Enrichment of the less polar ginsenoside(Rg3)from ginseng grown in New Zealand by post-harvest processing and extraction
Wei Chen,Wen-Liang Xu,Dan-Xia Shi,Prabhu Balan,David Popovich*
Abstract Background:Previous studies showed that New Zealand-grown ginseng contains an abundance of ginsenosides and that rare less polar ginsenosides,such as Rg3,exhibit more pharmacological activities than polar ginsenosides,which are the major components of ginseng.Methods:The ginsenoside profile of New Zealand-grown Panax ginseng was manipulated by treatment with acetic acid,sodium hydroxide,pH,and high temperature.The abundance of 23 ginsenosides extracted by different treatments was quantified using high-performance liquid chromatography.Results:Treatment with 0.5 mol/L acetic acid can stimulate the degradation of polar ginsenosides to less polar ginsenosides(5.6%Rg3 was accumulated,P<0.0001).Furthermore,when ginseng root was treated at 121°C for 100 min in a pH 3.0 acetic acid aqueous solution,the majority of the polar ginsenosides were converted into less polar ginsenosides.Specifically,83.46±3.69%(P=0.0360)of the less polar ginsenosides and 41.01±2.39%(P=0.0412)of Rg3 were enriched.In contrast,alkali treatment did not convert the polar ginsenosides into less polar ginsenosides at mild temperature and less conversion was observed compared with acid treatment at high temperature.Conclusion:This is the first attempt to manipulate the ginsenoside profile of New Zealand-grown ginseng.The conditions(high temperature with low pH)may be modified to produce and enrich the less polar ginsenoside fraction(especially Rg3)from the total ginseng extract.
Keywords:New Zealand grown ginseng,Less polar ginsenoside,Ginsenoside Rg3,Ginsenoside transformation
Background
Ginseng is a famous traditional medicinal herb that has been used in Eastern Asia(especially in China,Korea,and Japan)for thousands of years.Ginseng first appeared in theOracle Bone Script(1600-1100 B.C.E.),the earliest known form of Chinese writing engraved on oracle bones,and was later listed in the first Chinese pharmaceutical monograph,Shennong’s Classic of Materia Medica(around 200 C.E.,author unknown)[1].Zhang Zhongjing(150-219 C.E.)described the use of ginseng as an herbal prescription in theTreatise on Cold Pathogenic Diseases[2].Today,ginseng is not just used as a medicinal source,but it is increasingly being incorporated into health care products.It is widely planted,traded,and consumed throughout the world.Ginsenosides,a type of triterpene saponin that is a unique ingredient of the ginseng species,are thought to be the most valuable and main bioactive constituents of ginseng[3].Nearly 200 ginsenosides have been isolated and identified from ginseng plants and related ginseng products[4].Traditionally,ginsenosides are classified into three main groups based on their aglycone backbone:protopanaxadiol(PPD)type,protopanaxatriol(PPT)type,and oleanane-type ginsenosides.According to the polarity of ginsenosides,they may also be divided into two groups:polar ginsenosides and less polar ginsenosides.The major ginsenosides present in raw ginseng are the polar ginsenosides including ginsenosides Rb1,Rb2,Rb3,Rd,Re,Rg1,Rf,and malonyl ginsenosides.The less polar ginsenosides,including ginsenosides Rg3,Rh2,compound K,Rh3,Rh4,Rk1,Rk2,Rk3,Rg5,are rare in raw ginseng products.They are usually found in heat-processed ginseng products including red and black ginseng[5].The most abundant ginsenosides are the polar compounds,which constitute more than 90% of the total ginsenosides in white and red ginseng[6].
However,these major ginsenosides contain multiple sugar moieties with larger molecular structures and cannot be fully absorbed by the human body[7].They exhibit low bioavailability and are not conducive to exerting pharmacological activities[8].The less polar ginsenosides,such as Rg3 with its smaller molecule weight,can easily pass through the intestinal wall,and they exhibit relatively higher biological and pharmacological activities compared with polar ginsenosides[9].For example,the less polar ginsenosides are superior to the polar ginsenosides with respect to anti-tumor activity[10].Moreover,after oral intake of ginseng,digestive enzymes and intestinal bacteria hydrolyze the major polar ginsenosides into the less polar ginsenosides(Rg3,Rh2,compound K),which are more easily absorbed by the human digestive system.However,the concentration of less polar ginsenosides remains low,even after the conversion process[11,12]and may vary between individuals.Therefore,enriching less polar ginsenosides by transforming polar ginsenosides through post-harvest processing may significantly improve the oral bioavailability of ginseng.
Heat-processing is widely used to enrich for less polar ginsenosides.For example,two importantly commercial products of ginseng,red and black ginseng are produced through steam treatment.The amount of the less polar ginsenosides in red ginseng is still very low[5,13],although the concentration of the less polar ginsenosides has been largely improved for black ginseng[5,14].The process of making black ginseng requires nine cycles of steaming and drying,which is complex and time consuming.In addition,other degradation methods have been reported that yield less polar ginsenosides.These methods mainly include high temperature degradation[15],acid hydrolysis[16],alkaline treatment[17],enzymatic conversion[18],and microbial degradation[19].Currently,no robust methods to produce less polar ginsenosides exist because of the degradation that frequently occurs for polar ginsenosides,and these methods are time consuming,costly,and demonstrate a low conversion rate.
In New Zealand(NZ),Panax ginseng(Asian ginseng)andPanax quinquefolium(American ginseng)have been grown as a second crop under pine forests for more than 15 years.Fifty and 43 ginsenosides have been identified from NZ-grown Asian ginseng and NZ-grown American ginseng,respectively[20].Of these,ginsenosides Rb1,Rb2,Rd,Re,Rg1,and the corresponding malonyl ginsenosides account for the majority(more than 80%)[20].Moreover,the average content of the major ginsenosides in NZ is much higher compared with ginseng grown in China and Korea[21].Thus,converting these major ginsenosides into the less polar ginsenosides through post-harvest processing will significantly improve the bioavailability and pharmacological activity of NZ-grown ginseng.In this study,different extraction conditions including acidic extraction,alkali extraction,different pH extraction,and high-temperature extraction were used to manipulate the ginsenoside profiles for enriching less polar ginsenosides in NZ-grown ginseng extract.These studies will provide theoretical support for the application of NZ-grown ginseng in the food and healthcare industries.
Material and methods
Ginseng materials
The roots ofPanax ginseng(Figure 1)were collected in Rotorua,NZ in May 2019 and were identified by Mr.Glen Chen from the Kiwiseng Co.,Ltd.(Rotorua,NZ).The oven-dried ginseng roots were powdered using a CG2B coffee grinder(Sydney,Australia)before extraction.

Figure 1 The whole plant of NZ-forest grown Panax ginseng and its medicinal site(ginseng roots).NZ,New Zealand.
