Periodontal bioengineering with mesenchymal stem cells
Nerea Sanchez, Mariano Sanz
Etiology and Therapy of Periodontal and Peri-implant Diseases Research Group, Faculty of Odontology, Complutense University of Madrid, University Complutense, Madrid 28040, Spain.
Abstract Current periodontal regenerative therapies aim at restitution ad integrum of the periodontal attachment apparatus, which involves periodontal ligament, root cementum, and alveolar bone. Guided tissue regeneration, bioactive agents and bone replacement grafts have been utilized in an attempt to fully restore the lost periodontal tissues. But their predictability has been limited and dependent on patient- and defect-related factors. Consequently, the treatment of most periodontal defects still lacks satisfactory and predictable outcomes. Cell therapies, based on the use of mesenchymal stem cells (MSCs), represent a promising therapeutic strategy in light of recently available published preclinical investigations and clinical studies. The application of MSCs in humans is being performed by two different strategies: (1) the ex vivo culture of undifferentiated MSCs from autologous or allogeneic sources, subjected to specific cell expansion and characterization/differentiation tests to obtain the required cell counts for transplantation; and (2) the use of autologous tissue grafts and micrografts, which apart from MSCs, contain other biologically active cell populations and their extracellular matrix. This review evaluates the current status of MSCs therapy applied for periodontal regeneration, describing not only their mechanism of action, but also their efficacy and safety according to the published evidence.
Keywords: Periodontal regeneration, mesenchymal stem cells, cell therapy, tissue engineering
INTRODUCTION
Periodontitis is a chronic multifactorial inflammatory disease associated with dysbiotic plaque biofilms and characterized by progressive destruction of the tooth-supporting apparatus (periodontal ligament, cementum, and alveolar bone) induced by the alteration of the homeostasis between the subgingival microbiota and the host immune response[1].
According to a systematic review with data from 37 countries, aimed at consolidating the epidemiologic records about the most destructive forms of periodontal disease, severe periodontitis was the sixth most prevalent medical condition in world, with a prevalence of 11.2%. In addition, it shows a significant increase with age and a peak incidence at 38 years old[2]. Severe periodontitis is widely distributed throughout the world and remains a public health concern[3]. Besides, periodontitis is associated with several systemic diseases and conditions[4,5]and, therefore, periodontal treatment would not only arrest periodontal inflammation and allow recovery of oral health, but it may also influence the course of these associated systemic diseases, or at least, in promoting a relevant improvement in biomarkers associated with them[6-9]. Therefore, the control of the disease may influence the long-term general health of periodontitis patients[3].
Conventional therapeutic strategies for periodontitis have focused on the infection control of the disease and the arrest of chronic inflammation, but not on the restoration of the injured tooth supporting tissues[10]. However, many periodontal regenerative therapies have aimed to reconstruct these tissues[11]by the formation of a new connective tissue attachment with well-oriented collagen fibres attached to the newformed root cementum[12]. Since the 1970s, multiple investigations have evaluated different agents and biomaterials that would promote cells from the periodontal ligament (PDL) cells, as the only cell population capable of repopulate the affected root surface, and thus regenerating the lost periodontal attachment[13]These technologies were based on the application of barrier membranes, bone replacement grafts, biologically active agents, and combinations of them; although, not all these strategies have provided the same level of evidence with regards to the efficacy in creating a new connective tissue attachment[14].
Recent systematic reviews of randomized controlled clinical trials have reported that strategies based on guided tissue regeneration (GTR) and the use of biologically active agents, in particular, enamel matrix derivatives (EMD), when used in the treatment of intrabony and furcation defects, provided an added clinical benefit of 1.43 mm (0.76-2.22 mm) and 1.27 mm (0.79-1.74 mm), respectively, in terms of clinical attachment level (CAL) gains when compared to an open flap debridement (OFD) alone[15,16]. Furthermore, the data shows that the addition of a bone replacement graft may improve in some defect configurations, the results obtained with the application of either GTR or EMD[15,16]. Conversely, the use of strategies with a minimally invasive surgical approach like the minimally invasive surgical technique (MIST)[17], the modified MIST technique (M-MIST)[18,19], or the single-flap approach[20]have allowed maximizing CAL gain with a significant decrease of postoperative gingival recession[21]. In fact, the importance of microsurgical techniques with minimal flap raising and enhancement of primary wound closure and space maintenance could be greater than even the regeneration material employed. A randomized controlled clinical study, in which the M-MIST technique with and without regeneration material was analyzed in intrabony defects, no statistically significant differences were observed between both groups in terms of clinical and radiographic variables[22].
