Chronic intestinal failure and short bowel syndrome in Crohn’s disease
Aysegul Aksan, Karima Farrag, Irina Blumenstein, Oliver Schroder, Axel U Dignass, Jurgen Stein
Abstract Chronic intestinal failure (CIF) is a rare but feared complication of Crohn’s disease. Depending on the remaining length of the small intestine, the affected intestinal segment, and the residual bowel function, CIF can result in a wide spectrum of symptoms, from single micronutrient malabsorption to complete intestinal failure. Management of CIF has improved significantly in recent years.Advances in home-based parenteral nutrition, in particular, have translated into increased survival and improved quality of life. Nevertheless, 60 % of patients are permanently reliant on parenteral nutrition. Encouraging results with new drugs such as teduglutide have added a new dimension to CIF therapy. The outcomes of patients with CIF could be greatly improved by more effective prevention,understanding, and treatment. In complex cases, the care of patients with CIF requires a multidisciplinary approach involving not only physicians but also dietitians and nurses to provide optimal intestinal rehabilitation, nutritional support, and an improved quality of life. Here, we summarize current literature on CIF and short bowel syndrome, encompassing epidemiology, pathophysiology, and advances in surgical and medical management, and elucidate advances in the understanding and therapy of CIF-related complications such as catheter-related bloodstream infections and intestinal failure-associated liver disease.
Key Words: Chronic intestinal failure; Short bowel syndrome; Crohn's disease; Inflammatory bowel disease; Parenteral nutrition; Intestinal failure-associated liver disease
INTRODUCTION
Chronic intestinal failure (CIF) is a rare but feared severe complication of Crohn’s disease (CD). Sixty percent of patients with CD and CIF are permanently dependent on parenteral nutrition (PN). According to the recommendations of the European Society for Clinical Nutrition and Metabolism (ESPEN)[1 ], CIF is defined as “a reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such that intravenous supplementation is required to maintain health and/or growth”. ESPEN suggests classifying CIF on the basis of anatomical, functional, pathophysiological, or clinical characteristics (Figure 1 )and has defined five major pathophysiological conditions causing CIF: Short bowel,intestinal fistula, intestinal dysmotility, mechanical obstruction, and extensive mucosal disease[1 ]. In CD, the most common cause of CIF is short bowel syndrome (SBS), in which the small bowel length, by definition, is less than 200 cm[1 ]. Concerning nutritional-medicinal management, patients with CIF are most frequently subdivided according to the type and extent of bowel resection and additional surgical procedures(e.g., stomata).
CLASSIFICATION
According to the recommendations of ESPEN, the classification of CIF can be based on pathophysiological, anatomical, functional, or clinical criteria (Figure 1 ).
From a pathophysiological point of view, a classification into five types (of which SBS is one) has been proposed based on the presence of various gastrointestinal and/or systemic diseases (Table 1 )[2 ].
Anatomically,according to the type and extent of bowel resection and additional surgical procedures (stomata), three different categories of short bowel can be distinguished as prognostic criteria for future disease progression (Figure 2 )[1 ,3 ]: Type I:Terminal jejunostomy (“very SBS” if remnant bowel length < 50 cm); Type II: Jejunoascendostomy, jejunotransversostomy, or jejunodescendostomy (rarely with colostoma);Type III: Jejunoileotransversostomy (very rarely with colostoma).
The functional classification of intestinal failure, first described by Shaffer[4 ], is based on its time frame, metabolic course, and long-term progression: Type I: Acute and short-term. Usually a self-limiting condition, often observed in the perioperative setting and/or in association with critical illness, where patients require PN for a few days or weeks; Type II: Prolonged acute condition, often arises in patients with metabolically unstable conditions and requires complex multidisciplinary treatment and intravenous supplementation over a period of weeks to months; Type III: Chronic condition found in metabolically stable patients who need to be intravenously substituted for months or years. May be (partially) reversible or irreversible.
The clinical classification is based on the patient's energy and fluid requirements for PN in the context of clinical management. It does not aim at risk stratification and is defined according to 16 categories (Figure 1 ).
EPIDEMIOLOGY
While intestinal failure can occur due to a number of different underlying conditions,CD has been found to be the most common reason for intestinal failure (Figure 3 )[5 ].However, incidence of CIF as a complication of CD can only be indirectly evaluated based on data from home PN registries. Recent reports from national intestinal failure units in the United Kingdom show that CD is the underlying disease in 30 %-32 % of patients with long-term intestinal failure[6 ,7 ]. Longitudinal data from Japan indicate an incidence of CIF in CD of 8 .5 %-18 .2 % during the first 20 years after initial presentation[8 ,9 ].
Mechanisms of CIF in CD are poorly understood. Among recognized pre-operative risk factors are systemic steroid treatment[10 -13 ], recent weight loss[11 ,14 ], intraabdominal abscess[15 -17 ], and smoking[14 ,18 -20 ]. Whether recent administration of antitumor necrosis factor medication is associated with post-operative morbidity in CD is currently under investigation.
The peri-/post-operative events that most frequently lead to CIF in CD have been purported to be multiple resections, enterocutaneous fistulation, and malabsorption with multiple resections, leading to cumulative loss of the small bowel. However,Soopet al[21 ], in a longitudinal cohort study of 121 patients referred to a national intestinal failure unit from 2000 to 2018 who were diagnosed with CD and subsequently treated with PN for at least 12 mo, identified septic complications following abdominal surgery to be the most frequent event leading directly to CIF.
ANATOMICAL-PHYSIOLOGICAL ADAPTATION
Three phases of intestinal adaptation can be distinguished. Although it is not possible to define specific biological or functional demarcation points between the hypersecretion, adaptation, and stable (chronically adapted) phases, their differentiation can be a useful aid in therapy planning.
Phase I: Acute (hypersecretory) phase
The hypersecretory phase occurs during the immediate post-operative period and may last for 2 or 3 mo, and in some cases even longer. This phase is characterized by poor absorption of almost all nutrients, including water, electrolytes, proteins,carbohydrates, fats, vitamins, and trace elements. Depending on the extent of resection, daily fluid loss of up to 5 L may occur, while some patients with jejunostomy may lose as much as 6 -8 L[22 ].
Gastric hyperacidity (GH) — increased stomach acidity due to a transient increase in gastric acid secretion (over a period of weeks to months) — affects over half of patients with small bowel resection of 30 % or more. GH has been suggested to be caused by the temporary discontinuation of an indirectly or directly acting intestinal inhibitor of gastric secretion (e.g., vasoactive intestinal peptide or gastric inhibitory polypeptide). Alternatively, it may arise due to the diminished breakdown or increased production of a stimulant. Serum levels of gastrin, for example, have been found to be increased after surgery.
Alongside diminished contact time, which leads to inadequate mixing with the chyme, GH-induced inactivation of pancreas ferments is considered vitally important.Following resection of the proximal small bowel, serum levels of cholecystokinin and secretin have been found to be substantially diminished. This generates, in effect, a disturbed positive feedback that leads to a reduction in pancreas stimulation. On theother hand, a reduction in enterokinase activity, observed mainly following duodenum resection, is of no significance.
