Synergistic Effect of Cation and Anion for Low-Temperature Aqueous Zinc-Ion Battery
Tianjiang Sun, Shibing Zheng, Haihui Du, Zhanliang Tao?
ABSTRACT Although aqueous zinc-ion batteries have gained great development due to their many merits, the frozen aqueous electrolyte hinders their practical application at low temperature conditions. Here,the synergistic effect of cation and anion to break the hydrogen-bonds network of original water molecules is demonstrated by multi-perspective characterization.Then, an aqueous-salt hydrates deep eutectic solvent of 3.5 M Mg(ClO4)2 + 1 M Zn(ClO4)2 is proposed and displays an ultralow freezing point of - 121 °C. A high ionic conductivity of 1.41 mS cm-1 and low viscosity of 22.9 mPa s at - 70 °C imply a fast ions transport behavior of this electrolyte. With the benefits of the low-temperature electrolyte, the fabricated Zn||Pyrene-4,5,9,10-tetraone (PTO) and Zn||Phenazine (PNZ) batteries exhibit satisfactory low-temperature performance.For example, Zn||PTO battery shows a high discharge capacity of 101.5 mAh g-1 at 0.5 C (200 mA g-1) and 71 mAh g-1 at 3 C (1.2 A g-1) when the temperature drops to - 70 °C. This work provides an unique view to design anti-freezing aqueous electrolyte.
KEYWORDS Low-temperature aqueous zinc-ion battery;3.5 M Mg(ClO4)2 + 1 M Zn(ClO4)2 electrolyte; Synergistic effect;Pyrene-4,5,9,10-tetraone; Phenazine
1 Introduction
The temperature in surface of earth is unevenly, which results in a great challenge for energy storage devices. For example, there are abundant wind and solar in severe cold regions, but how to store these energies becomes a problem. Aqueous zinc-ion batteries (AZIBs), with merits of high theoretical specific capacity and low redox potential of Zn anode, low cost and high ionic conductivity of aqueous electrolyte, and various cathode materials, have attached tremendous attention from researchers and have shown great potential for large-scale energy storage devices [1—6]. Unfortunately, AZIBs show terrible electrochemical performance at low-temperature condition, which hinders their application in harsh environments. For AZIBs, the ultralow and ultrahigh activation energies of the anodic and cathodic reactions result in insensitive electrode kinetics to varied temperatures based on the Arrhenius Equation [7]. While the aqueous electrolytes are extremely sensitive to temperature.As we know, the thermodynamic freezing point of solvent water is 0 °C. When temperature further drops, the aqueous electrolyte will freeze, and the ionic conductivity and interface wettability will rapidly deteriorate, which causes AZIBs cannot work normally [8, 9]. Therefore, inbibition of aqueous electrolyte freezing is an effective strategy to improve the low-temperature performance of AZIBs.
The formation of ice crystal is driven by hydrogen bonds(HBs) between water molecules [9, 10]. Modulating the chemical environment of O and H atoms in water to break the HBs network is possible to induce the freezing point depression of water. To date, several strategies have been reported including hybrid electrolyte with cosolvents or antisolvent additives, high-concentration electrolyte, and hydrogel electrolyte, etc. [11—16]. Although these methods improve the low-temperature performance of AZIBs to some extent, some inhere problems still hinder their practical application, such as low ionic conductivity and environmental unfriendliness of organic additives, high viscosity,and cost of high-concentration salt, and complex synthesis and assembly processes of hydrogel electrolyte. Essentially,these strategies alter coordination environment of H atoms by introducing HBs acceptors and further lower its freezing point. The researches about adjust chemical environment of O atoms to suppress ice up are ignored, which are worthy of further study.
Deep eutectic solvents (DES), as a kind of “green” solvent, have been studied to protect the Zn metal anode for AZIBs [17—19]. Meanwhile, it also exhibits a certain antifreezing property due to the interaction with water molecules. However, conventional DES systems (for example,aqueous-organic DES mixture) commonly show low ionic conductivity and high viscosity at low temperature due to the low water content. In contrast, the aqueous-salt hydrates DES, without organic compound, is worth to be considered for application in low-temperature AZIBs. Aqueous-salt hydrates possess rich hydrogen-bond (HB) acceptor and hydrogen-bond donor, which effectively destroy the HBs network of original water molecules [20, 21]. By simultaneously regulating the coordination environment of O and H atoms in water, this DES system can obtain a low freezing point.
