Aqueous extract of freeze-dried Protaetia brevitarsis larvae promotes osteogenesis by activating β-catenin signaling
Jayasingha Arachchige Chathuranga Chanaka Jayasingha, Kyoung Tae Lee, Yung Hyun Choi, Chang-Hee Kang, Mi-Hwa Lee, Gi-Young Kim?
1Department of Marine Life Science, Jeju National University, Jeju 63243, Republic of Korea
2Research Institute for Basic Sciences, Jeju National University, Jeju 63243, Republic of Korea
3Forest Biomaterials Research Center, National Institute of Forest Science, Jinju 52817, Republic of Korea
4Department of Biochemistry, College of Korean Medicine, Dong-Eui University, Busan 47227, Republic of Korea
5Nakdonggang National Institute of Biological Resources, Sangju 37242, Republic of Korea
ABSTRACT
Objective: To investigate the effect of an aqueous extract of Protaetia brevitarsis (AEPB) on osteogenesis using preosteoblast MC3T3-E1 cells and zebrafish larvae.
Methods: Flow cytometric analysis was used to measure the cytotoxicy. Alkaline phosphatase activity was detetmined using p-nitrophenyl phosphate as a substrate. Calcium deposition was detected using alizarin red staining along with osteogenic marker expression in preosteoblast MC3T3E1 cells. In addition, vertebral formation in zebrafish larvae was detected using calcein staining and osteogenic gene expression.
Results: AEPB highly promoted the expression of osteogenic markers including runt-related transcription factor 2, osterix, and alkaline phosphatase, along with elevated levels of mineralization in MC3T3-E1 cells. Moreover, AEPB accelerated vertebral formation in zebrafish larvae accompanied by upregulated expression of osteogenic genes. FH535, an inhibitor of Wnt/β-catenin, suppressed AEPB-induced osteogenic gene expression and vertebral formation,indicating that AEPB stimulates osteogenesis by activating the Wnt/β-catenin signaling pathway.
Conclusions: AEPB stimulates osteoblast differentiation and bone formation by activating β-catenin. Therefore, AEPB is a promising material that induces osteogenesis, and is useful for the treatment of bone resorption diseases.
KEYWORDS: Protaetia brevitarsis; Osteoblast differentiation; Bone formation; β-catenin; MC3T3-E1
Significance
Protaetia brevitarsis is a nutritional supplement and possesses various pharmaceutical properties such as neuroprotective and antioxidative activity. However, whether Protaetia brevitarsis improves bone formation has not been elucidated. In the present study, we found that Protaetia brevitarsis promotes osteoblast differentiation and bone formation by activating the Wnt/β-catenin signaling pathway.
1. Introduction
Bone is a highly dynamic organ system that maintains homeostasis through the balance between bone-forming osteoblasts and boneresorbing osteoclasts[1]. Disruption of the balance abnormally impairs bone architecture or function and leads to bone metabolic diseases such as osteoporosis or osteopetrosis[1,2]. Recently, many studies have demonstrated that the incidence rate of osteoporosis has been increasing in elderly men and postmenopausal women with low bone mass, resulting from the inactivation of osteoblasts and activation of osteoclasts[3,4]. Mahmoud et al.[5] reported that osteoblast-based therapy is a promising strategy for the treatment of osteoporosis through bone remodeling. Therefore, a potential boneforming platform, which increases bone density and integrity, would be a great avenue and solution against bone-resorbing diseases such as osteoporosis[6].
Osteogenesis is multiple processes of osteoblast development that involve the formation of new bone material through the activation of many transcription factors such as Wnt/β-catenin, runt-related transcription factor 2 (RUNX2), and osterix (OSX), accompanied by mineralization[7]. At the early development stage, the Wnt/β-catenin pathway promotes the differentiation of bone marrow-derived mesenchymal stem cells (BM-MSCs) to osteoprogenitors, and the activation is maintained in mature osteoblasts accompanied by mineralization. However, the differentiation capacity of the pathway significantly decreases in osteoporosis, resulting in a reduction of bone formation, which indicates that the Wnt/β-catenin pathway regulates early differentiation from BM-MSCs to preosteoblasts[8]as well as completes differentiation to mature osteoblasts. In addition, Wnt/β-catenin canonically activates RUNX2 and OSX,which stimulates the differentiation of preosteoblasts from BMMSCs[7,9]. Alkaline phosphatase (ALP) is also one of the key regulatory enzymes in differentiation to fully mature osteoblasts, and a significant increase in ALP activity in the tissue or cells represents potential evidence of mature osteoblasts and mineralization[10].More specifically, ALP catalyzes the hydroxylation of inorganic pyrophosphate (PPi) to generate inorganic phosphate (Pi),confirming the balance between PPi and Pi concentrations in the mineralization process[11]. Many phytochemical compounds have been developed as bioactive strategies for bone tissue regeneration by activating osteoblast differentiation[12].