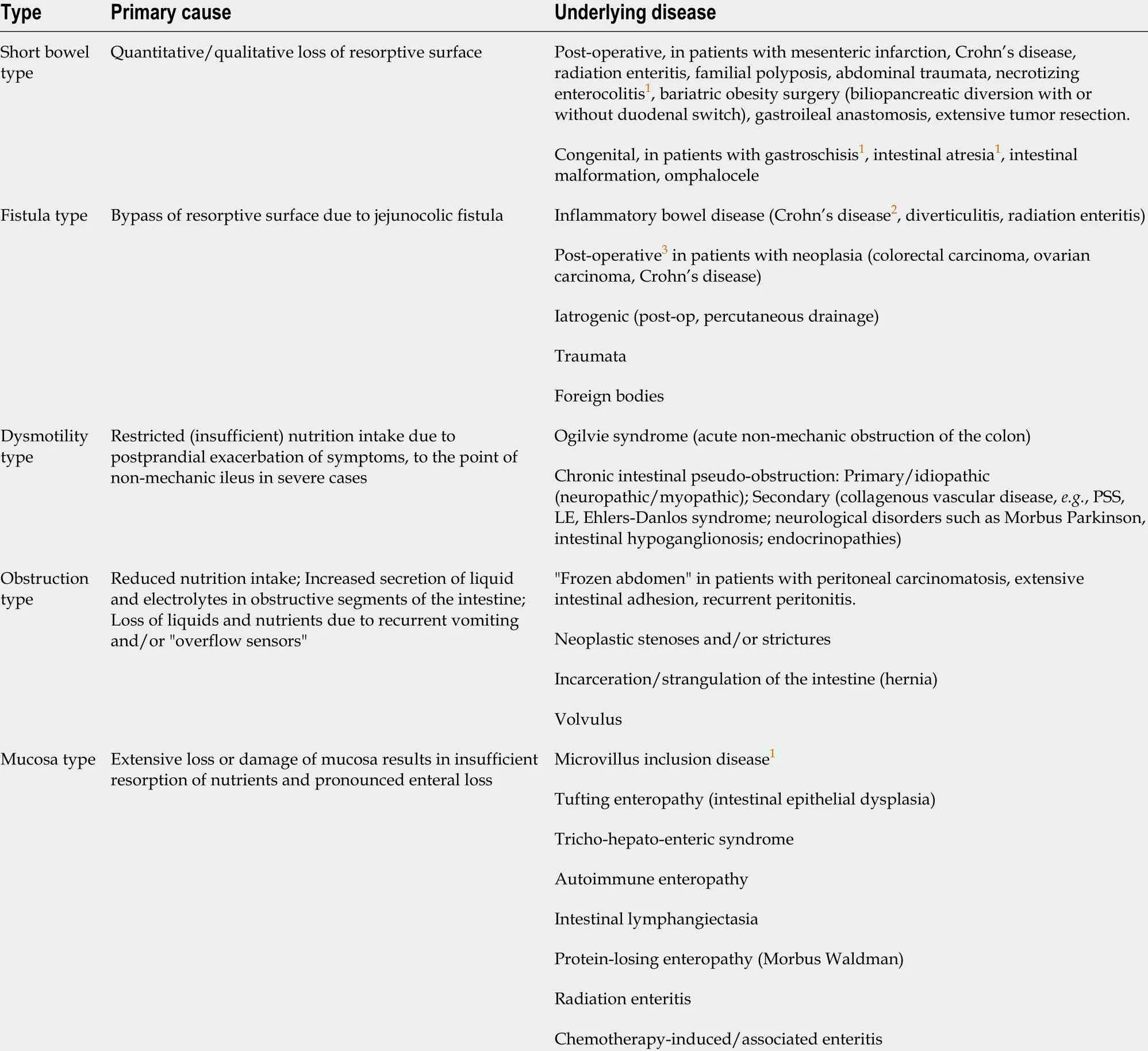
Table 1 Pathophysiological classification of intestinal failure (adapted from Pironi et al[2 ], 2015 )
In patients whose terminal ileum and proximal colon have been surgically removed(type I), a lack of L-cells leads to reduced synthesis of enterohormones [peptide tyrosine-tyrosine, glucagon-like peptide (GLP)-1 , GLP-2 ], effecting an acceleration of gastrointestinal transit duration[23 ].
Phase II: Adaptation of the residual intestine
Following intestinal resection, structural and functional adaptation processes in the remnant bowel begin even during the early post-operative hypersecretion phase.Distal resection tends to trigger a greater adaptive response than more proximal resection; during this phase, fluid losses should fall to less than 2 .5 L. Several years can pass before a stage of maximal adaptation is reached. Generally, 90 %-95 % of adaptation potential of the remnant bowel is realized within 2 years after resection surgery. In the stabilization phase, improvement of bowel efficiency due to enhanced adaptation leads to a reduction in diarrhea and steatorrhea. Intestinal adaptation is induced by enterotrophic hormones and growth factors, such as epidermal growth factor, growth hormone, insulin-like growth factor-1 (ILG-1 ) and glucagon-like peptide-2 (GLP-2 ), an amino acid peptide created by specific post-translational proteolytic cleavage of proglucagon. In addition, intraluminal nutrient supply and the resultant secretion of biliary and pancreatic enzymes play a vital role (for review,see[23 ]).

Figure 1 Four domains of chronic intestinal failure. Disease severity is defined by the content of the supplementation — fluid and electrolytes (FE) only, or admixture containing energy (parenteral nutrition (PN)). Each group is subdivided into four categories of mean daily intravenous supplementation (IVS), with volume calculated as average infusion volume per day × number of infusions per week/7 .
Phase III: CIF
Around 50 % of patients with prolonged acute intestinal failure go on to develop CIF[24 ]. Patients with CIF are metabolically stable but need intravenous nutritional support for months or years (reversible CIF) or even lifelong (irreversible CIF). Homebased PN (HPN) is the mainstay of treatment. During this phase, symptoms of SBS are usually addressed with medication. Overall, the probability of weaning off HPN has been reported to be about 50 % in adults and up to 73 % in children. Complete commutation off HPN in patients with SBS is unlikely[24 ]. PN can be necessary either to substitute total caloric-nutritional needs when patients are fasting and totally dependent on HPN (total PN) or as supplemental intake in patients who partially satisfy energy requirementsviaoral/enteral feeding (supplemental PN).
DIAGNOSIS AND WORK-UP
Diagnostics include on the one hand the correct diagnosis of SBS/CIF,i.e.,determination of the post-operative bowel anatomy (intraoperative bowel length, radiological assessment of bowel length) and the resorptive capacity of the remnant bowel, and on the other hand, the determination of specific nutrient deficiencies and their symptoms and, if applicable, any complications of enteral nutrition (EN)/PN.
As a first step, diarrhea is quantified and further characterized by stool frequency,stool weight, and 24 -h steatorrhea/creatorrhea[25 ,26 ]. The resorptive surface area can be calculated using the D-xylose absorption test and/or serum citrulline[27 ,28 ].However, data published on the validity of serum citrulline determination as a biomarker for bowel length and a prognostic parameter for successful intestinal adaptation are the subject of controversy. Assessment of early onset nutrient deficiencies requires analysis of iron (ferritin), folic acid, calcium, phosphate, and copper in serum in addition to urine concentrations of zinc and magnesium. In addition, if steatorrhea is present, serum concentrations of the fat-soluble vitamins (A,D, and E) can be utilized for dose-finding and/or correction (cave: Hypervitaminosis).Symptoms of late onset deficiencies, which often remain unnoticed for years before clinical manifestations become apparent, include megaloblastic anemia as a consequence of vitamin B12 deficiency (the liver’s stores are sufficient for 3 -5 years). In this context, and also as an early marker of folic acid or vitamin B6deficiency,homocysteine levels serve as a simple and efficient screening parameter (Table 2 )[29 ].
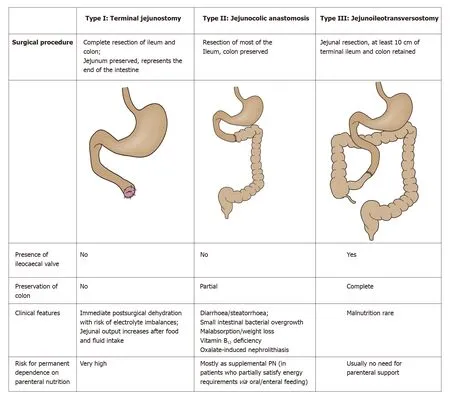
Figure 2 Main characteristics of different types of short bowel syndromes according to the anatomical criteria (adapted from Massironi et al[3 ], 2020 ).
MANAGEMENT OF CIF
Management of CIF focuses on optimizing nutritional status and fluid balance and achieving and/or maintaining adequate weight, while simultaneously minimizing risks associated with long-term complications[30 ]. The choice of therapy is contingent upon the underlying disease, concomitant disorders, and complications of therapy as well as the localization and extent of resection. Both strategies aim to compensate the diminished resorptive capacity of the remnant bowel, striving in the long-term for oral autonomy. For optimal management, ESPEN recommends that patients with CIF are supported by an expert multidisciplinary team (MDT) addressing all relevant domains of care, such as underlying disease therapy, catheter care, psychological well-being,and PN monitoring[1 ]. Thus, the ideal MDT should include physicians, surgeons,nurses specializing in stoma and catheter care, dieticians, pharmacists, and psychologists[31 ].
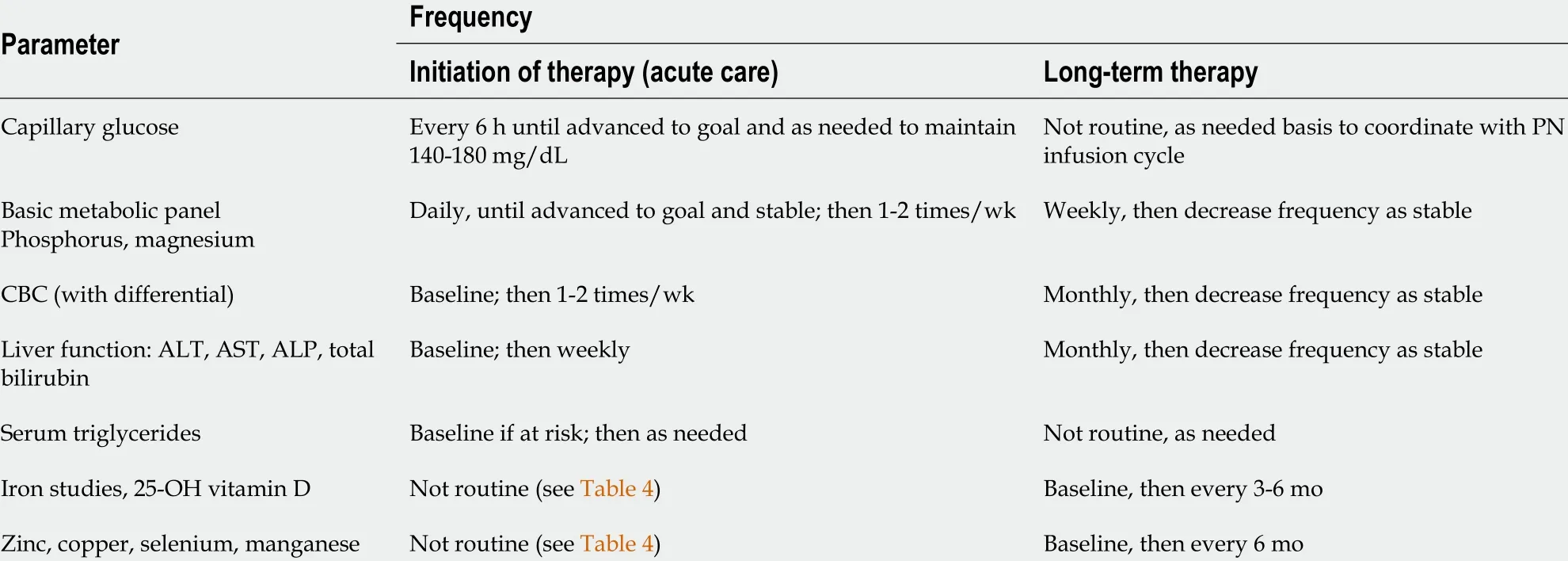
Table 2 Recommended laboratory monitoring for patients receiving parenteral nutrition (adapted from Lappas et al[29 ], 2018 )
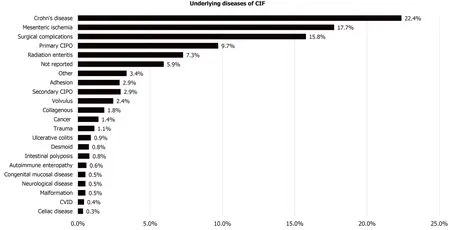
Figure 3 Underlying diseases of chronic intestinal failure. CIF: Chronic intestinal failure; CIPO: Chronic intestinal pseudo-obstruction; CVID: Common variable immunodeficiency.