Here, an anti-freezing dual-cations EDS electrolyte of 3.5 M (mol L-1) Mg(ClO4)2+ 1 M Zn(ClO4)2is reported for low-temperature AZIBs. It is discovered that the ratio of HBs in water molecules is significantly decreased by introducing oxygen-ligand Mg2+and hydrogen-ligand ClO4-,resulting in an ultralow solidifying point of - 121 °C. The novel aqueous-salt hydrate shows high ionic conductivity,low viscosity, and activation energy at - 70 °C due to the absence of organic additive. The excellent low-temperature physicochemical properties and good compatibility with Zn metal of this electrolyte give fabricated Zn||pyrene-4,5,9,10-tetraone (PTO) battery and Zn||Phenazine (PNZ) battery a satisfactory low temperature performance. For example,when at - 70 °C, the Zn||PTO battery exhibits a high discharge capacity of 101.5 mAh g-1at 0.5 C (200 mA g-1) and excellent rate performance (71 mAh g-1at 3 C (1.2 A g-1)).
2 Experimental Section
2.1 Preparation of Pyrene-4,5,9,10-tetraone (PTO)Sample
Pyrene-4,5,9,10-tetraone (PTO) was prepared by a previous reported method. 8.08 g pyrene was added into 160 mL CH2Cl2and 160 mL acetonitrile, followed by adding 72 g NaIO4, 200 mL H2O, and 1.0 g RuCl3·xH2O successively.The mixture was heated at 45 °C overnight and then organic solvents of which were filtrated and washed with CH2Cl2several times. Obtained filtrate was further washed with H2O and CH2Cl2and removed by rotary evaporation treatment.The pure golden needle-like product PTO was obtained going through column chromatography (using CH2Cl2as mobile phase).
2.2 Material Characterizations
DSC (NETZSCH, TG209 DSC204 DMA242 TMA202) was carried out in the procedure of + 25 ~ - 150 °C with a cooling rate of 5 °C min-1and scanned from - 150 to + 25 °C at 5 °C min-1. The polarizing microscope was using Olympus BX51TRF, liquid nitrogen as refrigerant, and the cooling rate was 4 °C min-1and standing 10 min at specific temperature. The viscosity of electrolytes was tested by Rotary viscometer (Ji Chang, NDJ-8S), and anhydrous ethanol was used as refrigerant. The characteristics of electrolyte were conducted by Raman spectroscopy (Renishaw, InVia Reflex microscope with 532 nm excitation laser, 100 ~ 4000 cm-1)and Fourier transform infrared spectroscopy (FTIR,BRUKER TENSOR II (FTS6000), 400 ~ 4000 cm-1).1H NMR analysis was carried out on an AVANCE III 400 MHz equipment. The morphologies and structures of the PTO were examined by scanning electron microscopy (SEM,JEOL JSM-7500F) and X-ray diffraction (XRD, Rigaku SmartLab 9 KW, Cu Kαradiation). All low-temperature tested were performed at ultra-low-temperature storage box(MELNG, DW-HW50).
2.3 Electrochemical Measurement
The PTO and PNZ electrodes are prepared by mixing PTO or PNZ, Ketjen black (KB), and polytetrafluoroethylene(PTFE) at an appropriate weight ratio of 5:4:1 and are pressed onto stainless-steel mesh (Φ 12 mm). Then, the electrode films are dried at 80 °C for 12 h under vacuum. The active materials mass loading is 1—2 mg cm-2. The 2032-type coin cells are assembled by PTO or PNZ cathode, 3.5 M Mg(ClO4)2+ 1 M Zn(ClO4)2electrolyte, Zn metal (0.05 mm,Φ 12 mm) anode and glass fiber separator. CV tests are carried out on an electrochemical workstation (CHI660E). The galvanostatic charge/discharge tests are implemented after resting 5 h by using a battery test system (LAND CT2001A).The tested voltage range of Zn||PTO is 0.3 ~ 1.5 V (vs. Zn2+/Zn). The tested voltage range of Zn||PNZ is 0.3 ~ 1.5 and 0.2 ~ 1.5 V (vs. Zn2+/Zn) at 25 and - 70 °C, respectively.The current density and specific capacity of full battery are based on the active mass of cathode in each electrode. The ionic conductivity is tested by fabricated coin cell, which includes filled electrolyte, cathode, and anode stainless-steel case (Φ 20 mm).
2.4 Calculation Details
The ionic conductivity is calculated follow Eq. 1:

σ: Ionic conductivity;T: temperature;Ea: activation energy;k: Boltzmann constant (1.3807 × 10—23J K-1). The -Ea/kwas fitted by different temperature ln (σT) and 1/T. TheEa was obtained.
