJ.Fetal programming and epigenetics.
2020;13:1-6[DOI:10.1016/j.coemr.2020.07.005]
119 Goyal D,Limesand SW,Goyal R.Epigenetic responses and the developmental origins of health and disease.
2019;242:T105-T119[PMID:31091503 DOI:10.1530/JOE-19-0009]
120 Vickers MH.Developmental programming of the metabolic syndrome - critical windows for intervention.
2011;2:137-148[PMID:21954418 DOI:10.4239/wjd.v2.i9.137]
121 Gluckman PD,Hanson MA,Cooper C,Thornburg KL.Effect of in utero and early-life conditions on adult health and disease.
2008;359:61-73[PMID:18596274 DOI:10.1056/NEJMra0708473]
122 Lillycrop KA,Slater-Jefferies JL,Hanson MA,Godfrey KM,Jackson AA,Burdge GC.Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications.
2007;97:1064-1073[PMID:17433129 DOI:10.1017/S000711450769196X]
123 Drake AJ,Walker BR,Seckl JR.Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats.
2005;288:R34-R38[PMID:15178540 DOI:10.1152/ajpregu.00106.2004]
124 Hoile SP,Lillycrop KA,Thomas NA,Hanson MA,Burdge GC.Dietary protein restriction during F0 pregnancy in rats induces transgenerational changes in the hepatic transcriptome in female offspring.
2011;6:e21668[PMID:21750721 DOI:10.1371/journal.pone.0021668]
125 Hardikar AA,Satoor SN,Karandikar MS,Joglekar MV,Puranik AS,Wong W,Kumar S,Limaye A,Bhat DS,Januszewski AS,Umrani MR,Ranjan AK,Apte K,Yajnik P,Bhonde RR,Galande S,Keech AC,Jenkins AJ,Yajnik CS.Multigenerational Undernutrition Increases Susceptibility to Obesity and Diabetes that Is Not Reversed after Dietary Recuperation.
2015;22:312-319[PMID:26166746 DOI:10.1016/j.cmet.2015.06.008]
126 Zheng J,Xiao X,Zhang Q,Wang T,Yu M,Xu J.Maternal Low-Protein Diet Modulates Glucose Metabolism and Hepatic MicroRNAs Expression in the Early Life of Offspring ?.
2017;9[PMID:28264458 DOI:10.3390/nu9030205]
127 Mericq V,Martinez-Aguayo A,Uauy R,I?iguez G,Van der Steen M,Hokken-Koelega A.Long-term metabolic risk among children born premature or small for gestational age.
2017;13:50-62[PMID:27539244 DOI:10.1038/nrendo.2016.127]
128 Soto N,Bazaes RA,Pe?a V,Salazar T,Avila A,I?iguez G,Ong KK,Dunger DB,Mericq MV.Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year:results from a prospective cohort.
2003;88:3645-3650[PMID:12915649 DOI:10.1210/jc.2002-030031]
129 Deng HZ,Li YH,Su Z,Ma HM,Huang YF,Chen HS,Du ML.Association between height and weight catch-up growth with insulin resistance in pre-pubertal Chinese children born small for gestational age at two different ages.
2011;170:75-80[PMID:20734204 DOI:10.1007/s00431-010-1274-8]
130 Wigmore PM,Stickland NC.Muscle development in large and small pig fetuses.
1983;137(Pt 2):235-245[PMID:6630038]
131 Patel HP,Jameson KA,Syddall HE,Martin HJ,Stewart CE,Cooper C,Sayer AA.Developmental influences,muscle morphology,and sarcopenia in community-dwelling older men.
2012;67:82-87[PMID:21357193 DOI:10.1093/gerona/glr020]
132 Confortim HD,Jer?nimo LC,Centenaro LA,Felipe Pinheiro PF,Brancalh?o RM,Michelin Matheus SM,Torrejais MM.Effects of aging and maternal protein restriction on the muscle fibers morphology and neuromuscular junctions of rats after nutritional recovery.
2015;71:7-13[PMID:25597842 DOI:10.1016/j.micron.2014.12.006]
133 Jousse C,Muranishi Y,Parry L,Montaurier C,Even P,Launay JM,Carraro V,Maurin AC,Averous J,Chaveroux C,Bruhat A,Mallet J,Morio B,Fafournoux P.Perinatal protein malnutrition affects mitochondrial function in adult and results in a resistance to high fat diet-induced obesity.
