Effects of enzymatic and non-enzymatic antioxidants in diluents on cryopreserved bull epididymal sperm
Hasan Sakhdary, Abbas Farshad?, Jalal Rostamzadeh, Fateme Bahri Binabaj, Keyvan Sobhani
1Laboratory of Reproduction Biology, Department of Animal Sciences, Faculty of Agriculture, University of Kurdistan, Sanandaj, Kurdistan, Iran
2Department of Animal Sciences, Faculty of Agriculture, Gonbad Kavous University, Gonbad Kavous, Gorgan, Iran
ABSTRACT
Objective: To evaluate the supplementation effects of vitamin E,vitamin C, superoxide dismutase (SOD), and catalase to diluents on bull cryopreserved epididymal sperm.
Methods: Sperm were retrieved from 20 bull testes and were then supplemented with 0.1 mM vitamin E, 5.0 mM vitamin C, 100.0 IU/mL SOD, and 100.0 μg/mL catalase alone, or in a combination. The control treatment contained no addition. After supplementation, samples were frozen and stored in liquid nitrogen.The sperm parameters including motility, progressive motility,viability, acrosome integrity, plasma membrane integrity, kinematics and DNA damage were evaluated following the thawing process.
Results: Vitamin E alone significantly increased the parameters of acrosome and membrane integrity compared to the control treatment (P<0.05). While compared to the control treatment,vitamin C had no improvement effect on sperm characteristics except for membrane integrity. Treatment of vitamin E+vitamin C had a significant improvement in total motility, progressive motility, viability, membrane and acrosome integrity compared to the control and other treatments (P<0.05). Compared to the control treatment, addition of SOD or catalase alone significantly improved the percentages of total motility, progressive motility,viability, membrane and acrosome integrity (P<0.05). Furthermore,SOD+catalase significantly increased total motility, progressive motility, viability, acrosome and membrane integrity characteristics compared to the catalase treatment (P<0.05). Vitamin E alone,vitamin E+vitamin C, and SOD in diluents decreased DNA damages and thereby improved the rate of intact sperm heads.
Conclusions: Addition of 100.0 IU/mL SOD alone and 0.1 mM vitamin E+5.0 mM vitamin C, and also 5.0 mM vitamin C+100 μg/mL catalase in a combination improves the quality of cryopreserved bull epididymal sperm and could be used for cryopreservation.
KEYWORDS: Antioxidant; Cryopreservation; Spermatological characteristics; DNA damage; Bull; Vitamin E; Vitamin C;Superoxide dismutase; Catalase; Oxidative stress
Significance
Cryopreservation process reduces the number of internal antioxidants in mammalian sperm cells. Moreover, during the freezing process, large volumes of reactive oxygen species are formed, so the antioxidant system is not enough to protect sperm. Evidence is recommended to strengthen the semen diluent by adding antioxidants to improve and increase the quality and fertility of semen. Therefore, this study indicated that the enzymatic and non-enzymatic antioxidants together could present significantly a better effect to epididymal bull sperm and are available to use for freezing bull epididymal sperm.
1. Introduction
Although bull semen is frequently cryopreserved, there are many deleterious effects on frozen-thawed sperm such as the decrease in motility, damage in the plasma membrane, DNA integrity and sperm mitochondrial membrane potential, and also the generation of lipid peroxidation (LPO)[1,2]. Considering the effect of diluents on the quality of cryopreserved sperm, there is a strong impetus to improve the effectiveness of the diluent and establish the freezing method[3]. Due to their lipid composition and fatty acid ratios, sperm membranes are very sensitive to temperature changes during the cryopreservation process which demonstrates a damaging potential to cells[1,2]. Therefore, various antioxidants have been used to increase the resistance capacity of sperm against lethal temperature changes and damage due to free radicals and oxidative stress[4-7].
In regard to types of used antioxidants, there are enzymatic and non-enzymatic antioxidants. Enzymatic antioxidants are known as natural antioxidants including glutathione peroxidase, glutathione reductase, superoxide dismutase (SOD) and catalase (CAT)[8], all participating in sperm natural antioxidant defence system[8]. Nonenzymatic antioxidants are also referred to as synthetic antioxidants or dietary supplements including glutathione, vitamin C (VC),vitamin E (VE), urate, ubiquinone, carotenoids (β-carotene), taurine and hypo-taurine, selenium, zinc, ubiquinone[8] and butylated hydroxytoluene[9,10] as well as plant extract[11].
