Hidradenitis Suppurativa:An Exploration of Genetic Perturbations and Immune Dysregulation
Ana Preda-Naumescu,Hana N.Ahmed,Tiffany T.Mayo,Nabiha Yusuf,*
1University of Alabama at Birmingham,Birmingham,AL 35294,USA;2Department of Dermatology,School of Medicine,University of Alabama at Birmingham,Birmingham,AL 35294,USA.
Abstract Hidradenitis suppurativa(HS)is a chronic,inflammatory skin condition that poses a significant diagnostic and therapeutic challenge for clinicians,as the underlying etiology and pathogenesis remains unclear.The host of genetic mutations and immune dysfunction has been identified to be involved in the pathogenesis of HS during recent years.These genetic defects,including monogenetic mutations altering subunits ofγ-secretase,a protease that functions through Notch signaling to maintain skin appendages,promote epithelial stability,suppress/terminate innate immune responses(ie,Toll-receptors),further have the propensity to induce aberrant cytokine responses that create to a proinflammatory environment,consequently induce hyperkeratosis and promote expression of pro-inflammatory,locally destructive matrix metalloproteinases.Cytokine-driven inflammation propagates the disease state of HS and contributes to the formation of painful subcutaneous nodules,abscesses,and eventually,fistulas and draining sinus tracts.A closer look at genetic mutations linked to the disease may explain the immune perturbations seen in HS.An understanding of the immune cells and inflammatory markers expressed in affected individuals provides insight into disease pathogenesis and can help identify therapeutic targets.
Keywords:hidradenitis suppurativa,genetic perturbations,immune dysregulation,monogenetic mutations,notch signaling,cytokine responses
Introduction
Hidradenitis suppurativa(HS)is a debilitating,chronic,inflammatory skin condition characterized by painful subcutaneous nodules and difficult-to-heal abscesses that occur in apocrine gland-bearing skin of the axillae,perineum,and inframammary regions.1Patients experience unrelenting cycles of relapse and remission with increasing severity of cutaneous sequelae.1As the disease progresses,the abscesses coalesce into draining sinus tracts that heal with dermal fibrosis and disfiguring contracture of the overlying skin.1
For many years,HS was considered a relatively rare disorder;however,its estimated prevalence in the United States ranges from 1% to 4%.2Misdiagnosis and underreporting are common,making the true prevalence of the disease difficult to discern.3As a result,many patients are undermanaged or not managed at all,leaving them to suffer both the physical and psychological consequences of this scarring disease.
Disease onset is generally post-pubertal.4Most patients first experience symptoms in the second and third decades of life.4Once believed to be a bacterial or inflammatory process of the apocrine glands,HS is now widely accepted as a disease of follicular occlusion.1Follicular hyperkeratosis and comedo formation result in occlusion,dilation,and eventual rupture of hair follicles.1Although inflammation of apocrine glands does not play a significant role in the propagation of the disease,it may occur subsequent to follicular occlusion.1Rupture of the follicular infundibulum leads to significant subcutaneous dermal inflammation that contributes to disease progression.4As the dermal inflammation progresses,abscesses form.4Eventually,the abscesses coalesce to form draining sinus tracts which,in addition to being difficult to heal,are inevitably a nidus for bacterial colonization and secondary infection.4
The currently available data concerning the natural history and prognosis of HS are limited.This is likely due in part to a lack of information regarding the disease etiology.Although the pathogenesis of HS as it relates to follicular occlusion is understood,the origin of this occlusion and the subsequent inflammation seen in HS is unclear.HS is not bacterial in origin because aspirates of unruptured lesions are sterile.3This understanding of the pathogenesis of HS has left clinicians and investigators with more questions than answers;nevertheless,they have achieved significant progress in recent years.Notably,many immunological and genetic perturbations have been linked to development of the disease(Fig.1).Genetic predisposition is suggested to play a significant role in disease development because familial clustering has been well documented and several genetic mutations have been identified.5Elevated levels of a host of inflammatory markers have also been identified in HS lesions,suggesting that immune dysregulation has a significant role in disease development.3
Herein,we conduct this review to summarize the advance of Genetic Perturbations and Immune Dysregulation in hidradenitis suppurativa.The review was conducted using the UAB School of Medicine library databases(Pubmed,Scopus,CINAHL,Google Scholar,Embase),limiting the search to articles containing keywords“hidradenitis suppurativa”and“genetics”O(jiān)R“genes”O(jiān)R“immune”O(jiān)R“immune system”from 2000 to 2020.

