Cross-talk between hepatic stellate cells and T lymphocytes in liver fibrosis
Hao Li,Peng Ding,Bo Peng,Ying-Zi Ming
Research Center of Chinese Health Ministry on Transplantation Medicine Engineering and Technology, the Third Xiangya Hospital of Central South University, Changsha 410 0 03, China
Keywords:Hepatic stellate cells T lymphocytes Inflammatory cytokines Fibrosis
ABSTRACT Background:Fibrosis results from inflammation and healing following injury.The imbalance between extracellular matrix (ECM) secretion and degradation leads to the ECM accumulation and liver fibrosis.This process is regulated by immune cells.T lymphocytes,including alpha beta ( αβ) T cells,which have adaptive immune functions,and gamma delta ( γ δ) T cells,which have innate immune functions,are considered regulators of liver fibrosis.This review aimed to present the current understanding of the cross-talk between T lymphocytes and hepatic stellate cells (HSCs),which are the key cells in liver fibrosis.Data sources:The keywords “l(fā)iver fibrosis”,“immune”,and “T cells” were used to retrieve articles published in PubMed database before January 31,2020.Results:The ratio of CD8 + (suppressor) T cells to CD4 + (helper) T cells is significantly higher in the liver than in the peripheral blood.T cells secrete a series of cytokines and chemokines to regulate the inflammation in the liver and the activation of HSCs to influence the course of liver fibrosis.In addition,HSCs also regulate the differentiation and proliferation of T cells.Conclusions:The cross-talk between T cells and HSCs regulates liver fibrosis progression.The elucidation of this communication process will help us to understand the pathological process of liver fibrosis.
Introduction
Chronic liver injury and inflammation lead to liver fibrosis.The most common causes of liver fibrosis include hepatitis B virus(HBV) and hepatitis C virus (HCV) infections,alcoholic liver disease (ALD),nonalcoholic fatty liver disease (NAFLD),and autoimmune liver diseases.Liver fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) which replaces/destroys the normal liver architecture and leads to liver cirrhosis and liver failure [1].Cirrhosis is the end stage of liver fibrosis and is estimated to affect 60 to 120 million people,up to 1 million deaths per year worldwide [2,3].Although liver fibrosis can be reversed by treating the primary cause of liver injury [4],the therapies targeting liver fibrosisperseis not available.
There has been increasing attention on the role of hepatic stellate cells (HSCs) in liver fibrosis [5].HSCs are resident mesenchymal cells located in the subendothelial space of Disse and account for approximately one-third of the mesenchymal cells of the human liver [1].HSCs are the major source of myofibroblasts in the liver and have the capacity to produce ECM when activated [6].The activation and function of HSCs are regulated by multiple cytokines such as interleukin 17 (IL-17),IL-10 and transforming growth factorβ1 (TGF-β1),which are secreted by immune cells in the liver,including T helper cell 17 (Th17 cells),effector memory T cells,regulatory T cells (Tregs),Th1/Th2 cells,and nonclassical gamma delta (γ δ) T cells [7-9].HSCs also play roles in regulating immune functions to maintain immune homeostasis in the liver [10-14].
This review aimed to analyze the cross-talk between HSCs and T lymphocytes,the known inflammatory mediators involved,and the potential therapeutic approaches to prevent or control liver fibrosis.
The unique immune environment of the liver
The liver is considered an immune privileged organ that possesses a unique form of immune homeostasis.Half of a century ago,surgeons discovered that allograft liver transplants exhibited spontaneous immune tolerance not only for the liver tissue but also other organs from the same donor [15-17].The liver is the most important metabolic organ of the human body,as it is exposed to large amounts of antigens as part of its physiological functions.For example,microbe-associated molecular patterns(MAMPs) and damage-associated molecular patterns (DAMPs),also known as alarmins,are continuously transferred from the intestine to the liver through the hepatic portal vein,and the concentration of MAMPs in portal venous blood has been reported to be 100 times greater than that in the peripheral blood [18].Therefore,the liver is crucial in the detection and elimination of blood-borne infections [14].On the other hand,nonpathogenic exogenous bloodborne molecules are included in this process.This requires the liver to “switch” between a tolerant state and a responsive state to prevent an inappropriate immune response [14].
