TiO2-Supported Single-Atom Catalysts for Photocatalytic Reactions
Xuemei Zhou
School of Chemical Engineering, Sichuan University, Chengdu 610065, China.
Abstract: Titania (TiO2) has been among the most widely investigated and used metal oxides over the past years, as it has various functional applications. Extensive research into TiO2 and industrial interest in this material have been triggered by its high abundance, excellent corrosion resistance, and low cost. To improve the activity of TiO2 in heterogeneous catalytic reactions, noble metals are used to accelerate the reactions.However, in the case of nanoparticles supported on TiO2, the active sites are usually limited to the peripheral sites of the noble metal particles or at the interface between the particle and the support. Thus, highly dispersed single metal atoms are desired for the effective utilization of precious noble metals. The study of oxide-supported isolated atoms, the so-called single-atom catalysts (SACs), was pioneered by Zhang’s group.The high dispersion of precious noble metals results helps reduce the cost associated with catalyst preparation. Because of the presence of active centers as single atoms, the deactivation of metal atoms during the reaction, e.g., by coking for large agglomerates, is retarded. The unique coordination environment of the noble metal center provides special sites for the reaction, consequently increasing the selectivity of the reaction, including the enantioselectivity and stereoselectivity. Hence, supported SACs can bridge homogenous and heterogeneous reactions in solution as they provide selective reaction sites and are recyclable. Moreover, owing to the high site homogeneity of the isolated metal atoms, SACs are ideal models for establishing the structure-activity relationships. The present review provides an overview of recent works on the synthesis, characterization, and photocatalytic applications of SACs (Pt1, Pd1,Ir1, Rh1, Cu1, Ru1) supported on TiO2. The preparation of single atoms on TiO2 includes the creation of surface defective sites, surface modification, stabilization by high-temperature shockwave treatment, and metal-ligand self-assembly.Conventional characterization methods are categorized as microscopic imaging and spectroscopic methods, such as aberration-corrected scanning transmission electron microscopy (STEM), scanning tunneling microscopy (STM), extended X-ray absorption fine structure analysis (EXAFS), and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS).We attempted to address the critical factors that lead to the stabilization of single-metal atoms on TiO2, and elucidate the mechanism underlying the photocatalytic hydrogen evolution and CO2 reduction. Although many fascinating applications of TiO2-supported SACs in photocatalysis could only be addressed superficially and in a referencing manner, we hope to provide interested readers with guidelines based on the wide literature, and more specifically, to provide a comprehensive overview of TiO2-supported SACs.
Key Words: Titanium dioxide; Single atom catalyst; Stabilization; Characterization; Photocatalytic H2 evolution
1 Introduction
Titanium dioxide (TiO2) is among the most widely investigated metal oxides over the past years based on the various functional applications1, such as pigments in paints,dye-sensitized solar cells2,3, photocatalysts4,5, self-cleaning coatings6, biomedicine7,8, ion-insertion batteries9,10, and electrochromic devices11,12. The extensive research and industrial interest on titania is promoted by its high abundance,high resistance against corrosion, and low-cost13–16. The photocatalysis of titania is determined by its unique semiconductive properties. It shows a band-gap of 3.0 eV for titania rutile and 3.2 eV for titania anatase1, that suggests the electron at the valence band of titania can be excited to the conduction band by ultraviolet (UV) light (λ< 413 nm for rutile,andλ< 388 nm for anatase), leaving a hole at the valence band.The photogenerated electrons can diffuse in micrometer length range in anatase, enabling the photocatalytic reaction to occur in a short distance. The relative band-edge positions, valence band maximum (VBM) and conduction band minimum (CBM),determine the occurrence of a photocatalytic redox reaction,exploiting electrons in the CBM or holes in the VBM.
A considerable amount of work has been focused on modifying the band gap and band-edge positions of titania to trigger a broad range of redox reactions and to harvest the full spectrum of solar light. One way to regulate the band-edge positions of titania is to form a junction with a secondary semiconductor17,18, such as a Z-scheme19–24or an S-scheme25junction. An alternative approach is to decorate TiO2with noble metal particles26, as a co-catalyst to accelerate the separation of photogenerated electron and holes27. Specific noble metals, e.g.,Ag or Au, work as plasmonic centers to generate hot electrons28.
Upon the attachment of a nanoparticle on a support, the active sites are usually limited on the peripheral sites of noble metal particles or at the interface between the particle and the support29–31. The highly dispersed single metal atoms are thus desired to enhance the utilization efficiency of precious noble metals. The study of oxide-supported isolated atoms, so called single atom catalysts (SACs), has been pioneered in 2011 by Zhang’s group32, who has developed single atom dispersion of Pt on FeOxby a tuned co-precipitation method. Fig. 1 summarizes the advantages of supported single metal atoms in heterogenous reactions.
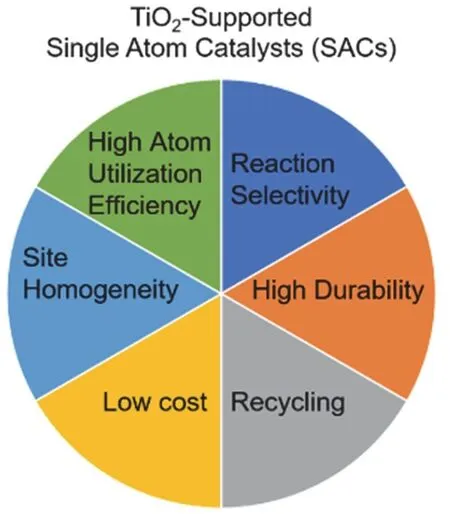
Fig. 1 A summary of advantages using TiO2-supported single atoms in heterogenous catalysis.
The high dispersion of precious noble metals results in a low cost upon catalyst preparation. Due to the presence of active centers as single atoms, the deactivation of metal atoms during the reaction, e.g., coking, is slowed down. The unique coordination surrounding the noble metal center provides a special reaction site for the reaction, which consequently increases the selectivity of the reaction, such as enantioselectivity and stereoselectivity33. The supported SACs are hence supposed to bridge the homogenous reactions in solution and heterogeneous reactions, by providing a selective reaction site and becoming recyclable. Moreover, owing the high site homogeneity of the isolated metal atoms, the SACs are ideal models to establish the structure-activity relationship. For example, for Pt SACs on nitrogen-doped graphene support, the partially unoccupied density of states of the Pt atoms’ 5dorbitals are responsible for the excellent performance in the electrochemical hydrogen evolution reaction34.