VC, a water-soluble analogue of VE, promotes the peroxidation of unsaturated lipids in the cell membrane and protects intracellular phospholipid capacity[12]. The positive effects of VE analogue on membrane integrity have been also reported during the freezing process[12]. Moreover, VE is a highly potent chain-breaking lipophilic antioxidant residing on the cell membrane which can break the covalent links that radical oxygen species (ROS) have formed between fatty acid side chains in membrane lipids[13].
CAT is a common enzyme found in all living organisms exposed to O2. CAT, an enzymatic antioxidant exists in peroxisomes, which can convert H2O2metabolism to H2O and O2and neutralize the toxic effects of free radicals[14]. For instance, CAT antioxidant can protect the sperm from oxidative damage during the cryopreservation process[8] and thereby enhance the rate of viability in different species such as red deer[15], boar[16], and dog[17].
SOD is an important enzyme that helps protect cells from oxidative damage by ROS, and it has also been found that a molecule of SOD can convert millions of molecules of hydrogen peroxide to water and oxygen[18]. In this context, much evidence reported from different antioxidants in freezing media showed the negative effects caused by ROS on sperm could be reduced[18-20].
Studies of scientific sources show that different antioxidants have been used in the cryopreservation of bovine sperm which often contain ejaculate semen. In regards to epididymal sperm, evidence shows few reports using the above-mentioned protectants for cryopreservation. In addition, there are currently very few reports on the effect of enzymatic and non-enzymatic antioxidants on sperm characteristics. Therefore, the present study was conducted to evaluate the supplementation effects of VC, VE, CAT, and SOD alone or in combinations with a basic diluent on bull cryopreserved epididymal sperm characteristics.
2. Materials and methods
2.1. Chemicals
All chemicals used in the experiment were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany).
2.2. Collection of testes and separation of epididymal sperm
A total of 20 testicles from ten healthy mature bulls were obtained from a local abattoir and transferred within 30 min using a cooled insulated container to the laboratory. The epididymis has been removed from the testicular tissue making several longitudinal incisions. The samples were putted into the Tyrode lactate solution consisting of 100 mM NaCl, 3.1 mM KCl, 25 mM NaHCO3, 0.29 mM NaH2PO4H2O, 21.6 mM Na lactate, 2.1 mM CaCL22H2O, 0.4 mM MgCL26H2O, 10 mM HEPES buffer,0.000 6 g/mL bovine serum albumin, 1 mM sodium pyruvate,25 μg/mL Gentamycin, and 10 mg/L phenol red for 15 min at 37 ℃.Sperm suspension was centrifuged at 700 ×g for 10 min and then the sperm were used to continue the experiments. Sperm with total motility >80%, morphological normalities >90% and a final concentration 250×106/mL were used for cryopreservation.
2.3. Preparation of separated epididymal sperm and treatments
The treatments included a basic diluent composed of 3.07 g Tris 108382, 1.26 g fructose 104007, and 1.64 g citric acid 818707 in 100 mL distilled water containing 10% (v/v) egg yolk and 5%(v/v) glycerol. The pH and the osmolality of diluents were set at 7.2 and 320 mOsm/kg, respectively. The preparation of experimental treatments (n=11) was carried out as follows: the basic diluent was supplemented with 0.1 mM VE, 5.0 mM VC, 100.0 IU/mL SOD and 100.0 μg/mL CAT, based on our previously finished and not published study, alone and in a combination with each other. The control contained no antioxidant. Each treatment consisted of 8 replicates. Diluted samples were loaded into 0.25 mL French straws and exposed to 4°℃-5°℃ for a period of 2 h. The equilibration straws were frozen in liquid nitrogen vapours, 3-4 cm above liquid nitrogen within 12 min and then plunged into liquid nitrogen for storage. And 24 h after storage, the cryopreserved samples were thawed at 37 ℃ for 35 s using a water bath and were evaluated for different parameters.