Nutrient supplements derived from insects or their derivatives are known to be good sources of proteins, fatty acids, minerals, and vitamins[13]. More than 2 000 species of insects are consumed in the world as edible and dietary supplements with nutrients comparable to other meat sources[14]. In particular, Protaetia brevitarsis (P.brevitarsis) larvae are considered promising sources for the treatment of many human diseases, such as stomatitis, tetanus, hepatic cancer,liver cirrhosis, and cerebral stroke in Korean traditional medicine[15].Recently, scientists reported that freeze-dried P. brevitarsis larva contained 58% crude protein, 17% crude fat, and 11% total carbohydrate with crude ash and fiber, and showed no evidence of mutagenic and carcinogenic potential in genotoxic studies[16,17].Additionally, the extract of P. brevitarsis larvae possessed antioxidant and anticancer activities[18,19]. In a recent study, we demonstrated that an aqueous extract of freeze-dried P. brevitarsis larvae (AEPB)stimulates the immune response in RAW 264.7 macrophages by activating the NF-κB signaling pathway[20]. However, whether AEPB promotes osteoblast differentiation and bone formation has not been evaluated.
In the present study, we investigated whether AEPB promotes the differentiation of preosteoblast MC3T3-E1 cells and enhances bone mineralization and formation in zebrafish larvae.
2. Materials and methods
2.1. Preparation of AEPB
Huimang-Gonchung Farm (2626-11 Gajogaya-ro, Gaya-myeon,Hapcheongun, Gyeongsangnamdo, Republic of Korea) supplied freeze-dried P. brevitarsis larva powder. The specimens were authenticated and deposited at Nakdonggang National Institute of Biological Resources (Sangju, Gyeongsangbukdo, Republic of Korea). AEPB was obtained according to the previous study[20]. The total yield was approximately 23%.
2.2. Reagents and antibodies
Alizarin red, calcein, β-glycerophosphate (GP), and FH535 were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).Santa Cruz Biotechnology (Santa Cruz, CA, USA) supplied specific antibodies against RUNX2 (sc-390715), ALP (sc-398461), OSX (sc-393325), β-catenin (sc-7963), β-actin (sc-8432), and nucleolin (sc-55486). All antibodies were used at 200-fold dilutions. Minimum Essential Medium Alpha Modification (α-MEM), fetal bovine serum (FBS), and antibiotics mixture were obtained from WelGENE(Gyeongsan, Gyeongsangbukdo, Republic of Korea).
2.3. Cell culture and flow cytometry
Mouse preosteoblast MC3T3-E1 cells were obtained by the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in α-MEM supplemented with 10% FBS and antibiotics mixture in a humidified incubator at 5% CO2and 37 ℃. In order to determine viable and dead cell population, MC3T3-E1 (1 × 104cells/mL) were treated with different concentrations of AEPB (0-40 μg/mL) for 12 d. Fresh media were replenished with AEPB every 3 d.Hydrogen peroxide (H2O2, 300 μM) was used as a positive control for inducing cell death and treated for the last 24 h. Then, the cells were stained using a Cell Count & Viability Kit (Luminex, Austin,TX, USA) for 5 min and analyzed using a Muse Cell Analyzer(Luminex).
2.4. Alizarin red staining
MC3T3-E1 (1 × 104cells/mL) were incubated for 12 d after treatment with different concentrations of AEPB (0-20 μg/mL).Fresh media were replenished with AEPB every 3 d. In vitro calcium deposition was measured by staining with 2% alizarin red. Briefly,MC3T3-E1 cells were fixed with 4% paraformaldehyde for 30 min at 37 ℃ and, then, stained with 2% alizarin red solution for 30 min. Images of each well were captured using a phase-contrast microscope (Ezscope i900PH, Macrotech; Goyang, Gyeonggi-do,Republic of Korea).