Though excellent reviews and perspectives have discussed the history, current state and future of single atom catalysts in preparation and industrial applications35–38, in this mini review,the author tries to summarize the current progress on the titania supported single atoms and the representative applications for photocatalytic energy production. Other configurations of the single metal atoms are beyond the scope of this mini-review,such as SACs embedded in metal-organic frameworks39–42, in single atom alloys43–45, or supported on zeolites46,47, on other metal oxides32,48–51.
The employment of an economic metal oxide support, TiO2,and highly dispersed noble metals, becomes a promising approach to design low-cost and efficient photocatalysts, that are feasible to be scaled up. The demands from fossil energy to sustainable clean energy, is promotional to the investigation of photocatalysts, that convert the solar light to chemical energy,e.g., the fuels for the future, hydrogen.
2 Evaluation of the character of TiO2-supported single metal atoms
In this section, the characterization techniques for titaniasupported single atoms will be assessed, to give a brief overview on the evaluation of the single atoms. Two approaches are included here, microscopic techniques to visualize the metal atoms and spectroscopic techniques to reveal the metal surrounding bond information. Table 1 gives an overview on these techniques and the key experimental conditions.
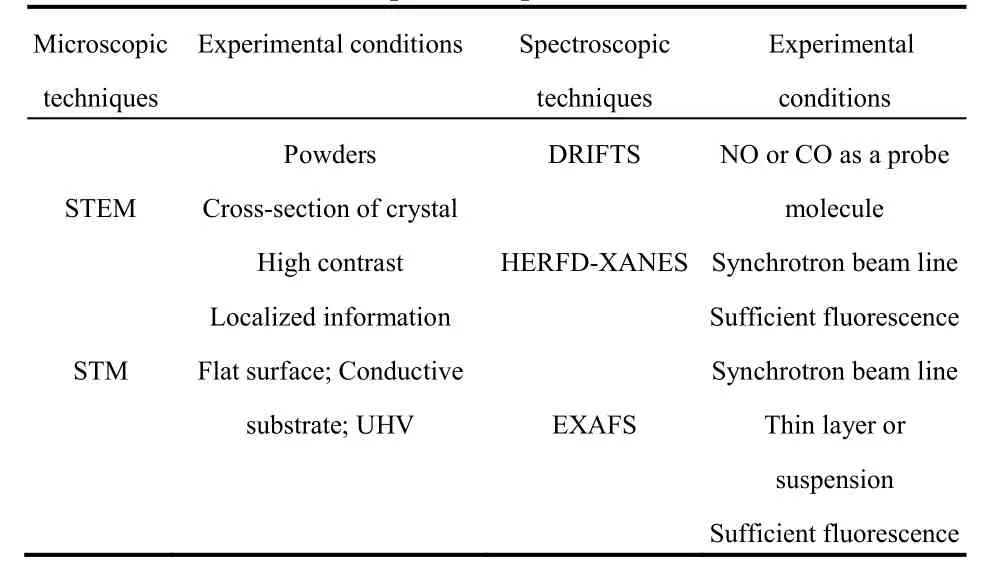
Table 1 Techniques used to characterize the single atoms on TiO2 surfaces and respective experimental conditions.
2.1 Microscopic techniques
Aberration-corrected scanning transmission electron microscopy (STEM)52,53, that can minimize noise issues, has been the breakthrough in recent years to acquire images of heavy metal atoms on titania supports. To obtain atomic resolution images, the contrast between the supported heavy metals and the titania support should be sufficient. Noble metals, Pt54,55, Rh56,Ir57, Au58, and Pd59can be clearly resolved on titania (Fig. 2).In addition, elemental mapping enables to reveal the dispersion profile of the supported metal. Electron energy loss spectroscopy(EELS) offers information on the chemical oxidation states.
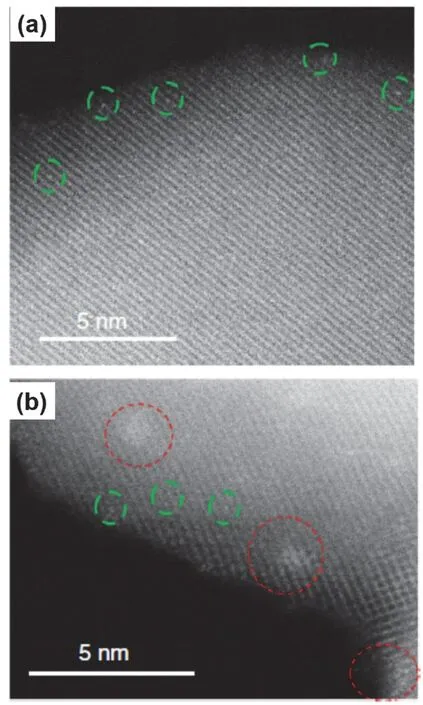
Fig. 2 Representative high-angle annular dark-field (HAADF)STEM images of TiO2-supported Rh catalyst reduced at (a)100 °C and (b) 300 °C.
For SACs on a flat surface, scanning tunneling microscopy(STM) is a frequently used technique. When a fine tip is rastered across the surface, species as small as atoms can be imaged.Therefore, STM offers a chance in the characterization of adsorbates and single metal atoms on single crystals43,44,60–63.For example, for the Pt1Cu alloy catalyst, STM reveals that Pt atoms exist as individual, and isolated species on the Cu surface at the substituted sites of Cu, with a low Pt coverage (0.02 monolayer)44.
2.2 Spectroscopic techniques
X-ray absorption spectroscopy (XAS)32,64, operated at a synchrotron beamline, is a powerful tool to characterize thestructural information of the catalyst. XAS simultaneously acquires the extended X-ray adsorption fine structure spectroscopy (EXAFS) and X-ray absorption near edge structure spectroscopy (XANES). The electrons, ejected from the metal atoms, immediately interact with the atoms neighboring the absorbing atoms and get scattered. EXAFS region reveals the neighboring bond information of the absorbing metal atoms, that contains the coordination numbers and distances between atoms after Fourier transform (FT) of the spectrum. Besides conventional XANES, high-energy resolution fluorescence detection XANES (HERFD-XANES)64is a highly sensitive technique to investigate the surroundings of metal atoms on the support. It is determined with high-energy fluorescence detection and allows the resolution of spectral features that can be directly assigned to specific coordination of ligands to a metal atom.