2.5 DFT Calculation
All of the calculations are carried out using the Gaussian 16 program. Geometry optimization and frequency analysis are performed in water solvent with the SMD solvation model. C, H, O, N using B3LYP functional and 6—31 + G(d, p) basis set. Zn2+and Mg2+using B3LYP functional and def2tzvp basis set.
3 Results and Discussion
3.1 Synergistic Effect of Mg2+ and ClO4-
As mentioned above, it is a key that finding suitable salt to construct anti-freezing aqueous-salt hydrates EDS electrolyte. Aqueous electrolytes are constituted by various anions and cations. Matching type of anion and cation can simultaneously modulate the chemical environment of O and H atoms of H2O. Anions, such as BF4-, Cl-, and CF3SO3-, can form weak HBs with water molecules to suppress ice up of water [8—10, 22, 23]. Besides, ClO4-, as a kind of chaotropic anion, also has ability to form a lot of HBs with water molecules due to contain four HBs-acceptor O atoms [24—26]. Cations commonly exist in aqueous electrolyte in the form of hydration [27, 28]. Among of various divalent cations, Mg2+with smaller ionic radius(0.72 nm) and concentrated surface charge density displays strong electrostatic attraction with O atom of H2O. Thus,aqueous-Mg(ClO4)2salt solution shows satisfactory antifreezing ability at a certain concentration.
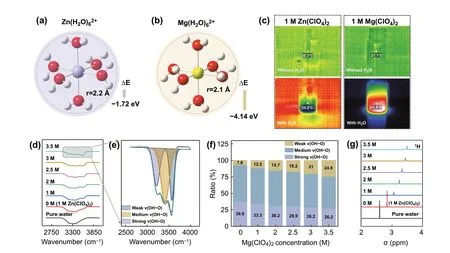
Fig. 1 The calculated formation energy and hydrated radius of a Zn2+ solvation configuration. b Mg2+ solvation configuration. c Photographs of infrared thermometry of different electrolytes. d FTIR spectra for O—H bond. e The fitted O—H stretching vibration representing the strong,medium weak OH…O HBs. f The ratio of different types of HBs. g 1H NMR spectra of different concentration electrolytes
Firstly, the hydrated interactions of Mg2+and Zn2+are investigated by density functional theory (DFT) calculations. As shown in Fig. 1a, b, the solvation configurations of Mg2+and Zn2+with six water molecules are simulated.The hydrated radius of Mg(H2O)62+(2.1 ?) is smaller than Zn(H2O)62+(2.2 ?). In addition, the binding energy of Mg(H2O)62+is - 4.14 eV, which is obviously lower than Zn(H2O)62+(- 1.72 eV) and H2O-H2O (- 0.13 eV, Fig. S1).The result indicates that the Mg2+has stronger interaction with O atom of H2O, which can hinder the formation of HBs network by competing with the H of H2O. Fourier transform infrared spectroscopy (FTIR) is utilized to observe the change of HBs in different electrolytes. As shown in Fig. S2a, the peaks at 2900 ~ 3700 cm-1correspond to O-H stretching vibration of H2O [29]. Notably, the intensity of high-wavenumber peak in 1 M Mg(ClO4)2solution is increased in comparison with that in 1 M Zn(ClO4)2solution. While the stretching vibration of Cl—O at 1093 cm-1has no shift (Fig. S2b) [30]. The peaks of O—H stretching vibration are further divided into three components, corresponding to strong HBs, medium HBs, and weak HBs,respectively (Fig. S3). As detected, the ratio of weak HBs in 1 M Mg(ClO4)2solution is higher than that in 1 M Zn(ClO4)2solution, suggesting that Mg2+has stronger ability to break HBs network of water molecules (Fig. S4). The1H nuclear magnetic resonance (1H NMR) also confirms reduced HBs effect of water molecules and content of free water in 1 M Mg(ClO4)2solution (Fig. S5). In addition, the dissolution Mg(ClO4)2salt in water is a violent exothermic process,which implies the bond-formation energy between Mg2+and O atoms is higher than bond-cleavage energy of HBs(Fig. 1c). Thus, Mg2+is easier to combine with O atoms in H2O and breaks up the hydrogen-bond arrangement. Thus,the freezing point of 1 M Mg(ClO4)2solution is lower than 1 M Zn(ClO4)2solution (Fig. S6).