Ginsenoside standards,chemicals,and reagents
Twenty-three reference standards of ginsenosides(Rb1,Rb2,Rb3,Rc,Rd,Re,Rf,Rg1,Rg2,20S-Rg3,20R-Rg3,Rh1,20S-Rh2,20R-Rh2,Rh3,Rh4,Rk2,Rk3,F2,20S-PPD,20R-PPD,20S-PPT,and 20R-PPT)were purchased from Star Ocean Ginseng Co.,Ltd.(Suzhou,China).The purities of all reference standards were no less than 98.0%.High-performance liquid chromatography(HPLC)-grade methanol,and acetonitrile were purchased from Fisher Chemical(Pittsburgh,PA,USA).Water(deionized)was obtained from a Milli-Q Ultra-pure water system(Billerica,MA,USA).Sodium hydroxide(NaOH),acetic acid(HAc),and n-butyl alcohol(n-BuOH)were purchased from Fisher Chemicals(Pittsburgh,PA,USA).The reagents used in this study were of analytical grade.
Preparation of ginseng extracts with acidic treatment
Approximately 1 g ginseng powder was weighed and added to 20 mL 0.5 mol/L HAc aqueous solution.The sample was extracted in an ultrasonic bath at 50°C for 1 h.The supernatant was collected after centrifugation at 4,000 rpm(Thermo Scientific Multifuge 1S-R Centrifuge,Marshall Scientific Co.,Ltd.,Hampton,NH,USA)for 10 min,and the sediments were extracted twice with 20 mL of 0.5 mol/L HAc aqueous solution.The three extracts of the supernatant with the same HAc concentration were mixed and adjusted to a neutral pH by NaOH aqueous solution.Then,the ginsenosides were subjected to liquid-liquid extraction usingn-BuOH[22].Briefly,the aqueous layer was extracted three times with 80 mL water-saturatedn-BuOH.The resultingn-BuOH layer was washed three times with 80 mL distilled water to remove impurities.The remainingn-BuOH solution was transferred to a tared round-bottom flask,and then-BuOH fraction was evaporated at 60 °C using a rotary vacuum evaporator.After evaporation,the concentrated extract was freeze-dried into powder.Another three experiments were designed,and extracts were prepared using 0.25,0.125,and 0 mol/L HAc aqueous solutions,as described above.
Preparation of ginseng extracts with alkali treatment
The ginseng powder was extracted with 0.4,0.2,0.1,and 0 mol/L NaOH aqueous solution as described in above section,except the alkali extraction solution was adjusted to a neutral pH with an HAc aqueous solution before liquid-liquid extraction usingn-BuOH.
Preparation of ginseng extracts with high temperature
Approximately 1 g ginseng powder was weighed and added to 40 mL deionized water in a 100 mL flask tube.The tube was closed by a filtered paper and extracted in an autoclave(121 °C,0.1 Mpa)for 10,20,and 40 min,respectively.The extracts were filtered using a filter paper,and ginsenosides from the supernatant were subject to liquid-liquid extraction usingn-BuOH,as described in above section.
Preparation of ginseng extracts with high temperature under different pH
Approximately 1 g ginseng powder was weighed and added to 40 mL of aqueous solutions with different pH values(pH 3.0,5.0,7.0,9.0,and 11.0)in a 100 mL flask tube.The tube was closed by a filtered paper and extracted in an autoclave(121 °C,0.1 Mpa)for 100 min.The extracts were filtered using a filter paper,and the supernatants were adjusted to a neutral pH with NaOH or HAc aqueous solution.Ginsenosides from the aqueous layer were subject to liquid-liquid extraction usingn-BuOH,as described in above section.
HPLC analysis
The ginseng extracts were prepared at approximately 1 mg/mL in 70% methanol aqueous solution and filtered through a 0.22-mm syringe filter before HPLC injection.The HPLC analysis,including validated calibration curves,was conducted as described previously[23].The HPLC instrument used in this study was a Shimadzu Prominence LC-20A UFLC stack HPLC system(Kyoto,Japan)equipped with a DGU-20A3 degasser,LC-20AD pump,CTO column oven,SPD-20A detector,and SIL-20A autosampler.A Waters Symmetry Shield RP18 column(4.6 mm×250 mm,5 μm,100?)was also used.The mobile phase consisted of deionized water(A)and acetonitrile(B),using a gradient elution of 20% B at 0-15 min,20-31% B at 15-25 min,31-35% B at 25-35 min,35-36% B at 35-40 min,36-39% B at 40-45 min,39-95%B at 45-75 min,95-20%B at 75-85 min,and 20% B for 5 min.The column temperature was set at 25 °C,and the flow rate was 1 mL/min.The sample injection volume was 20 μL,and the detection wavelength was set at 203 nm.
The content of the 23 individual ginsenosides in the various extracts was quantified according to the 23 ginsenoside standard curves.The percentage of ginsenoside was calculated by the following formula:percentage of ginsenoside X=content of ginsenoside X/total ginsenoside content×100%,total ginsenoside content was the sum amount of the 23 quantified ginsenosides.
Statistical analysis
Samples were evaluated three times,and the data are expressed as the mean±standard deviation.One-way analysis of variance test and Sidak’s multiple comparisons test were performed to analyze the experimental data using GraphPad Prism 8 software.Differences were considered statistically significant atP<0.05.
Results
Effects of different treatments on HPLC profiles of ginsenosides of ginseng root extracts
An HPLC-ultraviolet detection method was used to profile the ginsenoside constituents resulting from the various treatments.Twenty-three ginsenoside standards were employed to establish the HPLC method.The ginsenosides were well separated from each other from 20 to 75 min(Figure 2A).The polar ginsenoside peaks appeared between 20 and 45 min,and the less polar ginsenoside peaks were distributed between 50 and 75 min.For the natural ginseng root(untreated,Figure 2B),the abundance of the peaks came from the polar ginsenosides Rg1,Re,Rf,Rb1,Rc,Rb2,Rb3,and Rd.Essentially no less polar ginsenosides were present in the profile.Compared with the untreated ginseng root(Figure 2B),the HPLC profiles of all the treated ginseng root extracts were changed to varying extents(Figures 2C-H).
The profile of ginseng extracted with 0.5 mol/L HAc(Figure 2C)was similar to that of untreated ginseng in which ginsenosides appeared at retention times between 20 and 45 min(polar ginsenosides),but it was different between 50 and 75 min(less polar ginsenosides).Between 50 and 75 min,the peaks of the less polar ginsenosides,especially Rg3,appeared.They were not present in the naturally occurring ginseng root.This indicates that acidic treatment results in less polar ginsenoside transformation,although the abundance of the less polar ginsenosides was very low.When ginseng roots were extracted with 0.4 mol/L NaOH(Figure 2D),the profile was very similar to that of ginseng roots extracted with water between 20 and 45 min as well as between 50 and 75 min.This indicates that alkali treatment did not involve many ginsenoside transformations.When ginseng was extracted at 121 °C for 40 min(Figure 2E),numbers and individual peak areas of the less polar ginsenosides between 50 and 75 min were markedly increased.However,the transformation was not complete,and significant amounts of polar ginsenosides resolving between 20 and 45 min were still present.
Compared with the profile of ginseng extracted at 121 °C for 40 min,when ginseng roots were extracted at 121 °C for 100 min at pH 3.0(Figure 2F),pH 7.0(Figure 2G),and pH 11.0(Figure 2H),the profiles changed to a large extent.The numbers and individual peak areas of the less polar ginsenosides between 50 and 75 min further increased,whereas those of the polar ginsenosides between 20 and 45 min further decreased.In particular,the peaks of the polar ginsenosides Rg1,Re,Rf,Rb1,and Rd disappeared when extracted at 121 °C and pH 3.0,suggesting that the polar ginsenosides degraded completely under these conditions.However,the peaks of these polar ginsenosides emerged,and the peak areas of the polar ginsenosides increased when the pH of the extraction solvent was increased to 7.0 and 11.0.Thus,compared with the neutral extraction solvent,the acidic solvent enhanced polar ginsenoside transformation into less polar ginsenosides,whereas the alkali solvent reduced this transformation.