2.5. ALP activity
MC3T3-E1 cells were treated with different concentrations of AEPB (0-20 μg/mL) for 12 d. The media were freshly replaced with AEPB every 3 d. GP was used as a positive control for inducing osteoblast differentiation. ALP activity was measured using a tartrate-resistant acid phosphatase (TRAP) & ALP double-stain Kit (Takara Bio Inc., Kusatsu, Shiga, Japan). Briefly, the cells were incubated with the fixation buffer for 5 min, and ALP substrate was incubated at 37 ℃ for 45 min. The images of each well were captured using a phase-contrast microscope (Ezscope i900PH).
2.6. Reverse transcription-polymerase chain reaction (RTPCR)
MC3T3-E1 cells were treated with the different concentrations of AEPB for the indicated days, and total RNA was extracted using easy-BLUE Total RNA Extraction Kit (iNtRON Biotechnology,Sungnam, Gyeonggido, Republic of Korea). Two micrograms of RNA were reverse-transcribed using MMLV reverse transcriptase(Bioneer, Daejeon, Republic of Korea). The target genes were amplified using the specific primers[21]; mRUNX2 with 171 bp at 60 ℃ (forward: 5′-CAT GGT GGA GAT CAT CGC GG-3′, and reverse: 5′-GGC CAT GAC GGT AAC CAC AG-3′), mALP with 198 bp at 60 ℃ (forward 5′-TTG TGG CCC TCT CCA AGA CA-3′ and reverse 5′-GAC TTC CCA GCA TCC TTG GC-3′), mOSX with 194 bp at 60 ℃ (forward 5′-AAG GCG GTT GGC AAT AGT GG-3′ and reverse 5′-GCA GCT GTG AAT GGG CTT CT-3′) and mGAPDH with 123 bp at 61 ℃ (forward 5′-ACC ACA GTC CAT GCC ATC AC -3′ and reverse 5′-CAC CAC CCT GTT GCT GTA GC-3′). In a zebrafish model, cDNA was synthesized and amplified using the zebrafish primers[22]; zRUNX2a with 173 bp at 58 ℃ (forward 5′-GAC GGT GGT GAC GGT AAT GG-3′ and reverse 5′-TGC GGT GGG TTC GTG AAT A-3′), zOSX with 153 bp at 56 ℃ (forward 5′-GGCTATGCTAACTGCGACCTG-3′ and reverse 5′-GCT TTC ATT GCG TCC GTT TT-3′), and zALP with 149 bp at 44 ℃ (forward 5′-CAA GAA CTC AAC AAG AAC-3′ and reverse 5′-TGA GCA TTG GTG TTA TAC -3′), zβ-actin with 154 bp at 61 ℃ (forward 5′-CGA GCG TGG CTA CAG CTT CA-3′ and reverse 5′-GAC CGT CAG GCA GCT CAT AG-3′).
2.7. Protein extraction and Western blotting
MC3T3-E1 cells (1 × 104cells/mL) were lysed with RIPA lysis buffer (iNtRON biotechnology) with protease inhibitors (Sigma-Aldrich). Then, proteins were collected and quantified using Bio-Rad Protein Assay Reagents (Bio-Rad, Hercules, CA, USA). In a parallel experiment, nuclear proteins were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL,USA). An equal amount of protein was separated by sodium dodecyl sulfate-polyacrylamide gel and transferred onto nitrocellulose membrane (Schleicher & Schuell, Keene, NH, USA). Specific antibodies were used to detect the expression of each protein.
2.8. Bone mineralization in zebrafish larvae
Zebrafish were raised and handled according to standard guidelines of the Animal Care and Use Committee of Jeju National University(Jeju Special Self-Governing Province, Republic of Korea; approval No.: 2021-0065). All experiments were carried out in accordance with the approved guidelines[23]. For evaluating vertebral formation in zebrafish larvae, calcein green was used. To visualize vertebrae,zebrafish larvae (n=20 in each group) at 3 days post-fertilization(dpf) were treated with 0-200 μg/mL AEPB for 9 d. GP (4 mM)was used as a positive control for stimulating vertebral formation in zebrafish. The E3 culture media (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4) containing 2 mg/L methylene blue was changed every 3 d. The larvae were immersed in 0.05% calcein solution for 10 min at 12 dpf and then rinsed to allow diffusion of the free calcein. After rinsing, the larvae were anesthetized in 0.03% tricaine methanesulfonate solution and mounted on depression slides using 2% methylcellulose. In a parallel experiment, zebrafish larvae at 3 dpf were treated with 10 μM FH535 for 24 h prior to treatment with AEPB (200 μg/mL) to evaluate the Wnt/β-catenin pathway in bone mineralization.