2.4. Sperm motility and velocity parameters
The sperm motility and standard motion parameters were determined by computer-assisted sperm motility analysis (CASA:IVOS version 12; Hamilton-Thorne Biosciences, MA, USA) system.The software was also equipped with a positive phase-contrast microscope (Nikon, Tokyo, Japan), a digital video camera (Samsung,SDC-313B, Korea), a hot plate (37 ℃), and a computer for the analysis of sperm motility (Hoshmand Fanavaran, Tehran, Iran). The assessment of cryopreserved sperm characteristics was progressive motility (%), total motility (%), curvilinear velocity (VCL, μm/s),straight-line velocity (VSL, μm/s), average path velocity (VAP,μm/s), the amplitude of lateral head displacement (ALH,μm), beat/cross frequency (BCF, Hz), linearity (LIN%=VSL/VCL), wobble VAP/VCL (WOB), and straightness (STR%=VSL/VAP). Straws were expelled from liquid nitrogen and thawed at 37 ℃ for 1 min. Then, 5 μL of samples were placed on pre-warmed slides (37 ℃) and covered with a coverslip. Images were analysed under a phase-contrast objective microscope at ×100 magnification.The CASA software captured 50 images per second, and finally 150 images from eight fields and 25 sperm per field were evaluated.
2.5. Assessment of sperm viability
The rate of sperm viability was evaluated by using the eosinnigrosine staining as follows: In brief, 5 μL diluted sperm was mixed with 10 μL prepared eosin-nigrosine-stain solution smearing on a slide which was then let to air-dry in a dust-free environment. Next,200 sperm were counted under a bright-field microscope (Nikon PCM 2000TMPersonal Laser Scanning Confocal Microscope, USA)at 400× magnification and the unstained sperm were determined as live sperm. The counted cells were given in the percentage values.
2.6. Acrosomal integrity and hypo-osmotic swelling test (HOST)
The morphological intact acrosome of sperm was evaluated using the formalin-citrate buffer (96 mL 2.9% sodium citrate+4 mL 37.0% formaldehyde) according to the description by Daramola and Adekunle[21]. A drop of semen-buffer mixture was placed on slides and covered with a cover glass and observed under the optical microscopy using an oil-immersion objective with a magnification of ×100. Spermatozoa was at a density of 200 cells/slide. The results were expressed as percentages.
The functional membrane integrity of cryopreserved sperm was assessed based on HOST. The evaluation was carried out by the addition of 20 μL of sperm samples to 200 μL hypo-osmotic solution (9.0 g fructose+4.9 g sodium citrate/1 liter distilled water)and incubated at 37 ℃ for 60 min. Then, 100 μL of the mixture was smeared by coverslips on slides which were prewarmed to 37 ℃. And 200 sperm were counted from 5 different fields and the sperm with swollen and curled tails were counted and expressed as percentages.
2.7. Determination of DNA damage
Sperm DNA fragmentation caused by the cryopreserved process was investigated using the Sperm Chromatin Dispersion test. In brief, five slides were prepared for each of the treatment protocols. Slides were soaked in 150 μL of 0.65% agarose, covered with a slip and stayed to solidify at 4 ℃ for 20 min. Next, the coverslips were removed carefully following solidification, and 30 μL of sperm samples were mixed with 70 μL of 0.7% low-melting-point agarose, covered again with slips and air-dried. Subsequently, the coverslips were removed and the slide samples were instantly immersed horizontally in a freshly prepared acid denaturation solution (0.08 N HCl) at 37 ℃ for 7 min in a dark medium to generate restricted single-stranded DNA motifs from DNA breaks. The denaturation was stopped, and the protein contents were removed by transferring the slides into a tray with neutralizing and lysis solutions (0.4 M Tris base, 0.8 M DTT, 50 mM EDTA, 2 M NaCl and 1% SDS, pH 7) at room temperature for 25 min. The slides were completely rinsed in distilled water for 5 min, then dehydrated in sequential concentrations of ethanol (70%, 90%, and 100%) for 2 min, and finally air-dried. Cells were stained with 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) at a concentration of 0.2 mg/mL for counterstaining spermatozoa to visualize them under fluorescence microscopy (Olympus BX5l, Japan) and using Comet score software.