2014;9:e104896[PMID:25118945 DOI:10.1371/journal.pone.0104896]
134 Oreffo RO,Lashbrooke B,Roach HI,Clarke NM,Cooper C.Maternal protein deficiency affects mesenchymal stem cell activity in the developing offspring.
2003;33:100-107[PMID:12919704 DOI:10.1016/s8756-3282(03)00166-2]
135 Raja JS,Hoffman ML,Govoni KE,Zinn SA,Reed SA.Restricted maternal nutrition alters myogenic regulatory factor expression in satellite cells of ovine offspring.
2016;10:1200-1203[PMID:26856892 DOI:10.1017/S1751731116000070]
136 Zhu MJ,Ford SP,Means WJ,Hess BW,Nathanielsz PW,Du M.Maternal nutrient restriction affects properties of skeletal muscle in offspring.
2006;575:241-250[PMID:16763001 DOI:10.1113/jphysiol.2006.112110]
137 Metges CC,G?rs S,Lang IS,Hammon HM,Brüssow KP,Weitzel JM,Nürnberg G,Rehfeldt C,Otten W.Low and high dietary protein:carbohydrate ratios during pregnancy affect materno-fetal glucose metabolism in pigs.
2014;144:155-163[PMID:24353346 DOI:10.3945/jn.113.182691]
138 Claycombe KJ,Roemmich JN,Johnson L,Vomhof-DeKrey EE,Johnson WT.Skeletal muscle Sirt3 expression and mitochondrial respiration are regulated by a prenatal low-protein diet.
2015;26:184-189[PMID:25483313 DOI:10.1016/j.jnutbio.2014.10.003]
139 Zisman A,Peroni OD,Abel ED,Michael MD,Mauvais-Jarvis F,Lowell BB,Wojtaszewski JF,Hirshman MF,Virkamaki A,Goodyear LJ,Kahn CR,Kahn BB.Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance.
2000;6:924-928[PMID:10932232 DOI:10.1038/78693]
140 El-Khattabi I,Grégoire F,Remacle C,Reusens B.Isocaloric maternal low-protein diet alters IGF-I,IGFBPs,and hepatocyte proliferation in the fetal rat.
2003;285:E991-E1000[PMID:12902319 DOI:10.1152/ajpendo.00037.2003]
141 Burns SP,Desai M,Cohen RD,Hales CN,Iles RA,Germain JP,Going TC,Bailey RA.Gluconeogenesis,glucose handling,and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation.
1997;100:1768-1774[PMID:9312176 DOI:10.1172/JCI119703]
142 Martin LJ,Meng Q,Blencowe M,Lagarrigue S,Xiao S,Pan C,Wier J,Temple WC,Devaskar SU,Lusis AJ,Yang X.Maternal High-Protein and Low-Protein Diets Perturb Hypothalamus and Liver Transcriptome and Metabolic Homeostasis in Adult Mouse Offspring.
2018;9:642[PMID:30619467 DOI:10.3389/fgene.2018.00642]
143 Ozanne SE,Smith GD,Tikerpae J,Hales CN.Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams.
1996;270:E559-E564[PMID:8928759 DOI:10.1152/ajpendo.1996.270.4.E559]
144 Ramadan WS,Alshiraihi I,Al-karim S.Effect of maternal low protein diet during pregnancy on the fetal liver of rats.
2013;195:68-76[PMID:22877887 DOI:10.1016/j.aanat.2012.05.006]
145 Blesson CS,Schutt A,Chacko S,Marini J,Balakrishanan M,Yallampalli C.Sex Dependent Dysregulation of Hepatic Glucose Production in Gestational Low Protein Programmed Insulin Resistant Rat Offspring.
2019[DOI:10.3389/fendo.2019.00538]
146 Mortensen OH,Olsen HL,Frandsen L,Nielsen PE,Nielsen FC,Grunnet N,Quistorff B.A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle;protective effect of taurine.
2010;17 Suppl 1:S38[PMID:20804614 DOI:10.1186/1423-0127-17-S1-S38]
147 D?ring F,Lüersen K,Schmelzer C,Hennig S,Lang IS,G?rs S,Rehfeldt C,Otten W,Metges CC.Influence of maternal low protein diet during pregnancy on hepatic gene expression signature in juvenile female porcine offspring.