Figure 1.Schematic of the theorized pathogenesis of hidradenitis suppurativa.Certain genetic defects affect the subunits ofγ-secretase,a protease that cleaves a number of type 1 transmembrane proteins,including the Notch intracellular domain.Mouse studies have shown that γ-secretase functions through the Notch signaling pathway to maintain skin appendages,promote epithelial stability,and suppress/terminate innate immune responses.Overexpression of TLRs induces an aberrant cytokine response that may alter the microbiome and create a proinflammatory environment.TNF-αreleased from keratinocytes and DCs induces hyperkeratosis,decreases the release of adiponectin from adipocytes,and promotes expression of proinflammatory,locally destructive matrix metalloproteases.Macrophages and DCs express high levels of TLR-2,which induces production of large amounts of IL-23.IL-23 drives Th-17 cell differentiation,which subsequently leads to the release of IL-17,a cytokine that readily mobilizes neutrophils and increases their survival in peripheral skin.Ultimately,this potentiates the inflammatory response.Elevated levels of IL-1β,a proinflammatory cytokine produced via proteolytic cleavage in the inflammasome of monocytes and macrophages,lead to an increased inflammatory response and contribute to overexpression of the anti-inflammatory cytokine IL-10.IL-10 functions to suppress the proinflammatory IL-22;however,while proinflammatory,IL-22 also contributes to wound healing,maintains epithelial barrier function,and has antimicrobial functions.Cytokine-driven inflammation propagates the disease state of HS and contributes to the formation of painful subcutaneous nodules,abscesses,and eventually fistulas and draining sinus tracts.DCs:Dendritic cells;HS:Hidradenitis suppurativa;IL:Interleukin;Th-17:T-helper 17;TLRs:Toll-like receptor;TNF-α:Tumor necrosis factor alpha.Adapted with permission from Goldberg et al.20.
Genetic perturbations in HS
Familial clustering is well documented in HS.Evidence suggests a pattern of inheritance via single gene transmission of an autosomal dominant trait,with one study demonstrating vertical disease transmission in all three generations of five families and in two consecutive generations of six families.6However,only 34% of first-degree relatives were affected in these studies,whereas an autosomal dominant condition requires a 50%frequency of affected first-degree relatives.6An argument for incomplete penetrance versus incomplete case analysis has been made,the latter supported by the inclusion of younger,unaffected relatives in the study whose propensity for developing HS later in life had not been explored.6A subsequent study was conducted to address these discrepancies.Fourteen of the surviving probands were reviewed,and 34 surviving first-degree relatives were included in the study.7Of these individuals,10(27%)were confirmed to have HS.7In addition,nine probable cases were further identified,resulting in a 51% potential combined percentage of affected first-degree relatives.The only contingency was that about half did not entirely meet the disease definition established by the researchers.7
The idea that HS might be linked to a single-gene mutation with incomplete penetrance was further assessed by genome-wide linkage analysis of a four-generation Chinese family in 2006 that demonstrated autosomal dominant inheritance.8The linkage analysis was unable to identify a single disease gene;however,a novel locus for HS on chromosome 1p21.1-1q25.3 was identified in this large Chinese family.8This locus was associated with alterations in genes known to be involved in theγ-secretase pathway.9Subsequent studies identified 41 sequence variants in theγ-secretase pathway that were associated with HS development,30 of which involved loss-offunction mutations in the nicastrin gene(NCSTN).9
In the continued search for a single gene locus,investigators mapped an HS locus to an interval on chromosome 19q13 in two Han Chinese families that demonstrated autosomal dominant features of disease.10More specifically,the locus was mapped to the region in which the presenilin enhancer 2(PEN2)-coding gene(PSENEN)is located.10Much like NCSTN,PSENEN is involved in proper functionality of theγ-secretase pathway.9Sequence analysis of all PSENEN exons and introns in the first family revealed a guanine deletion that produced a frameshift mutation and premature termination of PEN2.10In the second family,a cytosine deletion in PSENEN was identified that similarly caused a frameshift mutation and premature codon termination.10Both mutations had the propensity to change the distal and functionally important C-terminus of PEN2,and mRNA analysis of the mutants indeed showed marked reduction in transcript expression.10These findings suggest that in both families,a nonsense mutation was responsible for mRNA decay and complete loss of function of the PEN2 protein,resulting in the clinical picture of HS.10