2.8. Data analysis
Normality of data and homogeneity of variances were examined using the PROC UNIVARIATE by the SAS v8.0 software. The results showed that data for all investigated traits were normal and homogeneity of variances was assessed. Analysis of variance was conducted using Proc GLM of SAS (version 9.1, SAS Institute,2002, Cary, NC, USA) in a Completely Randomized Design. The model used is presented as follows:

Where Yij=Each individual observation for a given variable,μ=overall mean, Ti=Treatment effect, Eij=Residual error.
The differences between the treatments were determined using orthogonal contrasts. The independent groups’ comparisons included control vs. VE, control vs. VC, control vs. VE+VC, control vs. SOD,control vs. CAT, control vs. SOD+CAT, SOD vs. SOD+CAT, CAT vs. SOD+CAT, control vs. VE+SOD, control vs. VE+CAT, control vs. VC+SOD and control vs. VC+CAT. Means were compared using Duncan’s new multiple range test, and data were expressed as the mean±standard deviation (mean±SD). Values were considered significant when P<0.05.
2.9. Ethics approval
All experimental procedures in this study were performed according to the international guidelines and were approved with the number 9515005106 on 18 February 2018 by the Animal Care and Use Committee of the University of Kurdistan, Sanandaj, Iran.
3. Results
3.1. Sperm characteristics in semen supplemented with nonenzymatic antioxidants alone and in combination
The data presented in Table 1 show that VE (0.1 mM) significantly improved membrane and acrosome integrity compared to the control treatment (P<0.05). However, compared to the control treatment,addition of VC (5.0 mM) into the diluent showed no significant effect in acrosome integrity (P>0.05). VC had no improvement effect on sperm characteristics except for membrane integrity. Treatment of VE+VC had a significant improvement in total motility, progressive motility, viability, membrane and acrosome integrity compared to the control and other treatments (VE, VC alone) (P<0.05). The lowest DNA damage was observed in diluent supplemented with VE+VC compared to the control and other treatments. Regarding VCL, VSL,VAP, LIN, WOB, and STR characteristics, VE+VCT treatment had a significant improvement compared to the control and other treatments (P<0.05).
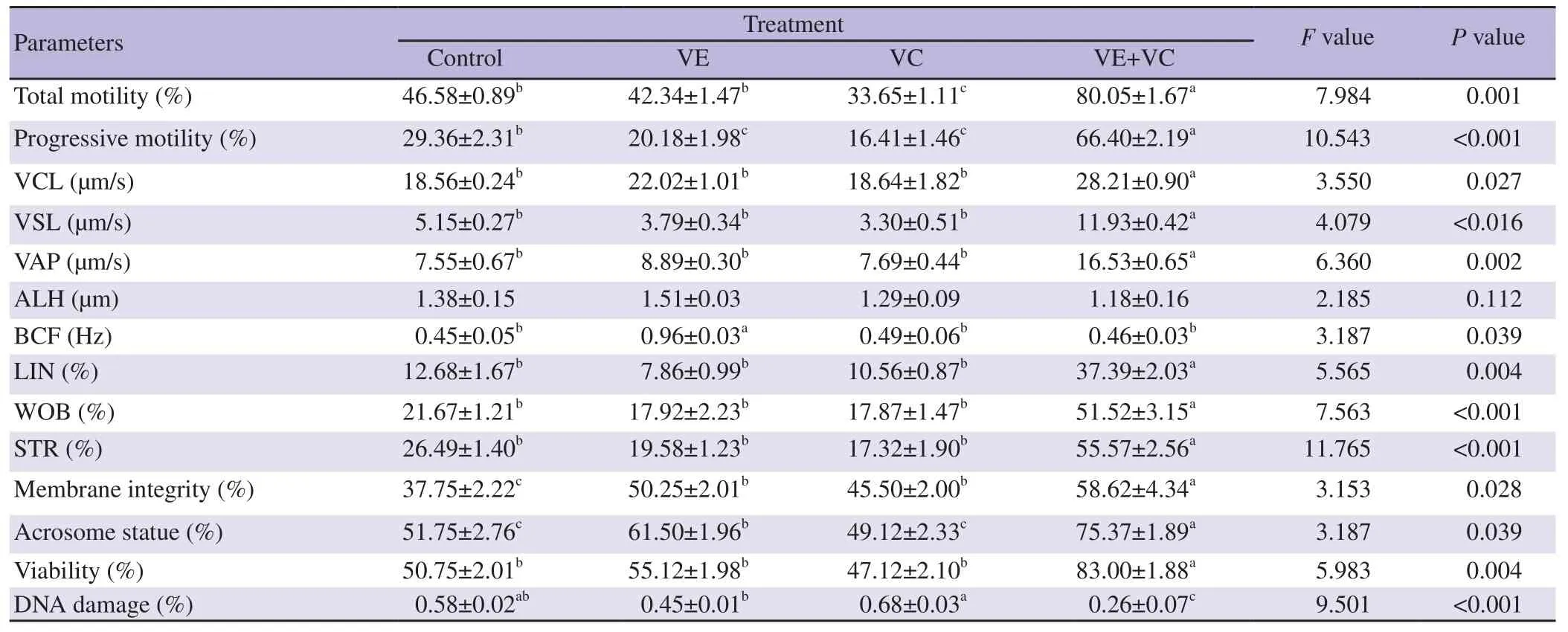
Table 1. Effect of the addition of non-enzymatic antioxidants in the freezing diluent on the post-thaw bull epididymal sperm characteristics.