Fig. 2 a V-shape relationship between the freezing temperature and concentration of Mg(ClO4)2 (0 M refers to 1 M Zn(ClO4)2). b DSC curve of 3.5 M electrolyte. c Polarizing light (PL) and non-polarizing light microscope observation of 0 M and 3.5 M electrolyte. d Ionic conductivity of 3.5 M electrolyte at different temperatures. e Viscosity of 3.5 M electrolyte at different temperatures. f Electric conductance activation energy of 3.5 M electrolyte
The interaction between anion and water molecules is systematically investigated by spectroscopic methods. The FTIR spectra of O—H stretching vibration for different concentration electrolytes are collected and summarized in Figs. 1d and S7 (xM:xM Mg(ClO4)2+ 1 M Zn(ClO4)2). As shown in Figs. 1d, e and S8, the fitting peaks of strong and medium HBs gradually weaken and shift to high wavenumber (blue shift) with increasing concentration of Mg(ClO4)2.On the contrary, the weak HBs peak gradually enhances and shifts to low wavenumber (red shift). DFT calculations show that the bond length of O—H in H2O (0.982 ?) is longer when forming weak HBs with ClO4-(0.970 ?) (Fig. S9),thus resulting in the peaks of strong and medium HBs have blue shift (Fig. S8). Meanwhile, the ratio of weak HBs is increased (Fig. 1f). In addition, the Cl—O stretching vibration at about 1093 cm-1has a red shift (Fig. S10), which is consistent with DFT calculations (Fig. S9, the bond length of Cl—O is getting longer). Raman spectra of aqueous-salt hydrates with different concentration Mg(ClO4)2are collected. As shown in Fig. S11, the wide peak at 3000 ~ 3700 cm-1is attribute to O—H stretching vibration,which is constituted with three type HBs [31]. Similarly, the strong and medium HBs peaks have blue shift, and weak HBs shifts to low wavenumber with the increasing concentration, and the HBs ratio of H2O—H2O has obvious decrease(Fig. S12).1H NMR is performed to study the HBs network in this system. As shown in Figs. 1g and S13, it can be seen that the1H chemical site shifts to low field with increasing concentration of Mg(ClO4)2, which is caused by reduced electron cloud density of H in H2O when formed weak ClO4-—H2O HBs (Figs. S1 and S9, the Mulliken charge of H in ClO4-—H2O is lower than in H2O—H2O). DFT and spectroscopic results demonstrate that ClO4-has ability to form weak HBs with water molecules and depresses HBs network formation among of water molecules. Benefitting from synergistic effect of cation and anion, the aqueous-Mg(ClO4)2/Zn(ClO4)2DES system has satisfactory anti-freeze potential in theory.
3.2 Low-Temperature Properties of 3.5 M Electrolyte
The freezing points of different concentration solution are tested by differential scanning calorimetry (DSC) (Fig.S14). Figure 2a shows the V-shape relationship between the liquid—solid transition temperature and concentration of Mg(ClO4)2. When the concentration of Mg(ClO4)2increases to 3.5 M, an ultralow glass transition temperature of - 121 °C is obtained (Fig. 2b) [32]. The freezing temperature below 3.5 M is mainly dominated by HBs ratio among of water molecules. However, above 3.5 M, the freezing temperature is raised because of increased ions interaction [9].Therefore, 3.5 M Mg(ClO4)2+ 1 M Zn(ClO4)2(3.5 M) solution can be used as electrolyte for low-temperature AZIBs.In situ polarizing light (PL) or non-PL microscope is applied to intuitively observe solidification state of different electrolytes. As shown in Fig. 2c, under PL, the 0 M electrolyte(1 M Zn(ClO4)2) shows clear ice crystal birefringence when temperature drops to - 20 °C. However, no signal is detected for 3.5 M electrolyte (3.5 M Mg(ClO4)2+ 1 M Zn(ClO4)2)even at - 130 °C, due to the fact that glass transition corresponds to the formation of the amorphous phase. Under non-PL, the crystalline state of 0 M electrolyte at - 20 °C is clearly observed (Fig. S15). By contrast, the 3.5 M electrolyte still maintains liquid state at - 70 °C, and an uneven boundary appears when temperature reduces to - 130 °C,indicating the solution transforms into brittle glass (Fig. 2c).Thus, 3.5 M solution shows a good freezing resistance and its physicochemical properties are further investigated. The ionic conductivity of 3.5 M electrolyte at different temperature from + 25 ~ - 70 °C is calculated by impedance of electrolyte. As shown in Fig. 2d, it shows a high ionic conductivity of 1.41 mS cm-1at - 70 °C. A low viscosity of 22.9 mPa s is achieved at - 70 °C, which enables fast ion transport (Fig. 2e). The conductive activation energy (Ea) of 3.5 M electrolyte is fitted by the relationship between ionic conductivity and temperature (Fig. 2f). TheEais calculated to be 0.23 eV, implying fast ions diffusion ability. The excellent physicochemical properties enable AZIBs to achieve a favorable low-temperature performance.