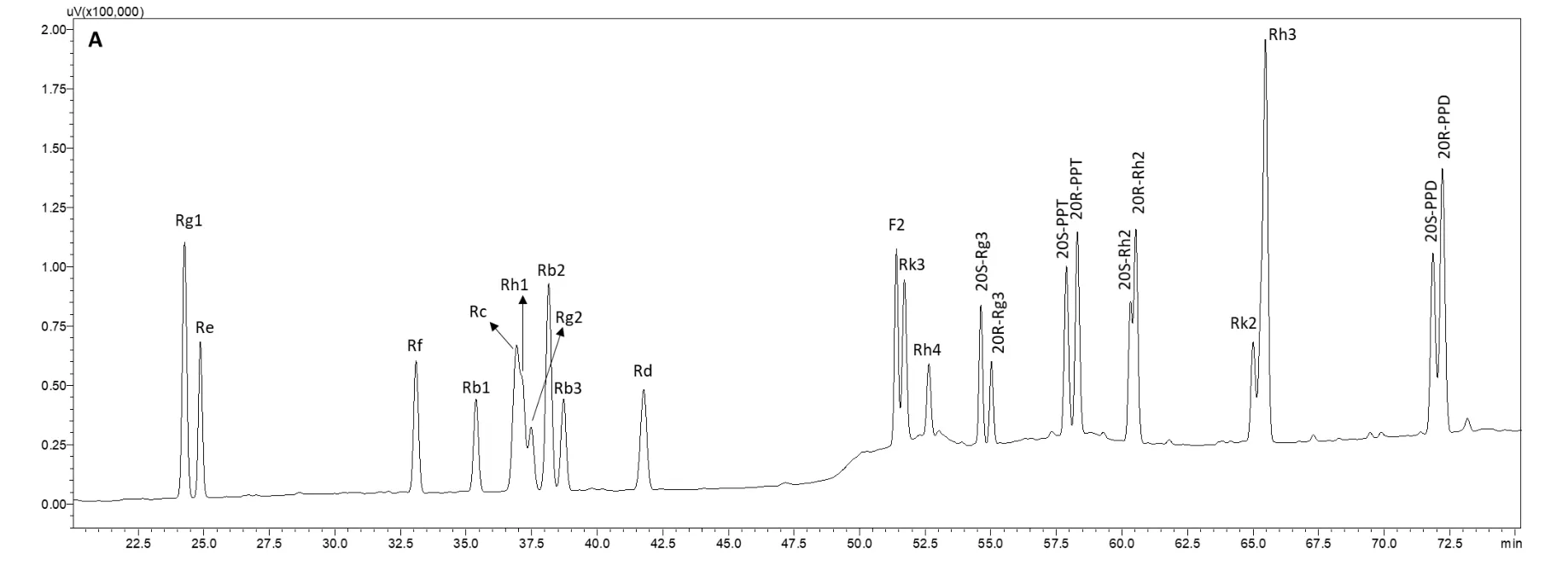
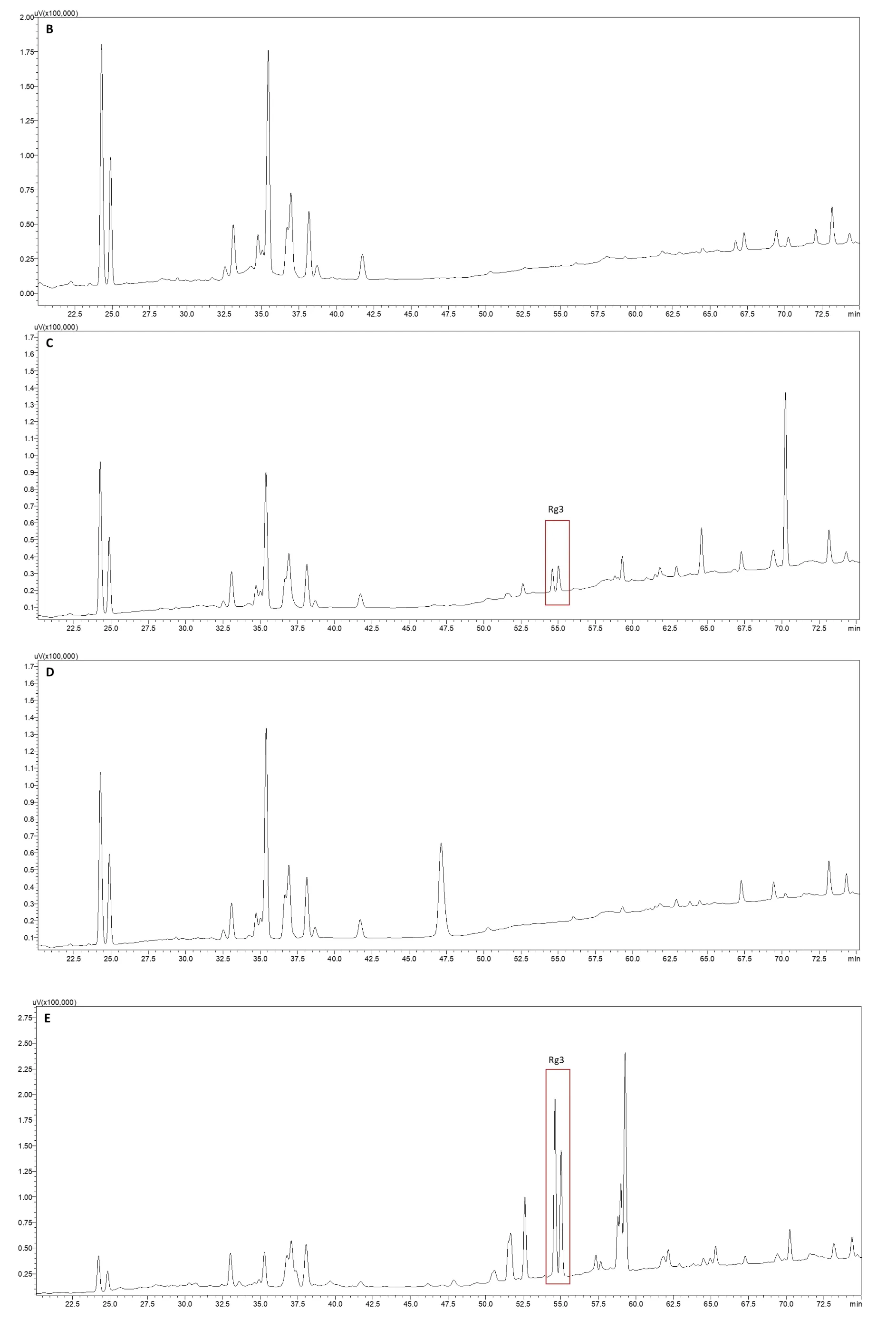
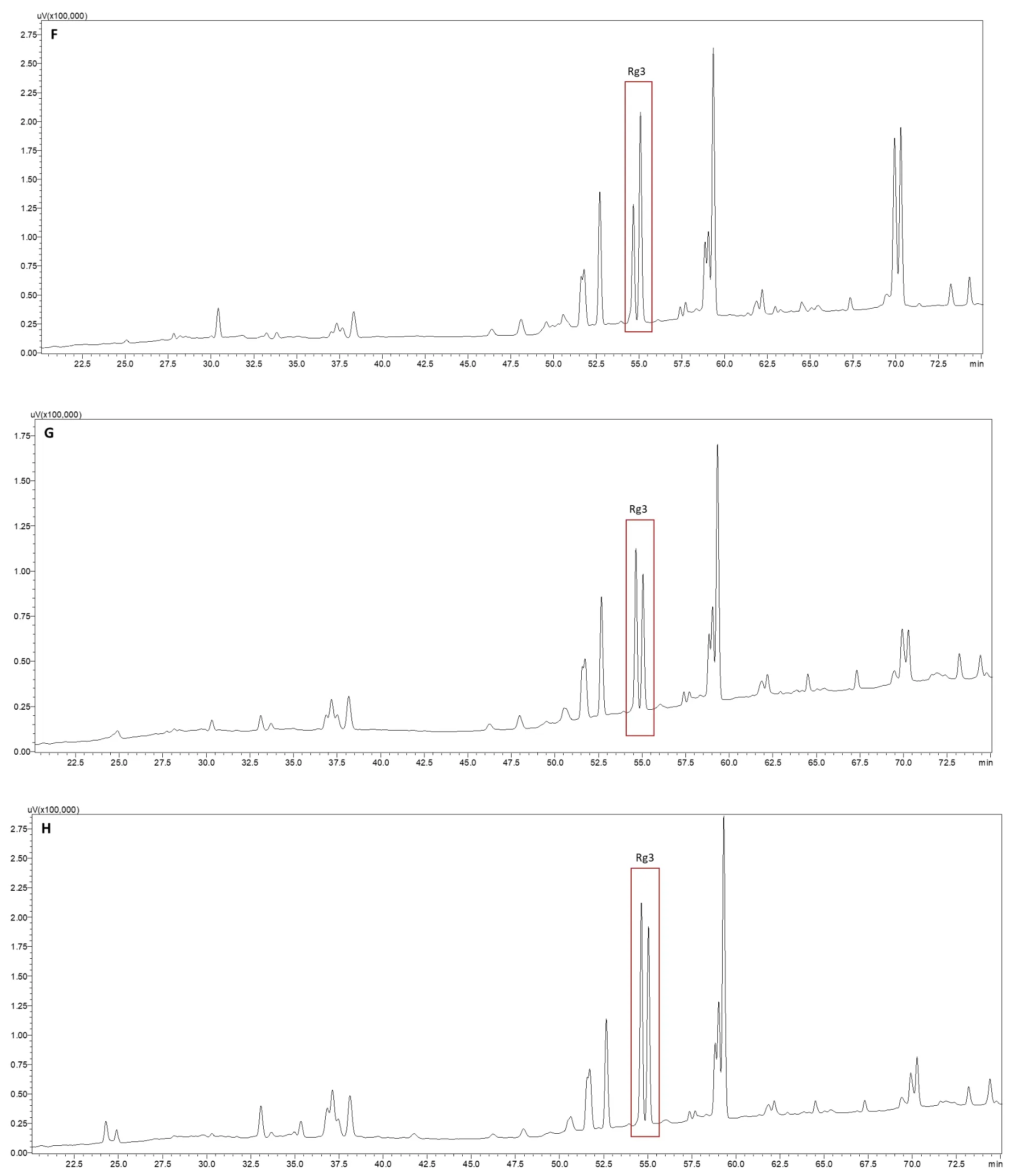
Figure 2 HPLC chromatograms of ginsenoside profiles resulting from different extraction conditions.A,ginsenoside standard mixture;B,ultrasonically extracted with deionized water at 50 °C for 1 h;C,ultrasonically extracted with 0.5 mol/L HAc aqueous solution at 50°C for 1 h;D,ultrasonically extracted with 0.4 mol/L NaOH aqueous solution at 50 °C for 1 h;E,extracted with deionized water at 121 °C(0.1 Mpa)for 40 min;F,extracted with pH 3.0 aqueous solution at 121 °C(0.1 Mpa)for 100 min;G,extracted with pH 7.0 aqueous solution at 121°C(0.1 Mpa)for 100 min;H,extracted with pH 11.0 aqueous solution at 121°C(0.1 Mpa)for 100 min.PPD,protopanaxadiol;PPT,protopanaxatriol;NaOH,sodium hydroxide;HAc,acetic acid.
Effect of ginsenoside conversion under different extraction conditions
To quantify the contents of ginsenosides in different treated extracts,calibration curves of ginsenoside standards were used to calculate each of the treated extracts(the HPLC validation is presented in our previous publication[23]).The contents of individual ginsenosides are provided in the supplementary data(Supplementary Table S1-S4).