The CO molecule is sensitive to the changes in metal oxidation states and the local environment upon chemical adsorption54,56,59,65,66. Provided with featured vibrational frequency, CO is an excellent probe to check the nature of the catalyst, for example, to distinguish isolated, coordinativelyunsaturated metal single-atoms from larger clusters (Fig. 3)67–69.In the diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), peaks with a vibrational frequency above 2100 cm?1are ascribed to the stretching band of linearly adsorbed CO on cationic Pt species (Pt coordinated with oxidizing ligands, oxidized Pt clusters, or isolated Pt species)65,67.The full-width at half maximum (FWHM) of CO stretching mode is an indication to discern the aggregation size of Pt. On TiO2, FWHM of CO band for homogeneously isolated Pt species is smaller than 15 cm?1(6–8 cm?1), by contrast, FWHM of CO band for metallic Pt particles is larger than 25 cm?1. This measure should be carefully applied to other oxide supports. For instance, the FWHM of CO band for isolated Pt on CeO2or FeOx(> 25 cm?1) is reported to be larger than that on titania29,32,70. In another work, NO is used as a probe molecule to verify the single metal species71. Solid-state magic-angle spinning NMR can provide bond information about the nature of the metal species as well. In a recent work, it reveals the information surrounding Al sites for an Al2O3-supported single Pt catalyst72. Nonetheless,such technique requires a high loading of supported metal that is sufficient to provide reliable spectroscopic information.
3 Stabilization of the single metal atoms on TiO2
In this section, we will introduce the methods to stabilize single metal atoms on the surface of titania, including anatase,rutile, TiO2(B), and their defective surfaces (see an overview in Fig. 4). The key factors to control the synthesis procedure will be discussed as well. Due to the high thermodynamic energy of isolated single atoms on titania, these dispersed atoms intend to move and aggregate during synthesis, also catalysis. One of the challenges, upon synthesis, is to stabilize these energetic single metal atoms on the surface of titania. The approaches include surface defective sites, surface modification, high temperature shockwave, metal-ligand self-assembly and others.
3.1 Surface defects
Defect engineering has been a successful technique to tune the optical and electric properties of titanium dioxide in devices and catalysis73,74. Recent work by Li’s group57,58has utilized the function of surface defects to stabilize noble metal atom (Au and Ir) on the oxygen vacancy (Ov) sites of titania. These vacancies are created by annealing titania anatase nanosheets in H2/Ar at 200 °C for 2 h. This annealing condition leads to the highest amount of Ti3+sites58. The Au atoms are deposited on the surface from HAuCl4precursor by deposition-precipitation method, with a loading of 0.3% (w). The loading of single metal atoms on the oxide surface is reported to be below 1.5% (w) to reach a homogeneous dispersion of metal atoms. The Au atoms are located at the oxygen vacancy sites and bond with Ti after post-calcination at 200 °C in Ar/H258. EXAFS spectrum shows the Au-Ti bond with a mean bond length of 2.79 ? (1 ? = 0.1 nm) and a coordination number of 1.8. This method can be extended to a TiO2(B) support57. A metal-support interaction, Ir-Ti bond, can be regulated, by the impregnation of H2IrCl6on defective TiO2(B) and the post-annealing. EXAFS spectrum shows the Ir is bonded with three surrounding Ti atoms and form an Ir1Ti3chemical bond (Fig. 5). Such coordination between metal atoms and the support Ti offers a minimized energy of supported isolated metal centers, thus establishes a firm structure for either isolated Au or Ir atoms.
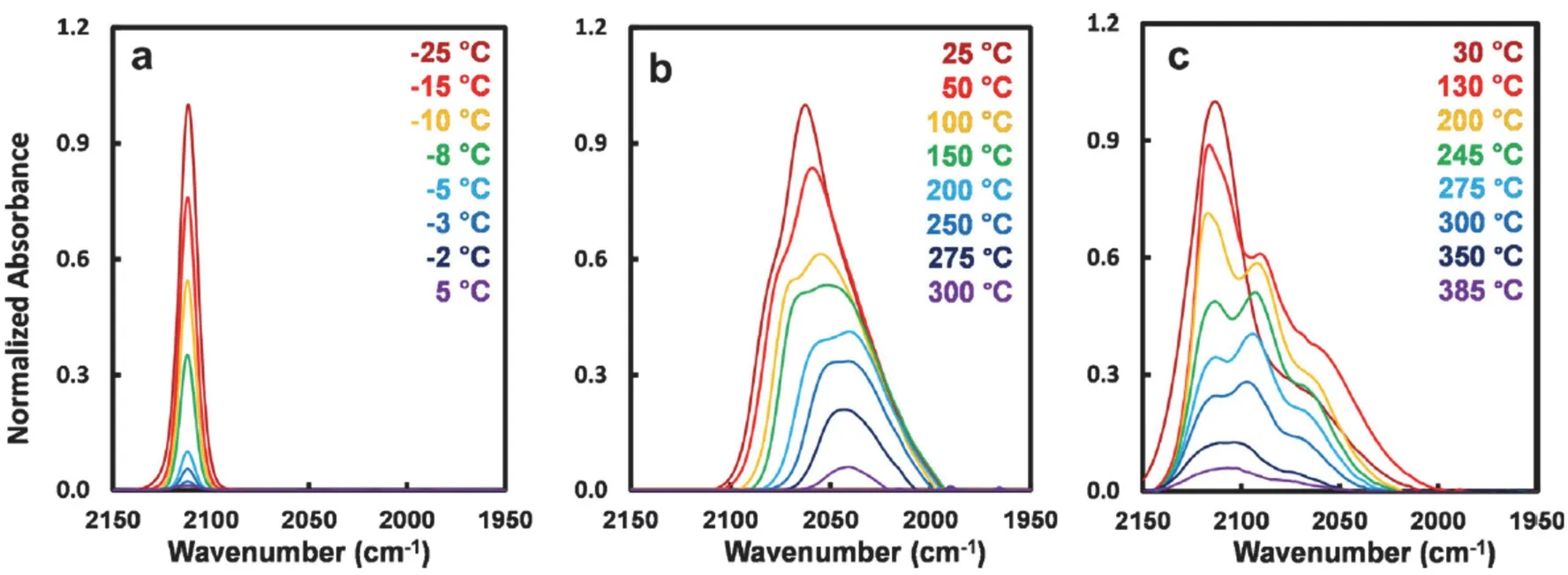
Fig. 3 Temperature-programmed desorption (TPD) of CO IR spectra on (a) single Pt, (b) metallic Pt and (c) oxidized Pt catalysts.