3.3 Compatibility Between 3.5 M Electrolyte and Zn Anode
The compatibility between 3.5 M electrolyte and Zn metal anode is further understood. It is well known that introduced metal cations in AZIBs electrolyte can promote uniform deposition Zn and alleviate its dendrite problem by electrostatic shield effect [33—35]. The Mg2+has concentrated surface charges on account of small ionic radius (0.72 nm)and high positive charge. Thus, the Mg2+has more distinct electrostatic shield effect than univalent cation in theory.The cyclic voltammetry (CV) curves Fig. S16a show excellent reversibility of Zn plating/stripping on stainless-steel mesh (SS) in 3.5 M electrolyte. Compared with 0 M electrolyte (1 M Zn(ClO4)2electrolyte), the Zn||SS half cell shows smaller voltage polarization in 3.5 M electrolyte (Fig. S16b).An obvious Zn plating peak (wide peak, not sharp peak)in 3.5 M electrolyte is detected, implying a depression of side reaction. In addition, the symmetric Zn||Zn battery in 3.5 M electrolyte exhibits a long-term cycling life of 500 h with a low and stable voltage polarization of 50 mV at 0.5 mA cm-2. While in 0 M electrolyte (1 M Zn(ClO4)2),the symmetric battery shows increased voltage polarization and finally shorted circuit at about 450 h (Fig. S17). The symmetric Zn||Zn battery also displays excellent cycling stability at 1 mA cm-2(Fig. S18), Scanning electron microscopy (SEM) image shows compact and smooth Zn surface after cycling 10 times at 3.5 M electrolyte (Fig. S19). These results suggest Mg2+has significant protect effect for Zn plating process, showing the potential feasibility of applying 3.5 M electrolyte for AZIBs.
3.4 Reaction Mechanism of PTO and PNZ Electrodes
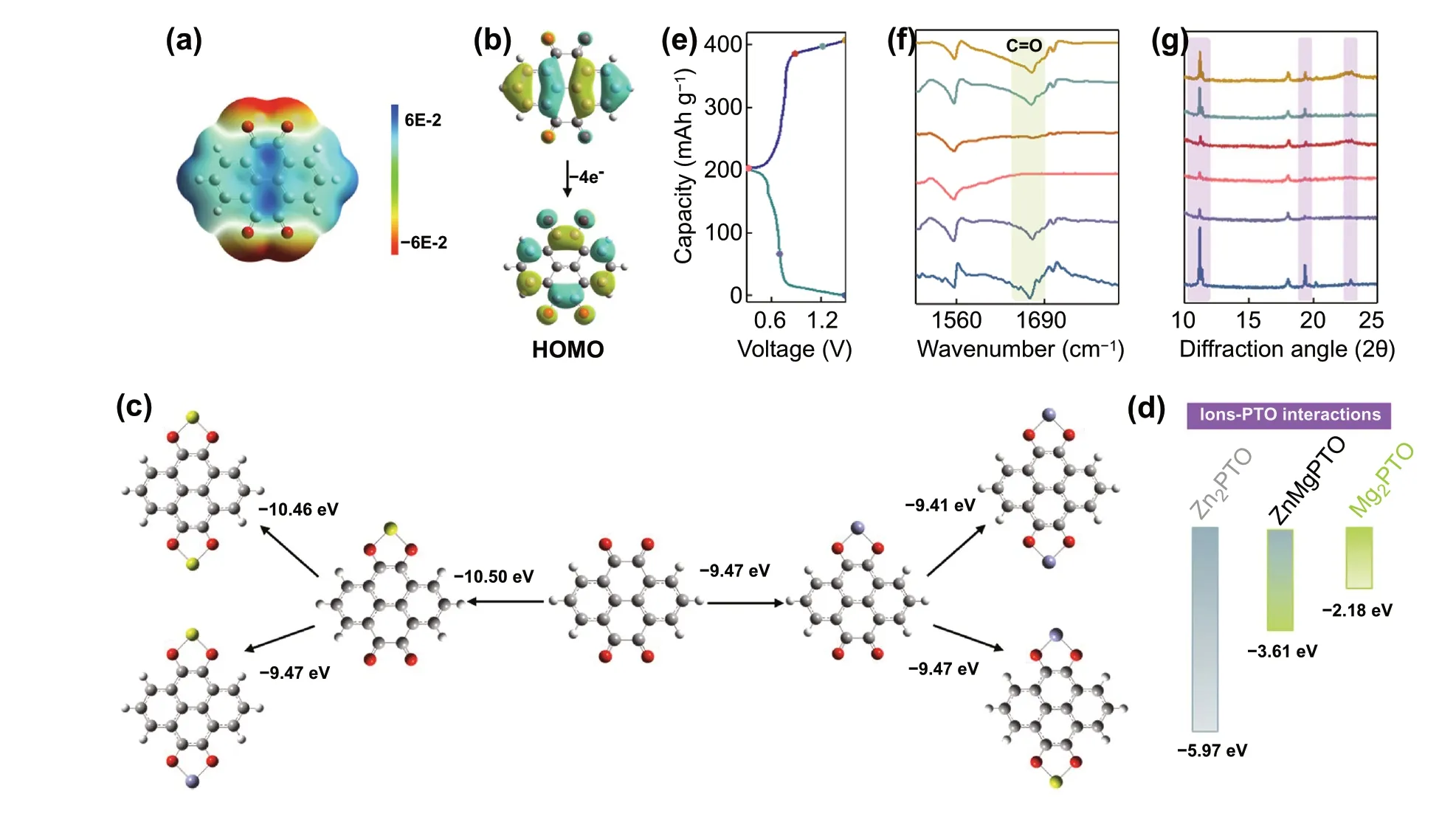
Fig. 3 a ESP of PTO molecule. b HOMO plots of PTO and PTO4-. c Binding energies between PTO and Zn2+ or Mg2+. d The corrected binding energy levels of Zn2PTO, ZnMgPTO, and Mg2PTO. e Charge—discharge curves of Zn||PTO battery. f Ex situ FTIR spectra of PTO electrodes. g Ex situ XRD patterns of PTO electrodes
To fabricate a high-performance low-temperature AZIBs,suitable electrode materials are selected. Organic electrode materials, with many advantages such as low cost,environmentally friendly, fast reaction kinetics, and high capacity independence of temperature, have been seen as a feasible choose [36—38]. Thus, pyrene-4,5,9,10-tetraone(PTO) with electroactive carbonyl groups and