Control strategy and methods for continuous direct compression processes
Yasur Suzu,Hrazu Suyaa,Maau Ka,Ryutar S,Gsu Saa,Ry Furuawa,E Ma,K Mtyaa,Tatsu K,Yasur Matsu,Kazu Kurasa,Iss Taayaa,Su Ha,Nr Katr,Masa Ku,Hrs Saa,Ysr Matsua
a Formulation Technology Research Laboratories,Daiichi Sankyo Co.,Ltd.,Kanagawa 254-0014,Japan
b Department of Chemical System Engineering,The University of Tokyo,Tokyo 113-8656,Japan
c Department of Systems Science,Kyoto University,Kyoto 606-8501,Japan
d CMC Sciences Department,Regulatory Affairs Division,R&D Division,Janssen Pharmaceutical K.K.,Tokyo 101-0065,Japan
e E uroTechno Ltd.,Kanagawa 223-0057,Japan
f P harmaceutical Research Department,Mitsubishi Tanabe Pharma Corporation,Osaka 532-8505,Japan
g Chemical Products,CMC Regulatory Affairs Area Japan Development,MSD K.K.,Tokyo 102-8667,Japan
h Graduate School of Pharmaceutical Sciences,Kumamoto University,Kumamoto 862-0973,Japan
i D ivision of Drugs,National Institute of Health Sciences,Kawasaki 210-9501,Japan
j T echnology Research & Development,Sumitomo Dainippon Pharma Co.,Ltd.,Osaka 564-0053,Japan
k Formulation Development Department,Chugai pharmaceutical Co.,Ltd.,Tokyo 115-8543,Japan
l O ffice of New Drug IV,Pharmaceuticals and Medical Devices Agency,Shin-Kasumigaseki Bldg.,Tokyo 100-0013,Japan
m Office of Generic Drugs,Pharmaceuticals and Medical Devices Agency,Shin-Kasumigaseki Bldg.,Tokyo 100-0013,Japan
n KOKANDO Co.,Ltd.,Toyama 930-8580,Japan
o Pharmaceuticals and Medical Devices Agency,Shin-Kasumigaseki Bldg.,Tokyo 100-0013,Japan
Keywords:Continuous manufacturing Solid drug products Process control Residence time distribution Loss in weight feeder Regulatory science
ABSTRACT We presented a control strategy for tablet manufacturing processes based on continuous direct compression.The work was conducted by the experts of pharmaceutical companies,machine suppliers,academia,and regulatory authority in Japan.Among different items in the process,the component ratio and blended powder content were selected as the items requiring the control method specific to continuous manufacturing different from the conventional batch manufacturing.The control and management of the Loss in Weight(LIW) feeder were deemed the most important,and the Residence Time Distribution (RTD)model were regarded effective for setting the control range and for controlling of the LIW feeder.Based on these ideas,the concept of process control using RTD was summarized.The presented contents can serve as a solid fundament for adopting a new control method of continuous direct compression processes in and beyond the Japanese market.
1.Introduction
In the last years,continuous manufacturing has been implemented in the commercial production of solid drug products.In 2018,the first approval was given for continuous manufacturing in Japan,which was based on direct compression process.Typically,direct compression process consists of three steps: raw material blending,tableting,and coating.These steps are interconnected in continuous processes,so that the raw materials are fed into the process,and the tablets are obtained from the process simultaneously.
The understanding of continuous direct compression is growing along with the increased presence of the technology.One topic often found in the literature is monitoring and control.Pawar et al.[1]applied near infrared (NIR) prediction of dissolution of tablets made by continuous direct compression towards real time release testing (RTRT).De Leersnyder et al.[2]developed an in-line NIR spectroscopic method for continuous blend potency determination in the feed frame of a tablet press.Almaya et al.[3]presented a control strategies of the process with a focus on the state of control,product collection,and startup/shutdown operations for clinical and commercial production.Moreno et al.[4]investigated sensor network robustness using model-based data reconciliation.Another well-investigated topic is quality variation measured by the residence time distribution (RTD).Van Snick et al.[5]applied inline NIRs to estimate the RTD in the mixer and to monitor blend uniformity for producing sustained release tablets.Escotet-Espinoza et al.[6]analyzed the effect of tracer material properties on the RTD in the blending step of the process.Bhaskar et al.[7]reported an RTD-based control system where non-conforming materials were diverted on a real time basis.Billups et al.[8]used RTD for analyzing material traceability for determining raw material batch changes.Besides,the role of continuous manufacturing and continuous direct compression processes has been analyzed from quality and economic perspectives [9-11].
Regulatory authorities,as the backbone to provide quality products to the patients,have been recognizing the relevance of continuous manufacturing including direct compression processes.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) documentations provided ICH Q8 (R2) [12],Q9 [13],Q10 [14],Q11 [15],and Q12 [16]as the basis for adopting Quality-by-Design (QbD) approaches.From November 2018,activities have been carried out toward the development of a new guideline for continuous manufacturing as ICH Q13 [17].Currently,each regulatory authority is working on the issues to apply the current regulations for continuous manufacturing based on the batch manufacturing method in parallel with the discussion in ICH Q13.From 2016 to 2018,a Japan Agency for Medical Research and Development (AMED)research project was performed on a "Research into Quality Assurance of Pharmaceutical Continuous Manufacturing".The project was led by the author Yoshihiro Matsuda in Pharmaceuticals and Medical Devices Agency (PMDA),the Japanese regulatory agency.As the outcomes,"Points to Consider Regarding Continuous Manufacturing" Document[18]was first published in March 2017.Then "State of Control in Continuous Pharmaceutical Manufacturing",an important concept in continuous manufacturing [19],was published in March 2018.These documents summarize the opinions of experts in industry,regulatory authority and academia on the concept to take actions to consolidate the environment where companies may easily apply continuous manufacturing.
In the fiscal year 2018,a new AMED project started for the"Study on Quality Assurance of Pharmaceutical Continuous Manufacturing",where the authors of the present paper are participating in this project with Yoshihiro Matsuda as the project leader.In order to promote continuous manufacturing in Japan,we have been conducting activities to present the measures to ensure the state of control in the process as well as the effective control methods to ensure the product quality.This time,we generally focused on process control,and summarized tools and points to consider for manufacturing control.For the continuous direct compression processes,RTD models were investigated particularly as a useful tool for process control.