2013;57:277-290[PMID:23197441 DOI:10.1002/mnfr.201200315]
148 Gong L,Pan YX,Chen H.Gestational low protein diet in the rat mediates Igf2 gene expression in male offspring
altered hepatic DNA methylation.
2010;5:619-626[PMID:20671425 DOI:10.4161/epi.5.7.12882]
149 Rees WD,Hay SM,Cruickshank M,Reusens B,Remacle C,Antipatis C,Grant G.Maternal protein intake in the pregnant rat programs the insulin axis and body composition in the offspring.
2006;55:642-649[PMID:16631441 DOI:10.1016/j.metabol.2005.12.006]
150 Malaisse WJ,Sener A,Herchuelz A,Hutton JC.Insulin release:the fuel hypothesis.
1979;28:373-386[PMID:36543 DOI:10.1016/0026-0495(79)90111-2]
151 Dumortier O,Blondeau B,Duvillié B,Reusens B,Bréant B,Remacle C.Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet.
2007;50:2495-2503[PMID:17882398 DOI:10.1007/s00125-007-0811-0]
152 Zhang L,Chen W,Dai Y,Zhu Z,Liu Q.Detection of expressional changes induced by intrauterine growth restriction in the developing rat pancreas.
2016;241:1446-1456[PMID:27190278 DOI:10.1177/1535370216638771]
153 Cox AR,Beamish CA,Carter DE,Arany EJ,Hill DJ.Cellular mechanisms underlying failed beta cell regeneration in offspring of protein-restricted pregnant mice.
2013;238:1147-1159[PMID:23986224 DOI:10.1177/1535370213493715]
154 Sparre T,Reusens B,Cherif H,Larsen MR,Roepstorff P,Fey SJ,Mose Larsen P,Remacle C,Nerup J.Intrauterine programming of fetal islet gene expression in rats--effects of maternal protein restriction during gestation revealed by proteome analysis.
2003;46:1497-1511[PMID:13680128 DOI:10.1007/s00125-003-1208-3]
155 Fernandez-Twinn DS,Wayman A,Ekizoglou S,Martin MS,Hales CN,Ozanne SE.Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring.
2005;288:R368-R373[PMID:15514105 DOI:10.1152/ajpregu.00206.2004]
156 Alejandro EU,Gregg B,Wallen T,Kumusoglu D,Meister D,Chen A,Merrins MJ,Satin LS,Liu M,Arvan P,Bernal-Mizrachi E.Maternal diet-induced microRNAs and mTOR underlie β cell dysfunction in offspring.
2014;124:4395-4410[PMID:25180600 DOI:10.1172/JCI74237]
157 Merezak S,Hardikar AA,Yajnik CS,Remacle C,Reusens B.Intrauterine low protein diet increases fetal beta-cell sensitivity to NO and IL-1 beta:the protective role of taurine.
2001;171:299-308[PMID:11691650 DOI:10.1677/joe.0.1710299]
158 Petrik J,Reusens B,Arany E,Remacle C,Coelho C,Hoet JJ,Hill DJ.A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II.
1999;140:4861-4873[PMID:10499546 DOI:10.1210/endo.140.10.7042]
159 Mateus Gon?alves L,Vettorazzi JF,Vanzela EC,Figueiredo MS,Batista TM,Zoppi CC,Boschero AC,Carneiro EM.Amino acid restriction increases β-cell death under challenging conditions.
2019;234:16679-16684[PMID:30815898 DOI:10.1002/jcp.28389]
160 Theys N,Clippe A,Bouckenooghe T,Reusens B,Remacle C.Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction.
2009;4:e6110[PMID:19568427 DOI:10.1371/journal.pone.0006110]
161 Ajuogu PK,Al-Aqbi MAK,Hart RA,McFarlane JR,Smart NA.A low protein maternal diet during gestation has negative effects on male fertility markers in rats - A Systematic Review and Meta-analysis.
2021;105:157-166[PMID:32654274 DOI:10.1111/jpn.13411]
162 Fabozzi G,Iussig B,Cimadomo D,Vaiarelli A,Maggiulli R,Ubaldi N,Ubaldi FM,Rienzi L.The Impact of Unbalanced Maternal Nutritional Intakes on Oocyte Mitochondrial Activity:Implications for Reproductive Function.