3.2. Sperm characteristics in semen supplemented with enzymatic antioxidants alone and in combination
The effects of the SOD (100 IU/mL) and CAT (100 μg/mL) on the bull epididymal sperm characteristics are illustrated in Table 2. The addition of SOD and CAT alone to the freezing diluent improved total motility, progressive motility, viability, acrosome and membrane integrity in comparison with the control treatment(P<0.05). Furthermore, SOD+CAT significantly increased total motility, progressive motility, viability, acrosome and membrane integrity characteristics compared to the control treatment (P<0.05).Our results showed higher spermatozoa total motility, progressive motility, viability, and acrosome, and membrane integrity in diluent supplemented with SOD+CAT antioxidants compared to CAT treatment alone (P<0.05). Regarding VSL characteristic, SOD+CAT treatment demonstrated a significant increase compared to the control and other treatments (P<0.05). However, the lowest DNA damage was observed in diluent supplemented with SOD alone compared to the control and other treatments.
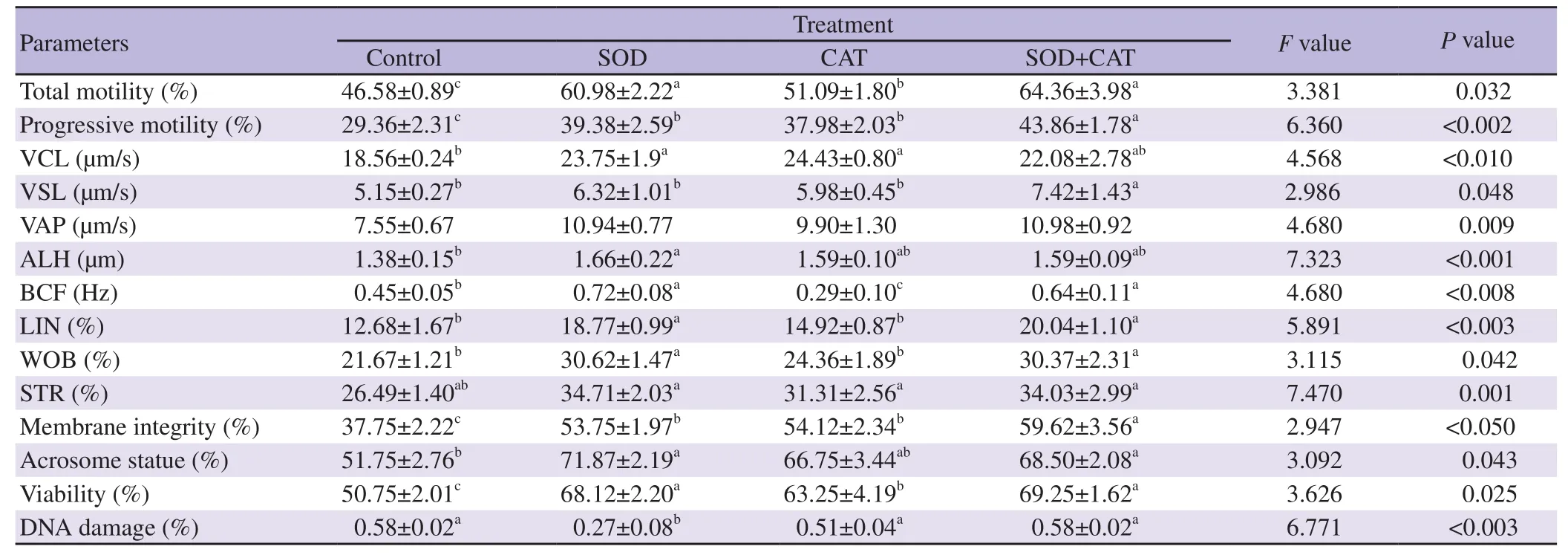
Table 2. Effect of the addition of enzymatic antioxidants in the freezing diluent on the post-thaw bull epididymal sperm characteristics.