Based on these activities,we present a control strategy of continuous direct compression processes.The paper first introduces the overview of the target process,followed by the items to be controlled in the process and the setting of the control range using RTD model.The presented contents were discussed and agreed by the experts from drug producers,machine suppliers,academia,and the Japanese regulatory authority,PMDA,which is the originality of the work.Thus,the presented contents can serve as a solid fundament for adopting a new control method of continuous direct compression in and beyond the Japanese market.
2.Target process
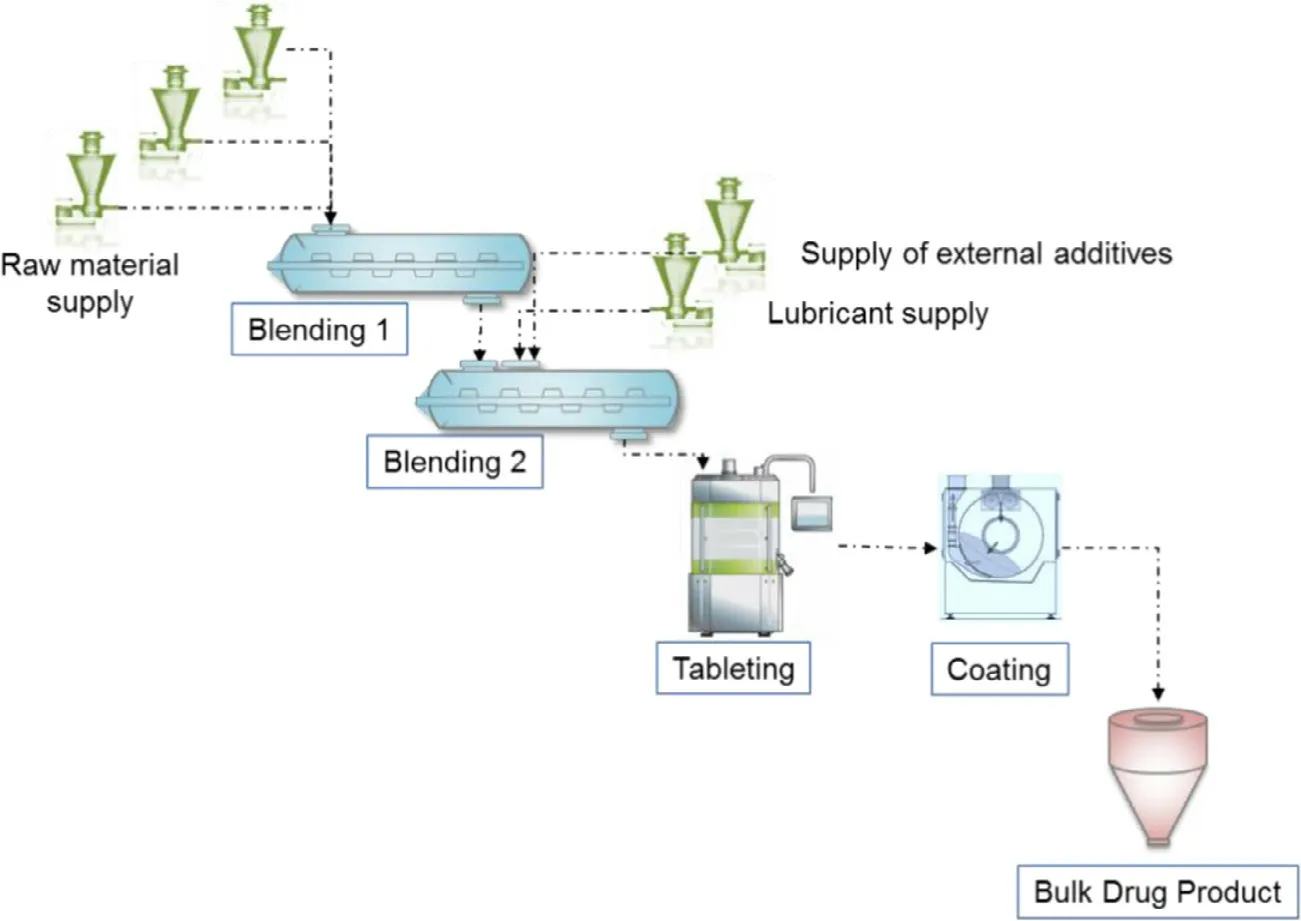
Fig.1–Schematic diagram of continuous direct compression process.
In the continuous direct compression process investigated in this study,as shown in Fig.1,the raw materials including the active pharmaceutical ingredient (API) are continuously supplied to Blender 1 by the Loss in Weight (LIW) feeder,and they are mixed by Blender 1 (Blending process 1).Then,the blended powder is transferred to Blender 2,and at the same time,external additives such as lubricant are supplied to Blender 2 by a LIW feeder and mixed (Blending process 2).The blended powder for tableting was transferred to a tablet press to make tablets (Tableting process),and the tablets are coated by a small amount of sub-batch using a semi-continuous film coating machine (Coating process).The characteristics of this process are as follows: the raw materials are supplied continuously by LIW feeders,the materials continue to move from the upstream process to the downstream process without remaining in the process,and they are formulated as a bulk drug product.
3.Items to be controlled in the process in continuous direct compression
3.1.Selection of the items to be controlled in the process
The continuous direct compression process has process parameters and material attributes that are specific to continuous manufacturing and must be controlled since raw materials and intermediate products continuously move in the process.The items to be controlled in the continuous direct compression process were discussed,focusing on the significant difference of operations between continuous manufacturing and batch manufacturing.As a result,the LIW feeding operation was selected as an item to be controlled.The LIW feeding operation,which corresponds to the weighing process in batch manufacturing,determines the component ratio of the drug product and affects the critical quality attributes (CQAs) of the drug product (i.e.,assay and content uniformity of dosage units)via the blended powder content.Thus,control of LIW feeders is crucial in the continuous direct compression process.The differences between batch and continuous manufacturing for the component ratio and blended powder content are shown in Table 1 .
3.2.Process control
3.2.1.Process variability to be considered for in-process control
In the continuous direct compression process,there are three types of typical variation patterns of process parameters and material attributes to be controlled.Table 2 shows such variation patterns and Cor111responding control methods.Since the actual variation may be a combination of these variation patterns,it is important to consider all of these variation patterns when developing control methods of the LIW feeders.