The importance of these loss-of-function mutations in PSENEN and NCSTN to HS development is best explained by first understanding the functional protein,γ-secretase,for which these genes encode.PSESEN encodes for one of four subunits of theγ-secretase protein,an intramembranous protease,whereas NCSTN is critical for integrating and stabilizing the different subunits of the γ-secretase complex.6,9Theγ-secretase protease comprises four separate protein subunits,namely presenilin,PEN2,nicastrin,and anterior pharynx defective,which are encoded by a total of six genes:PSEN1/PSEN2,PSENEN,NCSTN,and APH1A/APH1B,respectively.6
The function of theγ-secretase protein is to target and cleave type 1 transmembrane proteins,including amyloid precursor protein(APP),Notch receptors,and cadherins.6Prior to its association with HS,theγ-secretase protein was extensively studied in the context of familial Alzheimer disease(AD).6Missense mutations in PSEN1 and PSEN2,which code for the presenilin subunit of theγ-secretase protease,are implicated in the development of AD.6Mutations in these genes alter the ability ofγ-secretase to appropriately cleave APP,leading to longer,aggregationprone forms that result in the fibrillar plaques and neurotoxic species characteristic of AD.11
Studies concerning the role of this protease in AD may have inadvertently disclosed its importance in human skin.This association came to light when theγ-secretase inhibitor semagacestat was investigated as a possible treatment for AD.6In mice,the use of this inhibitor caused depletion ofγ-secretase.The mice subsequently developed conversion of hair follicles to epidermal cysts and destruction of sebaceous glands.6In human trials,semagacestat caused an increase in squamous and basal cell carcinomas.6These findings suggest that theγ-secretase protein plays a significant role in proper development and maintenance of both the hair follicles and the integumentary system as a whole.Whenγ-secretase was found to cleave a number of type 1 transmembrane proteins(ie,APP,Notch receptors,and cadherins),a new question arose:inhibition of which of these pathways leads to the development of skin abnormalities?
Mouse studies have demonstrated thatγ-secretase functions through the Notch signaling pathway to maintain skin appendages.12This signal transduction pathway comprises four Notch receptors(Notch 1-4)that may be engaged by one of five identified ligands[Jagged1,Jagged2,and three Delta-like proteins(DLL-1,DLL-3,or DLL-4)].6,13Activation of this pathway is necessary for epidermal skin and hair follicle differentiation as well as suppression of innate immune responses.13The role ofγ-secretase in this pathway is proteolytic cleavage of the intracellular Notch domain.13This allows translocation into the nucleus and formation/activation of a transcription complex that is necessary for expression of genes important in epidermal skin and hair follicle differentiation.6,13As previously mentioned,Notch signaling is also important in the suppression and termination of innate immune responses,particularly the termination of Toll-like receptor(TLR)-activated innate immunity.13
Monogenetic findings implicating the Notch singling pathway in development of HS are sufficient to demonstrate that familial HS can be an allelic disorder.6However,discrepancy between a positive family history andγ-secretase mutations in affected individuals implies that genes other than PSENEN are also likely involved in the pathogenesis.6Although theγ-secretase-Notch signaling pathway is the best described genetic association,HS is also a known component of other systemic autoinflammatory syndromes including the combination of pyoderma gangrenosum,acne,and suppurative hidradenitis,which is associated with monogenic mutations in the proline-serine-threonine phosphatase interacting protein 1 gene leading to defective inflammasome function.14
Although a positive family history has been noted in approximately one-third of patients with HS,suggesting a genetic basis of the disease,Pink et al.15found that only 7%of patients exhibited a monogenetic mutation with autosomal dominant inheritance.15,16The most commonly involved monogenic mutations are within either the Notch or phosphatase interacting protein 1 gene signaling pathway.15,16A 2019 comprehensive review of predisposing genetic mutations was performed in an effort to compile the research surrounding all known genetic mutations related to HS.16Researchers also found evidence for polygenetic inheritance of HS.15Current data related to genetic mutations in patients with HS suggest that the disease may be inherited as a monogenic trait affecting the Notch signaling pathway or inflammasome function or that it may be inherited in a polygenetic pattern because of defects in genes important for epidermal proliferation,barrier function,or immune system function.9
Genetic comorbidities
The role of genetics in the development of HS is further supported by associations of HS with a number of welldocumented genetic diseases.17Classification of these major comorbidities and their genetic background may be of great value to investigators and providers because they could suggest a host of chromosomal loci or genes that might serve as targets of future therapeutic interventions.17Genetic conditions including Dowling-Degos disease and Down syndrome are described in association with HS.17