To exhibit changes of the constituents during the various treatments,some terms were defined as follows:Sbcd was the sum saponin of ginsenosides Rb1,Rb2,Rb3,Rc,and Rd,referring to the polar PPD type ginsenosides;Segf was the sum saponin of ginsenosides Re,Rg1,and Rf,referring to the polar PPT type ginsenosides;the amount of Rg3 represented the content of 20S-Rg3 plus 20R-Rg3;the polar ginsenoside amount was Sbcd plus Segf;the less polar ginsenoside amount was the sum content of ginsenosides Rg3,F2,Rk3,Rh4,Rh2,Rk2,Rh3,PPT,and PPD.
The effect of acidic treatment on ginsenoside conversion
To explore the effect of acidic treatment on ginsenoside transformation,ginseng root powder was extracted with 0,0.125,0.25,and 0.5 mol/L HAc.As shown in Figure 3A,the percent of polar ginsenosides resulting from acidic extraction significantly(P=0.0023)decreased from 95.14±0.05% to 88.63±0.44%as the concentration of HAc increased from 0 to 0.5 mol/L.Meanwhile,the concentration of Rg3 increased significantly with increased HAc concentration(Figure 3B).No Rg3 was present when water was used for extraction.However,when ginseng was treated and extracted with 0.5 mol/L HAc,the percentage of Rg3 increased to 5.62±0.01%.These results indicate that acidic treatment can convert polar ginsenosides into less polar ginsenosides such as Rg3,and this conversion indicates an acid dose-dependent effect within the range of concentrations tested(0-0.5 mol/L).Although we observed that acid treatment enhances transformation, the acid-stimulating conversion is very low,thus making meeting the needs of industrial production difficult.
