Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells
Sunghyun Hong, Kandhasamy Sowndhararajan, Taewoo Joo, Chanmook Lim, Haeme Cho, Songmun Kim, Gur-Yoo Kim, Jin-Woo Jhoo*
1Kangwon National University, Department of Animal Products and Food Science, Chuncheon, Gangwon, Republic of Korea, 200-701
2Department of Process and Food Engineering, Faculty of Engineering, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 43400
3Kangwon National University, Department of Biological Environment, Chuncheon, Gangwon, Republic of Korea, 200-701
Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells
Sunghyun Hong1, Kandhasamy Sowndhararajan2, Taewoo Joo1, Chanmook Lim1, Haeme Cho3, Songmun Kim3, Gur-Yoo Kim1, Jin-Woo Jhoo1*
1Kangwon National University, Department of Animal Products and Food Science, Chuncheon, Gangwon, Republic of Korea, 200-701
2Department of Process and Food Engineering, Faculty of Engineering, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 43400
3Kangwon National University, Department of Biological Environment, Chuncheon, Gangwon, Republic of Korea, 200-701
ARTICLE INFO
Article history:
Received 15 November 2014
Received in revised form 20 February 2015
Accepted 25 March 2015
Available online 20 June 2015
Antioxidant enzyme
Cannabis sativa
Hemp seed
HepG2 cells
Catalase
Superoxide dismutase
Objective:To determine the gene expression of antioxidant enzymes by hemp seed extracts in human hepatoma (HepG2) cells.Methods:Ethanol and supercritical fluid (SF) extracts obtained from de-hulled hemp seed were used for the evaluation of in vitro antioxidant activity and gene expression of antioxidant enzymes. In vitro antioxidant activities of the samples evaluated using 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) radical scavenging assays. The expression of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) in HepG2 cells was evaluated by real-time PCR.Results:In the antioxidant assay, SF extract of hemp seed exhibited higher ABTS and DPPH radical scavenging activities (IC50of 66.6 μg/mL and 9.2 mg/mL, respectively) than ethanol extract. The results of antioxidant enzyme expression in real-time PCR study revealed the H2O2(200 μM) challenged HepG2 cells reduced the expression of enzymes such as SOD, GPx and CAT. However, the cells treated with ethanol and SF extracts were up-regulated the expression of antioxidant enzymes in concentration dependent manner. When compared to ethanol extract, the SF extract exhibited higher activity in the expression of all the antioxidant enzymes at the concentration of 500 μg/mL.
Conclusion:In conclusion, the findings of our study demonstrated that the hemp seed effectively inhibited H2O2mediated oxidative stress and may be useful as a therapeutic agent in preventing oxidative stress mediated diseases.
1. Introduction
Free radicals, generated in oxidation processes, are essential for the production of energy to fuel biological processes in most of the living organisms. However, the excessive productions of free radicals such as superoxide, hydroxyl and peroxy radicals etc., which responsible for the damage of lipids, proteins and DNA in cells, leading to several degenerative diseases, including inflammation, cardiovascular diseases, cancer, diabetes, and neurological disorders[1]. Generally, all the organisms are well protected against free radical damage by endogenous oxidative enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR) and catalase (CAT). However, these enzymes are commonly insufficient when it comes to completely preventing degenerative diseases and other health problems[2, 3].
In addition, several non-enzymatic antioxidant compounds such as ascorbic acid, tocopherol, glutathione and other dietary compounds play an important role in defending the body against free radicals damage by scavenge or neutralize the oxidizing molecules and maintaining redox balance[4]. The plant kingdom offers a widerange of natural antioxidant molecules including phenolic acids, flavonoids, and other secondary metabolites. These metabolites are commonly found in a variety of fruits, vegetables, herbs, cereals, sprouts and seeds[5]. In recent years, there has been increasing interest in obtaining natural dietary antioxidants especially from plants. Previous studies have been reported that the compounds from plants provide potential health benefits, such as antioxidant, anti-inflammatory, antitumor, anticarcinogenic, and antimicrobial activities. They can also be used for the treatment of various ailments including, atherosclerosis, arthritis and diabetes[6-9].