OPTIMIZING NUTRITIONAL STATUS
There is no specific diet for patients with CIF. Individual remnant intestinal anatomy is a crucial consideration when planning nutritional management and should be clarified prior to commencement of PN. As mentioned above, it is recommended that patients with SBS-IF are divided into three groups based on the absence or presence of the terminal ileum and the colon, since these are the main factors influencing the type and amount of nutrient supplementation required[32 ].
Specific caloric requirements will vary on an individual basis; however, observational studies have found that patients with SBS consume between 35 and 58 kcal/kg/d in order to meet their nutritional needs[33 ].
Depending on the adaptation stage, nutritional therapy measures can take the form of overlapping or combined therapy with oral and (long-term) PN/EN, with adjunctive medication as necessary. Patients with jejunostoma (type I) and a residual bowel length of less than 100 cm, as well as those with less than 50 cm continual remnant colon (Table 3 ), are almost certain to require PN on a permanent basis. If,however, a greater length of remnant bowel is present, it is usually possible to gradually discontinue parenteral feeding or at least progressively convert to partial PN or intravenous fluid supplementation[24 ,32 ,34 ].
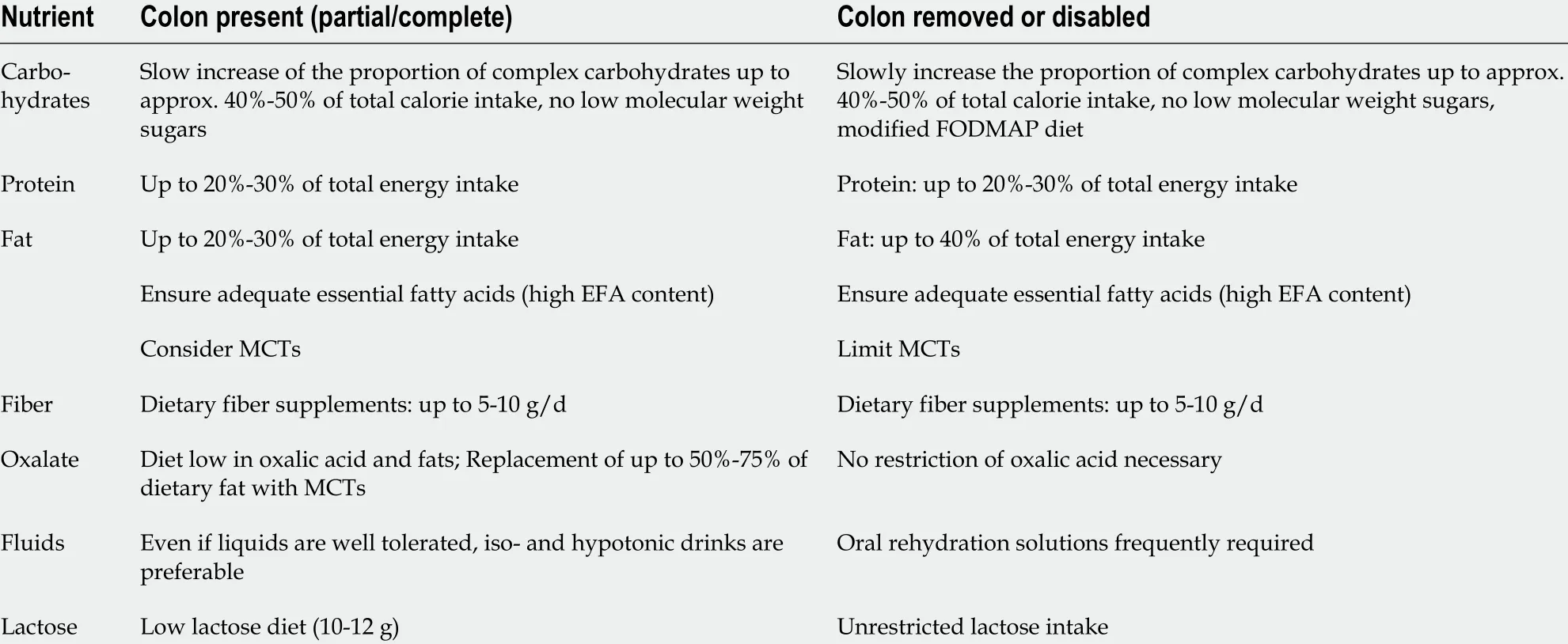
Table 3 Nutrition therapy (macronutrients) according to presence or absence of colon (modified from Pironi et al[46 ], 2016 )
There are no controlled data describing the protein requirements of adults with CIF.Prior studies show that protein absorption depends on the length of the small bowel remnant and may increase over time as the bowel adapts, ranging from 61 % to 80 %[33 ]. In patients with IBD, supplementation must additionally compensate protein lossviathe bowel[35 ]. Current ESPEN guidelines recommend protein substitution of 0 .8 -2 .0 g/kg body weight (BW) per day according to individual needs, representing 20 %-30 % of daily calorie requirements[1 ]. The supply of proteins (enteral) and/or amino acids (parenteral) should be chosen to offset undermet needs and ensure the prompt correction of existing deficits (catabolism). The sparse data available on the utility of peptide-based EN are contradictory. However, a considerable increase in protein supply can be achieved through continual nasogastric application of standard polymer feeds, either alone or supplementary to oral (ad libidum) nutrition, above and beyond the immediate post-operative phase. Peptide-based enteral feeding is possibly only of additional value in SBS type I[32 ].
Fat restriction is not necessary in patients who lack a colon. In patients with a preserved colon, it is important to limit fat intake to 20 %-25 % of total calories in order to reduce the risk of nephrolithiasis from calcium oxalate stones (Table 3 ). In these patients, a low-fat diet has been shown to increase absorption of calcium, magnesium,and zinc[36 ] and to improve utilization of medium chain triglycerides for nutrient absorption without increasing stool volume and diminishing calcium and magnesium absorption[37 ,38 ]. Fat assimilation can be improved through the addition of pancreas enzyme formulations in powder or granulate form. Controlled studies are not available for CIF[32 ].
MANAGEMENT OF FLUID AND ELECTROLYTE BALANCE
The primary challenge in patients with jejunostomy is the management of major losses of fluid and electrolytes, particularly zinc, copper, and magnesium (Table 4 )[39 ,40 ]. It is important for these patients to avoid drinking hypotonic solutions or tap water, as this can lead to worsening electrolyte disturbances by increasing stool sodium content.Conversely, hypertonic solutions such as soda and fruit juice should also be avoided as these solutions are hyperosmolar and can draw water into the gastrointestinal tract and worsen dehydration[33 ]. Ideally, fluids should be given in small portions between mealtimes, initially in the form of oral rehydration salts (ORS) that offer a sodiumglucose ratio optimized to enhance resorption[41 ]. In patients with a colon, ORS solutions based on rice starch have been shown to be particularly effective[42 ]. Sport electrolyte solutions, on the other hand, are not only costly but generally unsuitable,since they contain relatively large amounts of sugar and/or sweeteners in relation to their sodium content. Common sweeteners such as sorbitol or aspartame are known to cause osmotic diarrhea. In addition, fruits, fruit juice, and sweets/candies may increase the frequency of diarrhea. Glutamine-containing ORS solutions are not to be recommended, since glutamine diminishes the intake of sodium and fluids[43 ].In the adaptation phase (see above), filling foods such as potatoes, rice, oat flakes, or bananas can help solidify the stool. Fluid binding and swelling formulations with pectin may also be beneficial[44 ].