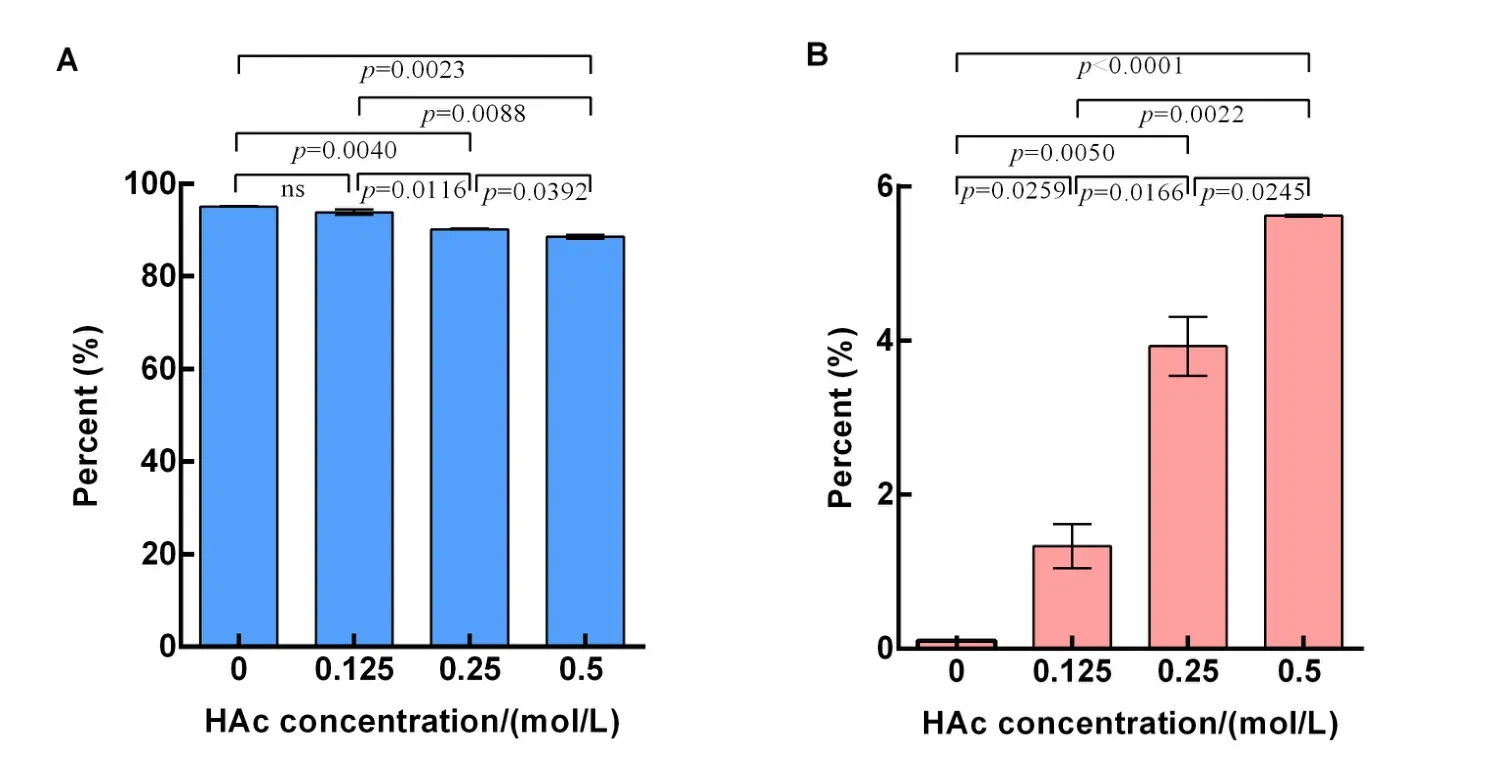
Figure 3 The polar ginsenoside percentage(A)and Rg3 percentage(B)extracted at different HAc concentrations.P<0.05 was considered to be statistically significant;HAc,acetic acid.
The effect of alkali treatment on ginsenoside conversion
Compared with the low conversion induced by acid,the conversion under alkali treatment was worse.Ginseng roots were treated and extracted with different concentrations of NaOH,but no significant change was found in the concentration of polar ginsenosides including Sbcd and Segf(Figure 4).The less polar ginsenosides,such as Rg3,were rare and hard to quantify in the extracts.These results suggest that few polar ginsenosides(neither Sbcd nor Segf)are converted into less polar ginsenosides in alkali solutions.Additionally,a large peak was found at 47 min in the HPLC profile of the alkali-treated ginseng extract(Figure 2D),which was not detected in other treated ginseng extracts.This may be a byproduct generated during NaOH treatment.
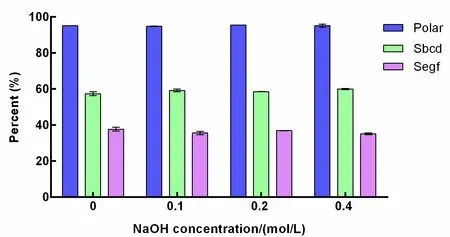
Figure 4 The percentage of polar ginsenosides,Sbcd,and Segf resulting from different NaOH concentration treatments.Sbcd is the sum saponin of ginsenosides Rb1,Rb2,Rb3,Rc,and Rd,referring to the polar PPD type ginsenosides;Segf is the sum saponin of ginsenosides Re,Rg1,and Rf,referring to the polar PPT type ginsenosides;polar refers to the total polar ginsenosides which consists of Sbcd plus Segf.NaOH,sodium hydroxide;PPD,protopanaxadiol;PPT,protopanaxatriol.
The effect of high-temperature extraction on ginsenoside conversion
Compared to the relatively mild extraction temperature with acid or alkali treatment,high-temperature treatment demonstrated a significant effect on the conversion of the polar ginsenosides into less polar ginsenosides.As shown in Figure 5A,no significant change was found in the concentration of Sbcd between a 10-and 20-min extraction.When the extraction was extended to 40 min,the percent of Sbcd decreased significantly from 34.71±0.59%to 25.76±1.44%(P=0.0121).For the polar PPT type ginsenosides,the Segf content decreased significantly with extraction time.Specifically,Segf accounted for 34.16±0.20%of the total saponins extracted at 10 min and was markedly reduced to 21.78±0.22%(P=0.0003)at 20 min and 13.52±0.80%(P=0.0008)at 40 min(Figure 5B).Conversely,the less polar ginsenosides significantly increased as treatment time increased(Figure 5C).The percentage of the less polar ginsenosides at 40 min of treatment(54.41±0.32%)was 2.3 times that at 10 min(23.23±0.38%).Rg3 exhibited a consistent trend within the concentration of less polar ginsenosides during high-temperature treatment.Rg3 accounted for 13.01±0.43% of the ginsenosides after incubating at 121 °C for 10 min,whereas this figure increased significantly by 1.18 times(28.35±0.57%,P=0.0011)when the treatment time was increased to 40 min(Figure 5D).Among the less polar ginsenosides,Rg3 accounted for more than half of the total amount of the less polar ginsenosides(55.8% at 10 min,58.1% at 20 min,and 52.1% at 40 min),which were obtained by degrading from Sbcd.Also,Segf was degraded to form the less polar ginsenosides,Rk3 and Rh4,and the proportion of these reached 16.11±0.51% when the ginseng was incubated at 121°C for 40 min.