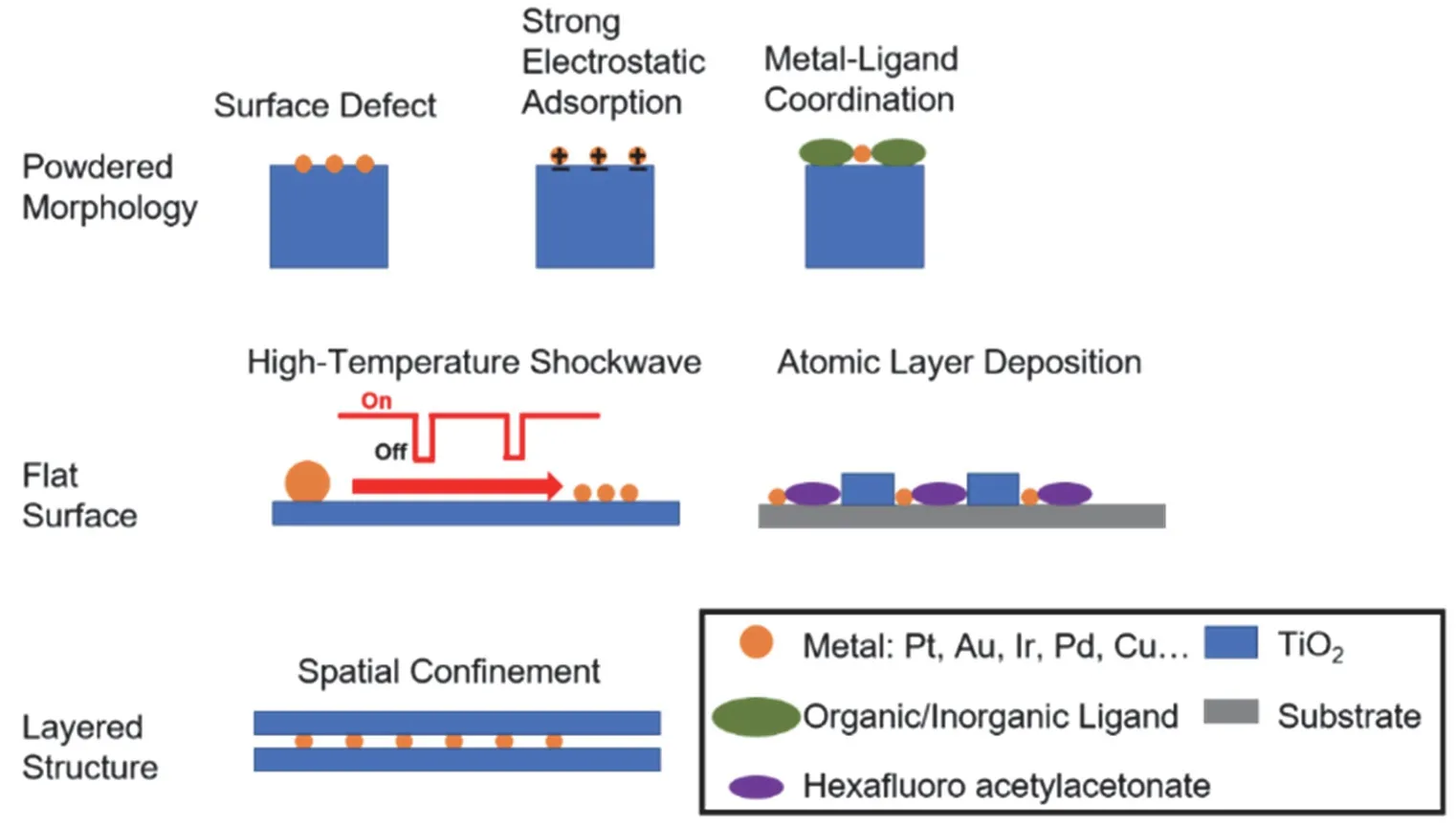
Fig. 4 An overview of the preparation methods for TiO2-supported single metal atoms.
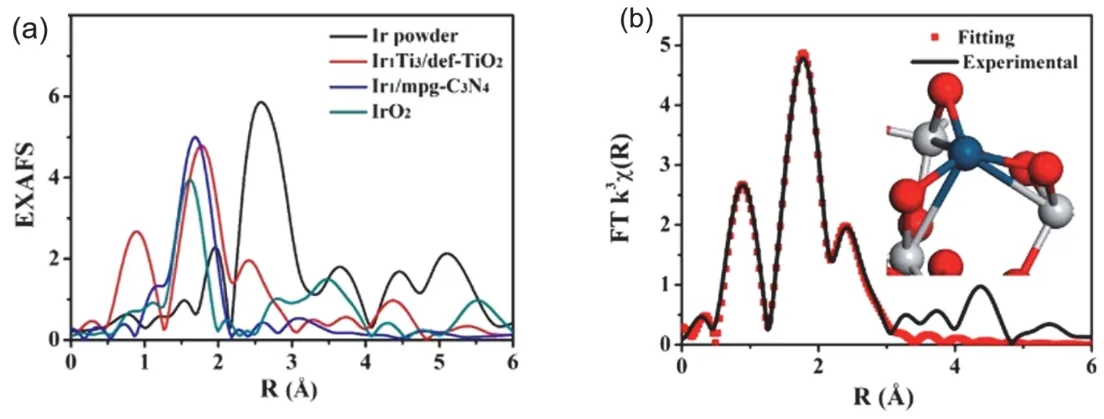
Fig. 5 (a) FT at the Ir L3-edge of Ir powder, Ir on defective TiO2(B), Ir on C3N4 and IrO2. (b) Corresponding fitting of the EXAFS spectrum of Ir1/def-TiO2(B) at the R space. The inset of panel (b) shows the local structure of the Ir1Ti3 obtained from the DFT simulations.