Dowling-Degos disease,a rare autosomal dominant genetic skin condition with hyperpigmentation of the flexural skin folds,has been reported in patients with HS.18Patients with concomitant Dowling-Degos and HS were found to have mutations in PSENEN and the gene encoding protein O-fructosyltransferase 1,both of which are necessary for functional Notch signaling.18
Loss of Notch signal transduction also links HS and Down syndrome.19Increased levels of APP are seen in people with Down syndrome as a result of overexpression of genes on chromosome 21(the genetic basis of Down syndrome).APP and the Notch receptor are competitive substrates for theγ-secretase protein,and overexpression of APP thus leads to preferential cleavage byγ-secretase protein,which impairs cleavage for Notch signaling.19The resultant decrease in effective Notch signaling can lead to the clinical manifestations of HS in these patients.19
Other genetic disorders associated with follicular occlusion and in which concomitant cases of HS have been described include pachyonychia congenita,a group of autosomal dominant ectodermal dysplasias characterized by hypertrophic nail dystrophy,and keratitis-ichthyosisdeafness syndrome,a rare congenital disorder of the ectoderm caused by genetic mutations that give rise to keratitis,erythrokeratoderma,and neurosensory deafness.17
Immune dysregulation in HS
Exploring the genetic perturbations of HS is critical to understanding the immune dysregulation described in this disease.Genetic influences,particularly loss-of-function mutations in PSENEN involved in Notch signaling,may explain the immune aberrations that permeate HS.
As previously discussed,the Notch signal transduction pathway is integral for epidermal skin and hair follicle differentiation as well as suppression and termination of innate immune responses,particularly the termination of TLR-activated innate immunity.13Innate immune cells are activated in a number of ways,one of which is activation of TLR on the cell surface with subsequent mitogen-activated protein kinase(MAPK)-mediated transcription of inflammatory markers.12The Notch pathway normally opposes MAPK activation of innate immune cells,providing feedback suppression of the TLR-MAPK immune response that is heavily implicated in autoinflammation.13
Loss of this critical feedback suppression in HS leads to aberrant TLR and innate immune system responses.13These claims are supported by examination of lesioned skin in patients with active HS.Inspection of these lesions demonstrates an abundance of macrophages,dendritic cells(DCs),and CD3+T cells.13Macrophages and DCs expressing high levels of TLR-2,a receptor known to induce large amounts of the inflammatory cytokine interleukin(IL)-23,have been identified.13IL-23 plays an important role in the induction of T-helper 17 cells(Th17 cells)that release IL-17,a cytokine that readily mobilizes neutrophils,increasing their survival in peripheral skin and ultimately contributing to inflammation.13These findings implicate both the innate and adaptive immune systems in the disease pathogenesis of HS.
Loss of Notch signaling not only impairs maturation of hair follicle cells and increases epithelial fragility in patients with HS20;defects in this pathway are key to understanding the immune dysregulation in HS.Failure of Notch signaling disrupts terminal hair follicle differentiation and the integrity of the outer root sheath cells,which leads to the formation of keratin-rich epidermal cysts.13This perturbation releases damage-associated molecular pattern molecules that activate TLRs on macrophages and DCs,stimulating the release of proinflammatory cytokines that promote an innate immune response.13Failure of the usually inhibitory Notch signaling pathway to suppress this response leads to tissue destruction,inflammation,and the clinical picture of HS.13
The role of the immune system in the pathogenesis of HS is derived from both clinical and experimental studies.Several studies have demonstrated relative therapeutic success with anti-tumor necrosis factor alpha(anti-TNF-α)agents,which suggests a role for cytokines in the disease,whereas others have described alterations of the innate immune system,such as elevated levels of TLRs,in the skin of affected patients.21
Immune cells
Investigation of the immune system’s role in HS begins with a thorough exploration of the cells found in lesional skin of patients with HS.Histologically,HS lesions contain both innate and adaptive immune cells.These include DCs,neutrophils,macrophages,CD3,CD4,and CD8 T cells as well as B cells.1,22
An extensively documented hallmark of HS lesions is severe dermal inflammation with prominent neutrophilic infiltration.22However,the role of neutrophils in the disease pathogenesis,as well as activation of other immune cells,has not been widely investigated.22In 2019,Byrd et al.22demonstrated that in patients with HS,neutrophils are primed to form neutrophil extracellular traps(NETs).These networks of extracellular fibers are primarily composed of DNA derived from neutrophils,and their function is to bind pathogens.Byrd et al.22found that increased production of NETs is associated with disease severity as well as activation of a type I interferon(IFN)response in the skin.