2.9. Statistical analysis
The images of RT-PCR and Western blotting were visualized by ImageQuant LAS 500 (GE Healthcare BioSciences AB, Uppsala,Sweden) and quantified using ImageJ 1.50i (National Institute of Health, Bethesda, VA, USA). Statistical analysis was performed and graphed using Sigma Plot 12.0 software (Systat Software, San Jose, CA, USA, www.systatsoftware.com) by unpaired one-way analysis of variance (ANOVA) with Bonferroni correction. All data were expressed as the mean ± standard error of the median (SEM).Statistical significance was set at P < 0.05.
2.10. Ethical statement
All animal experiments were conducted according to guidelines of the Institutional Animal Care and Use Committee, Jeju National University, Jeju, Republic of Korea (Approval No: 2021-0065; date of approval: 10-21-2021).
3. Results
3.1. AEPB has no toxicity in preosteoblast MC3T3-E1 cells
No significant toxicity was observed in MC3T3-E1 cells (Figure 1A). As shown in Figure 1B, the viable cell populations were (88.37± 0.15)%, (88.47 ± 0.42)%, (85.17 ± 0.27)%, and (82.94 ± 0.67)% at the concentrations of 5, 10, 20, and 40 μg/mL AEPB, respectively,compared with that in the untreated cells [(88.93 ± 0.67)%].However, H2O2treatment significantly decreased cell viability[(52.53 ± 3.76)%]. In addition, no significant increase in dead cell populations was observed in the presence of AEPB (11.63 ± 0.67)%,(11.53 ± 0.27)%, (14.83 ± 0.47)%, and (17.06 ± 0.67)% at 5, 10,20, and 40 μg/mL, respectively compared with that in the untreated cells [(11.07 ± 0.67)%]; however, H2O2significantly increased the dead cell population (47.47 ± 3.77)% (Figure 1C). These results indicate that low concentrations of AEPB did not induce toxicity in MC3T3-E1 cells; however, 40 μg/mL AEPB increased morphological irregularity with many small vacuoles in the cells(data not shown). Therefore, AEPB at concentrations below 20 μg/mL were used for further experiments.
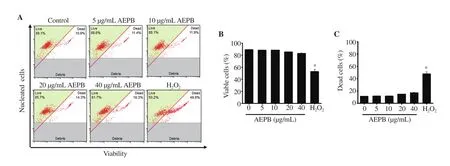
Figure 1. Effect of an aqueous extract of freeze-dried Protaetia brevitarsis larvae (AEPB) on viability of preosteoblast MC3T3-E1 cells. The cells (1 × 104 cells/mL) were treated with AEPB (0-40 μg/mL) for 12 d. The media were freshly replaced with AEPB for every 3 d. Hydrogen peroxide (H2O2; 300 μM) was used as the positive control. (A) The cell viability was determined using flow cytometry. (B) Viable and (C) dead cell populations. Each bar indicates mean ± SEM,n=3, *P < 0.05 vs. untreated cells.
3.2. AEPB promotes ALP activity and calcium deposition in preosteoblast MC3T3-E1 cells
To address whether AEPB induces mature osteoblast in preosteoblast MC3T3-E1 cells, ALP activity and mineralization were investigated. As shown in Figure 2A, AEPB at 5 μg/mL moderately increased ALP activity compared with that in the untreated cells,and AEPB at the increasing concentrations remarkably stimulated ALP activation. GP, as a bone-stimulating agent, significantly upregulated ALP activity. These data indicate that AEPB induces the maturation of osteoblasts. Furthermore, to evaluate whether AEPB increases Ca2+deposition in MC3T3-E1 cells, alizarin red staining was performed on day 12. Consistent with the data on ALP activity,AEPB markedly increased Ca2+deposition compared to that in the cells treated with GP (Figure 2B), suggesting that AEPB enhances calcification in MCT3C-E1 cells. Taken together, these data indicate that AEPB stimulates the full maturation of osteoblasts, leading to calcification.