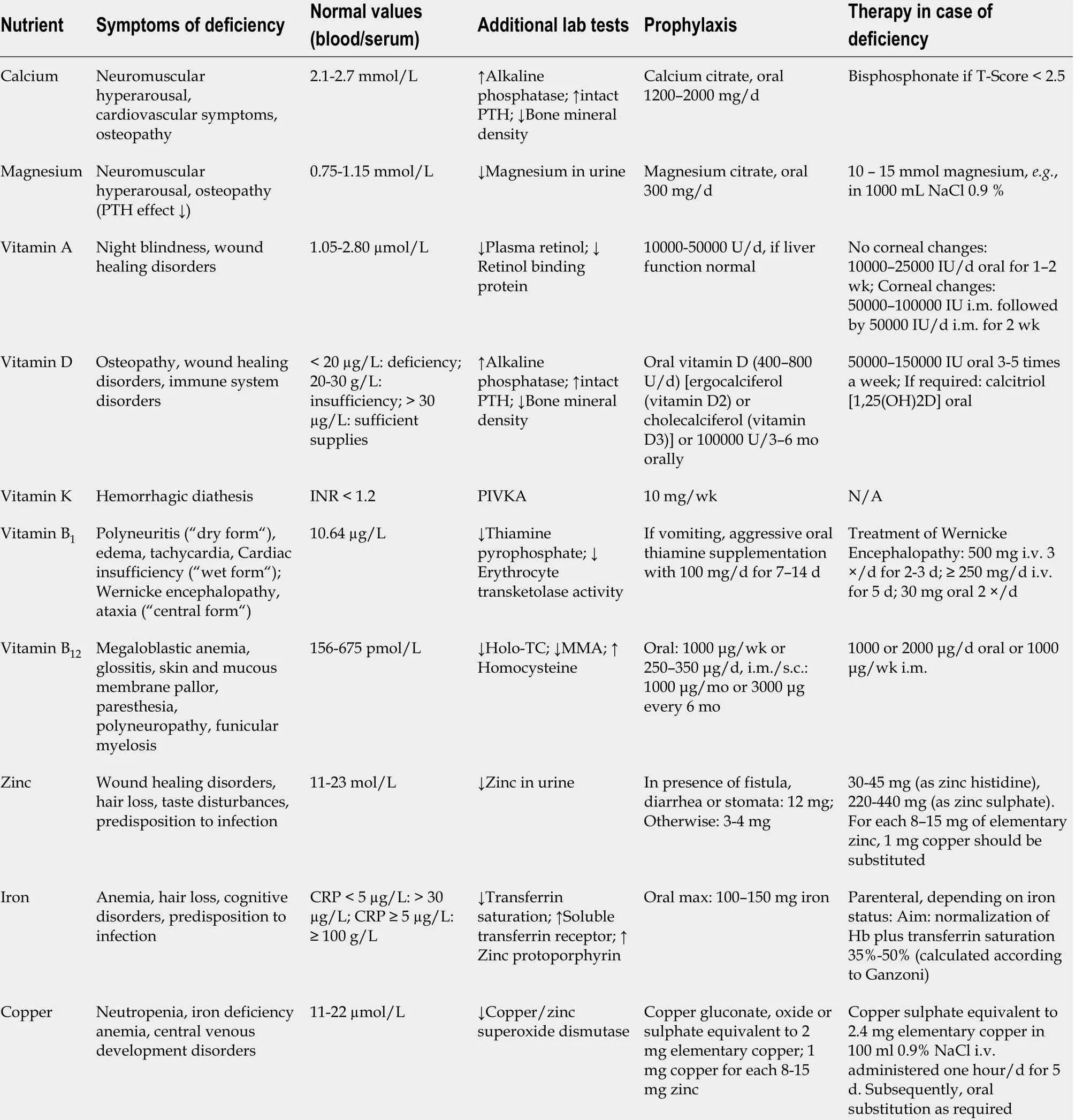
Table 4 Symptoms, prophylactic supplementation, and therapy of frequent (critical) nutrient deficiencies in chronic intestinal failure
Ostomy patients should be made aware that every intake of food can be expected to result in stool emptyingviathe ostomy outlet. These patients have a daily fluid requirement of approximately 3 L. Adequate fluid intake can be assessed on the basis of urine volume, with a urine volume of at least 1 L/d considered ideal (urine volume monitoring). Daily intake of 6 -9 g salt (e.g., salted meat or vegetable broth) and 25 -30 g dietary fiber are also recommended.
Cave: Ileo-/jejunostomy patients (type I) are extremely susceptible to thirst and increased fluid loss due to sweating, diarrhea,etc.The resulting rise in oral fluid intake can lead to increased stool volume. Thus, these patients are at increased risk of rapid dehydration, to the point of prerenal kidney failure.
MINERAL SUBSTITUTION
The electrolyte disturbance most frequently seen in SBS is sodium deficiency. As a rough guide, it can be assumed that about 100 mmol/L of sodium are lost with each liter of jejunostomy fluid. Sodium deficiency is better detected by measuring urinary sodium, which decreases before hyponatremia occurs: Urinary sodium concentration below 10 mmol/L is a diagnostic criterion for sodium depletion. Overall, the aims of treatment are to maintain normal hydration and a daily urine volume of at least 800 mL, with a urinary sodium concentration greater than 20 mmol/L[1 ,40 ,45 ,46 ].
A urine sodium to potassium ratio of ≤ 1 indicates secondary hyperaldosteronism caused by volume depletion as a result of inadequate fluid substitution[47 ,48 ]. This marker therefore requires close monitoring and therapy should seek to maintain a sodium to potassium ratio > 1 [49 ].
While hypokalemia may occur in patients with SBS, net intestinal loss occurs only in the case of end-jejunostomy with less than 50 cm remnant jejunum. The effluent from a jejunostoma with a longer remnant jejunum or an ileostoma contains approximately 15 mmol/L potassium. Low serum potassium is most commonly due to urinary losses of potassium associated with secondary hyperaldosteronism elicited by dehydration and sodium depletion. Hypokalemia may also be caused by magnesium depletion, which disrupts many of the potassium transport mechanisms and increases renal potassium excretion; in this case, hypokalemia is resistant to potassium substitution but responds to magnesium replacement[1 ,50 ,51 ].
Due to potassium lossviathe stool or stoma output, daily potassium requirements of patients with CIF are increased compared to healthy individuals, for whom the recommended intake is 1 -1 .5 mmol/kg BW per day. Potassium loss in patients with small bowel ostomy amounts to approximately 10 -30 mmol/L (Table 4 )[49 ].
Daily calcium requirements are estimated at 0 .15 -0 .25 mmol/kg BW (300 -400 mg).Calcium intake should be aimed at maintaining normal levels of parathyroid hormone,with calcium excretion in 24 h urine providing a useful additional marker[49 ]. Extreme depletion of magnesium stores can disrupt parathyroid hormone excretion, leading to false low measurements[52 ].
Magnesium requirements, estimated at 0 .1 -0 .2 mmol/kg BW (200 -300 mg) per day for healthy individuals, can be more than doubled as a consequence of magnesium lossesviastool or stoma (Table 4 ). Magnesium supply should be sufficient to maintain normal serum magnesium levels (0 .75 -1 .15 mmol/L). When interpreting serum magnesium values, it is important to consider that magnesium deficiency may be present even at serum levels of up to 0 .85 mmol/L[53 ]. Magnesium secretion of < 9 .7 mg/24 h in urine is regarded as a more sensitive parameter for magnesium deficiency than serum magnesium: 24 h urine levels should therefore be determined when serum magnesium is 0 .75 -0 .85 mmol/L[54 ]. Since magnesium is not well absorbed, oral magnesium substitution is often inadequate. Nocturnal infusions once or twice a week are recommended, each containing 10 -15 mmol magnesium, e.g., in 1000 mL NaCl 0 .9 %[55 ]. For oral substitution of calcium or magnesium, Ca2 + or Mg2+citrate are preferable due to their superior bioavailability[56 ].
Daily phosphate requirements of healthy persons are estimated at 0 .3 -0 .5 mmol/kg BW. Phosphate supply should be calculated to maintain normal serum phosphate levels while taking account of parathyroid hormone levels (there are no data from controlled trials). When initiating parenteral and/or enteral feeding in undernourished patients, the possibility of nutrition-associated hypophosphatemia must be borne in mind (refeeding syndrome)[57 ].
MICRONUTRIENTS
The most common micronutrient deficiencies are of vitamin D, zinc, iron, and vitamin B12 . These deficiencies can be observed even in patients receiving full (“total”) PN,especially during weaning from PN to EN[58 ].
Vitamin B12 malabsorption occurs frequently after terminal ileum resection, even after short resection of only 50 cm. Resection of the ileocecal valve reduces intestinal transit duration and increases the risk of bacterial miscolonization of the small bowel,increasing the likelihood of additional bile acid deconjugation and depletion of the vitamin B12 intrinsic factor complex. The resulting pernicious anemia manifests as megaloblastic anemia, thrombocytopenia, and Hunter (atrophic) glossitis, along with neurological disturbances consistent with funicular myelosis (except in folic acid deficiency). To prevent vitamin B12 deficiency, 1000 IE should be given prophylactically every 2 -3 mo. In case of manifest deficiency, treatment should commence with daily administration of B12 at a dose of 1000 IE intramuscular/subcutaneous for 5 d, followed by monthly applications of 1000 IE (Table 4 ).