These reported benefits from current biomaterials and MISTs, however, are restricted to a specific defect anatomy, mainly deep intrabony defects and mandibular degree II furcations[17,18,23-26]. Most periodontal lesions, such as supracrestal defects, class III furcation involvement lesions, or wide 1-wall intrabony defects still do not have a predictable outcome with standard regenerative technologies[27,28]. For this reason, new tissue engineering strategies are being investigated in order to overcome the limitations of current regenerative technologies. The growing evidence in the field of cell therapy research is stimulating the use of live cells in periodontal regenerative medicine.
The aim of this review is to summarize the current status of cell therapy in periodontal regeneration, presenting the particular characteristics and potentials of stem cells and the most recent evidence regarding the application of different modalities of cell therapy for the regeneration of the tooth-supporting structures both in the preclinical and in the clinical field.
WHAT IS CELL THERAPY?
Over the last few decades, the concept of cell therapy, as a new technology for the regeneration of organs and tissues, has been introduced in regenerative therapies in multiple areas of medicine, such as cardiology and traumatology, among others[29-31]. Its objective is to restore the function of damaged tissues and organs by the use of diverse cell populations such as resident stem cells, multipotent adult cells, or embryonic stem cells[32]which have proliferative and paracrine potential to promote the target tissue regeneration. These cell therapies usually include the use of three-dimensional matrices or “scaffolds” that guide cell growth and act as vehicles for the release of cells or bioactive molecules, thus supporting and enhancing their tissueinductive properties[33].
“Stem cells” are the cells with the greatest proliferation capabilities since they are the most undifferentiated progenitors. They are characterized by self-renewal capacity, potential for indefinite proliferation, and capacity for differentiation into several cell lineages. Traditionally, two categories of stem cells have been described according to their origin and differentiation capacities: embryonic stem cells, which are pluripotent cells (able to differentiated to cells from the three embryonic lineages), and adult or postnatal stem cells, which are multipotent (capacity to differentiate into cells from their same embryonic layer)[34,35].
Adult stem cells are located in most tissues and organs since they have important roles in tissue homeostasis and repair[35]. The use of these cells lacks the ethical and legal issues associated with embryonic stem cells and they have not been associated with tumor formation when transplantedin vivo. These facts have made this cell population as the preferred source of cells for current cell-based therapeutics[36,37]. Hematopoietic stem cells, have been employed during decades as therapeutic tool in bone marrow transplantations[38]. Mesenchymal stem cells or “mesenchymal stromal cells” (MSCs), were isolated in 1970s, from bone marrow aspirations, and were defined as a subpopulation of plastic-adherent fibroblast-like cells, that formed singlecell colonies under culture “colony-forming units fibroblasts”[39]. Several years later, these multipotent MSCs have become well characterized and their source is not only derived from bone marrow, but also from many other adult tissues, many from intraoral sources[39-43]. Among the extraoral tissues for collecting MSCs, the umbilical cord and the adipose tissue have been extensively used[44,45]. As for the intraoral sources, MSCs have been isolated from the periodontal ligament, the pulp from temporary and permanent teeth, the gingival connective tissue, the alveolar bone, the apical papilla, or the dental follicle[40-43,46-48].
Since the firstex vivopreclinical studies with MSCs in the 1970s, many publications have investigated the benefits of “putative” MSCs transplantation into animals and humans in several fields of medicine[49-52]. However, it was not until 2006, that the International Society for Cell Therapy established the minimal criteria for defining human multipotent MSCs forin vitroand preclinical investigations[53]. Thus, a general consensus worldwide about the characteristics of MSCs would allow comparing studies from many different research groups. According to that consensus, the requisites for being considered as MSCs were: (1) plasticadherence under standard culture conditions; (2) expression of specific mesenchymal phenotype markers by at least 95% of the MSCs population (CD90+, CD73+, CD105+), as measured by flow cytometry, and absence (≤ 2% expression) of CD34, CD45, CD45, CD79α or CD19, CD14 or CD11b and HLA-DR class II; and (3) differentiation potential to osteoblasts, adipocytes, and chondrocytes underin vitroculture,demonstrated by cell staining[53]. In addition, as the ultimate goal ofex vivoMSCs expansion is human transplantation, the biosafety of the cells in the clinical setting must be ensured through the previous analysis of their genomic stability and confirmation of the lack of tumorigenic potential in small animal models[37].