3.2.2.Concepts for control in continuous direct compression
In order to control the component ratio and the blended powder content in the continuous direct compression process,it is necessary to monitor the manufacturing state and control the process parameters to the target values/set values.Depending on the state,it is necessary to judge whether it should be treated as a conforming product,whether it should be diverted from the process as a defective product,or whether equipment or quality should be checked by stopping the equipment.Table 3 summarizes the concept of what tools are useful for monitoring and control and how to manage the process.
[Control of component ratio ]
Each material is consistently monitored and controlled to a target feeding rate by an independent LIW feeder.In the component ratio control,it is most important to control the feed rate of each LIW feeder so that it becomes the set value and consequently the component ratio becomes the target value as shown in No.1 in Table 3 .If the LIW feeding rate of each component is within an appropriate acceptance value/range (upper and lower limits),the component is judged as a conforming article.If it exceeds the acceptance value/range,judgment such as diversion from the system or shutdown of the equipment is made.It is appropriate to judge diversion from the system or equipment shutdown,considering the variations shown in Table 2 .Even if fluctuations due to disturbances occur,stable manufacturing can be achieved by continuously monitoring the process states and suppressing the fluctuations through feedback/feedforward control.When a disturbance is larger or more frequent than assumed and the component ratio exceeds the upper or lower limit,diversion may be performed to avoid the impact on the process if the disturbance is temporary.However,in cases where the magnitude or frequency of the disturbance exceeds the capability of the process control,it is desirable to shut down the equipment rather than diversion.Equipment shutdown is appropriate when the control is not working normally,for example,when a diversion from the system continues in variation pattern 1,when it frequently occurs in variation pattern 2,or when considerable variation occurs in variation pattern 3.

Table 1– Differences between batch and continuous direct compression methods for the component ratio and blended powder content.
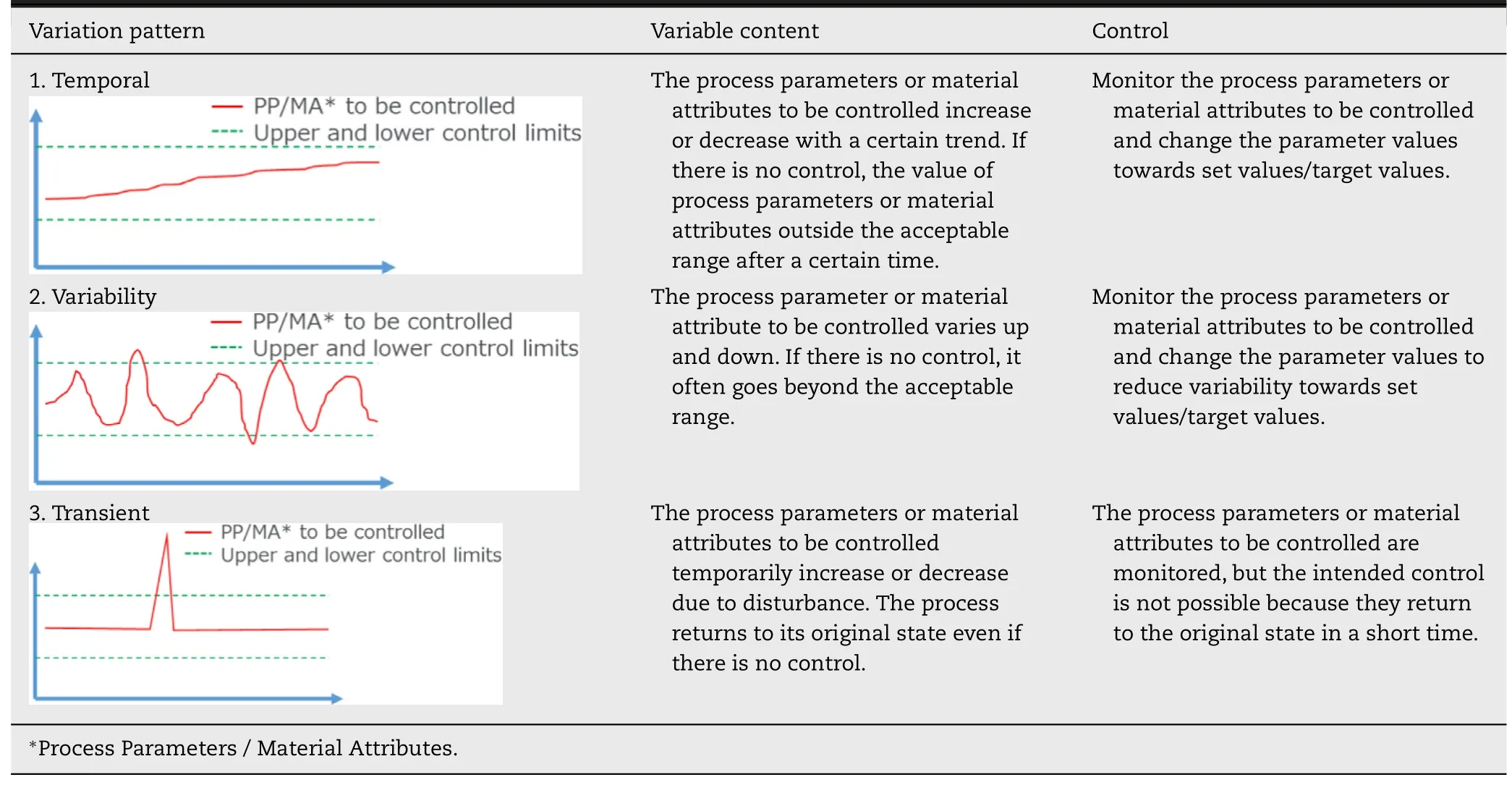
Table 2–Possible variation patterns of continuous direct compression process.
No.2 in Table 3 is to control the feed rate so that,for example,the excipient is supplied at a preset ratio against the API in accordance with the variation in the feed rate of the API,assuming that No.1 mentioned above is controlled.However,this control method is difficult to apply when the process parameters or material attributes to be controlled repeatedly move up and down,like variation pattern 2 in Table 2 .Therefore,the control of No.2 is considered to be useful as an auxiliary control of No.1.
The method shown in No.3 in Table 3 directly measures the concentration of components using NIR,Raman,etc.They are not used to control each component’s feed rate because the LIW feeder controls it.Instead,the NIR,Raman,etc.are used to detect process abnormalities in advance and to make decisions such as diversion from the system or shutdown of equipment,as a tool to support the control of the LIW feeder.Also,they are beneficial to acquire knowledge of time-series variation of each ingredient in the development stage and deepen the understanding of the process by using them for preparation and verification of the RTD model.