Deficiencies of fat-soluble vitamins arise as a direct result of disrupted fat resorption. If untreated, these deficiencies can lead to night blindness (vitamin A),coagulation disturbances (vitamin K), and, in the long term, bone metabolism disorders and even osteoporosis (vitamin D)[59 ] (Table 4 ).
Trace element deficiencies may also occur, especially of iron, zinc, and copper[58 ,60 ,61 ]. Patients with intestinal failure are prone to iron deficiency as result of malabsorption, gastrointestinal blood loss, and multiple surgical procedures.Accordingly, iron deficiency is the most common micronutrient deficiency during and after transition from TPN to EN, with reported incidences of 60 %-80 % for iron deficiency and 30 %-37 % for iron deficiency anemia[62 ,63 ]. A study from the Mayo Clinic (Rochester, MI, United States), including 185 patients, showed that iron deficiency anemia developed much more rapidly in patients with fistula and bowel obstruction than in those with SBS and dysmotility[63 ].
Despite the high prevalence of iron deficiency, iron is not routinely added to PN formulations because of the risk of anaphylaxis and concerns about incompatibilities.Although data describing the compatibility of iron supplementation with parenteral formulations are conflicting[64 ], iron dextran has been found to be compatible with lipid-free solutions at an amino acid concentration > 2 %. A safer approach would prescribe the intermittent infusion of therapeutic iron doses[65 ]. Dosage requirements for intravenous iron replacement should be calculated according to Evstatievet al[66 ].
In patients with diarrhea, large quantities of zinc are lost, with losses of 12 mg zinc per liter stoma output not unusual (Table 4 ). This considerably exceeds not only normal total zinc requirements but also the content of standard oral mineral supplements[67 ,68 ]. In the case of manifest zinc deficiency, 30 -45 mg zinc per day can be taken orally (approximately 1 h before breakfast) as zinc histidine or zinc gluconate[69 ]. However, response to oral supplementation is frequently inadequate, in which case parenteral substitution is indicated[68 ,70 ].
PHARMACOLOGICAL TREATMENTS
Analogous to the modified resorption of micro- and macronutrients in CIF, and depending on the underlying disease and the extent and localization of bowel resection, alterations in the bioavailability of any and every kind of drug therapy are to be anticipated. While the scale of these changes is subject to wide interindividual variation, larger drug doses may be required than are typically recommended[30 ,50 ].Data on specific drug classes are scarce, and those that have been published are either case reports or very small cohort studies[71 ,72 ]. Therefore, sublingual, transdermal, or transnasal drug application should be chosen whenever suitable options are available[41 ]. Pharmacological treatments for CIF are primarily based on antisecretory and antimotility treatments intended to minimize gastrointestinal fluid losses. Based on the identification of GLP-2 as a tissue-specific intestinal growth factor in the late 1990 s,hormonal treatment promoting intestinal hyperadaptation has been proposed, aimed at maximizing absorption in the remnant bowel, decreasing intestinal losses, and reducing the need for PN[73 ,74 ]. Table 5 shows current drug therapy options for the treatment of CIF.
Antisecretory treatment
For the majority of these medications, only scant scientific evidence is available, or none at all. For example, despite the complete absence of data from controlled studies to endorse the application of proton pump inhibitors in the hypersecretion phase, they are widely recommended as a means of reducing fluid volume and improving the effectiveness of pancreas ferments. Their use in the case of high output stomata is at least supported by two small monocentric studies, whereby intravenous is evidentiallysuperior to oral application[75 ,76 ]. Longer-term or permanent intake of proton pump inhibitors is, however, associated with increased risks for osteoporosis and vitamin B12 deficiency[41 ].
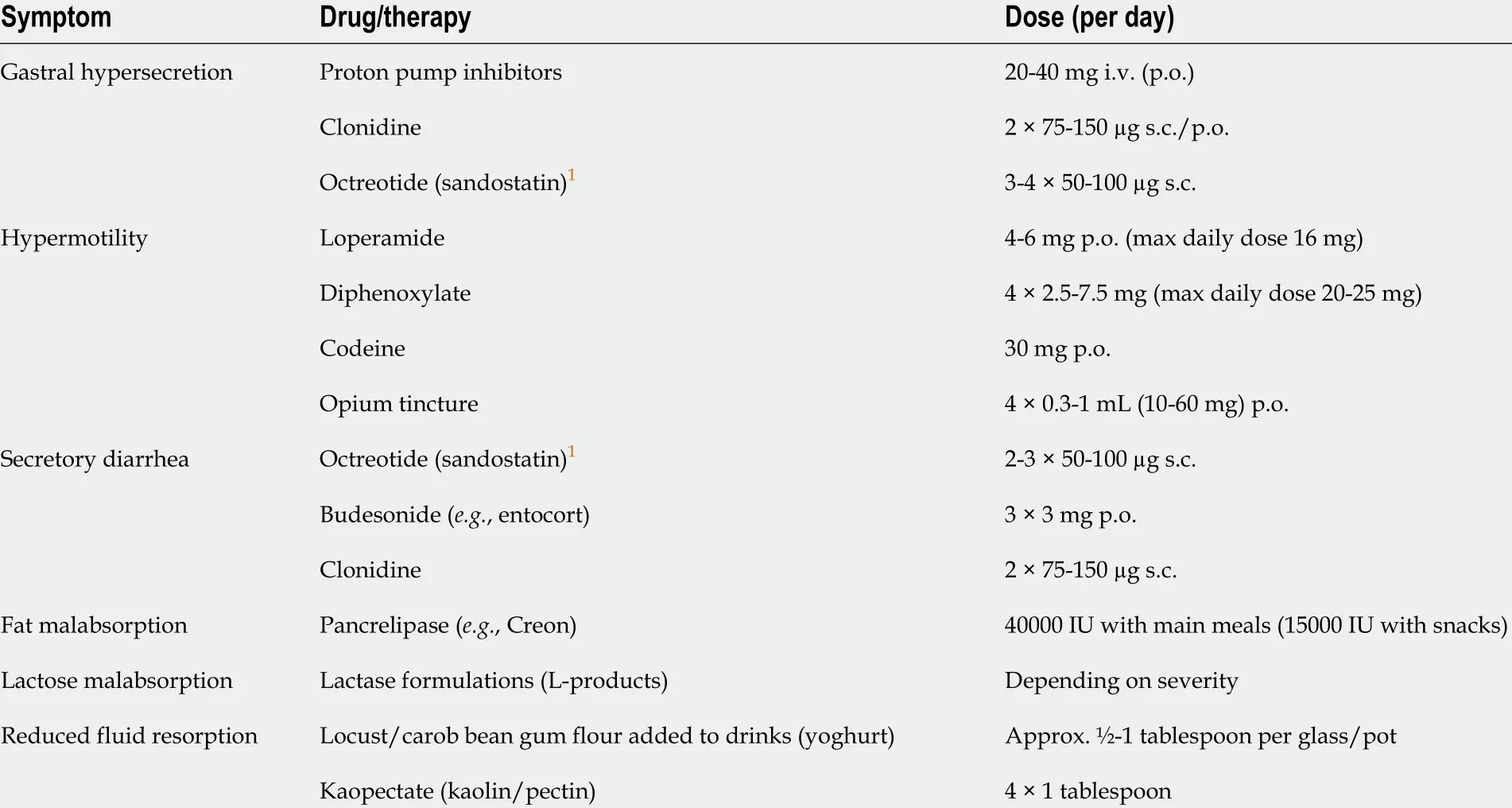
Table 5 Symptomatic treatment options in short bowel syndrome
Typical second-line agents used to combat gastric hypersecretion include histamine type 2 receptor (H2 ) antagonists (e.g., famotidine, ranitidine, cimetidine) and α2 -adrenergic receptor agonists (e.g., clonidine)[75 ,77 ]. As with antimotility agents, acid suppressors should be initiated at a low dose and titrated upward to yield maximal efficacy with minimal adverse events. The optimal duration of post-operative antacid therapy is unknown, but it should be discontinued in the event of worsening diarrhea[30 ].
Data from two smaller trials in patients with CD and ileostoma demonstrated that topically acting steroids may improve the absorptive capacity of the intestinal mucosa for water, independently of their anti-inflammatory effects[78 ,79 ].
A number of randomized controlled trials (RCTs) have been published examining the efficacy of crofelemer, a dual inhibitor of cyclic adenosine monophosphate- and calcium-mediated chloride secretion, in chronic acquired immunodeficiency syndrome-associated diarrhea (for reviews, see[80 ,81 ]).
Antimotility treatment
The use of antimotility drugs to control diarrhea in CIF has been validated mainly by small studies in patients with ileostomy[59 ,77 ]. Recommendations for their use are additionally based on extensive practical experience of their application in diarrhea of infectious and non-infectious etiology[49 ].