Hemp (Cannabis sativa L.) is an annual herbaceous plant belongs to Cannabaceae family and originated in Central Asia. The plant has been grown cultivated widely for the purposes of fiber, food and medicine[10]. The seed of hemp is a rich source of nutrition, containing 25%-35% of lipid, 20%-25% of protein, 20%-30% of carbohydrate, 10%-15% of insoluble fiber and a rich array of minerals[11, 12]. The hemp seed oil contains higher level of polyunsaturated fatty acid (70%-80%), particularly linoleic (ω-6) and α-linolenic (ω-3) acids. Most of essential amino acids contained in hemp protein are sufficient for the FAO/WHO suggested requirements of children[13, 14]. The cannabinoids are the most studied constituents from the hemp seed, in particular delta-9-tetrahydrocannabinol is the main psychoactive component. In addition, several other bioactive compounds have been reported from Cannabis include terpenes, sugars, steroids, flavonoids, nitrogenous compounds and non-cannabinoid phenols[15].
In south China, hemp milk is a popular traditional drink that obtained from the crashed hemp seed meal. In Chinese traditional medicine, the kernel of hemp seed (Huo Ma Ren in Chinese) is used for the treatments of constipation, gastrointestinal diseases, and antiageing[16]. Hemp seed has several positive health benefits, including the lowering of cholesterol and high blood pressure. Hemp seed oil produced significant changes in plasma fatty acid profiles and improved clinical symptoms of atopic dermatitis[12]. Further, Nissen et al[17] studied the antimicrobial activity of essential oil of industrial hemp seed and found that the essential oil effectively inhibited the growth of the food-borne and phytopathogens. Recent studies have reported that the hemp seed has been identified as a valuable antioxidant food[18-20]. However, there is no study in related to expression of antioxidant enzymes by hemp seed. Hence, the present study was carried out to investigate the in vitro antioxidant and expression of antioxidant enzymes (SOD, GPx and CAT) activities by ethanol and SF extract of hemp seed.
2. Materials and methods
2.1. Chemicals and materials
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypan blue, hydrogen peroxide (H2O2) and penicillin-streptomycin solution purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from HyClone Laboratories, Inc.(Utah, USA). RNeasy Mini kit and SYBR green master mix were purchased from Qiagen-GmbH (Hilden, Germany). SuperScript III First-Strand synthesis system was purchased from Invitrogen (Carlsbad, CA, USA). The hemp seeds (Cannabis sativa L.) were collected from the cultivated field in Sudong area (Pyungchang, Republic of Korea). The seed sample was authenticated and deposited at Sudong Agricultural Association (Pyungchang County, Republic of Korea) with batch No. PH-2012-011-S01.
2.2. Ethanol extraction of hemp seed
About 250 g of de-hulled hemp seed powder was macerated thrice over 48 h with ethanol (food grade 95%, Daehan Ethanol Life Co., Ltd., Seoul, Republic of Korea) at room temperature. The combined ethanol extract was concentrated by low-pressure evaporation (<40 ℃).
2.3. Supercritical fluid (SF) extraction of hemp seed
Extraction from de-hulled hemp seeds was experimentally determined using supercritical fluid extraction equipment (ISASCCO-S-050-500, Ilshin Co. Ltd., Republic of Korea). About 100 g of de-hulled hemp seeds were loaded into a stainless steel extraction vessel. CO2was pressurized with a high-pressure pump and then charged into the extraction column to desired pressure. Back pressure regulators are used to set the system pressure (in extractor and separator). The SF was conducted at the temperature of 40 ℃, and pressure of 400 bar. The CO2flow rate was maintained at 30 mL/min and the extract was collected in a glass tube. The extraction process was performed for 60 min and the yield of SF extract was expressed as percent of the dry weight of seeds.
2.4. Free radical scavenging activity on DPPH
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of ethanol and SF extracts of hemp seed was determined according to the method described by Blois with some modification[21]. IC50values of the sample i.e., concentration of sample necessary to decrease the initial concentration of DPPH by 50% was calculated.
2.5. Antioxidant activity by the ABTS·+ assay
Radical scavenging activity of ethanol and SF extracts of hemp seed was assessed spectrophotometrically by ABTS·+ cation decolorization assay and the absorbances were taken at 734 nm[22]. IC50values of the sample i.e., concentration of sample necessary to decrease the initial concentration of DPPH by 50% was calculated.