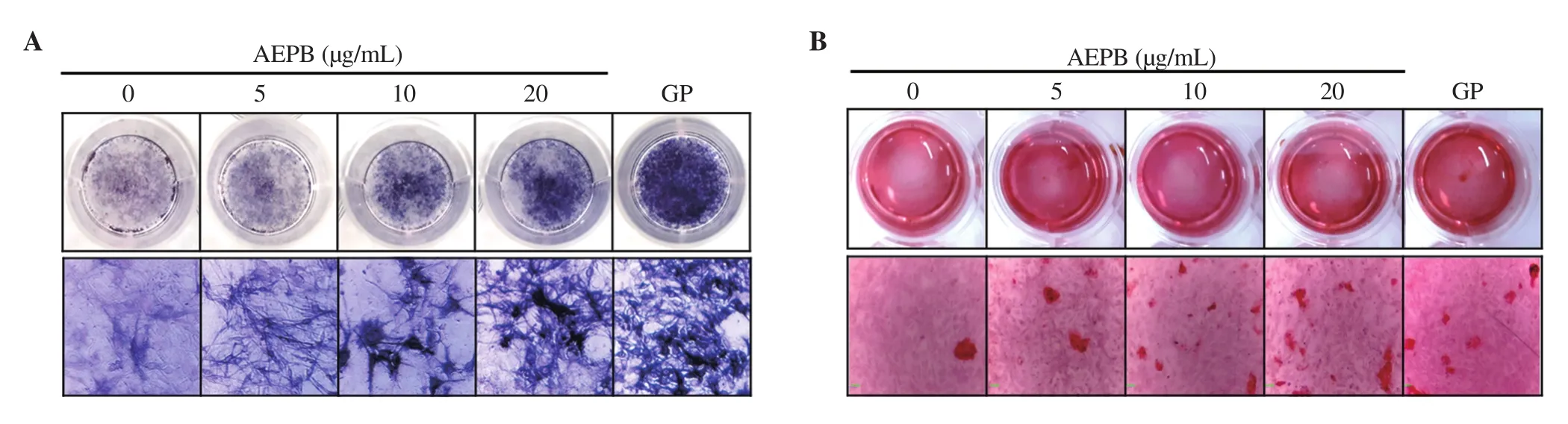
Figure 2. Effect of AEPB on osteoblast differentiation and calcification. MC3T3-E1 cells (1 × 104 cells/mL) were treated with APEB (0-20 μg/mL) for 12 d.β-Glycerophosphate (GP, 2 mM) was used as the bone-stimulating positive control. (A) ALP activity was detected using a TRACP & ALP Double Staining Assay Kit. (B) Calcification in MC3T3-E1 cells was measured using 2% alizarin red. The images were captured using a phase contrast microscope (×10).
3.3. AEPB enhances the expression of osteogenic markers including RUNX2, OSX, and ALP
To confirm whether AEPB increases the expression of osteogenic markers including RUNX2, OSX, and ALP, RT-PCR and Western blotting were performed 12 d after AEBP treatment. As expected,AEPB upregulated all genes tested in this study, including RUNX2,OSX, and ALP, in a dose-dependent manner, and the highest concentration of AEPB increased the expression compared to that in GP-treated cells (Figure 3A). Consistent with the RT-PCR data, all tested proteins gradually increased in MC3T3-E1 cells (Figure 3B).The data indicate that AEPB is an activator of osteogenic markers including RUNX2, OSX, and ALP.
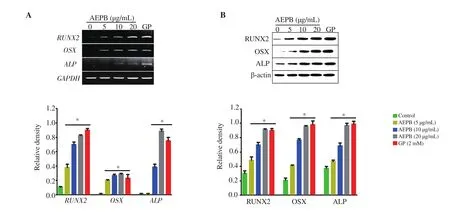
Figure 3. Effect of AEPB on the expression of osteogenic markers in preosteoblast MC3T3-E1 cells. MC3T3-E1 cells (1 × 104 cells/mL) were treated with the indicated concentrations of APEB (0-20 μg/mL) or β-glycerophosphate (GP, 2 mM) for 12 d. (A) Total RNA was extracted using an easy-BLUE Total RNA Extraction Kit. RT-PCR was performed to determine the expression of RUNX2, OSX, and ALP, while GAPDH was used as the internal control. (B) Protein expression was determined using Western blotting. β-Actin was used as the internal control. β-Glycerophosphate (GP, 2 mM) was used as the gene-stimulating positive control. Each bar indicates mean ± SEM, n=3, *P < 0.05 vs. untreated cells.