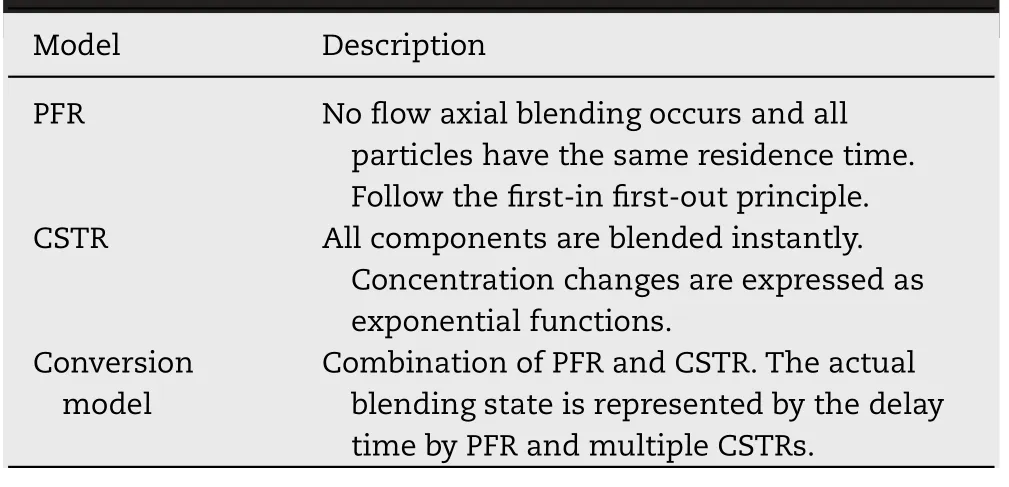
Table 4–Representative example of the RTD model used in continuous direct compression.
[Control of blended powder content ]
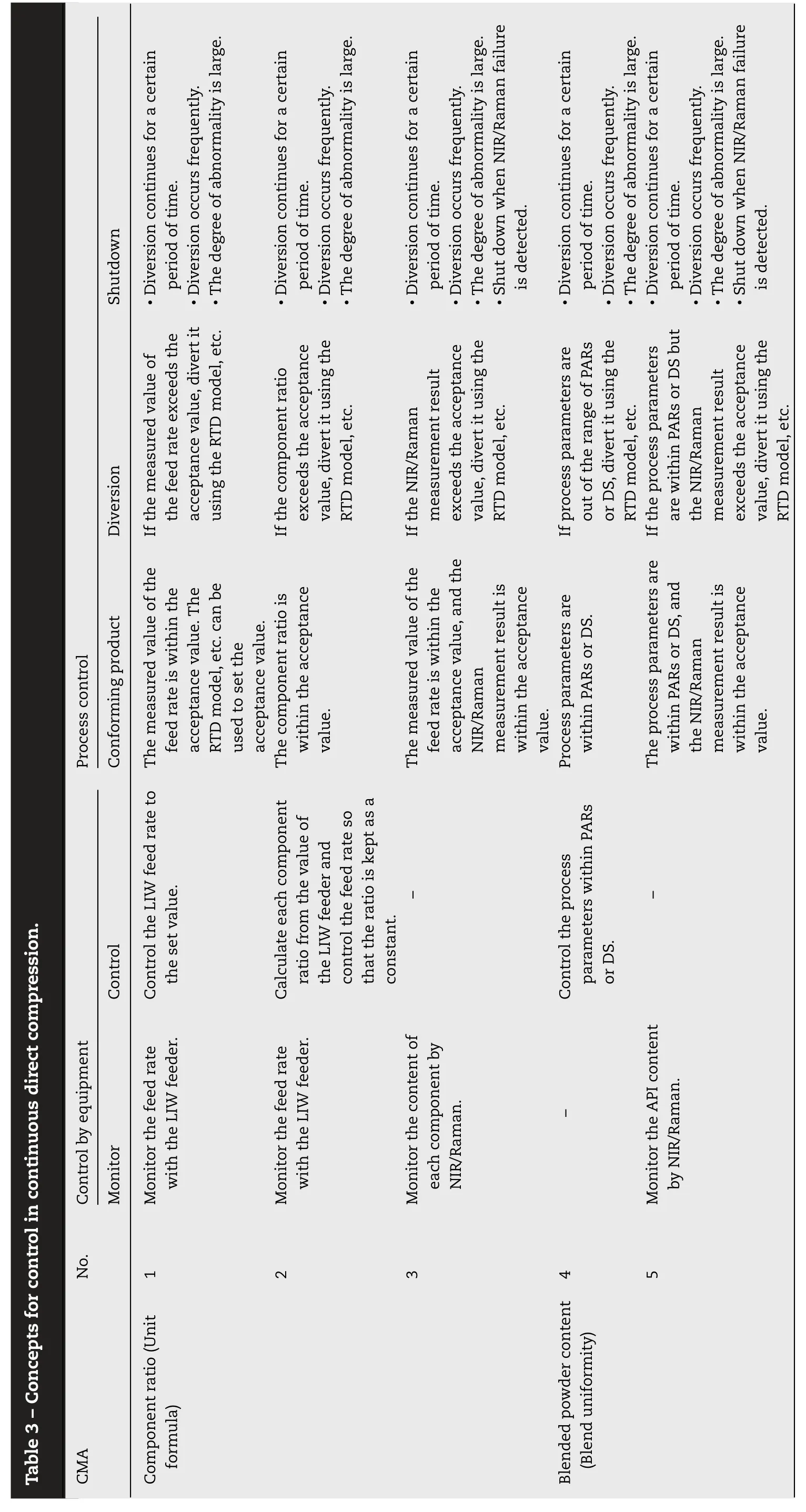
ality is large.ality is large.ality is large.ality is large.ality is large.Shutdown? Diversion continues for a certain period of time.? Diversion occurs frequently.? The degree of abnorm? Diversion continues for a certain period of time.? Diversion occurs frequently.? The degree of abnorm? Diversion continues for a certain period of time.? Diversion occurs frequently.? The degree of abnorm? Shut down when NIR/Raman failure is detected.? Diversion continues for a certain period of time.? Diversion occurs frequently.? The degree of abnorm? Diversion continues for a certain period of time.? Diversion occurs frequently.? The degree of abnorm? Shut down when NIR/Raman failure is detected.acceptance value, divert it If the measured value of the feed rate exceeds the using the RTD model, etc.If the component ratio exceeds the acceptance value, divert it using the RTD model, etc.If the NIR/Raman ent result exceeds the acceptance value, divert it using the RTD model, etc.If process parameters are out of the range of PARs or DS,divert it using the RTD model, etc.If the process parameters are within PARs or DS but the NIR/Raman ent result Diversion measurem measurem exceeds the acceptance value, divert it using the RTD model, etc.Process control Conforming product The measured value of the feed rate is within the acceptance value. The RTD model, etc. can be used to set the acceptance value.The component ratio is within the acceptance ent result is ent result is value.The measured value of the feed rate is within the acceptance value, and the NIR/Raman measurem within the acceptance value.Process parameters are within PARs or DS.The process parameters are within PARs or DS,and the NIR/Raman measurem within the acceptance value.feed rate to the set value.Table 3 –Concepts for control in continuous direct compression.Control Calculate each component Control the LIW ratio from the value of control the feed rate so the LIW feeder and that the ratio is kept as a constant.—Control the process parameters within PARs—or DS.ent Control by equipm feeder.feeder.—Monitor Monitor the feed rate with the LIW Monitor the feed rate with the LIW Monitor the content of each component by NIR/Raman.Monitor the API content by NIR/Raman.No.1 2 3 4 5 CMA Component ratio (Unit formula)Blended powder content(Blend uniformity)