This unique immune function of the liver benefits from the cellular microenvironment,which includes a large population of natural killer (NK) cells,natural killer T (NKT) cells,andγ δT cells.The ratio of CD8 + (suppressor) T cells to CD4 + (helper) T cells is significantly higher in the liver than in the peripheral blood [14],while B lymphocytes account for only 2% of the total lymphocyte population in the liver [19,20].Additionally,many resident lymphocytes in the liver are significantly different in number and function compared with circulating lymphocytes [21-26].Both HSCs and hepatocytes are involved in the recognition of pathogens,antigen presentation,cytokine production,and maintaining the balance between immune tolerance and immune response [11,12,14].
Therefore,the unique immune environment of the liver,including both T cells and HSCs,provides its unique immune response,which is involved in the regulation of immune homeostasis and has important roles in liver physiology and pathology.
Liver fibrosis as a wound healing response regulated by the immune system
Fibrosis is a highly conserved process that is the consequence of tissue repair and has a similar mechanism in multiple organs [27].The main feature of fibrosis is the accumulation of ECM [1].As shown in Fig.1,during acute inflammation,the deposition of ECM is associated with cell apoptosis,necrosis,and the release of lysosomal enzymes,which limit the spread of tissue damage in the liver.Within a few days to several weeks following injury,ECM proteins are degraded,leading to some restoration of the liver tissue architecture [28-30].However,in the context of chronic liver damage,due to inflammation caused by HBV,HCV,and cytomegalovirus (CMV) infections,cholestasis,ALD,or autoimmune liver disease,cells that have the potential to differentiate into myofibroblasts and produce ECM,which are mainly HSCs in the