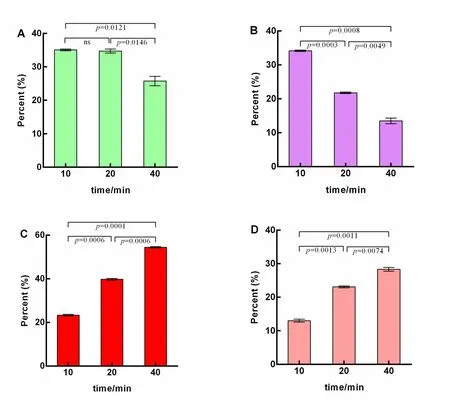
Figure 5 The percentage of Sbcd(A),Segf(B),less polar ginsenosides(C),and Rg3(D)extracted at 121 °C(0.1 MPa)for various times.Sbcd represents the sum saponin of ginsenosides Rb1,Rb2,Rb3,Rc,and Rd,referring to the polar PPD types;Segf was the sum saponin of ginsenosides Re,Rg1,and Rf,referring to the polar PPT types;the amount of Rg3 represents the content of 20S-Rg3 plus 20R-Rg3;the less polar ginsenoside includes the content of ginsenosides Rg3,F2,Rk3,Rh4,Rh2,Rk2,Rh3,PPT,and PPD;P<0.05 was considered to be statistically significant.PPD,protopanaxadiol;PPT,protopanaxatriol.
The effect of pH on ginsenoside conversion at high temperature
The above results indicate that both acid treatment and high temperature can convert polar ginsenosides into less polar ginsenosides to some extent.However,the effect of different pH levels on the conversion of ginsenosides during high-temperature extraction conditions is unknown.Therefore,ginseng roots were incubated with different pH solutions at 121°C for 100 min.The results are shown in Figure 6.
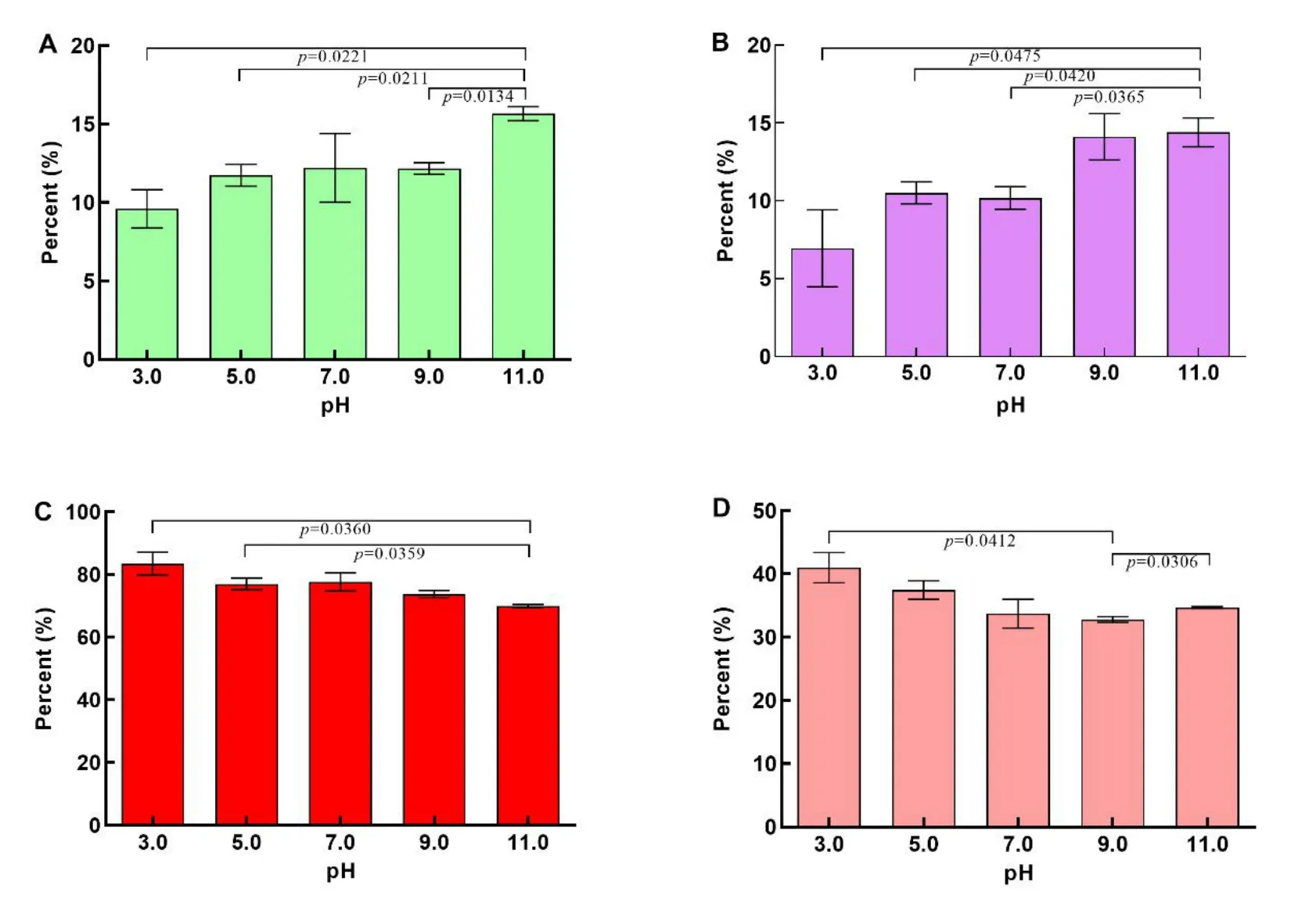
Figure 6 The percentage of Sbcd(A),Segf(B),less polar ginsenosides(C),and Rg3(D)extracted at 121 °C(0.1 Mpa)for 100 min at different pH values.Sbcd is the sum saponin of ginsenosides Rb1,Rb2,Rb3,Rc,and Rd,referring to the polar PPD type ginsenosides;Segf is the sum saponin of ginsenosides Re,Rg1,and Rf,referring to the polar PPT type ginsenosides;the amount of Rg3 represents the amount of 20S-Rg3 plus 20R-Rg3;the less polar ginsenoside amount was the sum of ginsenosides Rg3,F2,Rk3,Rh4,Rh2,Rk2,Rh3,PPT,and PPD;P<0.05 was considered to be statistically significant.PPD,protopanaxadiol;PPT,protopanaxatriol.
When ginseng was incubated with a neutral solution(pH 7.0)at 121°C for 100 min,the polar ginsenosides Sbcd(12.20±2.20%)and Segf(10.18±0.72%)were further degraded to form more less polar ginsenosides(77.625±2.92%),including Rg3(33.74±2.29%),compared with ginseng treated at 121 °C for 40 min.As the pH level of the extraction solution increased,the concentration of polar ginsenosides(Sbcd and Segf)increased,whereas the concentration of less polar ginsenosides(including Rg3)decreased.However,no significant changes were found in the proportions of Sbcd,the less polar ginsenosides,and Rg3 under acidic extraction conditions(pH 3.0 and pH 5.0)or in basic conditions(pH 9.0 and pH 11.0)compared with neutral conditions(pH 7.0).These figures show significant differences between ginseng extracted in a pH 3.0 solution and ginseng extracted in a pH 11.0 solution.For example,more than 80%of the less polar ginsenosides were obtained through degradation of polar ginsenosides at pH 3.0,whereas approximately 70% of the less polar ginsenosides were generated from a pH 11.0 extraction solution.These results suggest that high-temperature extraction is an effective method to convert polar ginsenosides into less polar ginsenosides.Moreover,a certain acidic extraction solvent can promote this conversion,whereas an overly basic extraction condition results in the opposite effect.