2021;10[PMID:33440800 DOI:10.3390/antiox10010091]
163 Rodríguez-González GL,Vigueras-Villase?or RM,Millán S,Moran N,Trejo R,Nathanielsz PW,Larrea F,Zambrano E.Maternal protein restriction in pregnancy and/or lactation affects seminiferous tubule organization in male rat offspring.
2012;3:321-326[PMID:25102260 DOI:10.1017/S2040174412000360]
164 Zambrano E,Rodríguez-González GL,Guzmán C,García-Becerra R,Boeck L,Díaz L,Menjivar M,Larrea F,Nathanielsz PW.A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development.
2005;563:275-284[PMID:15611025 DOI:10.1113/jphysiol.2004.078543]
165 Kotsampasi B,Balaskas C,Papadomichelakis G,Chadio SE.Reduced Sertoli cell number and altered pituitary responsiveness in male lambs undernourished in utero.
2009;114:135-147[PMID:18814977 DOI:10.1016/j.anireprosci.2008.08.017]
166 Oliveira JSd,Silva AADN,Magalh?es CP,Souza SLd,Silva Junior VAd,Melo ENd.Protein restriction during intrauterine and lactation periods:effects on testicular development in pre-puberty rats.
2015;37[DOI:10.4025/actascibiolsci.v37i1.21929]
167 Oliveira JSd,Silva AAdN,Souza SLd,Morais RNd,Neves de Melo E,Maia FCL,Júnior VA.Histomorphometric evaluation of the testicular parenchyma of rats submittedto protein restriction during intrauterine and postnatal life.Turkish Journal of Biology 2017;41:428-438[DOI:10.3906/BIY-1610-20]
168 Guzmán C,Cabrera R,Cárdenas M,Larrea F,Nathanielsz PW,Zambrano E.Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny.
2006;572:97-108[PMID:16497715 DOI:10.1113/jphysiol.2005.103903]
169 Winship AL,Gazzard SE,Cullen-McEwen LA,Bertram JF,Hutt KJ.Maternal low-protein diet programmes low ovarian reserve in offspring.
2018;156:299-311[PMID:30306601 DOI:10.1530/REP-18-0247]
170 Bernal AB,Vickers MH,Hampton MB,Poynton RA,Sloboda DM.Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring.
2010;5:e15558[PMID:21179452 DOI:10.1371/journal.pone.0015558]
171 C?rtes LS,Silveira HS,Lupi LA,de Mello Santos T,Cavariani MM,Domeniconi RF,Gaiotte LB,de Morais Oliveira DA,Justulin LA,de Almeida Chuffa LG.Maternal protein restriction impairs nutrition and ovarian histomorphometry without changing p38MAPK and PI3K-AKT-mTOR signaling in adult rat ovaries.
2021;264:118693[PMID:33130082 DOI:10.1016/j.lfs.2020.118693]
172 Chan KA,Jazwiec PA,Gohir W,Petrik JJ,Sloboda DM.Maternal nutrient restriction impairs young adult offspring ovarian signaling resulting in reproductive dysfunction and follicle loss.
2018;98:664-682[PMID:29351580 DOI:10.1093/biolre/ioy008]
173 Lisboa PC,Oliveira E,Fagundes AT,Santos-Silva AP,Concei??o EP,Passos MC,Moura EG.Postnatal low protein diet programs leptin signaling in the hypothalamic-pituitary-thyroid axis and pituitary TSH response to leptin in adult male rats.
2012;44:114-122[PMID:22314332 DOI:10.1055/s-0031-1299747]
174 Guzmán C,García-Becerra R,Aguilar-Medina MA,Méndez I,Merchant-Larios H,Zambrano E.Maternal protein restriction during pregnancy and/or lactation negatively affects follicular ovarian development and steroidogenesis in the prepubertal rat offspring.
2014;45:294-300[PMID:24819035 DOI:10.1016/j.arcmed.2014.05.005]
175 da Silva Faria T,de Bittencourt Brasil F,Sampaio FJ,da Fonte Ramos C.Effects of maternal undernutrition during lactation on estrogen and androgen receptor expressions in rat ovary at puberty.
2010;26:993-999[PMID:20097538 DOI:10.1016/j.nut.2009.09.027]
176 Barsky M,Merkison J,Hosseinzadeh P,Yang L,Bruno-Gaston J,Dunn J,Gibbons W,Blesson CS.Fetal programming of polycystic ovary syndrome:Effects of androgen exposure on prenatal ovarian development.