liver,are continuously stimulated due to persistent liver damage.Additionally,portal vein fibroblasts,bone marrow precursor cells,and hepatocytes have the potential to differentiate into myofibroblasts and continuously produce large amounts of ECM [6],which result in architectural distortion of the liver,with areas of regeneration and fibrosis leading to liver cirrhosis.
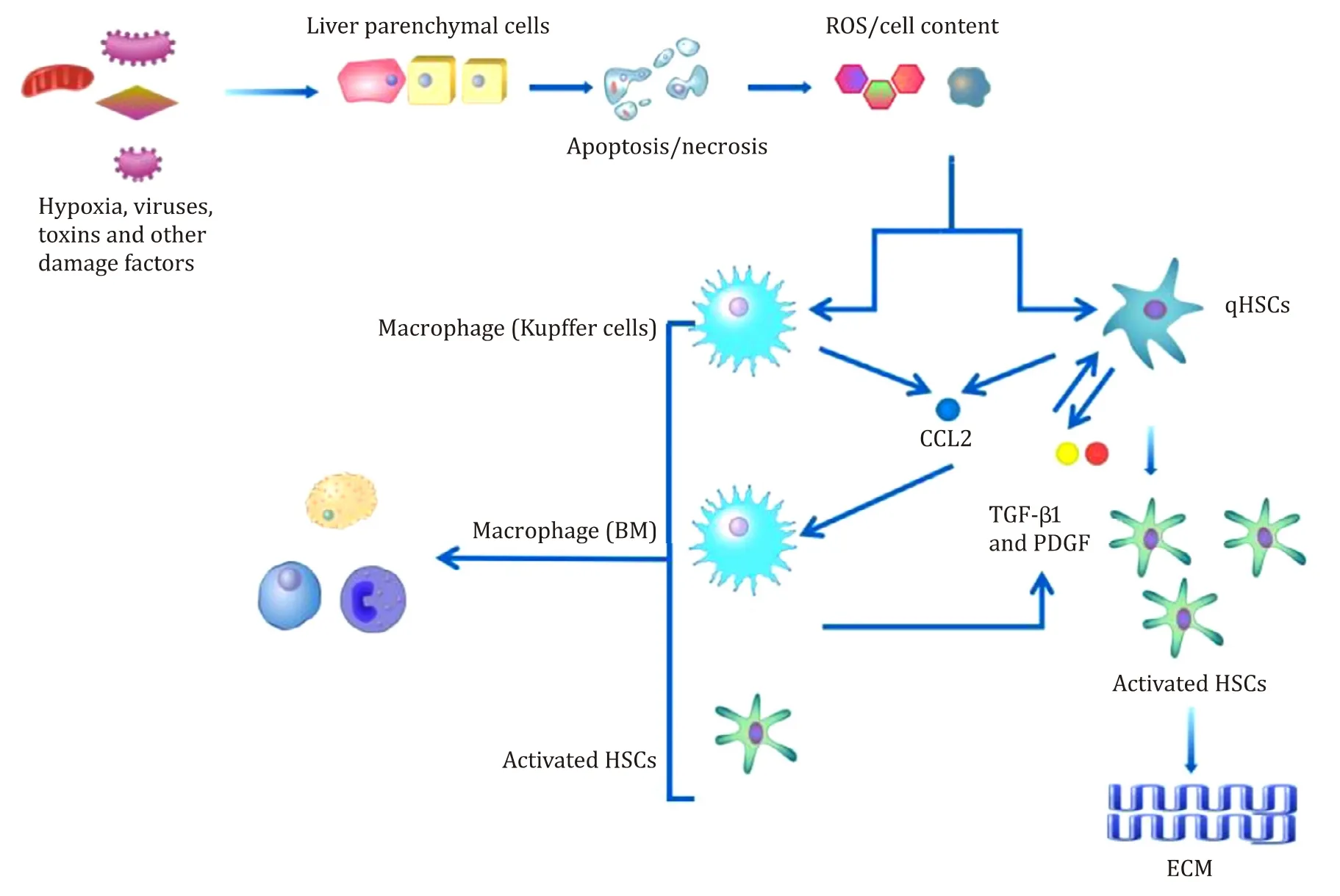
Fig.1.The role of hepatic stellate cells (HSCs) in inflammation resulting from liver injury.Damaged liver parenchymal cells release reactive oxygen species (ROS) and cellular factors that directly induce the activation of HSCs.Kupffer cells in the liver are recruited to the area of liver damage,phagocytose damaged and apoptotic cells,and secrete a series of chemokines,leading to an inflammatory environment that promotes the activation of HSCs and results in liver fibrosis.qHSCs: quiescent hepatic stellate cells;CCL2: C-C motif chemokine 2; TGF- β1: transforming growth factor β1; PDGF: platelet-derived growth factor; ECM: extracellular matrix.
Liver fibrosis is initiated by the release of inflammatory cytokines and damage signals,also known as alarmins,by injured hepatocytes,resulting in the production of profibrotic reactive oxygen species (ROS) and other cellular contents [31,32].These signals from injured hepatocytes directly activate HSCs to phagocytose damaged cells [33,34].The signals from damaged cells recruit and activate immune cells that secrete fibrogenic chemokines and cytokines,which further activate HSCs and lead to the secretion of ECM [35,36].Activated HSCs can also secrete regulatory factors for liver fibrosis and immunoregulatory factors [1,10,37,38].The interaction between HSCs and immune cells,predominantly T cells,is required to maintain the chronic inflammatory environment that provides signals for HSC survival and sustained activation [39-41].