The blended powder content is controlled by the LIW feeder similarly to the component ratio control,under the assumption that the blending process uniformly blends each component.Therefore,the proven acceptable ranges (PARs) and design space (DS) for the critical process parameters (CPPs) of the blending process are established in the development stage,and the blended powder content is controlled by the LIW feeder while controlling the CPPs within the established PARs and DS.When the LIW feeder is controlled within the acceptance value/range,the intermediate product is regarded as conforming if the process parameters of the blending process are within PARs and DS,and a judgment of diversion from the system or equipment shutdown should be made if they exceed the PARs and DS.The same concept as in the component ratio control applies to the decision on equipment shutdown.Monitoring and process control using NIR and Raman are of the concept similar to the control of the component ratio.
4.Setting of the control range of the LIW feeder using the RTD model
4.1.What is the RTD model?
The RTD model is a well-known theory in reaction engineering and can provide information about material flow and component concentration within the reactor.In the continuous direct compression process,the RTD model enhances the understanding of how the API and each excipient component flow in the blender and how they are blended; thus,it has been investigated as an important tool for process understanding and control.There are three major types of RTD models: plug flow reactor (PFR),continuous stirred tank reactor (CSTR),and laminar flow reactor (LFR).In the continuous direct compression process,PFR and CSTR shown in Table 4 and Fig.2,as well as a model combining both (Conversion model) can be used.
If a blender or a stirring feeder is modeled by one PFR and two CSTRs,the relationship between the concentration at the inlet (C) and the concentration at the outlet (C ) is expressed by Eq.1 based on mass balance.Where θ,τ,and τrepresent the lag time and the average residence time of the first and second CSTR,respectively,which are the specific parameters of the equipment.
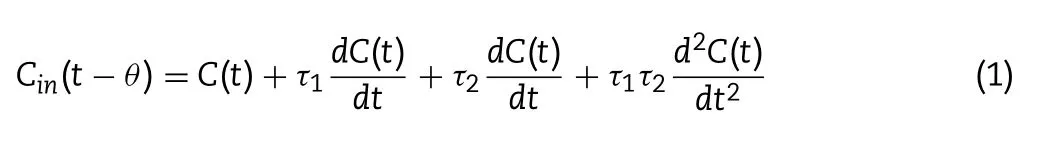
The RTD model is a function,which depends on the size of the equipment,blending conditions,powder fill level in the equipment,and powder feed rate,and it is not affected by variations in environmental conditions such as temperature and humidity in the production room.
4.2.How to develop the RTD model
The RTD model parameters (θ,τ,and τ) can be determined by fitting them with the actual concentration of the API or tracer measured continuously by in-line NIR,etc.Representative examples of how to develop the RTD models include an impulse response test (spike test),a step response test,and a multistep response test (relay tests) as shown in Table 5 .All the methods are available for developing an RTD model of the continuous direct compression process.However,in actual application,it is necessary to select an optimal method according to the formulation,process,and so forth.
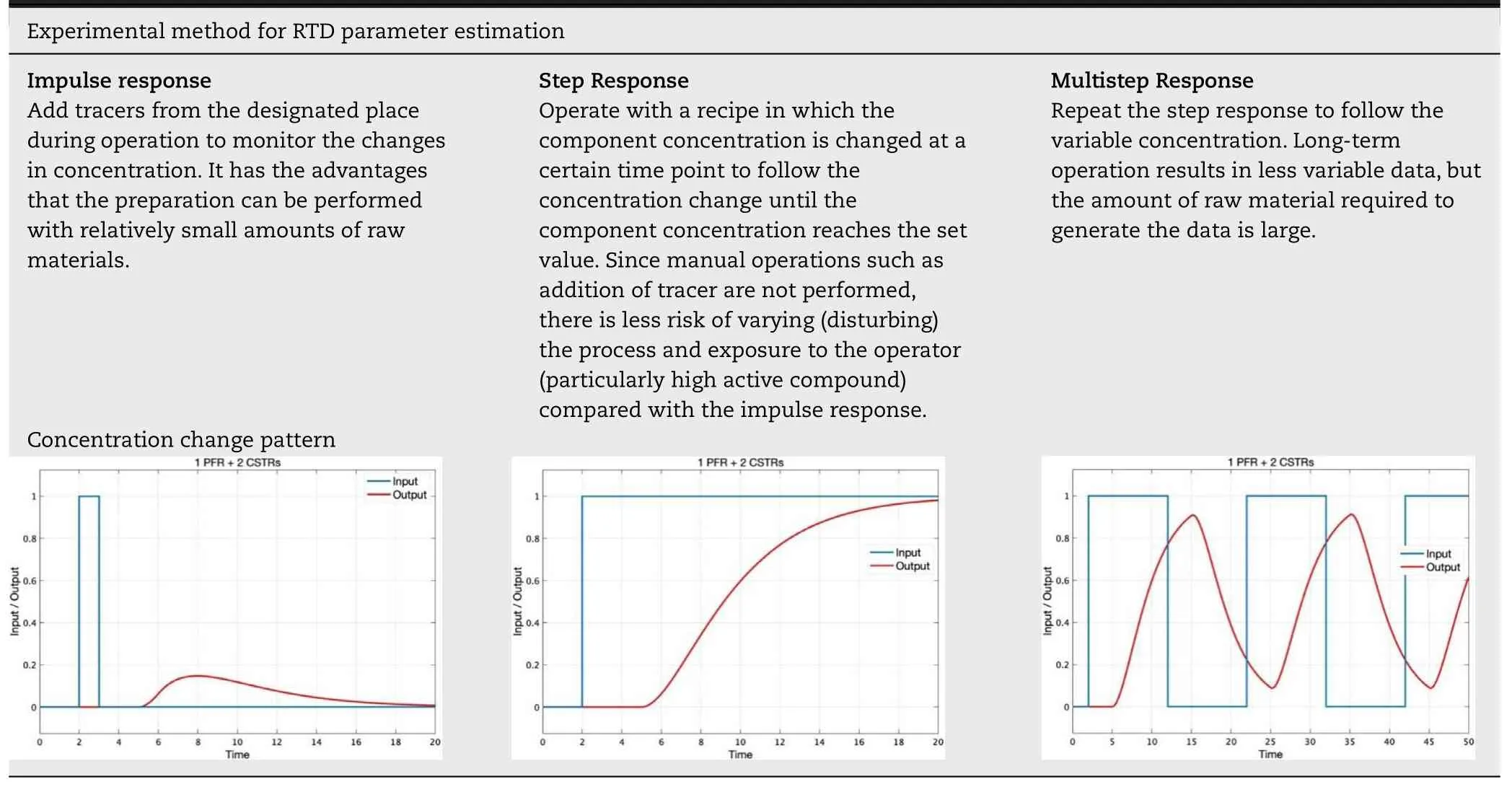
Table 5– Example of the experimental method for the RTD parameter estimation and concentration change pattern obtained.