Discussion
Acid and alkali treatment are the two most common methods for degrading large molecules.Major polar ginsenosides can be converted into rare less polar ginsenosides under acidic conditions using formic,acetic,citric,lactic,tartaric,and hydrochloric acids[24].In one study,the polar PPD type ginsenosides,Rb1,Rb2,and Rc,were incubated at 60°C in different acids including acetic acid,citric acid,lactic acid,tartaric acid,and hydrochloric acid.The hydrochloric acid increased the yield of ginsenosides Rg3 and△20-ginsenosides Rg3 compared with lactic acid and citric acid.Additionally,the transformation of ginsenosides to the△20-ginsenoside,Rg3,by 1% acid was significantly greater than that using a 0.1% acid concentration[25].Another study tested different concentrations of formic acid(0.01%,0.1%,0.5%,2%,and 5%)and found that ginsenosides cannot be converted under neutral conditions,but less polar ginsenosides could be produced under strong acidic conditions[26].Less polar PPT type ginsenosides,including prosapogenin of Re and Rg1,were obtained when polar PPT type ginsenosides,Re and Rg1,were treated with 0.1N HCL at 37 °C for 120 min[27].Consistent with these results,we found that the less polar ginsenosides,particularly Rg3,was not detected in then-BuOH extract from deionized water treatment,whereas the amount increased significantly at increased HAc concentrations.However,the yield of Rg3 was not high even when treated with 0.5 mol/L HAc for 3 h(3 times,each 1 h).This may result from an insufficient treatment time or mild temperature.As reported in the literature,pH,time,and temperature are the primary factors that control the conversion of ginsenosides under acid conditions[25].In an acidic environment,the polar ginsenosides are converted into less polar ginsenosides by losing sugars at the C3,C6,and C20 positions.Additionally,these ginsenosides specifically lose a sugar at the C20 position to form the Rg3 ginsenoside(Figure 7).
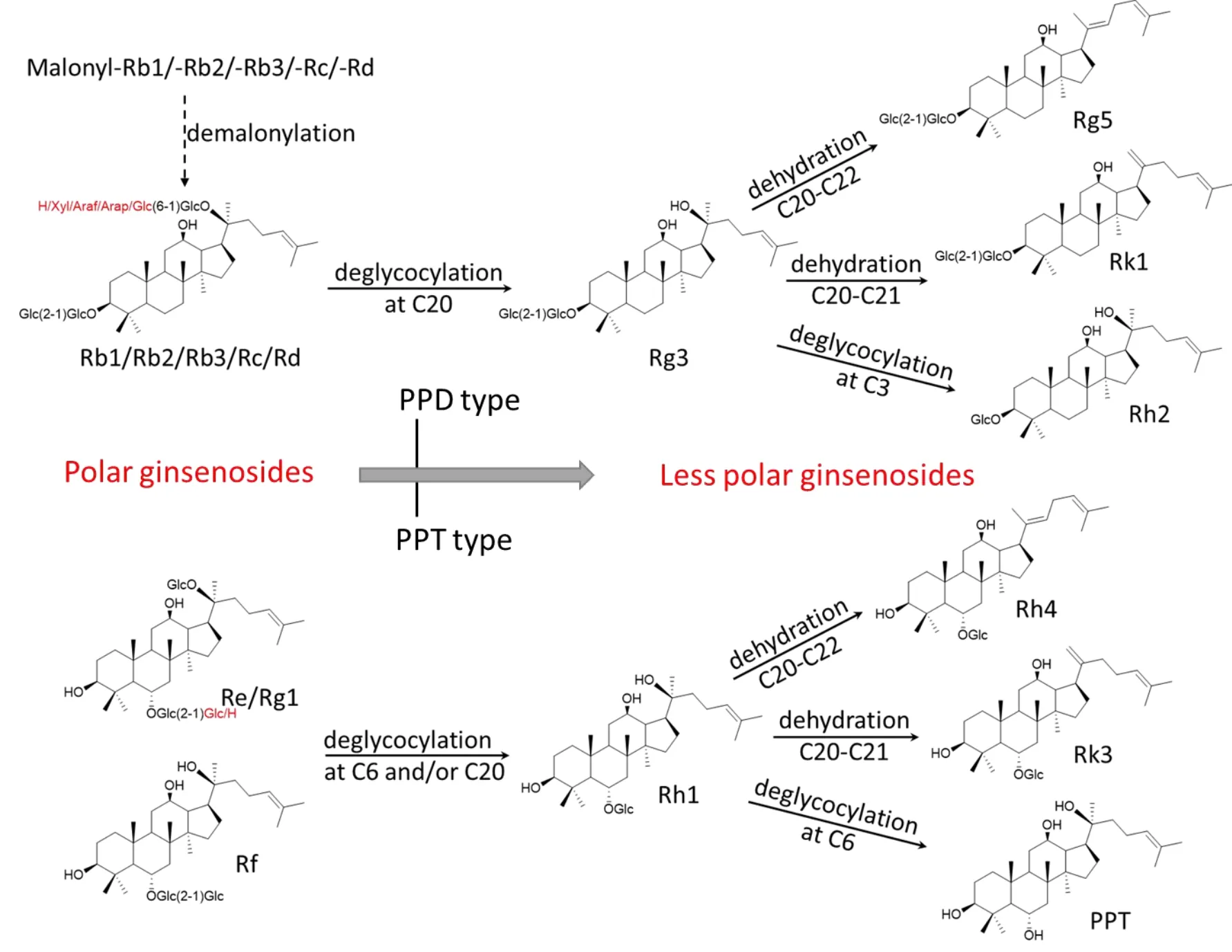
Figure 7 The proposed mechanism of polar ginsenosides converting into less polar ginsenosides at high temperature.PPD,protopanaxadiol;PPT,protopanaxatriol.
Researchers reported that ginsenosides can also degrade into less polar ginsenosides under alkali conditions.A new compound(ginsenoside Rh19)and several rare ginsenosides were isolated and identified from an ethyl acetate extraction after the total saponins were incubated in a boiling water bath with 2 mol/L NaOH aqueous solution for 8 h[28].Compared to the aqueous solution,the use of organic solutions,such as glycerol and isoamyl alcohol,resulted in a higher yield during the alkaline hydrolysis of ginsenosides.When isoamyl alcohol was used as the solvent,refluxing for 24 h at normal pressure produced a high yield of less polar PPD from NaOH hydrolysis.However,the yield was reduced when the reaction time was longer than 32 h in isoamyl alcohol[29].Another study used glycerol as the solvent and explored the factors affecting the alkali hydrolysis of ginsenosides.The results showed that the amount of NaOH,reaction time,reaction temperature,and the volume of glycerol were important factors for ginsenoside degradation.The optimal degradation conditions included the weight of NaOH two times that of the total saponins,the ratio of glycerol and total saponins at 15 mL/g,and incubation at 235 °C for 200 min[30].In a subsequent study,the total saponins from the stem and leaf ofPanax quinquefoliumwere converted into new compounds and rare ginsenosides in a 10% NaOH glycerol solution when incubated at 235 °C for 2 h[31].The literature indicates that compared with acid hydrolysis,high temperatures can be used to hydrolyze ginsenosides under alkalic conditions.The less polar ginsenosides were not detected at relatively high temperatures in this study.This suggests that ginsenoside conversion is more difficult to achieve under alkaline conditions compared with acidic conditions.This may be related to the presence of large amounts of acidic ginsenosides,such as malonyl ginsenosides.