phenazine(PNZ) with electroactive conjugated amino groups are selected to construct low-temperature AZIBs. It is worth noting that a great number of Mg2+exist in this EDS electrolyte (3.5 M electrolyte), which may be involved in organic electrode reaction. To distinguish it, DFT calculations are firstly carried out. The negative electrostatic potential (ESP) of the carbonyl groups in PTO molecule in Fig. 3a reveals its reaction sites. As shown in Fig. 3b,the effective electron delocalization occurs in the conjugated structure when PTO is reduced PTO4-with accepting four electrons, indicating it can occur to four-electron reduction [39]. Thus, the binding energy of two cations(Mg2+or Zn2+) one PTO molecule are calculated. As shown in Fig. 3c, the bind energy of PTO with two Mg2+(- 10.46 eV) is lower than with two Zn2+(- 9.41 eV), and it also is lower than ZnMgPTO (- 9.47 eV). However, it cannot be ignored that the metal cation binding to PTO requires a de-solvation process. As mentioned above, the hydrated energy of Zn2+(- 1.72 eV) is higher than Mg2+(- 4.14 eV). The Mg2+needs more energy to break the interaction with H2O molecules and then combine with PTO. Considering the de-solvation energy of metal cations,the bind energy of Zn2PTO is re-calculated to be - 5.97 eV,which is smaller than ZnMgPTO (- 3.61 eV) and Mg2PTO(- 2.18 eV) (Fig. 3d), suggesting that the PTO tends to bind to Zn2+not Mg2+. In addition, the CV curves of Zn||PTO battery in 3.5 M and 0 M electrolyte (1 M Zn(ClO4)2) show similar shape and potential, while it is different from in 3.5 M Mg(ClO4)2electrolyte (Fig. S20). The result implies that the redox reaction of PTO is independent of Mg2+.The PNZ also exhibits similar reaction mechanism by DFT calculations (Fig. S21). The reaction mechanism of PTO is further confirmed by ex situ FTIR and XRD patterns. As shown in Fig. 3f, the peak intensity of C=O groups shows a reversible weakening and enhancement during discharge and charge processes, suggesting that the C=O groups are the combining site of Zn2+. Meanwhile, the diffraction peaks of PTO at 11.18°, 19.36°, and 22.92° have same change, indicating a good reversibility. Noted that, no other by-produce diffraction peaks are observed (for example,basic zinc sulfate), suggesting only Zn2+participates in the redox process. This conclusion can also be demonstrated by ex situ SEM images and energy-dispersive spectroscopy(EDS) (Fig. S22 and Table S1).