2.6. Quantification of target gene expression by real-time PCR2.6.1. Cell culture and treatments
Human hepatoma cell line (HepG2) was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured and maintained DMEM supplemented with 10% FBS, 50 units/mL penicillin and 50 μg/mL streptomycin. The cells were incubated at 37 ℃ in a 5% CO2incubator (HERAcell 150, Thermo Electron Corp., Waltham, MA, USA). One day before treatment with extracts, HepG2 cells were trypsinized and seeded at a density of 5 105cells/well in a 24-well plate. The media were discarded after 24 h, and fresh FBS-free DMEM were added along with extracts with various concentrations. The 200 μM of H2O2was treated after 2 h exposure of extracts. After 24 h incubation, culture media were aspirated and the cells were collected.
2.6.2. RNA isolation and first-strand cDNA synthesis
Total cellular RNA was isolated with a commercial kit (RNeasy Mini kit, Qiagen), according to the manufacturer’s instructions. One microgram of total RNA was reverse-transcribed using oligo (dT) and SuperScript III Reverse Transcriptase (Invitrogen). cDNA synthesis was carried out according to the manufacturer’s instructions, and the resulting cDNA was stored at -20 ℃.
2.6.3. Quantification of mRNA levels by real-time PCR
Using cDNAs as the template, quantitative real-time PCR was carried out using the SYBR Green PCR Master Mix (Qiagen) in a real-time PCR (Rotor-gene Q, Qiagen), according to the manufacturer’s instructions, using specific oligonucleotide primers for human CuZnSOD, MnSOD, GPx and CAT genes (Table 1). β-Actin cDNA was used as an internal control. A dissociation cycle was performed after each run to check for non-specific amplification or contamination. After initial denaturation (95 ℃ for 5 min), 40 PCR cycles were performed using the following conditions: 95 ℃, 5 s; 60 ℃, 10 s at the end of PCR reaction, samples were subjected to a temperature ramp (from 60 to 95 ℃, 1 ℃/s) with continuous fluorescence monitoring. For each PCR product, a single narrow peak was obtained by melting curve analysis at specific temperature. Relative expression levels were estimated using the method described by Pfaff[23]. Analysis was performed with relative quantification software (Rotor-Gene Q series 2.0.3 software).
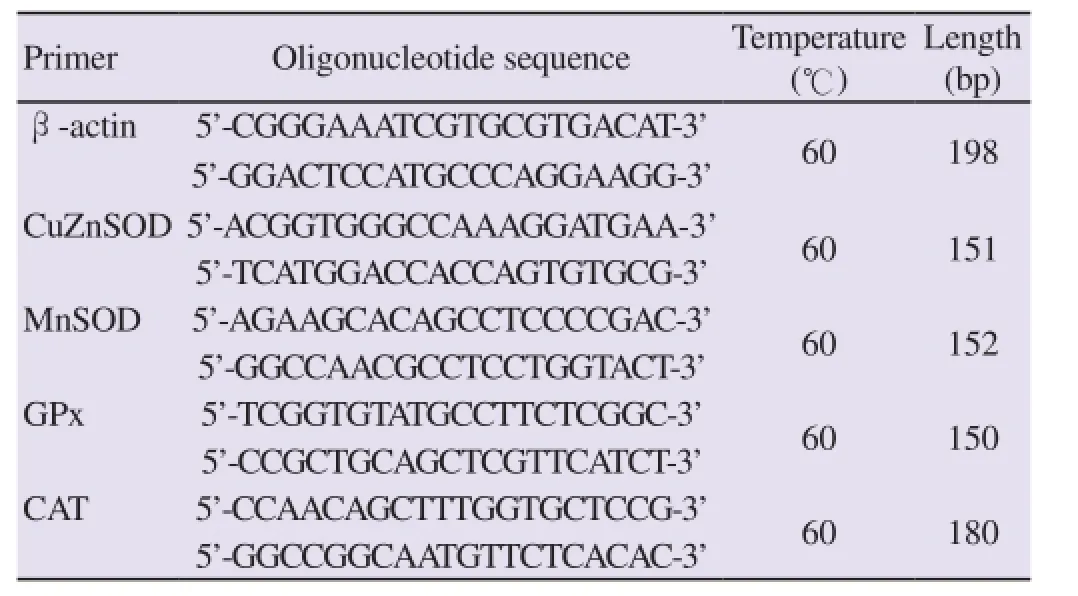
Table 1 Nucleotide sequences of PCR primers used for quantitative real-rime PCR.