Except for the powder morphology of titania, recent work from Schmuki’s group has shown that, on a thin sputtered TiO2layer, an atomic-scale defect engineering approach is able to form and control traps for single atom platinum75. The density of single Pt atoms on the TiO2layer is precisely controlled by the degree of reduction treatment, that is by annealing the catalyst composite (Pt decorated TiO2) in H2/Ar at different temperatures. Such atomic-scale defective sites pin single Pt atoms from a dilute aqueous Pt solution. Using a concentration of 120 μmol·L?1of H2PtCl6precursor, the density of single Pt atoms is the highest provided annealing at 500 °C. The anchored single Pt is supposed to be bonded with surface oxygen, giving a Ptδ+oxidation state. In the work by Leeet al.76, the Cu atoms are loaded on TiO2anatase (101) by a modified wrap-bake-peel process, in which the tetraethyl orthosilicate is employed to confine the Cu atoms during synthesis and has been removed at the last step. These single Cu atoms were site-specifically distributed at the Ti vacancy sites76.
It is worth noting that the formation path of oxygen vacancy varies on different titania crystal surfaces (anatase, rutile). The migration and diffuse of Ov on different titania crystal surfaces are impacted by the energy difference between the oxygen atoms neighboring the Ov, and oxygen at perfect sites. Therefore, the distribution profiles of Ov, which further determines the density of supported single metal atoms, should be considered on different surfaces. Additionally, the stability of these defects in the reaction environment is vital to be explored, particularly under an oxygen-containing reaction condition. Under the oxidative reaction atmosphere, the consumption of Ov may lead to the reconstruction of the titania surface. The defect engineering is applicable to other reducible metal oxide, like CeO2, FeO3, V2O5, or metal sulfide.
3.2 Surface modification
Here, external surface modifications are discussed (defect engineering, in this review, is considered to be an internal modification), which include introducing binding sites, e.g.,nitrogen77, changing the surface electrostatics56,65, or ion exchange55.
The strong electrostatic adsorption (SEA)78is among the impregnation method to prepare highly dispersed metals on silica. Various cationic metal ammine complexes are used as precursors, e.g., [Pd(NH3)4]+2, [Cu(NH3)4]+2, [Co(NH3)6]+3,[Ru(NH3)6]+2, [Ru(NH3)6]+3and [Ni(NH3)6]+2, that interact strongly with a negatively-charged surface78. Christopher’s group has reported the preparation of single noble metal atom per titania particle (~5 nm diameter), employing the SEA interactions (Fig. 6)65,67. The weight loading of Pt is tuned between 0.025% (w) to 0.15% (w). The large synthesis volume is beneficial for the homogeneous deposition of Pt on the titania.The pH of titania suspension, before adding of Pt (II) complexes,is regulated above 8 by adding NH4OH solution. The catalysts were then calcined in air to ensure the removal of remaining ammine ligands79.

Fig. 6 Illustration of isolated Pt on TiO2 surface by the strong electrostatic adsorption (SEA), and a linearly adsorbed CO molecule on an isolated Pt atom Pt, C, O and Ti atoms are shown in blue, gray, red and white, respectively.
The advantage of such a preparation method is assuring a high uniformity of isolated metal atoms on the surface. The isolated Pt is substituting into a six-fold coordinated Ti atom position and shows a formal oxidation state of 4+. EXAFS demonstrates the coordination number (CN) of Pt to be six for the first shell Pt―O54.The total CN of Pt―O drops to 2 after mild reduction and shows a formal oxidation state of 2+. The mild reduction pulls Pt atom out from the lattice, giving an adsorbed PtO2structure that is sitting on top of the Ti cation column. For both above-mentioned structures, the isolated Pt is locked along the axis without lateral motion. However, a harsh reduction leads to an adsorbed PtOH structure that shows a formal oxidation state of 1+. These PtOH species are attached to the defective sites of titania (steps and terrace) and are mobile on the surface. In a modified SEA approach, the ammonium aqueous solution, or ammonium carbonate solution is applied to adjust the pH to 8–9. With the addition of metal chloride precursor, homogeneously dispersed Pt80or Rh56on the titania support is prepared.
Alternatively, the spatial confinement on the titania surface is employed to stabilize the single metal atoms. The titania nanotubes with lamellar structures have been reported to trap cationic Pt in between the layers on the wall in the work by Liet al.55. In their experiments, through ion exchange with Na+ion,the cationic Pt complex is intercalated into interlayers of titanate sheets. The following calcination induces phase transformation from titanate to anatase TiO2, and the reduction treatment enhances the Pt-titania interaction. In this tubular layered Pt1δ+/TiO2, the isolated Pt is bond with surface oxygen, showing aδ+ oxidation state. During the reductive annealing treatment in H2/Ar, the hydrogen spillover effect generates surface defects on titania, forming a Pt―O―Ti3+structure81. Zhou’s group77has reported, in theory, a strategy to prepare Ru doped titania in the presence of nitrogen dopants. The nitrogen dopants promote the substitution of Ti by Ru1on anatase, which is interesting to be further explored experimentally.
3.3 High-temperature shockwave
Metal nanoparticles become oxidatively fragmented with oxygen at high temperature, e.g., platinum forms volatile PtO2at 1100 K. These Pt species in the gas phase are transported through the carrier gas phase and react with the support to form isolated cationic Pt on the surface82. In 2014, Datye and coworkers have reported the volatile mobile Pt from Pt particles is captured by PdO, forming bimetallic Pt-Pd nanoparticles. This is ascribed to the slowed Ostwald Ripening process of Pt during the high temperature annealing (650 °C) in air83. Such phenomena has been explored in the following work to synthesize ceriasupported isolated Pt with a high loading, named as hightemperature vapor-phase synthesis84–86. High temperature preparation promotes the bond formation between the Pt and CeO2with improved stability. But this method is not compatible with temperature-sensitive substrates.
Recently Yao and coworkers87have reported the preparation of Pt single atom on TiO2by applying a high temperature shockwave. The TiO2support is deposited as a thin layer (2.5 nm) on the carbon nanofibers by atomic layer deposition due to the low thermal conductivity of titania. A periodic on-and-off high temperature (1500–2000 K) heating treatment is applied,with a feature of 50 ms on-state and 600 ms off-state (Fig. 7).The on-state activates the TiO2by creating Pt-surface defect bonds, and the off-state assures the stability of such structure.Theoretical calculation shows that, on defective titania, the energy barrier for a single Pt escaping from Pt6cluster is thermodynamically favorable (ΔG= ?1.43 eV). However, on perfect TiO2surfaces, an energy barrier of +2.14 eV needs to be overcome to disperse Pt6cluster into Pt5+ Pt1. Datye88has mentioned that the key process is the short heating, followed by a rapid quenching. The multiple cycles of pulse allow the full dispersion of atomic volatile Pt into the mobile lattice defects that are created at high temperature on-state88. This method has been generalized to prepare isolated Ru and Co on titania.