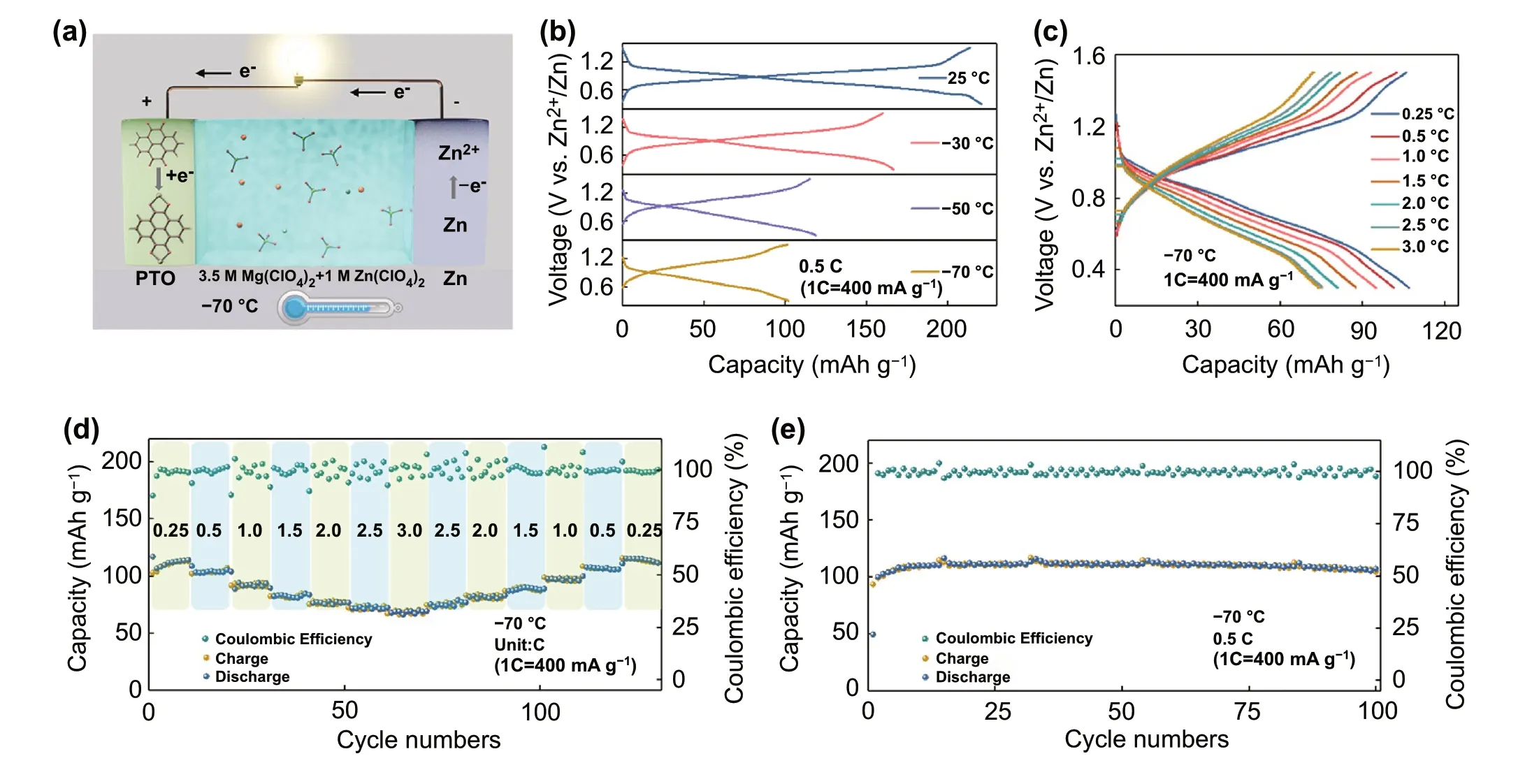
Fig. 4 a Schematic of low-temperature Zn||PTO battery. b Charge—discharge curves of Zn||PTO battery at different temperatures. c Charge—discharge curves of Zn||PTO battery at different current density. d Rate capacity of Zn||PTO battery at - 70 °C. e Cycling stability of Zn||PTO battery at - 70 °C
3.5 Low-Temperature Performance of Zn||PTO and Zn||PNZ Battery
The low-temperature AZIBs are constructed by 3.5 M Mg(ClO4)2+ 1 M Zn(ClO4)2electrolyte, PTO cathode, and Zn metal anode (Fig. 4a). The CV curves of Zn||PTO battery at + 25 ~ - 70 °C show a good reversibility(Fig. S23). The voltage polarization of Zn||PTO battery has gradually increased when temperature dropped, which may be caused by increased activation process of PTO material and concentration polarization of the electrolyte. Figure 4b shows the charge—discharge curves of Zn||PTO battery range from + 25 ~ - 70 °C. It can work well at - 70 °C and exhibit a high discharge capacity of 101.5 mAh g-1at 200 mA g-1.Even at ultrahigh current density of 3 C (1.2 A g-1), this system still maintains 71 mAh g-1discharge capacity, which is 67% of the capacity at 100 mA g-1(Fig. 4c). The discharge capacity recovers to initial level when current density increases to 0.25 C (100 mA g-1). The excellent rate performance of Zn||PTO battery benefits from 3.5 M electrolyte with high ionic conductivity, low viscosity and activation energy at low temperature. As shown in Fig. 4e, the Zn||PTO battery also can cycle 100 times with no obvious capacity fading at - 70 °C and achieve near 100% coulombic effi-ciency. In addition, the Zn||PNZ battery is fabricated and tested at + 25 ~ - 70 °C. The CV curves of Zn||PNZ battery at + 25 and - 70 °C are displayed in Fig. S24. This system obtained discharge capacity of 218.7 and 115.6 mAh g-1at + 25 and - 70 °C, respectively (Fig. S25). The battery also shows a good rate capacity at - 70 °C. A high discharge capacity of 68.3 mAh g-1is achieved at a current density of 1.5 C (435 mA g-1) (Fig. S26). Moreover, the Zn||PNZ battery exhibits an impressive cycling stability and maintains 100 mAh g-1discharge capacity after 100 times at - 70 °C(Fig. S27).
4 Conclusions