A series of cytokines and chemokines play an important role in the activation of HSCs.First,TGF-β1 is the most important activator of HSCs.TGF-βbinds to TGF-β1 receptors I and II to form a complex that phosphorylates Smad2/Smad3,which binds to Smad4 to form a nuclear heteropolymer that regulates gene transcription to stimulate the production of ECM.This process can be inhibited by Smad7 [42,43].Additionally,TGF-β1 promotes the generation of tissue inhibitor of metalloproteinase (TIMP),which inhibits the degradation of the ECM [44,45].TGF-β1 can be secreted by activated HSCs [1].Macrophages activated by the phagocytosis of apoptotic cells are also important sources of TGF-β1 during liver fibrosis [46].Second,platelet-derived growth factor (PDGF) activates HSC proliferation and activation via the PI3K/serine/threonine kinase (Akt),extracellular-signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK),and three JAK/STAT signaling pathways [47].The PDGFβreceptor (PDGFRβ) is upregulated during HSC activation and has been shown to affect inflammation and liver fibrosis in a mouse model through the NF-κB,ERK,and Akt pathways [48].PDGF is produced primarily by activated HSCs through an autocrine pathway [47].Third,vascular endothelial growth factor (VEGF) is involved in angiogenesis during the repair of liver damage.VEGF and its receptors,VEGFR-1 and VEGF-2,are upregulated,resulting in fibrogenesis,the proliferation of HSCs and collagen production [49,50].Fourth,A series of chemokines play important roles in liver fibrosis,including C-C motif chemokine 2(CCL2),CCL3,and CCL5,which are secreted by Kupffer cells [51].Additionally,the chemokines CCL5,monocyte chemoattractant protein 1 (MCP-1),and CCL21 directly promote the proliferation and migration of HSCs.HSCs also express cell surface chemokine receptors,such as CCR1 and CCR2,which are associated with HSC trafficking and fibrosis [52].Fifth,interferon-γ(IFN-γ) reduces HSC activity bothinvitroandinvivo[53,54].IFN-γinhibits the activation of HSCs induced by TGF-β,increases Smad7 protein expression in a STAT1-dependent way,and further reduces Smad2/Smad3 expression and activation [55].IFN-γalso promotes apoptosis in activated HSCs [56]and is synthesized and secreted by NK cells [57].Sixth,the six members of the IL-17 family (A to F)are primarily involved in mediating proinflammatory responses and the production of other proinflammatory cytokines and chemokines.In the liver,IL-17 contributes to the pathogenesis of alcoholic hepatitis and has a key role in neutrophil recruitment [58].In the pathogenesis of murine liver fibrosis,the expression of IL-17 and its receptor A (IL-17RA) is increased.IL-17 stimulates HSC to produce collagen type I by activating the signal transducer and activator of transcription 3 (STAT3) signaling pathway [59].In the liver,IL-17 is mainly produced by Th17 cells andγ δT cells.Seventh,interleukin-22 (IL-22) induces the senescence of HSCs through the STAT3,SOCS3,p53,and p21 pathways,to inhibit the progression and promote the regression of liver fibrosis [60].In the liver,IL-22 can be expressed by several types of immune cells,and Th17 cells are one of the most important sources of IL-22 in liver fibrosis [61].
Macrophages maintain the inflammatory environment required for liver fibrosis and regulate the progression and regression of liver fibrosis.A balance in favor of M1 macrophages over M2 macrophages has been recognized in liver fibrosis [27].There have been increasing studies on the role of macrophages and T cells in liver disease that have highlighted the importance of the adaptive immune response,with T cells playing a key role in fibrosis.There has been evidence for the role of T cells in liver fibrosis from clinical studies involving patients with immunosuppression.Alric et al.[62]reported that in patients with HCV infection who underwent kidney transplantation,the progression of liver fibrosis was significantly lower in patients who were not treated with immunosuppressive agents.Roth et al.[63]also showed that in patients with HCV infection,those who underwent kidney transplantation had a lower incidence of liver fibrosis progression and a higher survival rate than patients who were treated with dialysis.The inhibition of liver fibrosis by immunosuppressive agents has also been reported in animal models of either bile duct ligation- or carbon tetrachloride (CCl 4 )-induced liver fibrosis,and these studies have demonstrated the direct effect of immunosuppressants on liver fibrosis [64,65].However,in another group of patients with immunodeficiency,coinfection with human immunodeficiency virus(HIV) and HCV resulted in a more rapid progression of liver fibrosis than in those with a single infection [66,67].This seemingly contradictory clinical finding may be related to the complex function of T cells in the process of liver fibrosis.HIV infection is associated with a decrease in CD4 + T cells,and immunosuppressive treatment used to prevent transplant rejection often inhibits the activation and function of all T cell populations.The inhibition of different subgroups of T cells may result in the same degree of immunosuppression,but the progression of liver fibrosis can be affected differently.