2.7. Statistical analysis
The values expressed are means of three replicate determinations ± standard deviation. The data were evaluated with SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Yield and in vitro radical scavenging activities
In the extraction of hemp seed, ethanol extraction yielded 23.0% and the extracts obtained using SF extraction method yielded 29.8%. The efficacy of ethanol and SF extracts of hemp seed on DPPH and ABTS radical scavenging activity are presented in Figures 1 and 2. The ethanol and SF extracts showed considerable levels of DPPH (IC50of 10.7 and 9.2 mg/mL, respectively) and ABTS (IC50 of 99.9 and 66.6 μg/mL, respectively) scavenging activities in concentration dependent manner.
3.2. Expression of antioxidant enzymes in HepG2 cells
In addition to the radical scavenging activity, we evaluated the effects of ethanol and SF extracts on expression of antioxidant enzymes, including CuZnSOD, MnSOD, GPx and CAT, in intact HepG2 cells challenged with H2O2. The gene response for the CuZnSOD, MnSOD, GPx and CAT were monitored by quantitative real-time PCR in HepG2 cells were examined upon treatment with 200-500 μg/mL of ethanol and SF extracts for 24 h. The cells treated with H2O2(200 μM) alone significantly reduced the expressions of SOD, GPx, and CAT enzymes, when compared with the vehicletreated control (Figures 3 - 6). However, HepG2 cells pretreated with 200-500 μg/mL of ethanol and SF extracts exposure to H2O2demonstrated dose-dependently up-regulated the expression of SOD, GPx, and CAT enzymes (Figures 3-6). Further, the inductions of these enzymes by SF extract at 500 μg/mL were more potent than that by ethanol extracts.
4. Discussion
In the present study, ethanol and SF extracts of hemp seed were assayed for their total antioxidant activity as well as the ability to induce activity of antioxidant enzymes such as SOD, GPx and CAT. Da Porto et al[24] studied the optimization of extraction hemp seed oil using SF extraction method and reported that the maximum oil yield, 21.50% w/w, was obtained temperature at 40 ℃ and pressure at 300 bar. Interestingly, the yield of SF extraction from our study showed higher, when SF extraction conducted at the temperature of 40 ℃ and pressure of 400 bar. Nissen et al[17] studied the essential oil composition of different varieties of hemp seed and identified 55 compounds accounting for 95% of the whole GC profile. In these oil samples, β-caryophyllene, α-humulene, myrcene, α-pinene and β-pinene were detected as the main components.
The antioxidant properties of plant extracts or compounds can be evaluated using various in vitro chemical assays. Among them, DPPH and ABTS radical scavenging methods are the oldest and frequently used in vitro methods for the evaluation of the antioxidant potential of various natural products based on the transfer of hydrogen between the free radicals and the antioxidants[25]. The results of the in vitro antioxidant studies revealed that the SF extract showed higher DPPH and ABTS radical scavenging activities when compared to those of ethanol extract. It appears that the ethanol extract and SF extracts of hemp seed possess hydrogen donating capabilities to act as antioxidant. The chemical constituents are important factors governing the efficacy of natural antioxidants. Radical scavenging activities by the sample might be due to the presence of the hydroxyl groups in their structure and their electron donating ability. The scavenging properties of antioxidant compounds are often associated with their ability to form stable radicals[26]. In vitro radical scavenging of different varieties and extracts of hemp seed were determined by Chen et al[20] and reported that the IC50of DPPH ranged from 0.09 to 4.55 mg/mL and IC50of ABTS ranged from 0.012 to 0.485. Further, the authors isolated two potent free radical scavenging compounds, N-trans-caffeoyltyramine and cannabisin B from the seeds of hemp. Girgih et al[19] reported the antioxidant potential of protein hydrolysate fractions of hemp seed using various in vitro assays such as DPPH and hydroxyl radical scavenging, metal chelating, ferric reducing, and inhibition of linoleic acid oxidation. In addition, methanol extract of cold-pressed hemp seed oil possessed significant antioxidant and free radical scavenging activities[27].