3.3. Sperm characteristics in semen supplemented with nonenzymatic and enzymatic antioxidants in combination
Table 3 shows the effects of enzymatic and non-enzymatic antioxidants in combination on thawed bull sperm characteristics.Our data indicated that VE+SOD and VE+CAT treatments caused an increase in total motility, progressive motility, membrane and acrosome integrity compared to the control treatment(P<0.05). Acrosome integrity and viability were highest in semen supplemented with VC+CAT, compared to the control and other treatments. Moreover, the VC+CAT treatment indicated the lowest DNA fragmentation among all treatments, but there was no significant difference (P>0.05). A significant decrease in VCL, VSL,VAP, ALH, LIN and STR was observed in the treatment treated with VC+SOD compared to the other treatments (P<0.05).
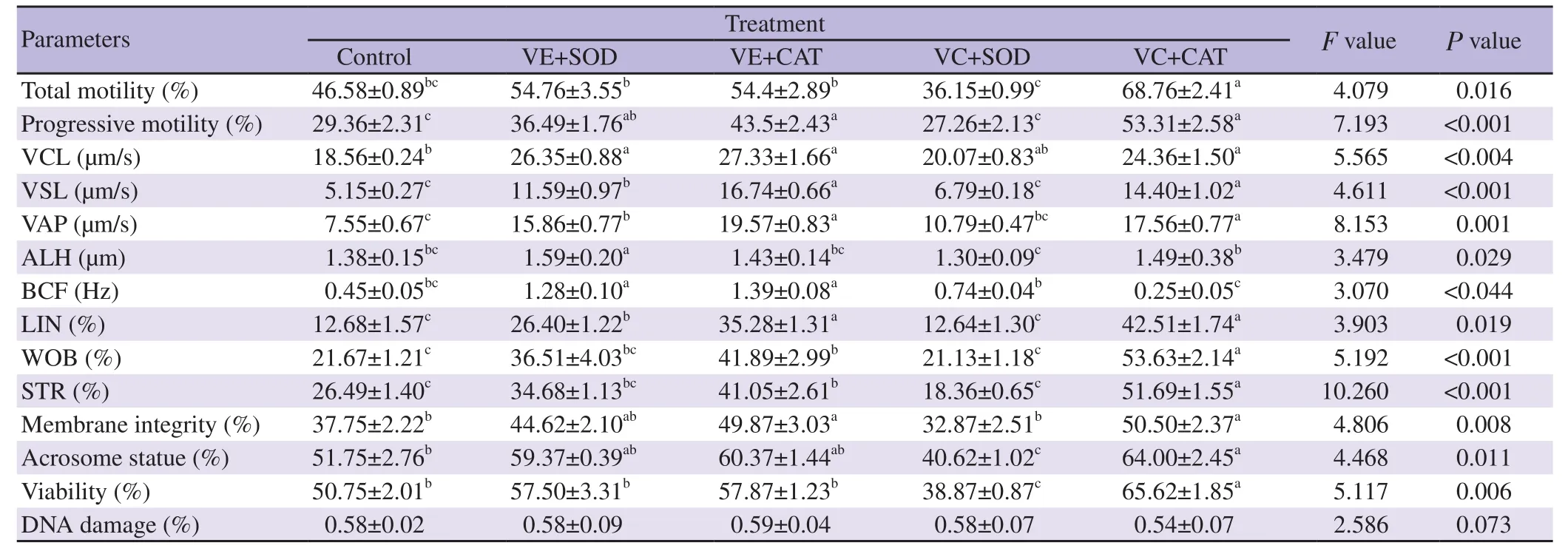
Table 3. Effect of the addition of non-enzymatic and enzymatic antioxidants in the freezing diluents on the post-thaw bull epididymal sperm characteristics.