Fig.3 is an example of an RTD model of a continuous blending process,constructed from an impulse response test,in which a tracer was added from the inlet of Blender 2,and the tracer concentration was measured by NIR placed between the Blender 2 and the tablet press force feeder.By obtaining the parameters θ,τ,and τthat minimize the difference between actual NIR data and the predicted values from the RTD model,the delay time of Blender 2 and the change in concentration in the blender can be calculated.By determining RTD parameters of Blender 1 and force feeder in a similar manner and integrating them,it becomes possible to grasp the changes in concentration throughout the process.
4.3.Process control using the RTD model
In the continuous direct compression process,the process variation caused by the LIW feeder is transmitted to the tablet content through the Blender 1,Blender 2,and tableting process (blending in the force feeder).Using the RTD model prepared by the method shown in 4.2.,it is possible to predict how the variation caused by the LIW feeder propagates in the process and how it affects the quality of the final drug product,and to set the acceptable range of variation of the LIW feeder.Fig.4 shows an image of predicting how the variation in feed rate due to disturbance in the LIW feeder would be transmitted to Blender 1,Blender 2,and the tablet press from the magnitude and duration of variation by the RTD model.
For example,a purple color pattern in Fig.4 indicates a considerable variation in size but a short variation in duration.On the contrary,a pattern shown by blue indicates a small variation in size but a long variation in duration.If this kind of variation occurs in the LIW feeder,it takes θfor the PFR model to propagate the variation to Blender 1,and then the time-series change in the concentration in the blender is calculated with the CSTR model.In Blender 2,the PFR model is further delayed by θto propagate the variation,and the CSTR model is used to calculate the time-series change in concentration.It was found that the magnitude became smaller and the duration of variation became longer after each of Blender 1 and Blender 2 than the variation caused by the LIW feeder.The RTD can express how the variation in the LIW feeder affects the CQA of Assay of the tablet finally after blending in the force feeder.
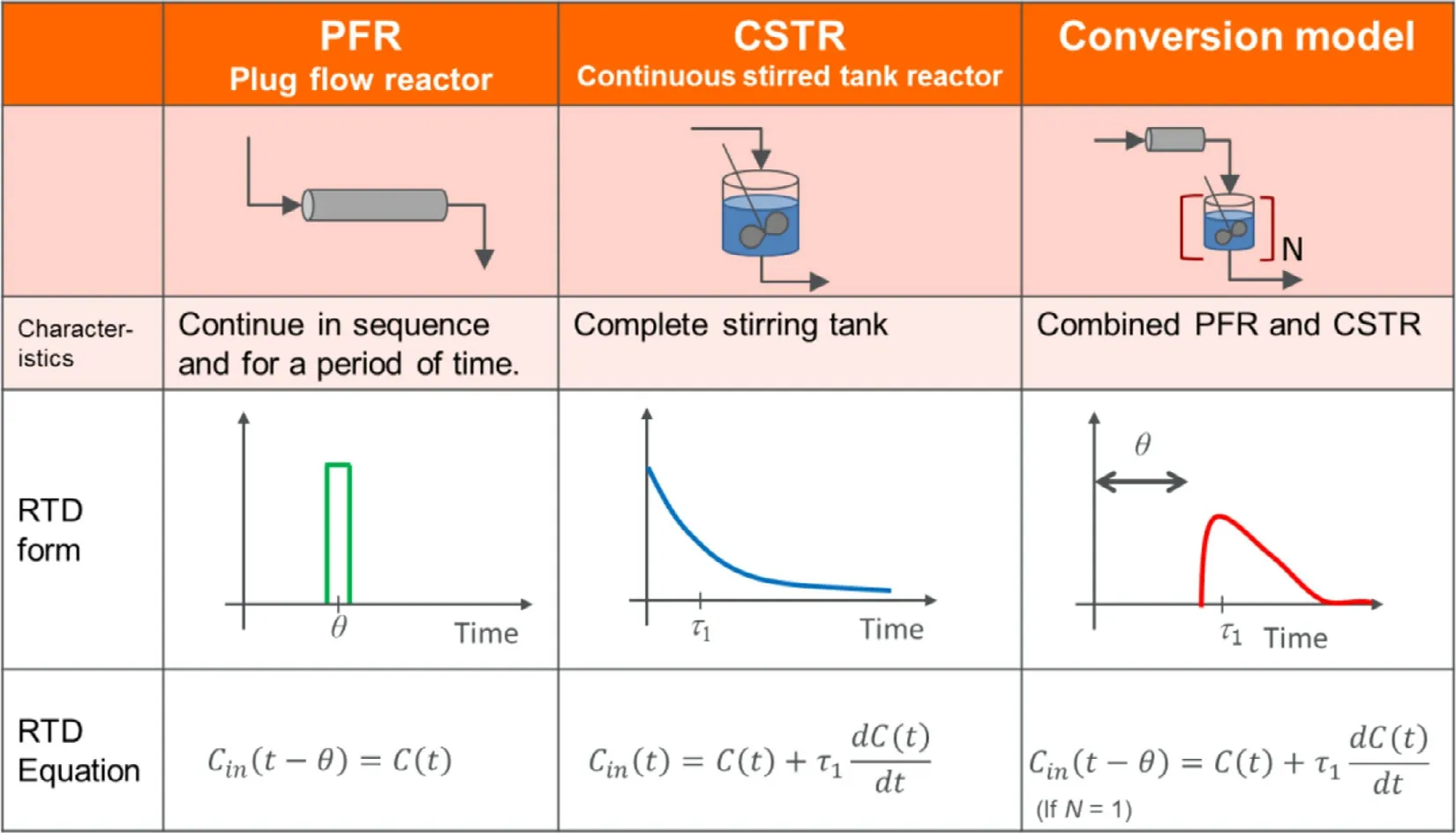
Fig.2–RTD model and its characteristics.