MESENCHYMAL STEM CELLS IN REGENERATIVE MEDICINE
MSCs-based strategies for regenerative therapies aim at stimulating the biological processes that lead to tissue regeneration through the privileged potential of undifferentiated progenitors[54]. Several pathways have been reported to describe the mechanisms by which MSCs exert their therapeutic effects.In vivostudies have demonstrated the capacity of MSCs to differentiate to cells from the damaged receptor tissue[55,56]; in fact, histological evidence has showed that green fluorescence protein-labeled MSCs, were present within the new-formed tissues several weeks after their transplantation[57,58]. Other proposed mechanisms of the action of MSCs is based through their paracrine effects, that is, the release of soluble bioactive molecules and signals that influence the host’s immune response and enhance the proliferation and differentiation of resident progenitor cells[59]. This mechanism based on the promotion of the transplanted cells to assist in the tissue self-repair potential have also been tested using the “conditioned medium”, that is, the medium where the cells have been cultured rather than transplanting the cells[60-62]. With this strategy, growth factors and other bioactive molecules that are released during cell expansion continue their activity when applied directly on the target tissues to regenerate by enhancing the cellular responses in the injured area in a similar way, but lacking the issues associated with the transplantation of living cells[60,61,63].
It has long been believed that the tissue source where the cells are isolated would predispose the cell lineage of MSCs differentiation[64]. However, the evidence supporting that cells epigenetics is regulated by the tissue of origin is limited[65]. On the contrary, the property termed “cell plasticity” as the capacity of demonstrating potential to extend beyond the differentiated cell phenotypes of their resident tissue has been ascribed for MSCs[66], which then may be considered as pluripotent cells[67]. Several investigations have evaluated this potential studying and whether these transdifferentiated cells are really able to perform identical functions as the cells of the tissue towards which they have been differentiated[68]. The first protocols differentiating BMSCs into neurons for nervous system repair were described by Woodburyet al.[69]. Subsequently, other investigations have applied epigenetic modifiers and neuronal induction signals being able to differentiate MSCs into neural-like cells[70], although the functionality of these cells is not completely elucidated[71]. MSCs also exhibit important immunomodulatory properties, since they reduce the proliferation of T helper lymphocytes and suppress the proliferation of activated T lymphocytes, B cells, “natural killers” cells, dendritic cells, and neutrophils[72,73], thus decreasing the production of proinflammatory cytokines. This property has allowed the use of cell therapies based on allogeneic transplantation of MSCs and also the development of cell therapies for autoimmune diseases[34,49,74,75]. Furthermore, MSCs have showed the capacity for homing into sites of injury after systemic infusion, a property orchestrated by certain chemotactic cytokines and integrins, that make MSCs interesting candidates for various medical disciplines[51].
Another advantage of MSCs-based regenerative technologies is their minimally invasive isolation and lower morbidity in comparison with standard regenerative therapies. This is particularly applicable to their use in the oral and maxillofacial area where the treatment of alveolar ridge atrophies requires oral rehabilitation with dental implants[76]. In these clinical situations, the gold standard therapy is the autologous bone graft, which not only entails a great morbidity, but also has limitations with respect to the bone availability of the donor site[77,78]. Besides a lower morbidity, the use of MSCs would also improve the perspectives regarding graft availability, as the number of cell counts may be controlled duringex vivoexpansion[37].
APPLICATIONS OF MESENCHYMAL STEM CELLS FOR PERIODONTAL REGENERATION
During the last decades, several attempts based on the use of stem cells for periodontal bioengineering have been published. They have been based on the application of cells with proliferative and paracrine potentials, in combination with three-dimensional matrixes, with the objective of maintaining the blood clot stability and the space for the regeneration, and at the same time, stimulating the regenerative process, thus resulting in improved clinical outcomes when compared with standard treatments[79]. These attempts have evaluated different modalities of MSCs therapy[80-84], some transplanting undifferentiatedex vivoexpanded MSCs into the periodontal defect and others transplanting tissue micro-grafts with potentiality to contain MSCs, but without anyex vivoprocessing and cell culturing[85].