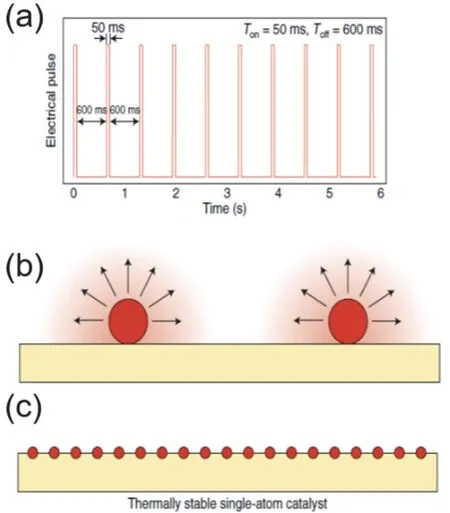
Fig. 7 Transformation of metal nanoparticles into embedded SACs by shockwave (upper row), into large particles by slow heating (lower row).
3.4 Metal-ligand coordination
The application of external ligands, coordinating with the metal centers, has been realized in the metal-organic frameworks39,40,89–93. Here we will particularly focus on the use of metal oxide as a support to load single metal atoms. The inorganic ligands, like ―O ligand oxo94, and organic ligands(e.g., bipyridyl groups)50,95have been reported. In our group, the metal ligand self-assembly has been observed and evidenced on a flat surface96,97. For example, Pt coordinates with bipyridyl tetrazine and forms planar chain structure on Au(100) surfaces98.Our following work shows that the metal-ligand coordination takes place on titania surface99, where the Pt is bond with surface oxygen and organic ligand bipyridyl tetrazine. Alternatively, the organometallic precursors react directly with functional groups(e.g., ―OH groups) on the metal oxide surface and form metal―O bond. Gateset al. have reported the loading of M(C2H4)2(acac) (M, Rh, Ir; acac, acetylacetonato) on titania surface100. For inorganic ligand, Flytzani-Stephanopoulos’s group has reported inorganometallic isolated gold complex on TiO2surface in alkaline solution94. The Au species are stabilized on the surface by forming a Au1―Ox―Na9―(OH)ycluster. For the metal-ligand approach, a strong coordination between metal and ligand is crucial to the stabilization.
3.5 Other approaches
Atomic layer deposition (ALD)101,102has attracted increasing attention and has been reported to conformally prepare single atoms on the surface. Lei’s group has shown that Pd1is decorated in the nanocavity of TiO2by ALD101. The precursor Pd(hfac)2(Pd hexafluoroacetylacetonate) is chemically adsorbed,following with the selective deposition of TiO2by ALD on the substrate, not on the Pd(hfac)2sites. The ligand hfac templates the formation of the nanocavity and isolates the Pd1sites. After annealing, the Pd1/TiO2shows a strong metal support interaction(SMSI) to stabilize the Pd atoms.
In another work, the photochemical reduction of frozen chloroplatinic acid solution by UV light is reported to generate single Pt atoms. This single Pt atom solution is then anchored on a substrate like TiO2, porous carbonetc103. On a porous substate,a high loading of the supported metal is reported, e.g., using a mesoporous titania allows a loading of Cu up to 12.33%. The Cu exists as mononuclear Cu (I) at low loadings and as CuO at high loadings104.
4 Use of TiO2-supported SACs in photocatalysis
Excellent reviews have summarized the applications of SACs in the heterogeneous catalysis105–108, electrochemical catalysis35,and photocatalysis109–111. Here we will focus on the application of titania-supported single atoms for photocatalysis and try to address the underlying mechanism for the improved photoactivity112. Different from nanoparticles, single atoms have unique electronic properties, e.g., the lack of the plasmonic effect under illumination36. The discussion will emphasize the photocatalytic H2production and cover the other photocatalytic reactions, like CO2reduction. These reactions are important applications for titania-based catalysts, that aim on solving the environmental and energy issues.
4.1 Photocatalytic H2 evolution
High purity hydrogen is used in various industrial applications, e.g., the fuel cell. H2evolution by photocatalysis is the most low-cost method to produce green hydrogen113, green hydrogen is the hydrogen generated in a green chemical process.Abundant metal oxides with tunable bandgap are ideal models to be employed to fabricate the catalysts114–116. Considering the promotional effect of noble metals to the reaction rate, the metal oxides are usually decorated with noble metals to reach a plausible hydrogen generation rate.
Due to the high dispersion of single atoms, the atom utilization efficiency of the noble metals is generally higher than the nanoparticles. Additionally, the single Pt atom is functionalized as a H atom recombination center, for the production of H2molecules75. Coupling with a surface defect state of titania, that serves as an electron transfer mediator74,117,the single Pt decorated titania shows the superior effectiveness on the activity, compared to the Pt nanoparticles (Fig. 8a). These isolated Pt species are anchored firmly on the surface of defective titania, by showing a high retention of the activity stability over the reaction time75.
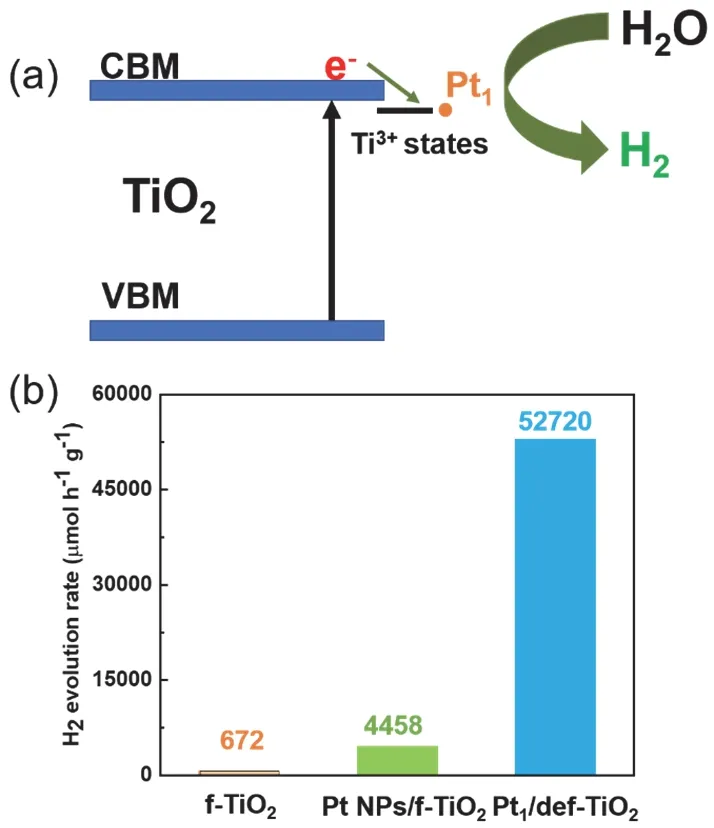
Fig. 8 (a) An illustration of electron transfer from TiO2 to single Pt,mediated by the defective states. (b) The hydrogen evolution rate of single Pt compared to supported Pt NPs.