The role of CD8 + T cells in liver fibrosis
Cytotoxic or CD8 + T cells can lyse target cells.Previously,CD8 +T cells have been regarded as a single functional T cell subset in liver fibrosis.However,in 2004,a study in a murine model of liver fibrosis induced by CCl 4 or thioacetamide (TAA) showed that adoptive transfer of CD8 + T cells extracted from the mouse spleen increased the degree of liver fibrosis,which was inhibited by IL-10 [68].Additionally,in a mouse model of liver fibrosis,CD8 + T cell deletion did not affect the progression of liver fibrosis in CCl 4 -treated mice [69]or in MDR2 gene-knockout mice [70].This result may indicate that CD8 + T cells do not act as a single functional T cell subset in the context of liver fibrosis but contain different subtypes that perform different functions.Because murine spleenderived CD8 + T cells have been shown to promote liver fibrosis,while peripheral and hepatic CD8 + T cell deletion did not affect liver fibrosis,it may be inferred that different CD8 + T cell subsets have different distributions in the spleen and liver in mice.Currently,this hypothesis requires further validation in mouse models,and studies in humans have yet to be undertaken to determine whether CD8 + T cells in the liver differ functionally from peripheral CD8 + T cells.A newly discovered CD8 + Treg subset has been described in which T lymphocytes tolerate foreign antigens and inhibit the proliferation of other lymphocytes that express specific CD8 + Treg markers,which suggests that CD8 + T cells have complex functions [71].The high proportion of CD8 + T cells in the liver highlights the unique and liver-specific immune environment that is associated with liver fibrosis [14].
The role of CD4 + T cells in liver fibrosis
Helper or CD4 + T cells also have complex functions and phenotypes.Based on different cytokine profiles and transcription factors,CD4 + T cells can be divided into multiple subgroups including Th17 cells,Tregs,T follicular helper (Tfh) cells,and Th22 cells.Different subpopulations play different or even opposite roles in liver fibrosis.For example,in a mouse model of liver fibrosis,deletion of CD4 + T cells has been shown to have no significant effect on the progression of liver fibrosis,reflecting its dual role [72].
Th1/Th2 cells
Early studies showed that the CD4 + T cell subpopulation could be divided into Th1 cells that inhibit fibrosis [73-75]and Th2 cells that exacerbate liver fibrosis [73,74,76].The ratio of Th1/Th2 cells was considered an important factor affecting the progression of liver fibrosis,as shown in Th2-dominant BALB/c mice that developed more advanced liver fibrosis than in Th1-dominant C57BL/6 mice [9,77].IL-13 production by Th2 cells has been shown to have a strong profibrotic effect,promoting the activation of HSCs by stimulating the synthesis and activation of TGF-β1 [78].Th1 cells secrete IFN-γ,which exerts antifibrotic effects by inhibiting TGFβactivation of HSCs and promoting HSC apoptosis [55,56].Additionally,IFN-γalso inhibits the production of Th2 profibrotic factors [78].
With an additional study on the function of CD4 + T cells in liver fibrosis,the simple subtyping of CD4 + T cells into Th1/Th2 cells has been expanded,and an increasing number of CD4 + T cell subsets have been defined [79].However,the concept of balance between individual cell subtypes remains the emphasis of ongoing research [79].The key CD4 + T cell subtypes that may play roles in liver fibrosis include Th17 cells and Tregs,and the ratio of Th17 cells to Tregs (Th17/Tregs) may also be important.