Antioxidant enzymes are considered to be most important in cellular defenses because they balance the redox status in cells by remove the excessive free radicals[28]. Previous studies have stated that intracellular generation of H2O2is an important mediator of apoptosis. Various enzymes including superoxide dismutase, peroxidases and catalase are effectively involved in H2O2modulation. Previous studies have been reported that up-regulating the expression of antioxidant enzymes in HepG2 cells promotes a protective effect against cytotoxicity or apoptosis induced by oxidative stress[29-31]. SOD reacts with superoxide anion radical to produce oxygen and the less-reactive H2O2. H2O2in turn can be neutralized by both GPx and catalase. Landis and Tower[32] reported that the level of catalase is lowered in several tumor cells, which results in a decreased detoxifying capacity for H2O2in tumors. Similar to our results, Bak et al[33] reported that the essential oil of red ginseng significantly restored the expression of antioxidant enzymes in HepG2 cells. Therefore, induction of expression of these antioxidant enzymes seems to be essential for prevention of various free radical-mediated diseases such as cancer, arthrosclerosis, and chronic inflammation. In addition, Valko et al[28] suggested that the expression of these antioxidant enzymes may also be regulated by upstream proteins such as mitogen-activated protein kinases like c-Jun N-terminal kinase, extracellular-regulated kinase and p38 etc.
The effect of ethanol and SF extracts of hemp seed on the expression activity of antioxidant enzymes that modulate H2O2levels, such as superoxide dismutase (CuZnSOD and MnSOD), glutathione peroxidase (GPx) and catalase (CAT) in HepG2 cells had not been previously investigated. Several authors have studied that the antioxidant and free radical scavenging potentials of hemp seed[18-20]. In addition, biological activities of the sample are directly related to their chemical composition. Previous investigations have been reported that the hemp seed and its oil contain numerous health promoting chemical substances including flavonoids, terpenes, steroids etc.[15]. Accordingly, the ethanol and SF extracts hemp seed appears to both scavenge the free radicals and also restore the expression of antioxidant enzymes. Hence, the expression of antioxidant enzymes activity by hemp seed is might be ascribed to the presence of bioactive metabolites.
This study is the first report on the expression of antioxidant enzymes by hemp seeds in HepG2 cells. The SF extract of hemp seed effectively scavenged the DPPH and ABTS radicals than ethanol extract. It also up-regulate the expressions of SOD, GPx and CAT enzymes in concentration dependent manner. These findings strongly suggest that ethanol and SF extracts of hemp seed may participate in cellular protection as an antioxidant molecule and stimulate the expression of antioxidant enzymes. It could be concluded that hemp seeds appear to be a useful of source of a therapeutic agent for the treatment of oxidative stress mediated disorders.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
This research fund was supported from Pyeongchang County, Gangwon, Republic of Korea.
[1] Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44-84.
[2] Borneo R, Leon A, Aguirre A, Ribotta P, Cantero J. Antioxidant capacity of medicinal plants from the province of Cordoba (Argentina) and their in vitro testing in a model food system. Food Chem 2009; 112: 664-70.
[3] Soares AA, Souza CGM, Daniel FM, Ferrari GP, Costa SMG, Peralta RM. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem 2009; 112: 775-781.
[4] Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem 2007; 103: 381-388.
[5] Slusarczyk S, Hajones M, Wozniak KS, Mathowski A. Antioxidant activity of polyphenols from Lycopus lucidus. Turcz. Food Chem 2009; 113: 134-138.
[6] Yoshimoto M, Okuno S, Yamaguchi M, Yamakawa O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweetpotato. Biosci Biotech Biochem 2001; 65: 1652-1655.
[7] Rice-Evans C. Flavanoids and isoflavones; absorption, metabolism, and bioactivity. Free Radical Biol Med 2004; 36: 827-828.
[8] Tsao R, Deng Z. Separation procedures for naturally occurring antioxidant phytochemicals. J Chromatogr B 2004; 812: 85-99.
[9] Proestos C, Boziaris IS, Nychas GJE, Komaitis M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem 2006; 95: 664-671.
[10] Flores-sanchez IJ, Verpoorte R. Secondary metabolism in cannabis. Phytochem Rev 2008; 7: 615-639.
[11] Pate DW. Hemp seed: a valuable food source. In: Ranali P. (ed.) Advances in hemp research. New York: The Haworth Press; 1999, p. 243-255.
[12] Callaway JC. Hempseed as a nutritional resource: An overview. Euphytica 2004; 140: 67-72.
[13] Tang CH, Ten Z, Wang XS, Yang XQ. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J Agric Food Chem 2006; 54: 8945-8950.