Accelerated intestinal motility is typically treated with opioids or the opioid receptor agonists loperamide and diphenoxylate-atropine as the first-line choices.Unlike diphenoxylate, which crosses the blood-brain barrier, loperamide, a peripherally restricted μ-opioid receptor agonist, does not engender undesirable central nervous system effects such as sedation, euphoria, or addiction[30 ,82 ,83 ].Because loperamide enters the enterohepatic circulation, higher doses (up to 16 tablets/d) may be needed in patients whose ileum has been shortened or removed.Other antimotility agents include codeine[82 ,84 ], morphine, and opium tincture[30 ].However, these opioids are not restricted to the peripheral nervous system and can thus generate central nervous system effects[30 ]. When used together, loperamide and codeine may have a synergistic effect[82 ].
Besides alleviating gastric hypersecretion, clonidine, a centrally acting α2adrenergic and imidazoline receptor agonist (which can also be administered transdermally), has been shown to diminish stomach and colon motility and inhibit intestinal chloride secretion. As with H2-antagonists, evidence supporting clonidine use in patients with CIF is based only on small case series[85 -88 ]. Dosage of the selected antimotility agentadministered 30 min before meals and at bedtime-should be escalated in a stepwise manner at intervals of 3 -5 d until benefit is observed, adverse events occur, or the recommended maximum dosage is reached[30 ]. Whereas the development of tolerance to analgesic properties of opioids or opioid receptor agonists is well recognized,tolerance to the antidiarrheal effect is rare and the effective dose may remain constant for months to years[30 ].
Hormonal treatment
In patients with SBS-type CIF, intestinal adaptation can be additionally stimulated by the application of enterotrophic hormones and growth factors, such as growth hormone with or without glutamine or the glucagon-like peptides GLP-1 and GLP-2 [89 -93 ]. Treatment with somatostatin analogues has also been studied.
Since the pilot trial of Jeppesenet al[94 ] in 2003 , a number of trials of teduglutide, a long-acting GLP-2 analogue, have shown an overall 20 %-40 % reduction in PN dependence, with some patients able to discontinue PN entirely. On the evidence of two 24 -wk RCTs, teduglutide is recommended to minimize the number of infusion days in patients with stable infusion-dependent CIF. Improvements in stool consistency and general condition of the patients were demonstrated in both of the aforementioned trials[95 ,96 ].
Most recently, first results from a European interdisciplinary center-based retrospective data analysis on teduglutide treatment for SBS in clinical practice have been published. The results show that, even when applied in routine medical care to patients with anatomically and clinically heterogeneous SBS-CIF, teduglutide induced functional and structural changes, allowing a gradual reduction of parenteral support.The data suggest that treatment with teduglutide results in an improved intestinal function and a compensatory effect on nutritional status[97 ].
It must, however, be kept in mind that all existing GLP-2 trials were conducted in patients at a late phase of intestinal adaptation, months or years after surgery. If therapy were initiated immediately after resection, adaptation would probably be induced more quickly (accelerated) and potentially to a greater degree (supraphysiologic). Conversely, and importantly, because GLP-2 is trophic to the intestinal mucosa, it may carry a risk of promoting or inducing the growth of localized polyps,or more ominously, malignancies. A recent systematic review indicated that treatment with teduglutide for up to 30 mo in individuals without known pre-existing cancer did not confer an increased risk of intestinal neoplasia[98 ]. However, based on animal data showing that GLP-2 may promote the growth of existing neoplasia, endoscopic examination of the remnant colon is mandatory before beginning teduglutide therapy[74 ].
In 2013 , an open-label, placebo-controlled study showed that GLP-1 decreased diarrhea and fecal excretion in SBS patients. More recently, liraglutide, a GLP-1 analogue, was administered daily subcutaneously in patients with end-jejunostomy over 8 wk in an open-label pilot trial[72 ]. Liraglutide reduced ostomy wet weight output (by 474 ± 563 g/d), increased intestinal wet weight output (by 464 ± 557 g/d),and improved intestinal energy absorption (by 902 ± 882 kJ/d), with statistical significance in all three instances. Combination therapy with GLP-1 and GLP-2 has been shown to be more effective than GLP-2 alone[99 ].
Over the past two decades, several clinical studies evaluating the effects of growth hormone,alone or in combination with a high-carbohydrate, low-fat diet and/or glutamine, on intestinal adaptation and absorption in pediatric and adult populations,have demonstrated conflicting findings[89 ,100 -107 ]. The positive effects of high-dose growth hormone treatment, mainly on intestinal water absorption, have been described in patients with SBS with a colon in continuity[74 ]. Although high-dose growth hormone therapy is already in use, no recommendation for routine application of growth hormone is given in the latest guidelines of either the German Nutrition Society[49 ] or ESPEN[108 ].
Octreotide, a long-acting analogue of the peptide hormone somatostatin, has been demonstrated to improve diarrhea in patients with CIF by inhibiting gastrin and other gastrointestinal hormones, by inactivating adenylate cyclase, thereby inhibiting intestinal ion secretion and prolonging intestinal transit time[109 -111 ]. Longer-term application of octreotide is associated with an increased risk of gallstones, a recognized complication of SBS, and impairment of intestinal adaptation[30 ,112 ].
COMPLICATIONS AND SPECIAL SITUATIONS
Nephrolithiasis and enteric hyperoxaluria
Enteric hyperoxaluria (EH) was first described as a complication in the early 1970 s,upon recognition of the association of small bowel resection and subsequent hyperoxaluria and nephrolithiasis: When significant lengths of the ileum are resected (> 60 cm), bypassed, or dysfunctional due to inflammation (e.g., in CD), both fatty acids and bile acids are delivered to the colon at an increased rate, subsequently complexing calcium and thereby reducing free calcium and increasing free oxalate in the colon.
The management of EH focuses on lowering diet oxalate intake and reducing colonic oxalate absorption. However, many patients find restricting oxalate intake to a therapeutic target level of approximately 50 mg/d (< 10 mg oxalate per mealtime)through avoidance of oxalate-rich foods (e.g., rhubarb, spinach, sorrel, cocoa,chocolate, and cola)[58 ] difficult on an ongoing basis. Since steatorrhea leads to the complexation of calcium by free fatty acids in the colon, and thus to an increase in free oxalate concentration, reducing fat intake is a useful additional dietary intervention.As an alternative, medium chain fatty acids can be used as a substitute for long chain fatty acids[22 ,113 ]. As the mainstay of EH therapy, calcium (1000 -1200 mg/d) should be orally substituted. If gastric suppression is required, calcium citrate supplements should be favored over the more commonly-used calcium carbonate salts, since the latter are insoluble at a neutral pH[113 ].
Choleretic diarrhea/bile acid loss syndrome
Colestyramine has been recommended on the basis of clinical consensus and practical experience to treat choleretic diarrhea associated with compensated bile acid malabsorption (i.e.,without significant steatorrhea)[49 ]. While few controlled studies of colestyramine have been conducted, a recent trial comparing the drug to hydroxypropyl cellulose (which is not a placebo) found colestyramine to have a significantly greater effect. A systematic review demonstrated a dose-response relationship between severity of bile acid diarrhea and treatment response, ranging from 96 % in patients with severe to 70 % in patients with mild bile acid diarrhea[114 ].
Newer bile acid sequestrants such as colestipol and colesevelam, which are much more selective bile acid binders than colestyramine[115 ], are reportedly associated with better compliance and fewer side effects[116 ,117 ]. The efficacy of colesevelam has now been confirmed in a first RCT[118 ]. Neither colesevelam nor colestyramine is suitable for the treatment of uncompensated bile acid loss syndrome, since the associated steatorrhea is exacerbated by both compounds (Table 6 ).
A promising therapeutic approach in the treatment of (decompensated) bile acid loss syndrome is the administration of cholylsarcosine, a conjugated bile acid that has no secretagogue effects. Smaller studies showed a significant increase in calcium and fat absorption in patients treated daily with 4 -6 g cholylsarcosine[119 -121 ].
A recent proof-of-concept study assessed the usefulness of obeticholic acid, a potent farnesoid x receptor agonist, in patients with bile acid diarrhea, and found statistically significant increases in fasting serum FGF19 , both in patients with idiopathic bile acid diarrhea and in those with secondary disease and ileal resection of less than 45 cm[122 ].
Gallstones
Gallstones have been reported in 31 %-45 % of patients with SBS (with or without a colon). Reported risk factors are prolonged periods of starvation, large fluctuations in BW, medications such as opiates, and lipid emulsions (in TPN). In addition, diseases affecting the ileum (e.g., CD) and absence of ileum and/or ileocecal valve following surgical resection alter enterohepatic circulation and cause a loss of bile salts (Table 7 ),thus leading to cholesterol supersaturation and sludge formation[123 -125 ].