The Prince was very unhappy when he heard these words, and begged the wolf to stay with them always; but this the good creature refused to do, though he thanked the Prince kindly47 for his invitation, and called out as he disappeared into the thicket48, Should any evil befall you, dear Prince, at any time, you may rely on my friendship and gratitude49
Th17 cells
Th17 cells are defined as a separate lineage of T helper cells in 2005 and are associated with the secretion of IL-17 [71,80].Studies have shown that the number of Th17 cells increased with the severity of liver fibrosis in humans and in mouse models of liver fibrosis [81,82].IL-17 secreted by Th17 cells can maintain the inflammatory environment required for liver fibrosis by recruiting neutrophils and monocytes [81-83].Additionally,IL-17 regulates the functions of HSCs [83,84].IL-17 activates HSCs via the ERK1/2 and p38 pathways and promotes the synthesis of type I collagen by HSCs via the STAT3 pathway [59].Additionally,IL-17 upregulates the expression of the TGF-β1 receptor on the surface of HSCs in a JNK-dependent manner and directly promotes the activation of HSCs through the JNK-SMAD3 pathway [84].
IL-22 is another cytokine that activates liver fibrosis and is secreted by Th17 cells.The expression levels of IL-22 were reported to be positively associated with liver fibrosis in patients with HBV infection [61].Animal study has shown that IL-22 can recruit Th17 cells [61].Additionally,IL-22 has recently been shown to enhance TGF-β1 signaling in HSCs via the p38 mitogen-activated protein kinase-dependent pathway,demonstrating that IL-22 has a direct profibrotic effect [84].Interestingly,Kong et al.[60]reported that overexpression of IL-22 induces the senescence of HSCs in mouse and therefore,IL-22 has an antifibrotic effect [60].
Tregs
Tregs are characterized by the expression of the transcription factor forkhead box P3 (FOXP3) [85].Tregs regulate the function of other immune cells and have the ability to secrete the negative immunoregulatory factors IL-10 and TGF-β.Although Tregs play a role in stimulating the secretion of TGF-β,they are not regarded as profibrotic T cells,and Tregs can inhibit liver fibrosis.In rats with liver fibrosis caused by bile duct ligation,the depletion of Tregs has been shown to exacerbate liver fibrosis [8,86],and Tregs were shown to inhibit profibrotic inflammation by inhibiting CD8 + T cells and IL-17 + T cells.Invitro,Tregs inhibit the activation of HSCs in a dose-dependent manner [87].Further study has shown that this function is associated with the inhibition of HSC activation by IL-17 through the TGF-β-SMAD pathway [88].IL-10 secreted by Tregs inhibits liver fibrosis induced by the adoptive transfer of CD8 + T cells [68].Additionally,IL-10 has been shown to inhibit the secretion of IL-17 by Th17 cells,preventing the establishment of the inflammatory environment required for liver fibrosis and the activation of HSCs [89].
Therefore,Tregs are often regarded as an inhibitory cell type that regulates Th17 cells [90].An imbalance between Th17/Tregs is now considered to play a key role in liver fibrosis [87].A significant increase in the proportion of Th17/Tregs is associated with poor prognosis and increased progression of liver fibrosis in patients with HBV infection [7].
HSCs regulate Th17 cells/Tregs
While the Th17/Treg balance affects the activation and function of HSCs,HSCs also downregulate the functions of Th17 cells and Tregs.HSCs have an important role in immune regulation and homeostasis in the liver.Activated HSCs express cell surface programmed death ligand-1 (PD-L1),which is associated with T cell apoptosis [91-93].TGF-βsecreted by HSCs can effectively inhibit the function of T cells [10].HSCs can also secrete a variety of cytokines,including IL-10 and IL-17 [13,94].