Use of undifferentiated ex vivo expanded MSCs for periodontal regeneration
Isolation andex vivoexpansion of undifferentiated MSCs have been the most common strategy for cell therapy applied in periodontal regeneration and tested in preclinical studies[54,86,87]. Although there are multiple variations depending on the specific methods used by each research group, the standard isolation protocol for MSCs is based on the aspiration [bone marrow mesenchymal stem cells (BMSCs) or adipose tissue-derived MSCs (ADSCs)][45,46], surgical harvesting [gingiva-derived MSCs (GMSCs)][37,40], tooth extraction and root scraping [periodontal ligament (PDL-MSCs), dental follicle (DFCs), and apical papilla MSCs (SCAPs)][41,48,88], or collection of the pulp tissue [dental pulp stem cells from permanent (DPSCs) or exfoliated deciduous teeth (SHEDs)][42,43]. After isolation, solid samples are fragmented and digested in enzymatic solutions, usually type I collagenase (3 mg/mL) and dispase (4 mg/mL) for 30-60 min at 37 °C[40,41,88]. Then, the suspension is centrifuged and filtered to obtain single cell suspensions that are seeded in tissue flaks with culture media, usually α-modified Eagle’s minimal essential medium (α-MEM) or Dulbecco’s modified minimum essential medium/nutrient mixture F-12 (DMEM/F12), containing 10% fetal calf serum, 2 mM L-glutamine and 100 U/mL penicillin and 100 μg/mL streptomycin[89,90]. Cells are then incubated (37 °C, 5% CO2, 95% humidity) until they reach 80%-90% confluence, and then they are trypsinized (trypsin-EDTA) and seeded again at a lower concentration until a proper cell count is attained[91,92].
Although most preclinical investigations have injected the MSCs suspensions in the target area or seeded them in tri-dimensional scaffolds, new approaches using cell sheets technology have been proposed[93-95]. This treatment consists of theex vivoexpanding of cell layers, which preserve, unlike standard culture techniques, their endogenous extracellular matrix, growth factors, and fibronectin molecules[81]. With this technology, no proteolytic enzymes, such as trypsin[96], are used to prevent alterations of the extracellular microenvironment, and cells achieve confluency, within 80-150 μm cell layers (usually 4-5 layers)[97], which can be directly transplanted into the defect without any scaffold[96]. Cell pellets or microtissues may also be tri-dimensionally manufactured from multilayer cell sheets; they are aggregates that increase the endogenous extracellular matrix secretion and exhibit improved mechanical properties and cell viability. These technologies aim to mimic the natural development of periodontal tissues by applying these PDLMSCs pellets to promote the complete reconstruction of cementum-periodontal ligament complexes[98].
In addition to their local transplantation, MSCs have been applied systemically, based in their capacity to migrate to damaged areas and stimulate tissue repair. Yuet al.[99]reported that BMSCs injected systemically moved to surgically created periodontal defects and contributed to the healing of the lesions.
New regenerative strategies in periodontal therapy, to be considered as appropriate and effective therapeutic tools, require reliable evidence derived from preclinical and clinical studies[100]. The first step involves the design of preclinical investigations in which periodontal defects that mimic the naturally occurring lesions in humans affected by periodontitis are experimentally created[54,86,101,102]. The selection of a particular preclinical model will depend on the type of defect (e.g., fenestration, furcation, or intrabony periodontal lesion), the study follow-up, and the main study endpoint. Large animal models, such as the non-human primate or the canine, despite their high cost, are preferred due to their biological and histological similarities to humans[102]. In these studies, the formation of a new periodontal ligament, bone, and cementum, as well as a new connective tissue attachment are histologically evaluated in a test group, in which the new material is assessed in comparison to a control group (the gold-standard therapy, a negative control device,etc.)[100]. Experimental research is needed for successful translation of new regenerative periodontal strategies to the clinical setting. Afterwards, clinical studies, and especially, randomized controlled clinical trials are needed to determine, not only the safety and efficacy of a certain biomaterial, bioactive agent, or cell population for periodontal regeneration under ideal or controlled conditions in patients, but also the effectiveness of the device in normal clinical conditions[100,103].
Preclinical studies
The first preclinical investigations evaluating the transplantation ofex vivoexpanded MSCs into periodontal lesions were published in the 1990s[104]. Thereafter, multiple experimentalin vivoinvestigations have provided histological evidence of the formation of new cementum, connective tissue attachment, and bone formation when MSCs from bone marrow[105], adipose tissue[106], dental pulp from temporary and permanent teeth[107], periodontal ligament[89,91], or gingival connective tissue[108]were applied into experimentally created periodontal defects[54,86,87].