3.4. AEPB promotes bone formation in zebrafish larvae accompanied by high expression of the osteogenic genes RUNX2a, OSX, and ALP
Next, we investigated whether AEPB enhanced osteogenic gene expression and bone formation in zebrafish larvae. The data based on calcein staining showed that AEPB accelerated vertebral formation in a dose-dependent manner (Figure 4A). GP also markedly increased vertebral formation. The median vertebral numbers were significantly increased in zebrafish larvae treated with 100 μg/mL AEPB (6.33 ± 0.33) compared with that in the untreated larvae(3.33 ± 0.33), and maximally reached (7.33 ± 0.33) in 200 μg/mL AEPB-treated larvae (Figure 4B). The vertebra-forming ability of GP was observed to be the strongest (9.33 ± 0.33). Although, 50 μg/mL AEPB slightly increased vertebral formation (5.33 ± 3.33), the effect was not significant. In addition, we evaluated osteogenic gene expression in zebrafish larvae at 12 dpf after AEPB treatment. AEPB significantly upregulated osteogenic genes including RUNX2a,OSX, and ALP in a dose-dependent manner (Figure 4C). These data indicate that AEPB is a bone-stimulating candidate that activates the expression of osteogenic genes.
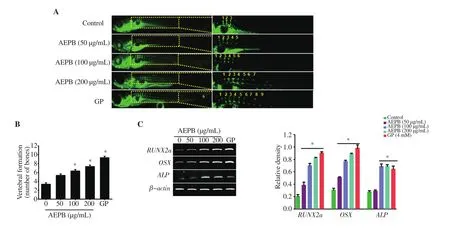
Figure 4. Effect of AEPB on vertebral formation and osteogenic genes in zebrafish larvae. (A) Zebrafish larvae (n=20) at 3 days post-fertilization (dpf) were treated with the indicated concentration of AEPB (0-200 μg/mL) and 4 mM β-glycerophosphate (GP) and stained with 0.05% calcein to visualize vertebral formation at 12 dpf. (B) The number of vertebrae was counted manually. (C) Total RNA was extracted from zebrafish larvae at 12 dpf, and RT-PCR was performed to determine the expression of RUNX2a, OSX, and ALP. β-Actin was used as the internal control. Each bar indicates mean ± SEM, n=3, *P < 0.05 vs.untreated zebrafish.
3.5. AEPB accelerates osteoblast differentiation and bone formation by activating the Wnt/β-catenin signaling pathway