Alternatively, Chenet al.81have reported that a Pt-O-Ti3+active center is responsible for the high hydrogen generation rate. During the photocatalytic reaction, the photogenerated electrons from Ti3+efficiently transfer to single Pt atoms through the Pt―O―Ti3+active centers. The electron-hole recombination rate is thus suppressed, and the overall charge transfer efficiency is enhanced. The atomic utilization efficiency of Pt, in this work,is reported to be ~591 fold higher than that for the supported Pt nanoparticles (Fig. 8b)81. The DFT calculation further pointed out that substituted Pt on a six-coordinated Ti site is the active Pt for the hydrogen production reaction due to the capability to dissociate H2O and form adsorbed H*81. By contrast, due to the formation of a depletion layer between TiO2and Pt NPs, the electrons transfer directly from the TiO2to the Pt sites and react with H* to form H2molecules. It has been reported in the hydrogen evolution reaction, the activation battier for the H*atom adsorption on Pt SACs is small than that on the Pt[111]34.
Moreover, the facet of titania impacts the activity of SACs in the photocatalytic hydrogen production. Weiet al.80have reported that the Pt single atoms on TiO2[001] shows 3 times higher activity in the H2evolution rate than that of Pt decorated on TiO2[101] facet. The improved activity is ascribed to the higher CBM and higher surface energy of TiO2[001], compared to TiO2[101] surface. On anatase [001] surface, Basset’s group95has reported the single Pt atoms coordinate with organic ligand (1,5-cyclooctadiene), forming a dimethylplatinum(II)[(CH3)2Pt(COD)] structure. In this configuration, the single Pt is significantly more active in the photocatalytic hydrogen evolution, compared to the conventional Pt particles. They attribute the enhanced reaction rate for single Pt to the strong suppression of the backwards reaction of H2and O2under dark conditions95.
In addition to the Pt single atom, titania-supported single Cu has shown to be active for the photocatalytic hydrogen generation76,104. The substitution of Pt by Cu atoms allows for the preparation of a low-cost, and eco-friendly photocatalyst.The catalyst experiences a different reaction path during the photocatalysis, in comparison to Pt. The single Cu atom needs to be activated by light and can be deactivated after the reaction by oxidation (Fig. 9)76.
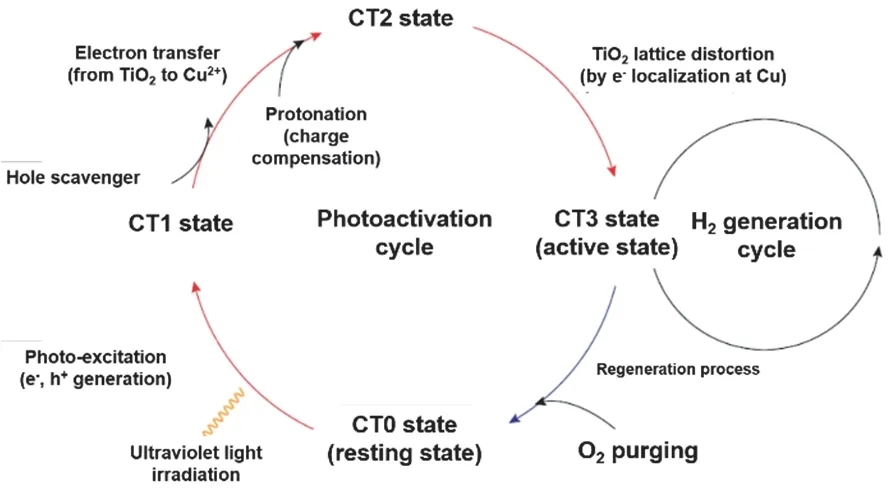
Fig. 9 Photoactivation cycle of Cu1/TiO2.
The as-prepared Cu1/TiO2catalyst is regarded as a non-active catalyst at the resting state (CT0)76, which gets photoactivated and generates electrons and holes (CT1). The electrons transfer from the CBM of TiO2to thedorbitals of the single Cu atoms(CT2), which results in a polarization field between the TiO2and the Cu atom. That is, around the single Cu atoms, a local lattice distortion occurs on titania (CT3). Such a state is completely different from the original resting state (CT1) and becomes significantly active for the hydrogen generation. The theoretical calculation shows that at a Ti vacancy site, a Cu atom provides mid-gap states with Cudx2?y2anddz2character. At state CT3, the electron is localized into the Cudz2anti-bonding state, that leads to the lattice distortion by elongating the backside oxygen coordination. As a result, the hydrogen evolution rate is enhanced by H2O reacting at the electron localized Cu sites.
However, Zhouet al.77have reported that it is difficult to accurately determine the single metal atom induced localized gap state in TiO2. They employed co-dopants, nitrogen, and Ru,to calculate the electronic structure and water splitting activity.It is suggested that the pre-doping with nitrogen stabilizes the thermodynamic and kinetic solubility of single Ru atoms in anatase TiO2. The chemical energy of H bonding changes and the light adsorption edge redshifts to the visible light range. This theoretical finding is interesting to be further explored experimentally using a photoelectrochemical or photocatalytic catalyst77.
4.2 Photocatalytic CO2 reduction
Reduction of CO2into value-added fuels by solar light-driven catalysis is supposed to be an economic approach118. Not only the products are valuable, e.g., methane, methanol, or long-chain hydrocarbons, also the vast amount of greenhouse gas CO2is consumed. Although p-type semiconductors, e.g., ZnTe, Cu2O,and GaP, are first studied and used in CO2photoreduction due to their relatively negative CBM, they often suffer photocorrosion problems. Therefore titania, as a highly stable, low-cost, and safe semiconductor, is currently widely investigated to reduce CO2when suitable modifications. In a photoelectrochemical configuration, the titania, modified with a p-type semiconductor or with dopants, has been applied as a photocathode119–125. The oxygen evolution from H2O by reacting with holes occurs on the counter electrode. Various single metal atoms have been applied,e.g., Co1126, Er1127, Ir189, Ru121,128, Cu1129, decorated on metal oxide, organic semiconductor or in a metal-organic-framework structure. The single atoms are usually immobilized in a metalligand complex, for example, [Ru(byp)3]2+21. Titania-supported single Ir appears to be active for the selectively thermal reduction of CO2to CO with IrO2active sites. The single Ir active site effectively blocks the over-reduction of CO2to methane128.