Monsarratet al.[54]reported that most of this preclinical research published until 2013, was performed in the dog model (49% of the studies), with MSCs with an autologous origin (63%vs.14% allogeneic and 28% xenogeneic) and harvested from intraoral sources (63%). MSCs-based therapies have been tested in different periodontal defect models, such as buccal dehiscence, 2 and 3-wall intrabony, and type II and III furcation lesions, as well as critical size supracrestal periodontal defects[75,94,109-111].
In the last years, the study of the tissue-inductive potential of allogeneic[75,94,111]and xenogeneic MSCs[109,110]has increased. These investigations have reported that the use of cells from other species or other individuals is safe, without eliciting relevant immunogenic reactions after their transplantations[109,111].
Table 1 shows the most recent preclinical investigations in which the effect of a test group, consisting of the use of MSCs from different tissue sources, was histologically evaluated for periodontal regeneration[75,94,109-116]. The results from these studies vary depending on the control used. When the comparative control was open flap debridement alone, or with an adjunctive negative control [i.e., sodium chloride (NaCl) solution injection], a clear and statistically significant superiority was reported in the test group applying a cell therapy[94,109,110,116]. Takewakiet al.[116]histologically observed epithelium invasion with inflammatory cell infiltrate and lack of periodontal ligament and cementum formation after OFD, but on the contrary, a successful periodontal regeneration with mature bone, new cementum and well-oriented Sharpey’s fibers when the OFD was combined with the application of autologous BMSCs clumps. Conversely, when the control group consisted of using the same three-dimensional scaffold without the cells, the results from different studies were heterogeneous[75,111,114]. The use of allogeneic and autologous ADSCs/fibrin gel constructs for the treatment of class II furcation models in minipigs provided new cementum, bone, and periodontal ligament, unlike the control group (fibrin gel), which showed lack of new tissues formation[75]. The micro-CT analysis revealed that the newly formed alveolar ratio in the allogeneic and autologous ADSCs groups was significantly greater (4.4 and 5.4 folds, respectively) than in the control group[75]. However, other publications did not report a significant added benefit with the application of MSCs[111,114]. Liuet al.[114]treated buccal dehiscencetype defects in Beagle dogs with either autologous BMSCs seeded in collagen-hydroxyapatite scaffolds or the scaffolds alone. The results of the histometric analysis showed very similar dimensions with respect to the height of new-formed cementum (64%-71%) and bone (71%-75%) in both groups. Similarly, Nu?ezet al.