RT-PCR data showed that FH535 markedly downregulated AEPB-induced high expression of osteogenic genes including RUNX2,OSX, and ALP, in MC3T3-E1 cells (Figure 5A). Western blotting showed that FH535 strongly inhibited AEPB-induced nuclear β-catenin in MC3T3-E1 cells (Figure 5B). Furthermore, calceinstained zebrafish larvae showed that FH535 markedly reduced AEPB-induced vertebral formation (Figure 5C). In particular, the number of vertebrae in zebrafish larvae showed that AEPB-induced vertebral formation was significantly reduced in the presence of FH535 from 7.33 ± 0.33 to 3.67 ± 0.33 (Figure 5D). The vertebral number in untreated and FH535-treated larvae had almost similar levels (3.00 ± 0.58 and 2.00 ± 0.33), respectively. Consistent with vertebral formation, RT-PCR results showed that AEPB activated the expression of RUNX2a, OSX, and ALP, while FH535 reduced their expression (Figure 5E). The above data indicate that AEPB-induced osteogenic gene expression and vertebral formation are regulated by the activation of the Wnt/β-catenin signaling pathway.
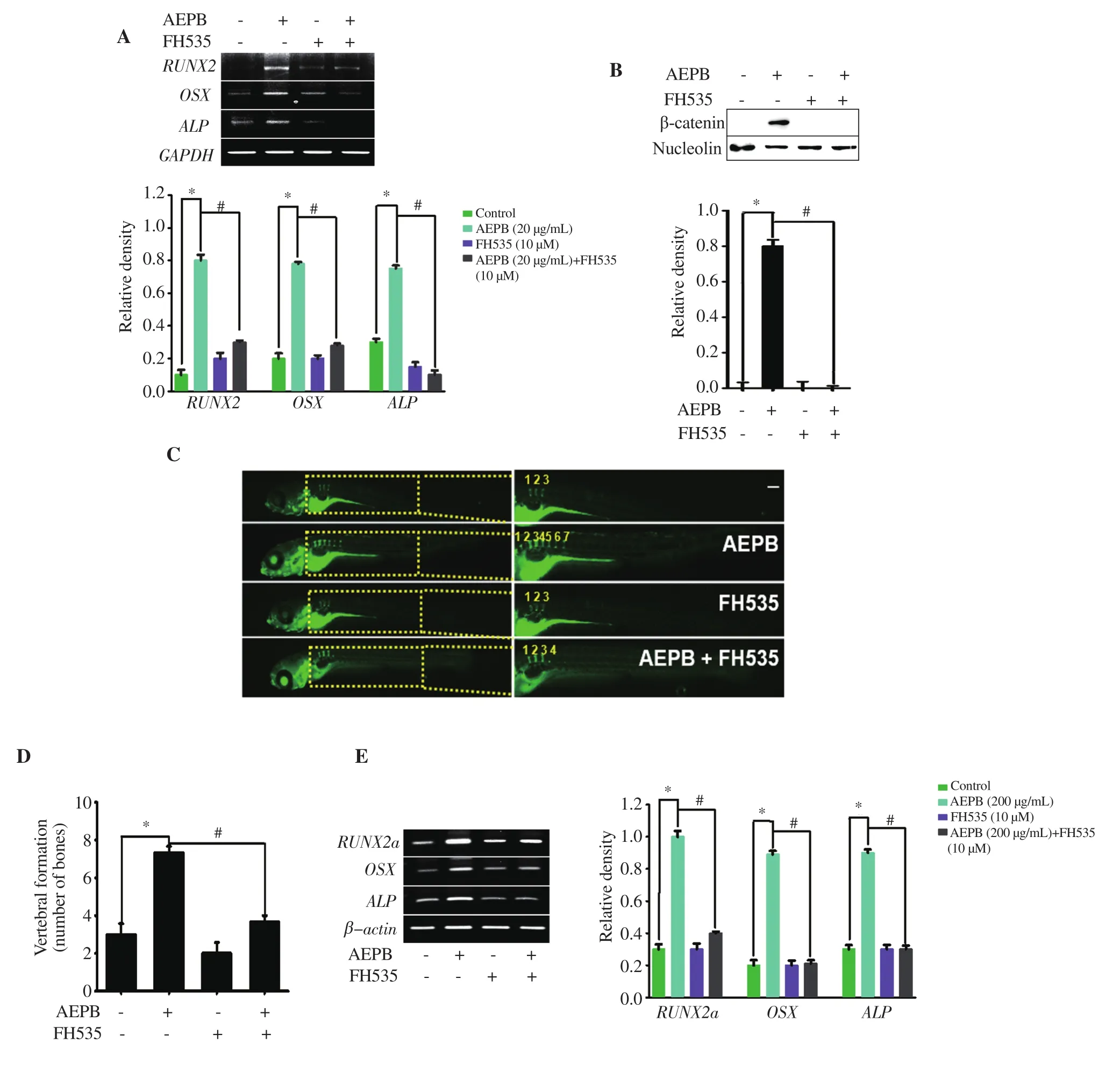
Figure 5. Effect of AEPB on osteogenic gene expression and vertebral formation related to the Wnt/β-catenin signaling pathway. MC3T3-E1 cells (1 × 104 cells/mL) were treated with 20 μg/mL AEPB in the presence and absence of 10 μM FH535 for 12 d. (A) Total RNA was extracted and RT-PCR was performed to evaluate expression of RUNX2, OSX, and ALP. GAPDH was used as the internal control. (B) Nuclear protein was extracted using a NE-PER Nuclear Protein Extraction Kit, and Western blotting was performed to determine the expression of β-catenin. Nucleolin was used as the internal control. (C) Zebrafish larvae at 3 days postfertilization (dpf) were treated with 10 μM FH535 24 h before treatment with 200 μg/mL AEPB. The zebrafish larvae at 12 dpf were stained with 0.05% calcein to visualize vertebral formation. (D) The number of vertebrae was counted manually. (E) In a parallel experiment, RNA was extracted from zebrafish larvae at 12 dpf, and RT-PCR was performed to detect expression of RUNX2a, OSX, and ALP. β-Actin was used as the internal control. *P < 0.05 and#P < 0.05 vs. untreated control and AEPB-treated group, respectively.