Compared with acidic and alkali treatments,heat treatment is widely used for manipulating the constituents of ginseng and has been for thousands of years.For example,steaming is a conventional method used to produce red and black ginseng.Raw ginseng is steamed at 95-100°C for 2-3 h to produce red ginseng and steamed at 95-100 °C nine times(each for 2-3 h)to produce black ginseng[32].Many less polar ginsenosides including Rg3,Rk1,and Rg5,which are absent from raw ginseng,accumulate in black ginseng.Furthermore,these less polar ginsenosides exhibit various anti-diabetic, anti-inflammation, and anti-cancer properties[33-35].However,a relatively high amount of polar ginsenosides still is found(24.1 mg/g accounts for about 33% of the total saponins)in black ginseng[32].The polar ginsenosides can be further transformed,and the less polar ginsenosides can be further enriched when ginseng is treated at a temperature over 100 °C.One study showed that steaming raw ginseng at 120 °C for 2 h significantly increased the radical-scavenging activity.Ginsenosides Rg3 and Rg5,which are not present in raw ginseng,are the most abundant ginsenosides in ginseng steamed at 120 °C,accounting for 39% and 19% of all ginsenosides,respectively[15].In our study,the profile of ginsenosides changed remarkably when ginseng root was heated at 121 °C in an autoclave.Although the less polar ginsenosides increased significantly during the first 20 min,especially Rg3(which increased to 24%of the total ginsenosides after steaming for 20 min),the concentration of Sbcd did not noticeably decrease during the first 20 min.Our previous study found that NZ-grown ginseng contains an abundance of malonyl ginseng[21],and these malonyl ginsenosides(mal-Rb1,-Rb2,-Rb3,-Rc,-Rd)may degrade to corresponding neutral ginsenosides(Rb1,Rb2,Rb3,Rc,Rd)upon high temperature treatment[36].This likely occurs because some malonyl ginsenosides are converted into the corresponding neutral ginsenoside,Sbcd,which compensates for the reduction of Sbcd degrading to Rg3(Figure 7).
The results described above demonstrate that acidic conditions and high temperature promote the conversion of polar ginsenosides to less polar ginsenosides.Moreover,the transformation may be further enhanced if the ginseng material is treated at a high temperature coupled with acidic conditions.In a study by Liu et al,ginsenosides Rb1 and Re were steamed at 120 °C at different pH 4.0 to 7.0 for 4 h.The content of Rb1 and Re decreased significantly and almost disappeared completely after steaming for 2 h under acidic conditions(pH 4.0-6.0)[37].This result agrees with our findings that the polar ginsenosides,Sbcd and Segf,are converted to less polar ginsenosides at 121 °C under acidic conditions(pH 3.0-5.0)compared with neutral conditions(pH 7.0).In contrast,researchers reported that the content of ginsenosides Rb1 and Re remained almost constant under neutral conditions even after steaming at 120 °C for 4 h[37].This may have occurred because during the steaming process of ginseng extract,some of the thermally unstable acidic ginsenosides are degraded to neutral ginsenosides and produce small organic acids,such as malonic acid or acetic acid,which in turn,promote the further conversion of neutral polar ginsenosides to less polar ginsenosides.The organic acids cannot be produced when the neutral polar ginsenosides are steamed alone.
Additionally,the polar saponins not only undergo deglycosylation at C20 to form the less polar ginsenosides Rg3 and Rh1,but they can also further dehydrate to form double bonds between C20 and C21 or C22.A large amount of Rk3 and Rh4,which are the products of Rh1 after losing an H2O at C20-21 and C20-22,are present in the extract after high-temperature treatment.Ginsenoside Rg3,Rg5 and Rk1 are the major components resulting from high temperature-treated ginseng products[5,38].They may have also been enriched in our study,and the peaks between 57.5 and 60 min may represent ginsenosides Rk1 and Rg5 according to their retention times and polarities[37,39],though they were not quantified because of a lack of reference standards.Conversely,the less polar ginsenosides,Rg3 and Rh1,continued to lose glucose at positions C3 and C6 to form ginsenoside Rh2 and PPT,respectively,except for the formation of double-bonded saponins through dehydration(Figure 7).However,small amounts of ginseng Rh2 and PPT were identified in the extract.In contrast,the concentrations of Rk3 and Rh4 were relatively large.The results suggest that the glucosidic bonds of C3(PPD type ginsenosides)and C6(PPT type ginsenosides)are stable for ginsenosides Rg3 and Rh1,and Rg3 is likely first to lose an H2O at the C20 position to form Rg5 and Rk1,rather than losing a glucose at the C3 position to form Rh2 at high temperatures.Similarly,ginsenoside Rh1 is more likely to dehydrate at the C20 position to produce Rk3 and Rh4,instead of undergoing deglycosylation at the C6 position to obtain ginsenoside PPT at high temperatures.
In addition to the transformation at low pH,we also explored the transformation of ginsenosides at high pH and high temperature.Although the polar ginsenosides could be converted into less polar ginsenosides at 121 °C under alkali conditions(pH 11.0),the efficiency was significantly lower than that under acidic conditions(pH 3.0).The conversion efficiency of Segf ginsenosides under alkaline conditions was also lower than that observed under neutral conditions(pH 7.0).This suggests that alkali exhibits a negative effect on polar ginsenoside conversion.However,some publications reported that alkali conditions may enhance the transformation of ginsenosides at high temperature[17,28,29].This transformation is probably caused primarily by high temperatures,rather than by alkali conditions,according to our results.
Conclusion
This study investigated the effects of various treatments(e.g.,acidic treatment,alkali treatment,pH treatment,and high temperature treatment)on the enrichment of less polar ginsenosides from NZ-grown ginseng.Our results show that acidic treatment stimulates the degradation of polar ginsenosides into less polar ginsenosides.Furthermore,the polar ginsenosides can be almost completely converted into less polar ginsenosides under low pH conditions at high temperature.In contrast,alkali treatment may have a negative effect on this conversion.This is the first attempt to manipulate the ginsenoside profile of NZ-grown ginseng and these conditions(high temperature with low pH)may be applied to produce and enrich for less polar ginsenosides(especially Rg3)from raw ginseng.
 Traditional Medicine Research2021年4期
Traditional Medicine Research2021年4期
- Traditional Medicine Research的其它文章
- Annual advances of integrative pharmacology in 2020
- Pharmacological research progress of ursolic acid for the treatment of liver diseases
- System pharmacology-based dissection of the potential effective material basis and mechanism of Xiaoxianxiong decoction for type 2 diabetes mellitus
- Network pharmacology-based approach to investigate the mechanism of Huang-Lian-Jie-Du-Decoction for treatment of type 2 diabetes mellitus
- Traditional Chinese medicine of Salvia miltiorrhiza Bunge:a review of phytochemistry,pharmacology and pharmacokinetics
- Study on possible synergistic anticancer cachexia effects of the main active compounds of Radix Sophorae flavescentis