4. Discussion
Evidence indicated that the cryopreservation process is partly associated with the oxidative attack and the generation of ROS.This condition is involved in an oxidative attack of the bis-allylic methylene group of membrane phospholipids leading to lipid peroxidation. Regarding lipid peroxidation, the sperm membrane characteristically contains a high level of polyunsaturated fatty acids lacking a significant cytoplasmic component containing antioxidants which can make the sperm cells more sensitive to LPO by free radicals such as superoxide, hydrogen peroxide, and hydroxyl radicals[22,23].
It was obvious from the data of this experiment that the addition of VE to the semen diluent resulted in a significant increase in sperm membrane and acrosome integrity of cryopreserved sperm during the freeze-thawing process. These results are in agreement with several studies[12,24-26], which reported that functional traits of sperm will increase when the addition of VE to the semen diluent. In another study, Michael et al[27] showed that inclusion of 0.1 mM VE in diluents significantly decreased LPO, and also significantly improved motility, progressive motility, viability, and membrane integrity in the cryopreserved canine sperm. Therefore, the higher values obtained in this research may be attributed to the beneficial effect of VE through its protective action on the sperm cell membrane against ROS and LPO during the cryopreservation process[28].
In the present study, it seems that the 5.0 mM VC level is not optimal for keeping sperm viability and motility. These results are in agreement with several studies (on ram and bull)[12,29,30]which reported the addition of VC to the diluent did not improve the motility of spermatozoa. The reason for the decrease may be the formation of intracellular ice crystals and ROS production[28].Moreover, low concentration, acidity, decrease in pH of semen after adding VC to the diluent can be possible reasons for not improving sperm parameters.
SOD leads to increased fluidity and membrane viability[31]. In the current study, SOD significantly increased viability, motility,and membrane and acrosome integrity parameters compared to the control. These results are in agreement with the findings of Amini et al[31], Roca et al[16], and Perumal[32] who demonstrated that the addition of SOD to the diluent increased sperm viability. In another study, a positive relationship was found between SOD activity and human sperm motility, so that a decrease in semen antioxidant capacity, especially SOD activity, could be responsible for male infertility[33]. In addition, improvement of sperm parameters,especially motility, in the SOD-containing treatment may be due to the positive effects SOD on the membrane and acrosome integrity[23,34] and its contribution to the integrity of the acrosome and plasma membrane, leading to improved motility with protecting sperm against oxidative stress[23,31].
In the present study, addition of CAT at 100.0 μg/mL into diluent was optimal for maintaining sperm motility compared to the control.These results are in agreement with several studies on boar, ram,bull, and dog[16,18,23,27]. Also, these results are in consistent with the findings of Gungor et al[35], in which the inclusion of CAT to bull sperm diluent did not protect sperm against freezing-induced defects.It has been demonstrated that adding CAT to semen diluent could improve sperm viability in several studies[23,27,36,37]. These findings underline our results which showed that CAT improved sperm viability. These results suggest that CAT can contribute greatly to the prevention of sperm membrane LPO. Therefore, CAT activity may not be directly responsible for fertility, but rather protects the sperm against the damaging effects of increased ROS.
Our findings demonstrated that adding VE+VC to diluent had a significant effect on functional sperm parameters. In agreement with our result, Mittal et al[30] reported that the combination of VE+VC has the most profound effect in protecting sperms against ROS production and cold shock when compared to VE and VC supplemented alone in the diluent for cryopreservation. In the present study, adding VE+SOD and VE+CAT to diluent led to higher motility, viability, acrosome integrity while VC+SOD only improved VCL, VAP, and BCF compared to the control. However,VC+CAT had better results than the alone use of VC, so that it could significantly improve the parameters of total motility, progressive motility, and acrosome integrity. To date, very few information has been available on the concomitant effects of an enzymatic antioxidant with a non-enzymatic antioxidant on the parameters of freezingthawing bull sperm.