In summary, an aqueous-Mg(ClO4)2/Zn(ClO4)2hydrates EDS electrolyte is reported and used for low-temperature AZIBs. Theoretical calculations and spectroscopic studies confirm the synergistic effect of cation and anion on freezing point reduction. Where Mg2+acts as HBs donor bonding O atom in H2O through strong electrostatic attraction to form stable hydration ions. And ClO4-as HBs acceptor can form weak HBs with H atom in H2O. By simultaneously regulating the chemical environment of O and H atoms in H2O,the 3.5 M Mg(ClO4)2+ 1 M Zn(ClO4)2(3.5 M) obtains an ultralow freezing point of - 121 °C, high ionic conductivity of 1.41 mS cm-1(- 70 °C) and low viscosity of 22.9 mPa s(- 70 °C). Based on significant anti-freezing characteristic of 3.5 M electrolyte, organic small molecules PTO and PNZ are developed to fabricate low-temperature AZIBs. Especially, the Zn||PTO battery delivers a high discharge capacity (101.5 mAh g-1at 0.5 C), excellent rate performance(71 mAh g-1at 3 C), and cycling stability (cycles 100 times with no obvious fading) at - 70 °C. This work highlights the design of low-temperature aqueous electrolyte and promotes the development of low-temperature AZIBs.
AcknowledgementsThis study was supported the National Natural Science Foundation of China (51771094 and 21835004), Ministry of Education of China (B12015), and Tianjin Natural Science Foundation (18JCZDJC31500).
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,adaptation, distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/.
Supplementary InformationThe online version contains supplementary material available at https:// doi. org/ 10. 1007/s40820- 021- 00733-0.
- Nano-Micro Letters的其它文章
- Recent Advances in Interface Engineering for Electrocatalytic CO2 Reduction Reaction
- Electrochemical Surface Restructuring of Phosphorus-Doped Carbon@MoP Electrocatalysts for Hydrogen Evolution
- Correction to: Boosting Transport Kinetics of Ions and Electrons Simultaneously by Ti3C2Tx (MXene)Addition for Enhanced Electrochromic Performance
- Zeptomole Imaging of Cytosolic MicroRNA Cancer Biomarkers with A Light-Controlled Nanoantenna
- Nanoengineered Shear-Thinning Hydrogel Barrier for Preventing Postoperative Abdominal Adhesions
- Dopant-Tunable Ultrathin Transparent Conductive Oxides for Efficient Energy Conversion Devices