[14] Wang XS, Tang CH, Yang XQ, Gao WR. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem 2008; 107: 11-18.
[15] ElSohly MA, Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci 2005; 78: 539-548.
[16] Cheng CW, Bian ZX, Zhu LX, Wu JC, Sung JJ. Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for functional constipation. Am J Gastroenterol 2011; 106: 120-129.
[17] Nissen L, Zatta A, Stefanini I, Grandi S, Sgorbati B, Biavati B, et al. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010; 81: 413-419.
[18] Tang CH, Wang XS, Yang XQ. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem 2009; 114: 1484-1490.
[19] Girgih AT, Udenigwe CC, Aluko RE. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J Am Oil Chem Soc 2011; 88: 381-389.
[20] Chen T, He J, Zhang J, Li X, Zhang H, Hao, J, et al. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem 2012; 134: 1030-1037.
[21] Doughari JH, Ndakidemi PA, Human IS, Benade S. Antioxidant, antimicrobial and antiverotoxic potentials of extracts of Curtisia dentate. J Ethnopharmacol 2012; 141: 1041-1050.
[22] Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 1999; 26: 1231-1237.
[23] Pfaffl MW. A new mathematical model for relative quantification in realtime RT-PCR, Nucleic Acids Res 2001; 29: 2002-2007.
[24] Da Porto C, Voinovich D, Decorti D, Natolino A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J Supercrit Fluid 2012; 68: 45-51.
[25] Stratil P, Klejdus B, Kuban V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables - Evaluation of spectrophotometric methods. J Agric Food Chem 2006; 54: 607-616.
[26] Shakirin FH, Nagendra Prasad K, Ismail A, Yuon LC, Azlan A. Antioxidant capacity of underutilized Malaysian Canarium odontophyllum (dabai) Miq. Fruit J Food Compos Anal 2010; 23: 777-781.
[27] Yu LL, Zhou KK, Parry J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem 2005; 91: 723-729.
[28] Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact 2006; 160: 1-40.
[29] Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem 1999; 274: 26217-26224.
[30] Bak MJ, Jeong JH, Kang HS, Jin KS, Jun M, Jeong WS. Stimulation of activity and expression of antioxidant enzymes by solvent fractions and isolated compound from Cedrela sinensis leaves in HepG2 cells. J Med Food 2011; 14: 405-412.
[31] Valdameri G, Trombetta-Lima M, Worfel PR, Pires AR, Martinez GR, Noleto GR, et al. Involvement of catalase in the apoptotic mechanism induced by apigenin in HepG2 human hepatoma cells. Chem-Biol Interact 2011; 193: 180-189.
[32] Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 2005; 126: 365-379.
[33] Bak MJ, Jun M, Jeong WS. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int J Mol Sci 2012; 13: 2314-2330.
ment heading
doi:10.1016/S2305-0500(15)30012-9
*Corresponding author: Jin-Woo Jhoo, Department of Animal Products and Food Science, Kangwon National University, KNU Ave 1, Chuncheon, Gangwon, Republic of Korea, 200-701.
Tel: +82-33-250-8649 Fax: +82-33-251-7719
E-mail: jjhoo@kangwon.ac.kr
Sunghyun Hong and Kandhasamy Sowndhararajan contributed equally.
Foundation project: This research was funded by Pyeongchang County, Gangwon, Republic of Korea.
 Asian Pacific Journal of Reproduction2015年2期
Asian Pacific Journal of Reproduction2015年2期
- Asian Pacific Journal of Reproduction的其它文章
- Successful triplet pregnancy in an African with pure gonadal dysgenesis: A plus for assisted reproduction
- Isolation and identification of phytoestrogens and flavonoids in an Ayurvedic proprietary medicine using chromatographic and Mass Spectroscopic analysis
- Recent advances on synchronization of ovulation in goats, out of season, for a more sustainable production
- Apogamous sporophyte development through spore reproduction of a South Asia’s critically endangered fern: Pteris tripartita Sw.
- The relationship between embryo quality assessed using routine embryology or time-lapse videography and serum progesterone concentration on the day of ovulatory trigger in in vitro fertilization cycles
- Effects of aqueous and ethanol extract of dried leaves of Pseudocalymma alliaceum (Bignonaceae) on haematological and biochemical parameters of wistar rats