HSCs also play a role in regulating the balance of Th17 cells/Tregs.In patients with advanced HBV-associated liver fibrosis,HSCs were reported to increase the number of Th17 cells and Tregs via the PGE2/EP2 and EP4 pathways [95].Similar findings have been obtained frominvitroculture experiments using human CD4 + T cells.Activated HSCs promote the proliferation of CD4 + T cells in a dose-dependent and time-dependent manner,which further promote the differentiation of na?ve CD4 + T cells into Th17 cells.This effect is associated with increased expression of cyclooxygenase (COX) and prostaglandin E2 (PGE2) and the secretion of IL-1βand IL-6 in HSCs [96].Additionally,in the mouse model of liver fibrosis induced by CCl4,IL-17 is mainly secreted by HSCs andγ δT cells in the early stage of liver fibrosis and promoted the secretion of IL-17 by Th17 cells [13].Four weeks after the injection of CCl4,Th17 cells became the primary IL-17-secreting cells [13].During the pathogenesis of liver fibrosis,HSCs regulate not only the proliferation and differentiation of Th17 cells and Tregs but also the function of Th17 cells.
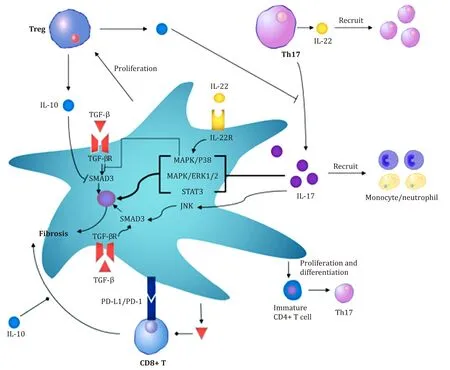
Fig.2.The interaction between hepatic stellate cells (HSCs) and T cells regulates liver fibrosis.Regulatory T cells (Tregs) and T helper 17 (Th17) effector memory T cells play essential roles in liver fibrosis.It is related to the formation of an inflammatory environment and the activation of HSCs by TGF- β1.In addition,HSCs also regulate the balance between Tregs and Th17 cells.During liver fibrosis,HSCs secrete IL-17 and induce the secretion of IL-17 by Th17 cells.HSCs directly promote the proliferation of Tregs,induce the proliferation of CD4 + T cells and promote the differentiation of CD4 + T cells into Th17 cells.TGF- β: transforming growth factor β; IL-17: interleukin 17;IL-10: interleukin 10; IL-22: interleukin 22; PD-1: programmed death 1; PD-L1: programmed death 1 ligand 1; MAPK: mitogen-activated protein kinase; ERK: extracellular regulated protein kinases; Treg: Regulatory T cells; STAT3: signal transducer and activator of transcription 3; SMAD3: sekelsky mothers against dpp 3.
The dual role of γδ T cells in liver fibrosis
γ δT cells are nonclassical T cells that are associated with innate immunity.Unlike classical T cells,the T cell receptor (TCR) ofγ δT cells is not composed of twoαandβchains but is composed ofγandδchains.Additionally,γ δT cells are enriched in the liver,and they account for 15%–25% of the T cells in the liver and 1%–10% of the T cells in the peripheral blood.However,γ δT cells play dual roles in liver infection.In patients with HBV infection,a negative correlation has been shown between the ratio ofγ δT cells in the liver and peripheral blood and disease severity [99].In patients with HCV infection,γ δT cells are enriched in the liver [100,101],andinvitroexperiments have shown thatγ δT cells have toxic effects on hepatocytes,indicating the role of these cells in liver damage during HCV infection [100].In the mouse model of liver fibrosis associated with murine hepatitis virus (MHV) infection,γ δT cells are dependent on the activity of TNF-α[102].These findings have been explained by the different functions of differentγ δT cell subsets [103].TCR Vδ1 + and Vδ2 + T cells mainly secrete IFN-γ,which is associated with the progression of immune liver disease,but theγ δT cell subsets that express Vγ4 + and IL-17 are protective against viral infection [103].Different causes of liver disease may be associated with differentγ δT cell subsets,which might explain the varied clinical findings.