2021;207:105830[PMID:33515680 DOI:10.1016/j.jsbmb.2021.105830]
177 Teixeira CV,Silandre D,de Souza Santos AM,Delalande C,Sampaio FJ,Carreau S,da Fonte Ramos C.Effects of maternal undernutrition during lactation on aromatase,estrogen,and androgen receptors expression in rat testis at weaning.
2007;192:301-311[PMID:17283230 DOI:10.1677/joe.1.06712]
178 Sui S,Jia Y,He B,Li R,Li X,Cai D,Song H,Zhang R,Zhao R.Maternal Low-protein Diet Alters Ovarian Expression of Folliculogenic and Steroidogenic Genes and Their Regulatory MicroRNAs in Neonatal Piglets.
2014;27:1695-1704[PMID:25358362 DOI:10.5713/ajas.2014.14335]
179 Khorram O,Keen-Rinehart E,Chuang TD,Ross MG,Desai M.Maternal undernutrition induces premature reproductive senescence in adult female rat offspring.
2015;103:291-8.e2[PMID:25439841 DOI:10.1016/j.fertnstert.2014.09.026]
180 Shimazu T,Minokoshi Y.Systemic Glucoregulation by Glucose-Sensing Neurons in the Ventromedial Hypothalamic Nucleus(VMH).
2017;1:449-459[PMID:29264500 DOI:10.1210/js.2016-1104]
181 Plagemann A,Harder T,Rake A,Melchior K,Rohde W,D?rner G.Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams.
2000;130:2582-2589[PMID:11015493 DOI:10.1093/jn/130.10.2582]
182 Kotsampasi B,Chadio S,Papadomichelakis G,Deligeorgis S,Kalogiannis D,Menegatos I,Zervas G.Effects of maternal undernutrition on the hypothalamic-pituitary-gonadal axis function in female sheep offspring.
2009;44:677-684[PMID:19642222 DOI:10.1111/j.1439-0531.2007.01046.x]
183 Langley-Evans SC,Gardner DS,Jackson AA.Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis.
1996;126:1578-1585[PMID:8648431 DOI:10.1093/jn/126.6.1578]
184 Marwarha G,Claycombe-Larson K,Schommer J,Ghribi O.Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring.
2017;45:54-66[PMID:28432877 DOI:10.1016/j.jnutbio.2017.03.005]
185 Rushmore RJ,McGaughy JA,Mokler DJ,Rosene DL.The enduring effect of prenatal protein malnutrition on brain anatomy,physiology and behavior.
2020;1-8[PMID:33314995 DOI:10.1080/1028415X.2020.1859730]
186 DeFronzo RA,Barzilai N,Simonson DC.Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects.
1991;73:1294-1301[PMID:1955512 DOI:10.1210/jcem-73-6-1294]
187 Backholer K,Peeters A,Herman WH,Shaw JE,Liew D,Ademi Z,Magliano DJ.Diabetes prevention and treatment strategies:are we doing enough?
2013;36:2714-2719[PMID:23637353 DOI:10.2337/DC12-2501]
188 Cefalu WT,Buse JB,Tuomilehto J,Fleming GA,Ferrannini E,Gerstein HC,Bennett PH,Ramachandran A,Raz I,Rosenstock J,Kahn SE.Update and Next Steps for Real-World Translation of Interventions for Type 2 Diabetes Prevention:Reflections From a Diabetes Care Editors' Expert Forum.
2016;39:1186-1201[PMID:27631469 DOI:10.2337/dc16-0873]
189 Ramachandran A,Snehalatha C.Diabetes prevention programs.
2011;95:353-372,viii[PMID:21281838 DOI:10.1016/j.mcna.2010.11.006]
190 Merezak S,Reusens B,Renard A,Goosse K,Kalbe L,Ahn MT,Tamarit-Rodriguez J,Remacle C.Effect of maternal low-protein diet and taurine on the vulnerability of adult Wistar rat islets to cytokines.