The results of this present study showed higher spermatozoa viability, acrosome, and membrane integrities in SOD+CAT-supplemented diluents compared to the control and CAT alone. In this context, using doses of 150 or 300 IU/mL SOD + 200 or 400 IU/mL of CAT led to a significant increase in both motility and progressive motility of boar sperm[16]. Indeed, Shafiei et al[38]stated that the use of 0.1 mM SOD + 400.0 IU/mL CAT significantly improved the viability, progressive motility, and membrane integrity of buck sperm. In the current study, SOD alone treatment increased viability, membrane integrity, and progressive motility, indicating the more effectiveness of SOD antioxidant compared to the control and CAT treatments. Moreover, the beneficial effects of SOD on semen are due to the fact that SOD is a very powerful antioxidant[39].
Recently, DNA degradation has also been linked to damage at the oxidation level in sperm, which is one of the causes of DNA fragmentation, leading to reduced fertility[40]. In this study, VE and SOD alone reduced DNA damage compared to the control. In agreement with our study, the inclusion of 5 mM VE[13] into human sperm diluents had significant improving effects on sperm DNA integrity. In contrast, Cabrita et al[41] reported no improvement in DNA integrity by the addition of VE (0.1 and 0.5 mM). This vitamin has been suggested to protect DNA from H2O2degradation in vitro and inhibit the production of lipid peroxides during the freezing-thawing process at the membrane surface and vital cell macromolecules, such as DNA[13]. In this context, an improvement in sperm DNA integrity from adding 10 mM VC to fish sperm diluent[42] and an increment of CAT to the human sperm diluent[14]have been reported. Moreover, the reduction of DNA damage in sperm supplemented with VE+VC and VC+CAT treatments could be due to the synergistic effects of antioxidants. In addition, the inclusion of antioxidants in sperm diluents improves DNA integrity and reduces the effects of oxidative stress[23,28] due to their defence against LPO.
There are some limitations in the study. Due to financial constraints,the availability of livestock, and the lack of laboratory facilities,we were not able to use the frozen-thawed sperm in the artificial insemination program. Given the existence of this condition, we could have a better analysis of the obtained results. Moreover, it is important to note that we do not know how the used antioxidants would affect the sperm from other species.
In conclusion, the present study results clarify that 100 IU/mL SOD antioxidant can protect cells effectively during cryopreservation processes when it is used alone to supplement bull epididymal sperm freezing diluents. According to these results, higher post-thawing sperm quality resulting from the addition of 0.1 mM VE combined with 5 mM VC and 100 μg/mL SOD antioxidants to diluents than that resulting from the addition of their alone reflects the existence of synergistic effects between these antioxidants. Finally, our findings showed using antioxidants in freezing diluents presents the ability to improve the performance of cryopreserved bull epididymal sperm. However,more information is needed to determine the precise effects of these antioxidants and to understand better their antioxidative protection mechanisms in combination during cryopreservation processes.
Conflict of interest statement
The authors declare there is no conflict of interest.
Authors’ contributions
Hasan Sakhdary and Abbas Farshad made substantial contributions to conception and design; Jalal Rostamzadeh and Fateme Bahri Binabaj conducted acquisition of data; Hasan Sakhdary, Abbas Farshad and Jalal Rostamzadeh made analysis and interpretation of data; Keyvan Sobhani, Hasan Sakhdary and Abbas Farshad drafted the manuscript; Abbas Farshad, Keyvan Sobhani critically revised the manuscript for important intellectual content. All authors had final approval of the manuscript for publication.
 Asian Pacific Journal of Reproduction2022年1期
Asian Pacific Journal of Reproduction2022年1期
- Asian Pacific Journal of Reproduction的其它文章
- Successful live birth after intracytoplasmic sperm injection using testicular sperm in non-mosaic Klinefelter syndrome
- Taurine in semen extender modulates post–thaw semen quality, sperm kinematics and oxidative stress status in mithun (Bos frontalis) spermatozoa
- Blepharis persica increases testosterone biosynthesis by modulating StAR and 3 β-HSD expression in rat testicular tissues
- Recurrent ovarian endometrioma after conservative surgery: A retrospective study
- Uptake of in-vitro fertilization among couples attending fertility clinic in a tertiary health institution
- An implementation study of barriers to universal cervical length screening for preterm birth prevention at tertiary hospitals in Thailand: Healthcare managers’ perspectives