Small intestinal bacterial overgrowth
Especially after ileocecal valve resection, patients with CIF are at risk of small intestinal bacterial overgrowth (SIBO),i.e.,the miscolonization of the upper small bowel by colon-derived bacteria. By promoting bile acid deconjugation, SIBO not only exacerbates steatorrhea, thereby disrupting the absorption of fat and fat-soluble vitamins[126 ,127 ], but also hinders intestinal adaptation[127 ,128 ]. SIBO is also perceived as a predisposing factor for intestinal failure-associated liver disease(IFALD), since hepatotoxins (e.g., lithocholic acid) produced by anaerobic bacteria in the small bowel may lead to hepatic injury. While diagnosis can be made using H2or C13 breath tests, their sensitivity is not very high[127 ]. Some authors therefore suggesta “therapeutic trial” approach on the basis of clinical suspicion, even in nonresponders[62 ,129 ,130 ]. Adjunctive intermitting-alternating antibiotic therapy should be administered for a period of 7 -10 d (Table 6 )[63 ].

Table 6 Major complications of short bowel syndrome: risk factors, prevention and treatment (adapted from Pironi et al[46 ], 2016 )
D-lactic acidosis
D-lactic acidosis is a rare, but often overlooked, condition observed only in patients with a preserved colon. It is caused by an increased intake of refined carbohydrates that, having been broken down by bacteria into short-chain fatty acids, lactate when they pass into the colon. The associated decrease in colonic pH promotes the growth of gram-positive, acid-resistant, D-lactate producing anaerobes (e.g.,Bifidobacterium,Lactobacillus, Eubacteriaceae). This results in insufficient metabolism of human D-lactate,and D-lactic acidosis occurs, with associated neurological symptoms such as vision disturbances, confusion, and gait insecurity. Such symptoms are often mistakenly assumed to be signs of alcohol abuse. Diagnosis is made by determining D-lactate levels in the blood[131 ,132 ]. The pathogenesis of neurological symptoms associated with D-lactate acidosis remains unclear. One hypothesis is that D-lactate itself is toxic to the brain or alters neuro-transmitter production. Other potentially neurotoxic substances or false neurotransmitters produced in variable quantities during periods of D-lactate elevation may also be involved[46 ] (Table 6 ). Due to the similarity of symptoms of D-lactic acidosis to those of Wernicke encephalopathy, prophylactic administration of thiamine has been recommended[131 ,132 ].
Diseases of the bone metabolism
Diseases of the bone metabolism, such as osteoporosis and osteomalacia, may occur aslong-term complications of TPN and have been reported to occur in 40 %-100 % of patients with CIF[133 -135 ]. The main causal factors include not only malabsorption of calcium, magnesium[136 ], and vitamin D but also the presence of underlying inflammatory disease (e.g., CD[137 ]), combined, as a rule, with many years of steroid intake[135 ]. Patients on long-term HPN are recommended to undergo bone density testing every 2 years, with frequent assessment of calcium and phosphorus balance, as well as vitamin D levels. Vitamin D substitution should be orientated at 25 -hydroxy levels >30 ng/mL[138 ].
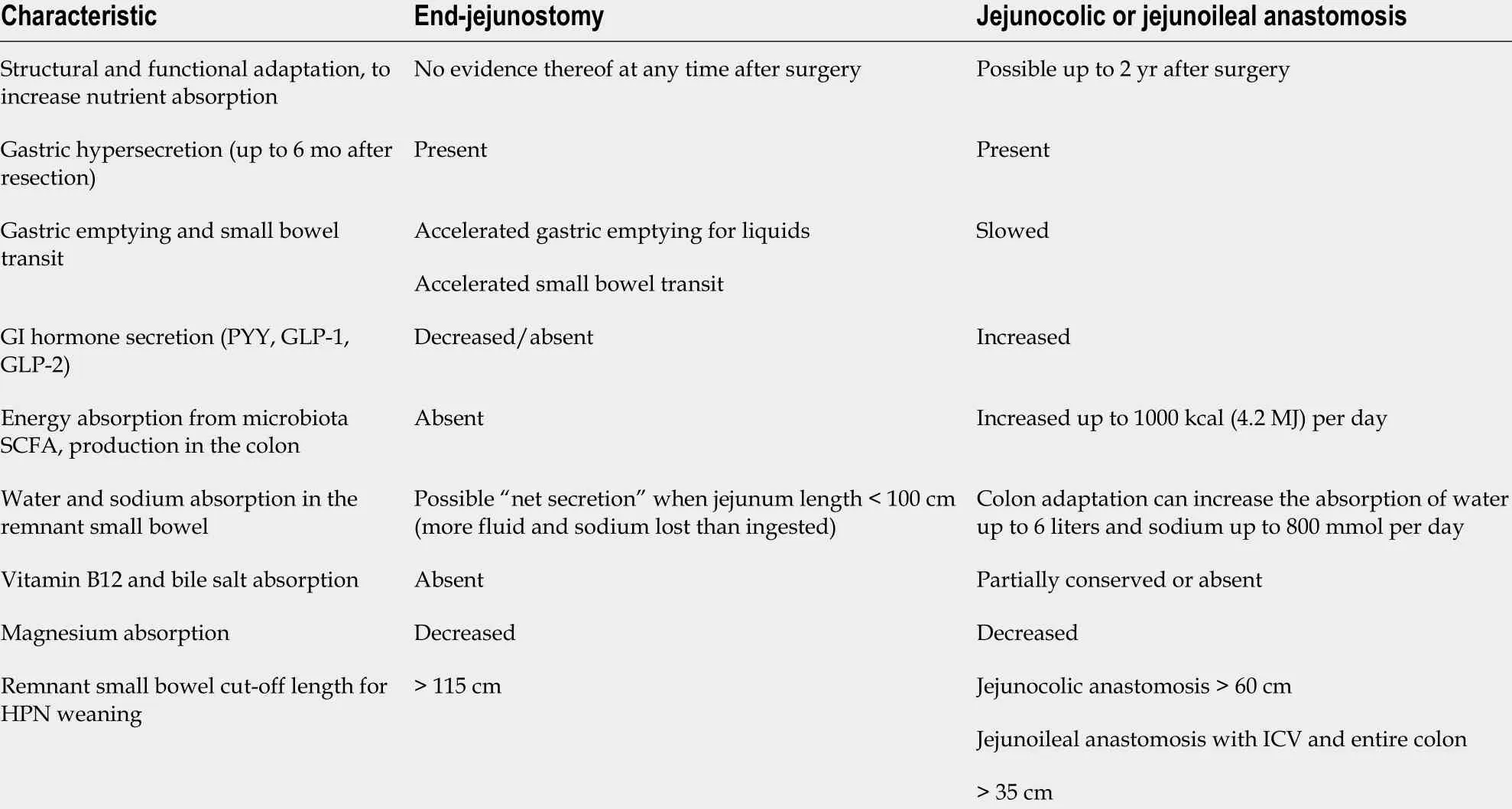
Table 7 Pathophysiological characteristics of short bowel syndrome with and without colon in continuity (adapted from Pironi et al[46 ],2016 )
MANAGEMENT OF THERAPY-RELATED COMPLICATIONS
Therapy-related complications most commonly arise from long-term HPN. While catheter-associated complications are frequent, a variety of organic and metabolic disorders may also occur (for detailed review, see[139 ]).
Catheter-associated complications
Direct catheter-associated complications in patients receiving long-term HPN can be characterized as either catheter occlusion or thrombotic or infectious complications.
Catheter occlusion can be partial or complete. Factors that influence its occurrence include duration of catheter placement, catheter size and material, type and meticulousness of care of the central venous access route, and the composition of applied infusion solutions. Signs and symptoms of occlusion include resistance when flushing,sluggish flow, inability to infuse fluids, frequent occlusion alarm on the infusion pump, infiltration, extravasation, swelling or leaking at the insertion site upon infusion or during flushing, inability to withdraw blood, and sluggish blood return upon aspiration of blood[140 -143 ].
Possible causes of catheter occlusion include: (1 ) mechanical problems; (2 ) nonthrombotic obstruction (precipitate of formulations such as medications or PN constituents within the catheter lumen); and (3 ) thrombotic obstruction (clot,thrombus, or fibrin deposition).
Any obvious mechanical obstruction should be ruled out before checking for nonthrombotic and thrombotic occlusion[144 ]. Mechanical occlusions arise from internal or external problems with the catheter, such as kinking in the catheter or tubing,catheter migration or malpositioning, clogging of the cap/needleless connector or filter, or excessive tightness of the retaining suture[145 ]. Depending on its underlying cause(s), mechanical occlusion can be remedied by removing the venous catheters,avoiding twisting/kinking when dressing, or, if occlusion or malpositioning of the needle is suspected, replacing the non-coring needle.
Catheter-associated infections (CAI) — in particular, catheter-related bloodstream infections (CRBSIs) — remain the "Achilles’ heel" of HPN treatment. Infections are classified as follows: Local infections: Catheter exit site infections, port recess infections, and tunnel infections in long-term subcutaneous tunnel catheters(Hickmann catheters); CRBSI: Defined as an infection in which the same organism can be isolated in cultures from the catheter and from peripheral blood, and clinical symptoms of sepsis are present, in the absence of an alternative focus of infection.CRBSI occurs in association with 5 %-10 % of all central venous catheters, equivalent to 0 .3 -30 cases/1000 catheter d, and accounts for approximately 70 % of all home PNrelated hospital admissions[146 -148 ].
More than 50 % of all CAIs are caused by gram-positive pathogens, 33 % by coagulase-negative staphylococci, and approximately 20 %-22 % by pathogenic fungi.About a quarter are mixed infections[149 ]. The chances of saving an infected(tunneled) catheter vary from 50 % to 85 %, depending on the pathogen responsible[49 ].