NETs contribute to increased disease severity by acting as autoantigens.22Byrd et al.22provided evidence that enhanced NET formation in patients with HS leads to increased release of autoantibodies,which then enter the circulation where they are detected by the host immune system,therefore propagating inflammation.Investigators hypothesized that increased levels of certain cytokines in these patients may promote NET formation.22Byrd et al.22also suggested a significant role for autoantibodies in the pathogenesis of HS;however,more definitive research is needed to determine their significance.
The innate immune system is not the only contender in HS;B cells and plasma cells are also seen in HS lesions.22The generation of autoantigens(ie,NETs)stimulates B cells to differentiate into plasma cells,key players in the adaptive immune system.22Plasma cells produce antibodies,which may also act as autoantigens and stimulate the host immune system to produce an inflammatory response.22Implication of the adaptive immune system in HS is supported by findings of elevated immunoglobulin G in patients with HS,deposition of immunoglobulin G in HS lesions,and increased plasma cells in these patients.22Cytokines will be described in greater detail in the subsequent section;however,it is important to note here that the existence of plasma cells in HS lesions may also be explained by the upregulation of the cytokine IL-10 seen in these patients.22
Cytokines
Studies of HS have consistently demonstrated elevated cytokine levels in patients’skin.23In 2018,Frew et al.23published a systematic review and critical evaluation of the inflammatory cytokine associations in HS.The review comprised 19 studies involving 564 patients with HS and 198 control patients.23Eighty-one discrete cytokines were examined.23The methodology varied among the studies,and the overall quality of the studies examined was generallylow;however,theanalysiswasabletoidentifydifferent cytokine profiles between lesioned and peri-lesioned skin in patients with HS.23Most of the elevated cytokines and inflammatory proteins identified were produced by keratinocytes,T cells,DCs,and innate lymphoid tissue.23
The cytokines and inflammatory proteins identified may be subdivided into those produced by keratinocytes versus other inflammatory cell types(T cells,DCs,neutrophils,and innate lymphoid tissue cells).24Table 1 shows a detailed summary of the cytokines and inflammatory proteins identified in lesioned HS skin by cell type.21-32
A large variety of cytokines and chemokines have been measured in patients with HS;however,their role in the disease pathogenesis remains unclear and requires further investigation.Although the list summarized in Table 1 is extensive,it is not exhaustive.A closer look at some of the best characterized proinflammatory cytokines implicated in HS provides insight into the immune aberrancy associated with this condition and gives researchers and clinicians options for therapeutic targets.
Tumor necrosis factor-α