Therefore,using the RTD model,the magnitude and length of acceptable variation of LIW feeder can be established from its impact on the CQA of Assay and can be incorporated into the control strategy of the continuous direct compression process.As shown on the left of Fig.5,even if a large transient change (blue line) occurs in the LIW feeder,if the API concentration in a tablet calculated by the RTD model is within the acceptance value/range (Fig.5,green line on the left),it can be judged to be conforming,and the control such as diversion from the system can be performed by the RTD model only when the calculated API concentration exceeds the acceptance value/range as shown in the part enclosed by the purple dotted line on the right of Fig.5 .
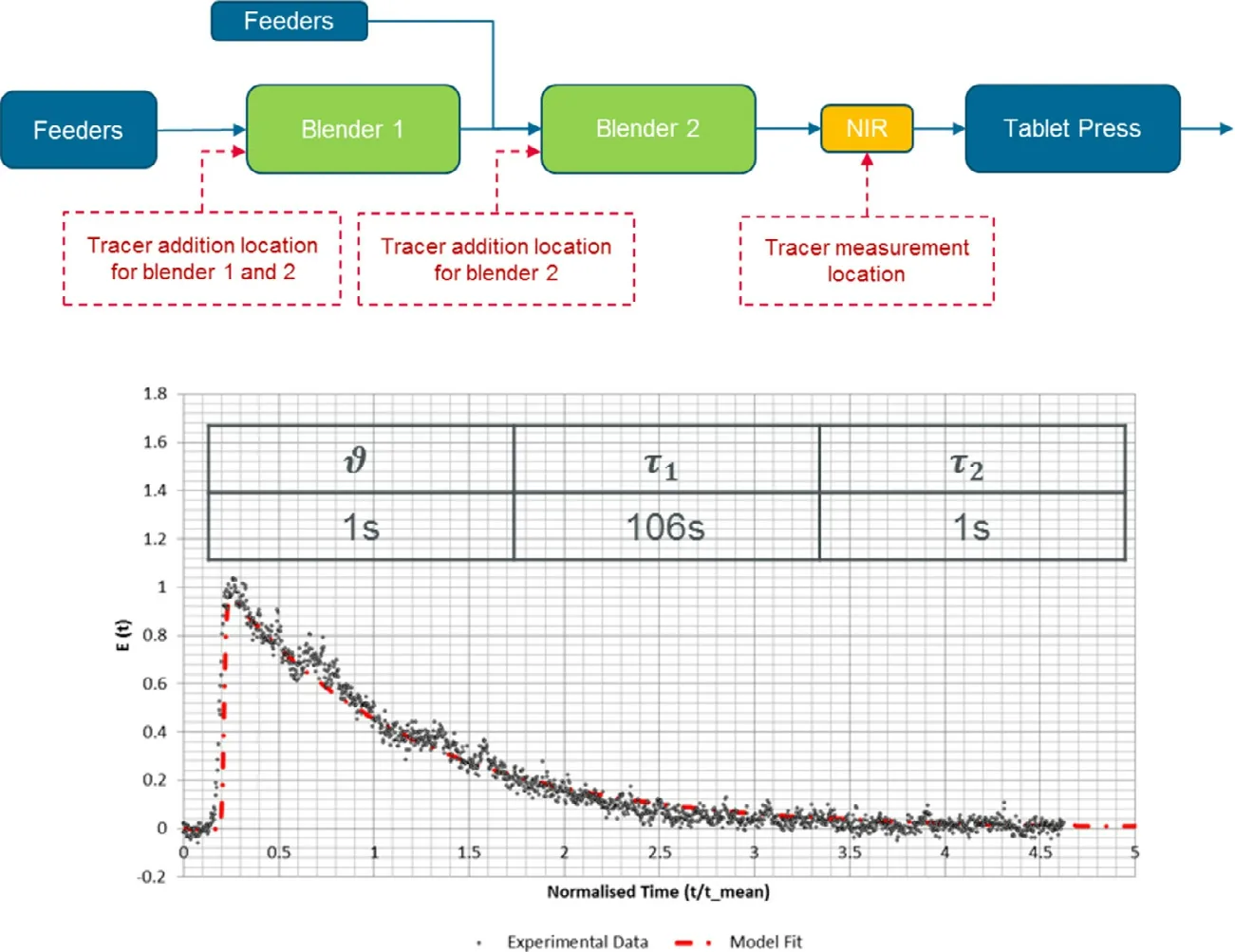
Fig.3–NIR data (black plot) from the impulse response method and RTD model prediction value (red dashed line).
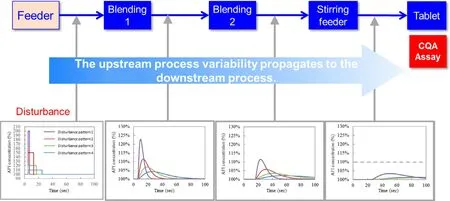
Fig.4–Variations in LIW feeder and image of propagation to each process.
Fig.6 shows the acceptable range of variation of the LIW feeder calculated by the RTD model based on the above concept.Theoretically,the blue area in the figure is within the specification,and therefore the product can be judged as a conforming product.On the other hand,in normal commercial production,it is common to set Alert Limit (Action Limit) inside the upper and lower limits of the acceptable range,and it is appropriate to control it inside the boundary between In spec and Out of spec in Fig.6 .In addition,as shown in "3.Items to be controlled and managed in the process in continuous direct compression," if the degree of variation is substantial,it is considered that the control is performed depending on the situation such as stopping the machine.