In the context of liver fibrosis,γ δT cells have been shown to have similar dual effects.In 2014,Hammerich et al.[104]reported the findings of studies in two mouse models of chronic liver injury due to liver fibrosis induced by CCl 4 and the methionine and choline-deficient diet.These murine models showed thatγ δT cells were recruited into the liver via the expression of chemokine receptor 6 (CCR6) and colocalized with activated HSCs,and reduced liver fibrosis was associated with HCS apoptosis mediated by Fas/FasL [104].A similar finding was reported by Zhou et al.[105],who showed that Vγ9Vδ2T cells lysed activated HSCs by direct contact.However,Seo et al.[13]showed that in CCl 4 -induced liver fibrosis in mice,γ δT cells were responsible for the secretion of IL-17 in the early stage of liver fibrosis and had a proinflammatory role.The secretion of IL-17 byγ δT cells is regulated by Toll-like receptor 3 (TLR3) and is mediated by IL-17A,IL-1β,and IL-23 in HSCs.Tedesco et al.[106]showed that the use of antibodies to deleteγ δT cells increased the degree of liver fibrosis in a mouse model.The study of liver fibrosis in mice infected withSchistosomajaponicumalso supports the view that Vγ2 T cells exacerbate liver fibrosis by secreting IL-17 [107].However,it has been shown that Vγ1 T cells secrete only IFN-γand are not involved in IL-17 secretion [107].In conclusion,γ δT cells have dual roles in liver fibrosis,and different cell subpopulations may have different functions.However,the effects ofγ δT cell subsets in different pathological processes remain poorly understood,and further research is needed.
Conclusions and future perspectives
In this review,the current status of the cross-talk between HSCs and T lymphocytes in liver fibrosis and the known inflammatory mediators involved have been discussed.The unique immune environment of the liver plays an important role in liver fibrosis,which may be the result of imbalances in the normal process of tissue repair following injury,and this unique cellular environment directly affects the progression or regression of liver fibrosis [27].Studies on the regulation of the cellular immune response in the liver provide insights into potential therapeutic approaches to reduce the severity of liver fibrosis.For example,renal transplant recipients with chronic HCV infection had long-term benefits from short-term treatment with anti-thymocyte globulin (ATG) and showed reductions in liver fibrosis [63],which indicates that a change in the immune environment in the liver may occur following ATG therapy.Both preclinical and clinical studies have investigated liver fibrosis resulting from single cause in animal models or in patients and have shown conflicting results.However,because there are complex interactions between immune cells,including T lymphocytes and HSCs,it is likely that limited studies will not reflect the complexity of these interactions.Therefore,relevant and comprehensive methods should be used to assess the cellular immune environment in the liver that leads to liver fibrosis,such as singlecell sequencing and other molecular techniques.Benefitting from its unprecedented resolution,scRNA-seq could map the transcriptomic landscape of tissues and organs at the single-cell level,identify and define new subsets of cells and delineate the interplay between multiple cell types [108].A unique immune-regulated HSC subpopulation has been identified by single-cell RNA sequencing [109],indicating the infinite potential of single-cell RNA sequencing technology in liver immune research.
Acknowledgments
We thank Profs.George B Stefano and Richard M Kream for their useful suggestion and help for this review.
CRediTauthorshipcontributionstatement
HaoLi:Conceptualization,Writing - original draft,Writing - review & editing.PengDing:Writing - review & editing.BoPeng:Writing - review & editing.Ying-ZiMing:Conceptualization,Funding acquisition,Project administration,Writing - review & editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81771722 and 81700658).
Ethicalapproval
Not needed.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2021年3期
Hepatobiliary & Pancreatic Diseases International2021年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- MEETINGS AND COURSES
- Reply to: Contrast-enhanced ultrasonography for intrahepatic cholangiocarcinoma: A cost-effective alternative for low-resource settings
- Contrast-enhanced ultrasonography for intrahepatic cholangiocarcinoma: A cost-effective alternative for low-resource settings
- Transarterial chemoembolization as adjuvant treatment after surgery:The cure of huge hepatocellular carcinoma?
- RELEVANT CONTENT
- Variations of the hilar biliary confluence from postoperative cholangiography in Chinese population