TNF-αis a proinflammatory cytokine well known for its role in inflammatory conditions.24It is produced by keratinocytes,macrophages,DCs,mast cells,and T cells.24Several studies focused on the cytokine profile of HS have shown that TNF-αexpression is significantly enhanced in both lesioned and peri-lesioned skin,identifying TNF-αas a key modulator of the disease state.1,24TNF-αreleased from keratinocytes and DCs has been shown to induce hyperkeratosis,decrease release of the anti-inflammatory hormone adiponectin from adipocytes,and increase the expression of proinflammatory,locally destructive matrix metalloproteases.20These findings have made anti-TNF-α biologics the most intensively studied drugs for patients with HS.25However,although several studies have shown increased levels of TNF-αprotein and TNF-αmRNA expression in the skin of patients with HS,other studies have shown decreased levels.1,25-27Interestingly,studies attempting to examine and quantify the levels of TNF-αin the peripheral blood of patients with HS have also shown similar discrepancies.1
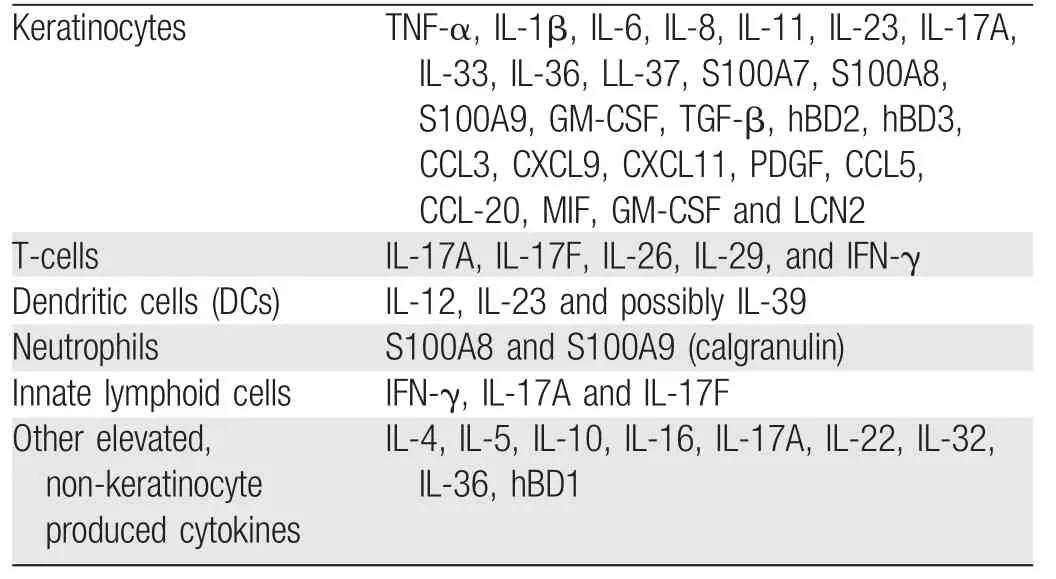
Table 1 Overview of cytokines and inflammatory proteins identified in lesioned hidradenitis suppurativa skin per cell type
The use of the anti-TNF-αagent adalimumab in patients with HS provides some insight into these conflicting results.In 2011,Van der Zee et al.28treated patients with adalimumab to investigate the correlation of TNF-αwith the HS disease presentation.Biopsies throughout the study demonstrated increased protein expression of TNF-αin the skin of patients with HS;however,other cytokines were amplified to a greater degree.28These findings suggest that the involvement of TNF-αin the disease pathogenesis was more minimal than previously expected.28Additionally,the use of adalimumab did not result in significant reduction in TNF-αexpression,supporting the idea that TNF-αwas only minimally elevated and suggesting a greater role of other inflammatory cytokines in disease development.28
Kelly et al.1sought to better understand the role of TNFαin the disease pathogenesis of HS because conflicting information has been published.Eight studies that focused on the role of TNF-αin HS were analyzed,and only six demonstrated increased levels of the cytokine in affected patients.1These findings support the idea that TNF-αis implicated in HS;however,the variability and lack of improvement with anti-TNF-αagents seen in some patients suggests that these findings are likely not consistent across patients.1In addition,these findings suggest that differences in the inflammatory cytokine profile of patients with HS must be considered.1
Interplay among IL-1β,IL-10,IL-22,and IL-20
Much like TNF-α,IL-1βis a proinflammatory cytokine that is elevated in the skin of patients with HS.1,23Mostly produced by monocytes and macrophages,IL-1βis biologically activated in response to a variety of triggers,including bacteria,viruses,and environmental irritants.25Activation occurs via proteolytic cleavage from pro-IL-1β to IL-1βwithin inflammasomes via caspase-1.25
Van Der Zee et al.25demonstrated a 31-fold increase in the IL-1βconcentration in patients with HS.This increase is even greater than that in the skin of patients with psoriasis,leading researchers to hypothesize that overactivation of the inflammasome may be involved in the disease pathogenesis of HS.25This theory lends itself to several therapeutic treatment options,including drugs that affect the IL-1βpathway(eg,colchicine)or biologics and neutralizing monoclonal antibodies against IL-1 receptors or IL-1β,respectively.25Analysis of the current literature concerning IL-1βin patients with HS reveals that the degree of expression of this proinflammatory cytokine in HS-affected skin is even greater than that of TNF-α.24,28These findings suggest a role of both IL-1βand the inflammasome in the immune dysregulation seen in HS.