4.4.Notes on the control of continuous direct compression process using the RTD model
As mentioned above,the RTD model can be a useful tool for controlling the continuous direct compression process.On the other hand,the points to consider when using this RTD model were summarized as follows.
[D evelopment of the RTD model ]
?The RTD model needs to be validated with actual experimental data.
?The RTD model may differ depending on the formulation of the product,machine,and manufacturing conditions;therefore,it is necessary to confirm the presence or absence of impact on the RTD model when they are changed.
?Since the RTD model is affected by the throughput of the process (kg/h),it is important to confirm in advance the upper and lower limits of the throughput within which the RTD model can be used.[V alidation of the RTD model ]
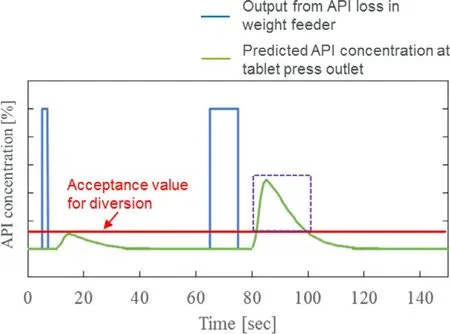
Fig.5–Process control image based on the concentration predicted by RTD.
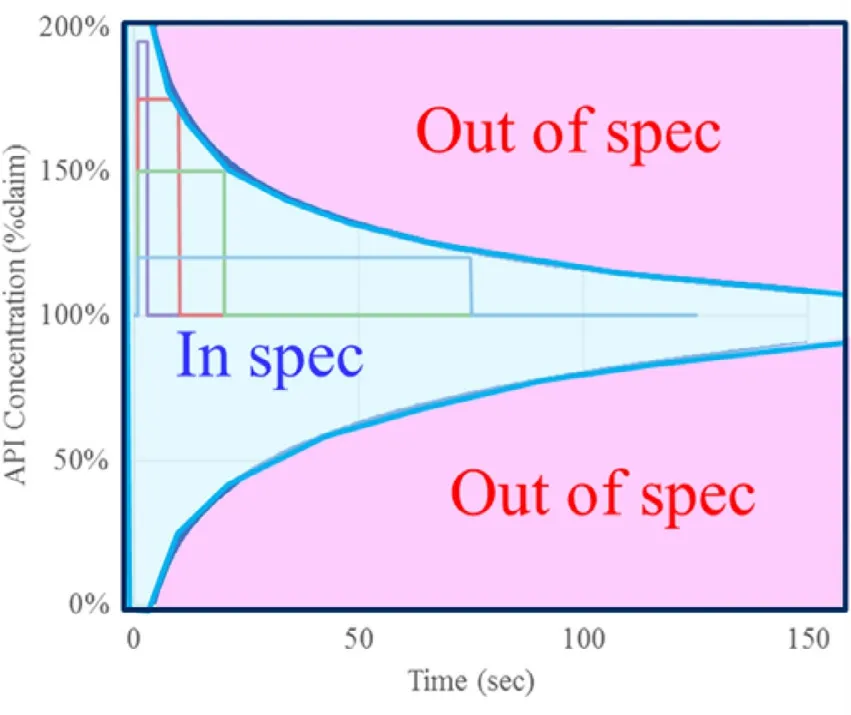
Fig.6–Image of acceptable variation range of the LIW feeder.
?It is necessary to validate the RTD model at an appropriate frequency during commercial production.The frequency and method of validation will depend on the application purpose of the RTD model (e.g.,Real Time Release,compensation of missing NIR data,understanding of process dynamics (raw material tracking)).
?For model validation,any appropriate method suitable for the process such as the impulse response test (spike test)and comparison with the content measurements during operation can be used.
[P rocess control ]
?It is useful to monitor the processes in multiple ways without relying on just one control method.For example,when NIR is used for the process control of the blended powder content,the content in the blank period of NIR measurement can be traced by using the RTD model if the measurement data are temporarily missing due to the washing of NIR probe during operation.
?The monitoring and control of the homogeneity of powder blend,which was out of scope of this study,is important in the actual manufacturing to assure the final drug product quality in the continuous direct compression process.
5.Conclusion
We presented a control strategy for continuous direct compression processes,as an outcome an industryacademia-regulatory authority research project in Japan.After discussions in the expert group,the component ratio and blended powder content were selected as the items requiring the control method specific to the continuous manufacturing different from the batch manufacturing.The control and management of the LIW feeder for each component were deemed the most important,and the RTD model was regarded effective for setting the control range and for controlling of the LIW feeder.Based on these ideas,the concept of process control using RTD model was summarized.The presented contents can serve as a solid fundament for adopting a new control method of continuous direct compression in and beyond the Japanese market.The content presented in this article is an example,and it should be noted that the use of RTD models varies depending on the product and process characteristics to be applied or the purpose of application,including how to control the manufacturing process,how to validate the RTD model and how often the RTD model should be verified.
Conflicts of interest
The authors report no conflicts of interest.The authors alone are responsible for the content and writing of this article.The views expressed in this manuscript by the authors are personal and do not necessarily reflect the official views of the Pharmaceuticals and Medical Devices Agency (PMDA).
Acknowledgments
This research was supported by AMED under Grant Number JP20mk0101106 .We would also like to express our appreciation to GEA Pharma Systems for their cooperation in explaining the RTD model and providing information.We would like to extend the acknowledgement to Mr.Kensaku Matsunami at The University of Tokyo for his support in the literature review.
 Asian Journal of Pharmacentical Sciences2021年2期
Asian Journal of Pharmacentical Sciences2021年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- Role of nanoparticle-mediated immunogenic cell death in cancer immunotherapy
- PH-responsive strontium nanoparticles for targeted gene therapy against mammary carcinoma cells
- Heterobifunctional PEG-grafted black phosphorus quantum dots: “Three-in-One”nano-platforms for mitochondria-targeted photothermal cancer therapy
- Intra-Articular injection of acid-sensitive stearoxyl-ketal-dexamethasone microcrystals for long-acting arthritis therapy
- A computer-aided chem-photodynamic drugs self-delivery system for synergistically enhanced cancer therapy
- Gas-blasting nanocapsules to accelerate carboplatin lysosome release and nucleus delivery for prostate cancer treatment