2004;47:669-675[PMID:15298344 DOI:10.1007/s00125-004-1357-z]
191 Taylor R,Al-Mrabeh A,Sattar N.Understanding the mechanisms of reversal of type 2 diabetes.
2019;7:726-736[PMID:31097391 DOI:10.1016/S2213-8587(19)30076-2]
192 Li G,Zhang P,Wang J,Gregg EW,Yang W,Gong Q,Li H,Jiang Y,An Y,Shuai Y,Zhang B,Zhang J,Thompson TJ,Gerzoff RB,Roglic G,Hu Y,Bennett PH.The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study:a 20-year follow-up study.
2008;371:1783-1789[PMID:18502303 DOI:10.1016/S0140-6736(08)60766-7]
193 Diabetes Prevention Program Research Group,Knowler WC,Fowler SE,Hamman RF,Christophi CA,Hoffman HJ,Brenneman AT,Brown-Friday JO,Goldberg R,Venditti E,Nathan DM.10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study.
2009;374:1677-1686[PMID:19878986 DOI:10.1016/S0140-6736(09)61457-4]
194 Lindstr?m J,Peltonen M,Eriksson JG,Ilanne-Parikka P,Aunola S,Kein?nen-Kiukaanniemi S,Uusitupa M,Tuomilehto J;Finnish Diabetes Prevention Study(DPS).Improved lifestyle and decreased diabetes risk over 13 years:long-term follow-up of the randomised Finnish Diabetes Prevention Study(DPS).
2013;56:284-293[PMID:23093136 DOI:10.1007/s00125-012-2752-5]
195 Pan XR,Li GW,Hu YH,Wang JX,Yang WY,An ZX,Hu ZX,Lin J,Xiao JZ,Cao HB,Liu PA,Jiang XG,Jiang YY,Wang JP,Zheng H,Zhang H,Bennett PH,Howard BV.Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance.The Da Qing IGT and Diabetes Study.
1997;20:537-544[PMID:9096977 DOI:10.2337/diacare.20.4.537]
196 Nanditha A,Snehalatha C,Raghavan A,Vinitha R,Satheesh K,Susairaj P,Simon M,Selvam S,Ram J,Naveen Kumar AP,Godsland IF,Oliver N,Johnston DG,Ramachandran A.The post-trial analysis of the Indian SMS diabetes prevention study shows persistent beneficial effects of lifestyle intervention.
2018;142:213-221[PMID:29859274 DOI:10.1016/j.diabres.2018.05.042]
197 Ramachandran A,Snehalatha C,Mary S,Mukesh B,Bhaskar AD,Vijay V;Indian Diabetes Prevention Programme(IDPP).The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance(IDPP-1).
2006;49:289-297[PMID:16391903 DOI:10.1007/s00125-005-0097-z]
198 Chmurzynska A,Stachowiak M,Pruszynska-Oszmalek E.Maternal protein and folic acid intake during gestation does not program leptin transcription or serum concentration in rat progeny.
2012;7:217-222[PMID:21735287 DOI:10.1007/s12263-011-0239-5]
199 Engeham SF,Haase A,Langley-Evans SC.Supplementation of a maternal low-protein diet in rat pregnancy with folic acid ameliorates programming effects upon feeding behaviour in the absence of disturbances to the methioninehomocysteine cycle.
2010;103:996-1007[PMID:19941678 DOI:10.1017/S0007114509992662]
200 Torrens C,Brawley L,Anthony FW,Dance CS,Dunn R,Jackson AA,Poston L,Hanson MA.Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction.
2006;47:982-987[PMID:16585422 DOI:10.1161/01.HYP.0000215580.43711.d1]
201 Burdge GC,Lillycrop KA,Phillips ES,Slater-Jefferies JL,Jackson AA,Hanson MA.Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition.
2009;139:1054-1060[PMID:19339705 DOI:10.3945/jn.109.104653]
 World Journal of Diabetes2022年3期
World Journal of Diabetes2022年3期
- World Journal of Diabetes的其它文章
- Beyond diabetes remission a step further:Post bariatric surgery hypoglycemia
- Free fatty acids,glucose,and insulin in type 2 diabetes mellitus
- Sodium-glucose co-transporter 2 inhibitors induced euglycemic diabetic ketoacidosis within four days of initiation
- Age at diagnosis of type 2 diabetes and cardiovascular risk factor profile:A pooled analysis
- Hemoglobin within normal range is negatively related to hemoglobin A1c in a nondiabetic American population aged 16 years and older
- Functional annotation and enrichment analysis of differentially expressed serum proteins in patients with type 2 diabetes after dapagliflozin