4. Discussion
Osteoporosis is a skeletal disorder characterized by increased bone resorption accompanied by a loss of bone mass and bone mineral density via the inactivation of osteoblasts and overactivation of osteoclasts[24]. As the elderly population increases worldwide,the number of osteoporotic patients steadily increases, and the prevention and management of osteoporosis is a social issue. Hence,many scientists have attempted to discover natural compounds and nutrients for osteoblast activation[25,26]. In the present study, we evaluated the potential of AEPB in osteoblast differentiation and bone formation because it is not known whether AEPB regulates osteogenesis, although P. brevitarsis has been used as an edible insect and traditional remedy with abundant nutrients[15,17,18,20]. In this study, we found that AEPB promotes osteoblast differentiation and bone formation by activating the Wnt/β-catenin signaling pathway.Osteoblast differentiation is characterized by the expression of transcription factors such as RUNX2, OSX, and ALP prior to the formation of extracellular matrix synthesis and mineralization[27].RUNX2 is a key factor for osteoblast differentiation, ECM production, and mineralization by stimulating major bone matrix component genes[28,29]. Previously, RUNX2-deficient mice completely inhibited OSX expression along with the loss of ALP activity and mineralization[30], whereas RUNX2 was normally expressed in OSX-deficient mice[31], indicating that RUNX2 is upstream of OSX in osteoblast differentiation. As a downstream transcription factor of RUNX2, OSX is specifically expressed in preosteoblasts to mature osteoblasts, and OSX-null embryos fail in bone formation[30]. In the present study, we found that AEPB promotes osteoblast differentiation characterized by elevated levels of RUNX2, OSX, and ALP concomitant with mineralization in MC3T3-E1 cells and vertebral formation in zebrafish. The data show the potential of AEPB as an osteogenic supplement. Nevertheless,Shrivats et al.[32] demonstrated that RUNX2 and OSX siRNA delivery significantly reduced mineralization in osteoblast cells accompanied by inhibition of ALP activity, which indicates that osteogenesis is regulated in a complicated manner. Therefore, the detailed effects of AEPB at each stage of osteoblast development should be evaluated.Additionally, the osteogenic activity of AEPB should be evaluated in higher organisms such as mammals because this study demonstrated that AEPB stimulated bone formation in osteoblast cell lines and zebrafish larvae.
The Wnt/β-catenin signaling pathway is known to promote preosteoblast differentiation from BM-MSCs and subsequently regulate the maturation and terminal differentiation of osteoblasts[8].The canonical Wnt signaling pathway inhibits ubiquitination and degradation of β-catenin and promotes the release of β-catenin from glycogen synthase-3β[33]. Free β-catenin translocates into the nucleus and binds to the N-terminal domain of the TCF/LEF transcription factor, which transactivates key osteoblastic genes,such as RUNX2 and OSX[34]. In this study, we found that AEPB increased the expression of osteogenic genes, including RUNX2,OSX, and ALP, and activated nuclear translocation of β-catenin in preosteoblast MC3T3-E1 cells. However, FH535, an inhibitor of Wnt/β-catenin, inhibited AEPB-induced activation. Furthermore,FH535 reduced AEPB-stimulated vertebral formation and osteogenic gene expression in zebrafish larvae, indicating that AEPB-stimulated osteogenesis is positively regulated by activating the Wnt/β-catenin signaling pathway. Nevertheless, FH535 cannot completely inhibit AEPB-induced osteogenic gene expression and vertebral formation,suggesting that other osteogenic transcription factors are also related to AEPB-induced osteogenesis. Apart from Wnt/β-catenin,there are many transcription factors, such as bone morphogenetic proteins and insulin-like growth factor, which are involved in osteogenesis[35], although β-catenin has been identified as a major mediator in AEPB-induced osteogenesis. Therefore, it is important to investigate whether AEPB regulates other transcription factors during osteogenesis in future studies.
In conclusion, we found that AEPB promoted osteoblast differentiation of MC3T3-E1 cells and vertebral formation in zebrafish larvae and elevated the expression of osteogenic markers by activating the Wnt/β-catenin signaling pathway. Although we report AEPB-induced osteogenic activity, further studies are needed to support the application of AEPB in treating bone diseases, such as osteoporosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Funding
This work was supported by Korea Environment Industry &Technology Institute through Project to Make Multi-ministerial National Biological Research Resources more Advanced funded by Korea Ministry of Environment (No.: 1485018221)and Basic Science Research Program to Research Institute for Basic Sciences of Jeju National University through the National Research Foundation of Korea funded by the Ministry of Education(2019R1A6A1A10072987).
Authors’ contributions
JACCJ, CHK, MHL, and GYK designed the project. JACCJ, KTL,CHK, and MHL performed the experiments and analyzed data.JACCJ drafted the manuscript. YHC and GYK performed critical revision of the article. GYK supervised all experiments. All authors have read and approved the final manuscript.
 Asian Pacific Journal of Tropical Biomedicine2022年3期
Asian Pacific Journal of Tropical Biomedicine2022年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress
- Phloretin-induced suppression of oxidative and nitrosative stress attenuates doxorubicin-induced cardiotoxicity in rats
- Non-alcoholic fatty liver disease: Epidemiology, pathophysiology and an update on the therapeutic approaches