It is important to note that elevation of IL-Iβis also correlated with IL-10,an anti-inflammatory cytokine.1Expression of IL-10 is induced by IL-1β,and the concentration of this cytokine is correlated with elevated expression of IL-1βin the skin of patients with HS as well.1IL-10 is produced by cells of the innate and adaptive immune systems.1It induces differentiation and propagation of regulatory T cells and suppresses production of various T-helper cells(Th1,Th1,and Th17)involved in inflammation and autoimmunity.1In addition,it selectively inhibits production of the proinflammatory cytokine IL-22.1,27While proinflammatory,IL-22 also has antimicrobial functions and contributes to wound healing and maintenance of epithelial barrier function.1In patients with HS,higher levels of IL-10 mRNA are seen in diseased skin than in healthy skin,which likely contributes to poor wound healing in these patients.1,26Because IL-10 is an anti-inflammatory cytokine,its increased expression in the skin of patients with HS may at first appear to be a defense mechanism against propagation of the disease.However,because of the selective inhibition of IL-22 and its suppressive role toward cells of the immune system,it may actually allow for proliferation of skin commensal microbes and propagation of the inflammatory cascade.1Targeting IL-10 may function as a potential treatment modality.In addition,because the expression of IL-10 is secondarily related to production of proinflammatory markers such as IL-1βand TNF-α,the role of therapeutic targets against these cytokines may indirectly reduce the IL-10 concentration and further decrease disease severity.
Much like IL-22,IL-20 is a proinflammatory cytokine that is increased in the skin of patients with HS.1IL-20 is produced by keratinocytes and DCs and is reciprocally involved in their proliferation.1In addition to its role in keratinocyte proliferation,IL-20 may also be involved in the production of adenosine monophosphates,nucleotides that have been found to play a critical role in the restriction of cutaneous infections.1,29Production of IL-20 is induced by IL-22,which explains their similar expression profile in the disease state.1
IL-17 and IL-23
An association between IL-23 and increased susceptibility to autoinflammation has been clearly defined in several genetic studies.21IL-23 is involved in the development of the T-helper cell subset(Th17)that produces IL-17.IL-17A,colloquially called IL-17,is an IL that stimulates chemokines involved in recruitment of neutrophils,macrophages,and lymphocytes to inflammatory sites.29The significance of IL-17 lies in its inherent ability to promote neutrophil migration and therefore to promote inflammation.22Studies of IL-23-deficient mice have shown that a deficiency of IL-23 correlates with a complete absence of Th17 cells,therefore solidifying the relationship between these markers.21In addition,IL-23-deficient mice were found to be resistant to autoinflammatory conditions,an interesting finding that implicates this pathway in a variety of human diseases.21
In 2011,Schlapbach et al.21demonstrated that IL-23 was overexpressed in patients with HS.The authors found that macrophages infiltrating the dermis of lesioned skin produced the IL-23.21These macrophages were found to have high levels of TLR-2,a TLR known to induce production of large amounts of IL-23 when activated by microbial agents.21Secretion of IL-23 subsequently activates Th17 cells,which leads to the production of inflammatory IL-17.21These findings led investigators to hypothesize that the chronic inflammation of HS may be the result of bacteria that enters lesioned skin,activates TLR-2,and triggers production of proinflammatory cytokines.21The aforementioned IL-23/Th17 pathway has also been implicated in the pathogenesis of noninfectious inflammatory skin diseases such as psoriasis and atopic dermatitis.