Liet al.129have reported that the defective titania supported Cu1species are active for the CO2photoreduction to CO under open circuit conditions. The presence of surface defects (Ti3+)provides active sites for the adsorption of the oxygen atoms from CO2molecules. Photogenerated electrons transfer from bulk TiO2to surface Ti3+sites and migrate to theπorbitals of adsorbed CO2. The surface adsorbed H2O also participates in the cycle as an electron donor and form HCO3?129.
4.3 Other reactions
One of the important applications of photogenerated holes is to oxidize methane to produce fuels, e.g., methanol130,131. The use of single atoms for the methane oxidation has been studied in heterogeneous thermal catalysis132,133, but has not been fully studied in photocatalysis. However, the photocatalytic reactions are worthy to be developed due to the high selectivity and utilization of single atoms. Pratsiniset al. have reported the removal of NOxusing titania-supported isolated Pd by photocatalysis71. The isolated Pd is resistant to nitrate poisoning and highly selective to the conversion of NO to nitrate.
5 Concluding remarks
While in the present overview many aspects of the fascinating application in photocatalysis for TiO2-supported SACs could only be addressed on the surface and in a referencing manner,we hope to provide interested readers with a guideline through the wide literature to the topic and, more specifically, to give a comprehensive overview of titania-supported SACs. However,several following issues need to be addressed on the application of single atoms (Fig. 10).
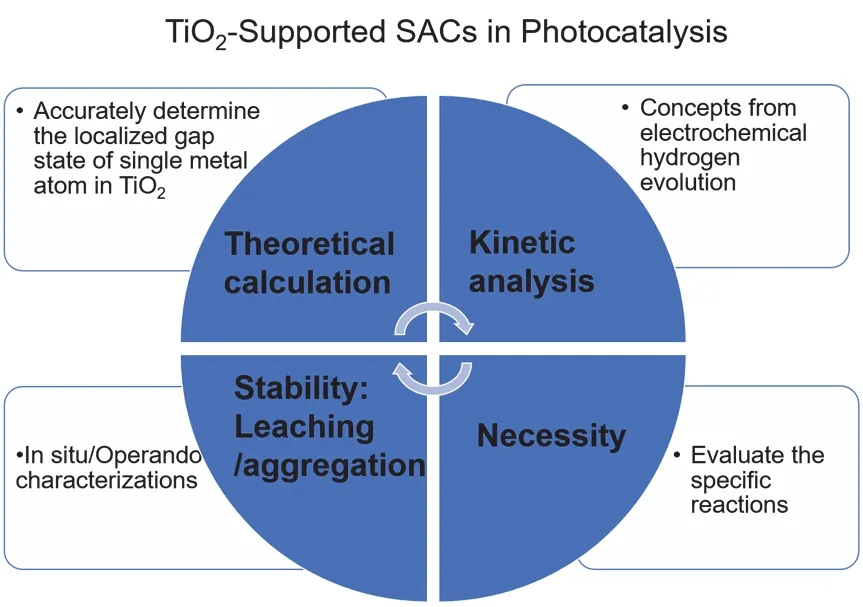
Fig. 10 Questions that are relevant to the TiO2-supported SACs in photocatalysis.
First, the Schottky junction is used to describe the junction between macroscopic metal/titania junction or the small nanoparticle/titania junction, which does not apply when much smaller nanoparticles (few nanometers, clusters) are placed on highly doped titania. These single metal atoms are considered as dopants. However, it is difficult to accurately determining the localized gap state of a single metal atom. As mentioned above,Zhouet al.77propose to use co-dopants in TiO2powders when performing the density functional theory calculation. The later work by Donget al. has shown that the Au SACs couple on the TiO2[110] surface by interfacial electronic interactions134,applying a combined first-principle calculation with STM study,the Au SACs introduce intermediate gap states that are located at ?0.85 eV (Au SA at the Ti 5c site) and ?1.3 eV (Au SA at the Ov site), below the Fermi level. These states provide a channel for the transfer of a photogenerated hole under UV illumination.The hole weakens the Ti―Au bonding and activates the diffusion of Au SACs.
Second, the kinetic analysis of supported single metal borrows the concepts and ideas from electrochemical theory. However,this may not work for a complicated photocatalytic system under open circuit conditions, e.g., with a Z-scheme, S-scheme junction, or with antenna dye molecules.
Third, under reaction conditions, the stability of the single atoms needs to be further explored135. For example, the holes weaken the Au―Ti bond that destabilizes the single Au atoms,134resulting in aggregation95or leaching during the photocatalysis in solution. Current characterization techniques are under development to meet the demands forin situ/operando photocatalytic measurements.
Last, for specific reactions, an active center as a dimer136, a trimer, or in a paired structure136,137may outperform the single metal center. For example, the neighboring noble metal facilitates the dissociation of hydrogen molecules in a hydrogenation reaction. It is worthy to consider whether a specific reaction benefits from the use of an isolated single metal center.
Nevertheless, the high demand in the industry (sustainable chemical processes) of low-cost catalysts and robust supports is promotional to the development of research on SACs. In photocatalysis, there are several aspects that are exciting to be further investigated, e.g., the establishment of the theory, the methodology on the synthesis, and the exploit of single atoms on other solar light-driven reactions.
Conflict of Interest:The author declares no competing interests.
- 物理化學(xué)學(xué)報的其它文章
- Recent Progress of Perovskite Oxide in Emerging Photocatalysis Landscape: Water Splitting, CO2 Reduction, and N2 Fixation
- Review of Z-Scheme Heterojunctions for Photocatalytic Energy Conversion
- Fluorinated TiO2 Hollow Photocatalysts for Photocatalytic Applications
- 微波輔助快速制備2D/1D ZnIn2S4/TiO2 S 型異質(zhì)結(jié)及其光催化制氫性能
- Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution
- Carboxyl-Functionalized Graphene for Highly Efficient H2-Evolution Activity of TiO2 Photocatalyst