Different catheter-locking solutions have been studied for their effectiveness in preventing CRBSIs, including antiseptic agents, antibiotics, and anticoagulants. Due to the supposed need for an anticoagulant, heparin has most commonly been used as a catheter-locking solution. Its use, however, is no longer recommended, as it has been shown to potentially increase the risk of CRBSI by promoting the formation of an intraluminal biofilm[1 ,150 ,151 ].
Based on its ability to prevent microbial adhesion to the inner catheter surface, and to destroy microbial cell membranes and toxins, use of the broad-spectrum antiseptic agent taurolidine as a catheter-locking solution was described in 1998 . Subsequent studies and a meta-analysis confirmed that, compared with heparin, taurolidine decreases CRBSI incidence in patients with catheters. Based on these data and the most recently published RCT by Wouterset al[152 ], and in view of its favorable safety and cost profile, taurolidine solution (e.g., 0 .2 %) can be applied as a secondary prophylactic locking solution as soon as infection occurs and may also be used for primary CRBSI prophylaxis. However, effective training of the patient (and/or their family) and nursing staff is recognized as the single most effective primary prophylaxis[1 ,153 ].
IFALD
Based on the multifactorial nature of IFALD, a recent international position paper suggested defining it as “l(fā)iver injury as a result of one or more factors relating to intestinal failure including, but not limited to, PN and occurring in the absence of another primary parenchymal liver pathology”[154 ,155 ]. Based on retrospective data in adult patients with CIF, Sasdelliet al[156 ] recently reported an IFALD prevalence of 13 %-40 % for cholestasis, 27 %-90 % for steatosis, 2 %-5 % for fibrosis, and 8 %-75 % for unclassified IFALD, depending on the criteria adopted.
The pathogenesis of IFALD is not yet fully understood and likely multifactorial,with both PN and patient-dependent associated factors playing a role (Table 8 )[123 ,157 ]. Recommended measures for the prevention and therapy of IFALD are[158 ,159 ]:(1 ) maintenance of suitable proportions of nutrients, avoiding in particular highcalorie and high-glucose feeds, and insulin application, with 20 % up to maximum 50 %of total energy intake as lipids (approximately 1 g fat/kg BW); (2 ) cyclical infusion feeding periods of 12 -16 h, maintaining an overnight fasting period of 8 -12 h; (3 )enteral feeding, even if the quantities are insufficient to cover requirements,e.g., as a supplement; (4 ) in patients with cholestasis and/or “sludge” production, administration of ursodesoxycholic acid (10 -15 mg/kg BW,e.g., as juice) or cholecystokinin(cave: possibility of anaphylactoid reaction), with additional dose reduction of fatsoluble vitamins, copper, and manganese; and (5 ) administration of “essential”substrates such as carnitine, taurine, or choline in infants and children. On the basis of affirmative results from a number of randomized studies, fish oil-based lipid solutions are recommended for IFALD therapy in children. Furthermore, an initial study showed a positive effect of fish oil on hepatopathy in adults with CIF[158 ] (for review,see[160 ]). Medium-chain triglyceride- and/or olive oil-based lipid solutions are not recommended either for primary or secondary prophylaxis of IFALD[158 ]. The same applies to probiotics.
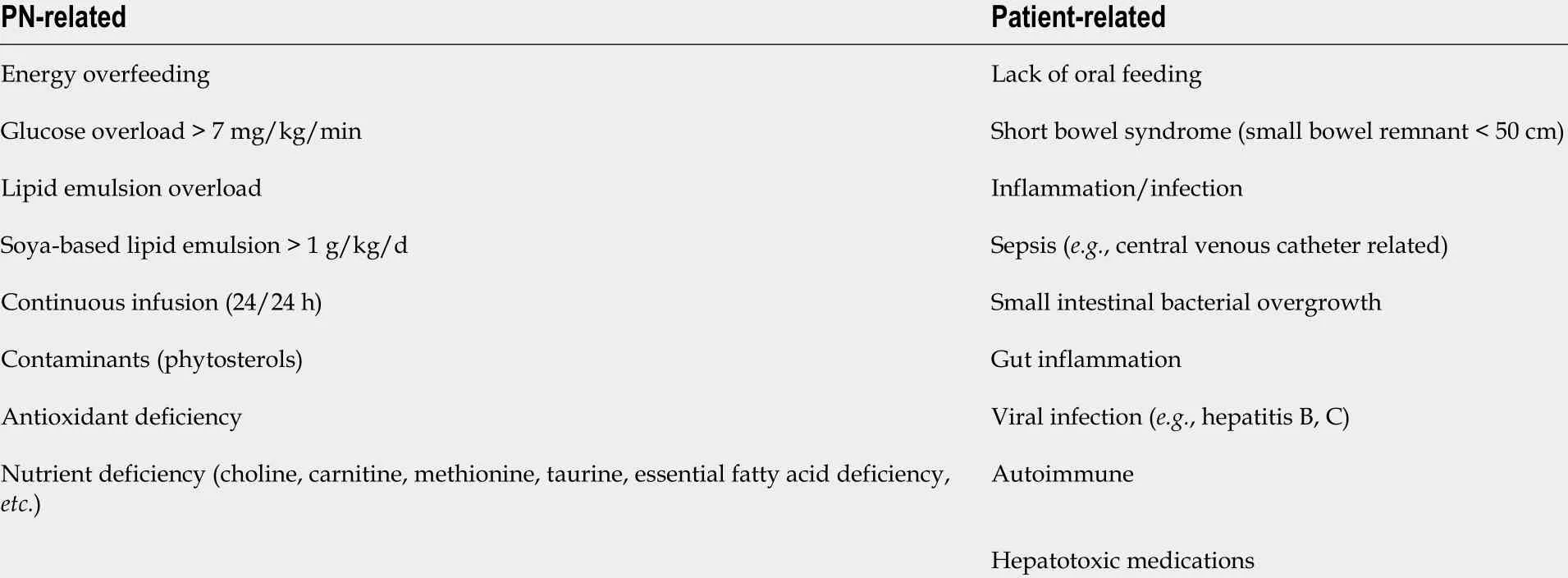
Table 8 Intestinal failure-associated liver disease risk factors in adult patients with home-based parenteral nutrition (adapted from Van Gossum et al[157 ], 2019 )
SURGICAL PROCEDURES
Reconstructive surgery
A range of operative techniques have been developed over the past years to allow patients with CIF at least the possibility of enteral feeding. These techniques have two common goals: To increase gastrointestinal transit duration and to increase the resorptive surface of the bowel. In principle, three surgical procedures may be applied(for review, see[161 -163 ]): (1 ) in cases where bowel length is critical, longitudinal lengthening and tailoring, first described by Bianchi in 1980 , accomplishes intestinal tapering without loss of surface; (2 ) in the serial transverse enteroplasty procedure described by Kimet al[164 ] in 2003 , the intestinal lumen is narrowed by firing a series of staples perpendicularly to the long axis of the bowel in a zig-zag pattern; (3 ) if the remaining small bowel is too short (i.e.,< 70 -80 cm) to achieve weaning from TPN, a reversed antiperistaltic intestinal segment (10 -15 cm in adults) can be placed to slow intestinal transit and thereby enhance nutrient and fluid absorption.
Intestinal transplantation
Intestinal transplantation, alone or in combination with other organs (visceral transplantation), is reserved for patients with life-threatening complications of PN defined by significant liver injury with elevated hepatic enzymes, multiple line infections, a single episode of life-threatening catheter-related sepsis, thrombosis of two of the central veins, or frequent episodes of dehydration (for review, see[165 ]).
CONCLUSION
While considerable advances have been made in recent years with regard to the understanding and management of CIF and its various related complications, it remains a challenging disorder. Home-based PN has brought distinct benefits, particularly in terms of higher survival rates and improved patient quality of life.Nevertheless, more than half of patients with CIF require PN on a permanent basis.While the traditional mainstays of pharmacological management are antimotility and antisecretory drugs, new hormonally acting drugs that stimulate intestinal adaptation,such as teduglutide, have had encouraging results, adding a new dimension to CIF therapy. More work is needed to improve understanding of CIF mechanisms, and to advance preventive and therapeutic approaches. Optimization of patient outcomes in complex CIF requires a dedicated multidisciplinary team of physicians, dietitians, and nurses, whose foci of care include maximizing intestinal rehabilitation, providing nutritional support, and improving quality of life.
ACKNOWLEDGEMENTS
The authors would like to thank Janet Collins (Interdisziplin?res Crohn-Colitis Centrum Rhein-Main, Frankfurt, Germany) for editing, correcting, and proof-reading the manuscript.
 World Journal of Gastroenterology2021年24期
World Journal of Gastroenterology2021年24期
- World Journal of Gastroenterology的其它文章
- COVID-19 and its effects on the digestive system
- Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: A systematic review and meta-analysis
- Disorders of the brain-gut interaction and eating disorders
- Stem cell injection for complex anal fistula in Crohn’s disease: A single-center experience
- Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis
- Early genetic diagnosis of clarithromycin resistance in Helicobacter pylori