A closer look at the role of Th17 cells in HS may provide investigators with more insight into this disease.IL-17,in addition to being produced by Th17 cells,is involved in their development and stimulation.29Growth and stimulation of Th17 cells leads to increased production of inflammatory cytokines,including IL-17 and the aforementioned IL-22.21These cytokines have inflammatory effects on keratinocytes,inducing hyperproliferation and epidermal acanthosis that may very well contribute to the follicular plugging and occlusion that is believed to induce this disease.21In addition,because IL-17 is known for its role in the mobilization of neutrophils to peripheral tissues,this may explain the neutrophil predominance characteristic of primary HS lesions.21Several studies have demonstrated impressive increases in IL-17 in the lesioned skin of patients with HS,with up to a 149-fold increase demonstrated.21,24,29More recent studies have supported these findings and shown an increased number of IL-17-producing cells,most of which were identified as neutrophils via immunolabeling,in the lesions of patients with HS.30,31The presence of such large amounts of IL-17 mRNA suggests a pathologic function of this IL.24
Ultimately,the proinflammatory properties of IL-17 combined with studies revealing enhanced IL-17 gene expression in HS provides a reason for targeting IL-17 and its receptors in treatment.In addition,IL-17 may play a role as a biomarker for monitoring clinical disease.31
IL-12
IL-12 is a proinflammatory cytokine that promotes differentiation of Th1 cells and influences production of proinflammatory IFN-γ.1Before the discovery of the IL-23/Th17 pathway,IL-12 was the cytokine most often implicated in the pathogenesis of autoimmune and inflammatory disease.1The role of IL-12 in HS requires further evaluation because conflicting data have been published.Whereas some studies have demonstrated abundant expression of IL-12 mRNA in lesioned HS skin,others have shown no significant protein expression.1The exact role of IL-12 in the pathogenesis of HS requires more research;however,treatment of patients with the monoclonal antibody ustekinumab,an IL-12/IL-23 inhibitor,has shown promising albeit variable results.32Three patients with significant disease were treated with the ustekinumab.33One patient achieved complete clearance of lesions,the second showed moderate improvement with eventual regression,and the third had no improvement at all.32These results suggest an important link between IL-12/IL-23 and the pathophysiology of HS in a subset of patients;however,the underlying cause(i.e.,IL-12 versus IL-23 versus both)requires further research.32
Type I IFNs
Byrd et al.22attempted to characterize the contributions of type I IFNs to HS.As previously discussed,NETs act as autoantigens that propagate inflammation in HS.In addition,NETs are known to be associated with activation of a type I IFN response in the skin because they have been shown to activate DCs to produce type I IFNs in patients with systemic lupus erythematosus.22This relationship has led investigators to explore whether patients with HS also display a type I IFN gene signature in the skin.22Western blot analysis of homogenized skin lysates taken from HS lesions and control skin showed enhanced IFN-αprotein expression only in lesioned skin,indicating that a type I IFN response may play an important role in HS.22These findings are significant because IFNs amplify both the innate and adaptive immune responses,and therefore,may significantly contribute to the pathogenesis of HS.23Investigators hypothesized that the enhanced NET formation seen in HS may activate DCs to produce type I IFNs in lesioned skin.22This idea is supported by finding netting neutrophils and DCs in the same area in HS-affected skin.22Ultimately,these findings suggest an important role for type I IFNs in the immune dysregulation of HS;however,further experiments are needed to fully understand the role of NETS and type I IFN dysregulation with respect to the clinical manifestations of the disease.
Conclusion and perspectives
HS is a chronic,inflammatory skin condition that can severely disrupt the lives of those it affects.The underlying etiology and pathogenesis of disease development remain unclear.This lack of understanding poses significant diagnostic and therapeutic challenges for clinicians.A better understanding of the factors underlying the onset and severity of HS is needed to optimize disease management.
Genetic mutations and a range of immune aberrations have been documented in both clinical and experimental studies.A closer look at the genetic influences affecting disease development may explain the immune perturbations in HS.In addition,an exploration of the immune cells and inflammatory markers seen in HS can provide researchers and clinicians with a deeper understanding of the disease pathogenesis and may aid in the identification of efficacious therapeutic targets.Although further research and investigation into the factors driving this disease are needed,the current literature provides us with stepping stones toward optimizing disease management and improving patient outcomes.However,literature review of this nature has following limitations:the method in which the research was conducted which required complete reliance on previously published research and the availability of studies using the databases herein discussed.Further limitations exist in the data collection process and the search methodology.Foremost,it is possible to have overlooked and not included literature on the topics considered with the limited keywords that were established.The timeline that was designated poses another limitation,as this could have further restricted the number and scope of articles used.The age of the data used,in particular older studies,could pose a further limitation as outdated information may be susceptible to misclassification or recall.The chances of the review being biased increases with literature reviews of this nature as the study relies on author synthesis and interpretation of previous works and data.
- 國際皮膚性病學雜志的其它文章
- The Power of Molecular Genetics in Understanding Heritable Skin Disorders:Introduction to the Special Theme on Genodermatosis
- Piloleiomyoma
- Eruptive Cutaneous Collagenoma:Report of Two Cases
- Urethral Orifice Genital Herpes Caused by HSV-1 in a Female Patient:A Case Report
- Clinical Manifestations of Adult Langerhans Cell Histiocytosis with Multisystem Involvement Successfully Treated Using Chemotherapy
- The Association of Psoriasis and Obesity:Focusing on IL-17A-Related Immunological Mechanisms