[111]found no statistically significant differences for histometric values between a test group consisted of hydroxyapatite-collagen scaffolds with embedded allogeneic PDL-MSCs and a control group (scaffold alone) for the regenerative treatment of critical-size supraalveolar periodontal defects in Beagle dogs. In this study, the authors suggest that the limited effects of the cells could be attributable to the early soft tissue recession and exposure of the furcation fornix, what could have prevented from space maintenance and a satisfactory regenerative process[111][Table 1].
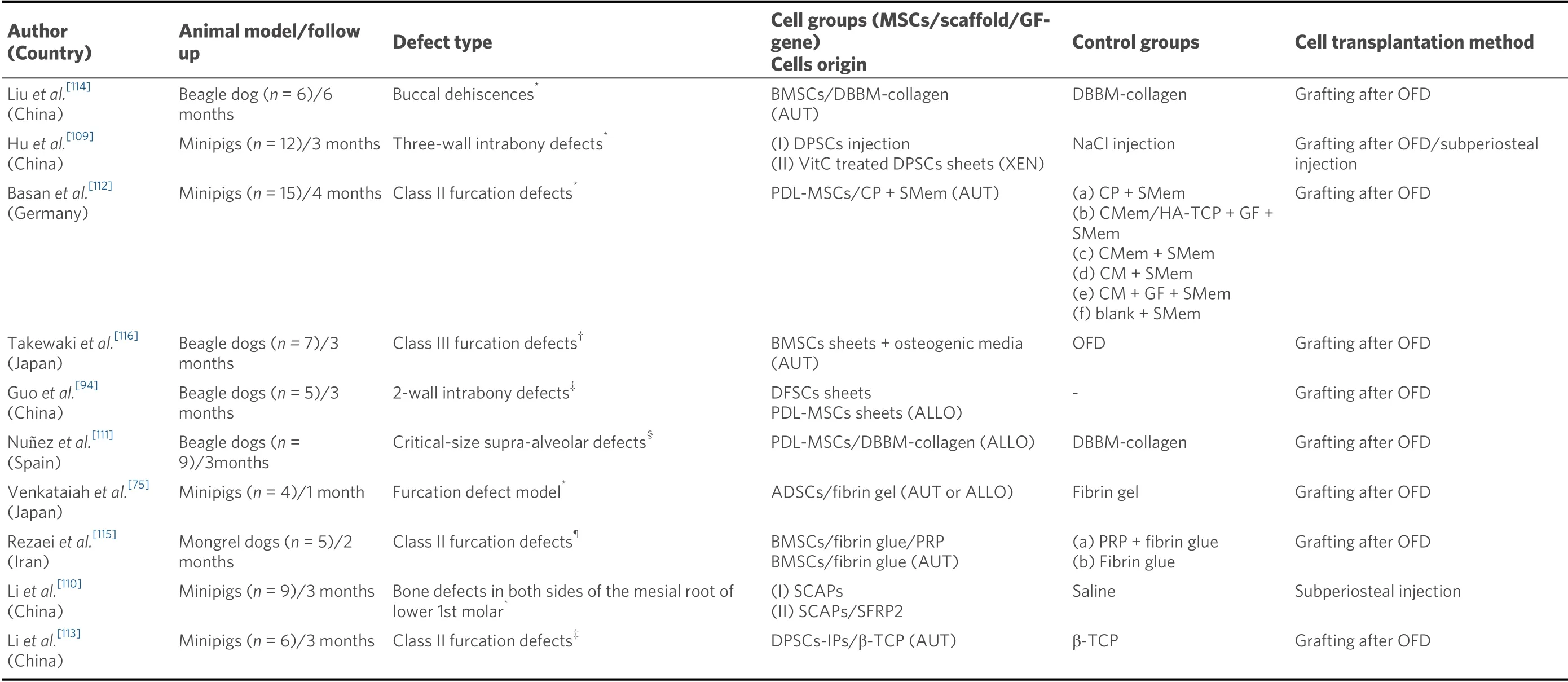
Table 1. Preclinical studies (2016-2020) with large animal models, in which mesenchymal stem cells are compared with a control treatment with no cells, for periodontal regeneration
In most studies the scaffold-cell construct has been applied directly into the periodontal defect after raising a full thickness flap and full debridement of the defect[92,112,116]. However, some authors have applied the cell therapy by a subperiosteal injection[110]. Huet al.[109]compared the regenerative efficacy of human DPSCs injections with DPSCs cell sheets placed after an OFD in a 3-wall intrabony defect model in minipigs. Although four months later, the histological results showed new alveolar bone and Sharpey’s fibers attached to the new cementoid layers in both groups, the histometric analysis revealed a greater new-formed alveolar bone height in the grafted sites (4.5 ± 0.3 mm) than in the areas treated by injected cells (3.8 ± 0.5 mm;P> 0.05). This low superiority should not necessarily be attributed to the transplantation strategy, but to other reasons, such as the supplementation of the culture media with vitamin C in the grafted group or the inherent benefits of the cell sheet technique[109]. These methods in which the extracellular matrix is preserved, as cell sheets or pellets/aggregates techniques, have been increasingly tested in preclinical studies for periodontal regeneration[94,109,116]. Guoet al.[94]found that allogeneic DFSCs sheets showed better results in terms of new cementum (5.16 ± 0.23 mm) and bone height (4.67 ± 0.35 mm) than allogeneic PDL-MSCs sheets (3.84 ± 0.30 mm and 3.42 ± 0.26 mm, respectively), although these differences were not statistically significant. As scaffolds for carrying the cells, the most recent preclinical investigations have used fibrin gel[75,115], collagen materials (membranes, matrixes, and powder)[112]and composite biomaterials made of hydroxyapatite and/or calcium phosphates mixed with collagen[111,112,114][Table 1].
Clinical studies
The safety and efficacy of the use of MSCs for periodontal regeneration demonstrated in preclinical studies, as well as their genomic stability shownin vitro, has prompted clinical investigations to assess their performance and efficacy in patients[37,54,86,87]. In the last decade, several clinical studies have studied the benefits of MSCs-based therapies in periodontal regeneration[58,74,81,82,84,85,117-119]. Their experimental design is shown in Table 2.
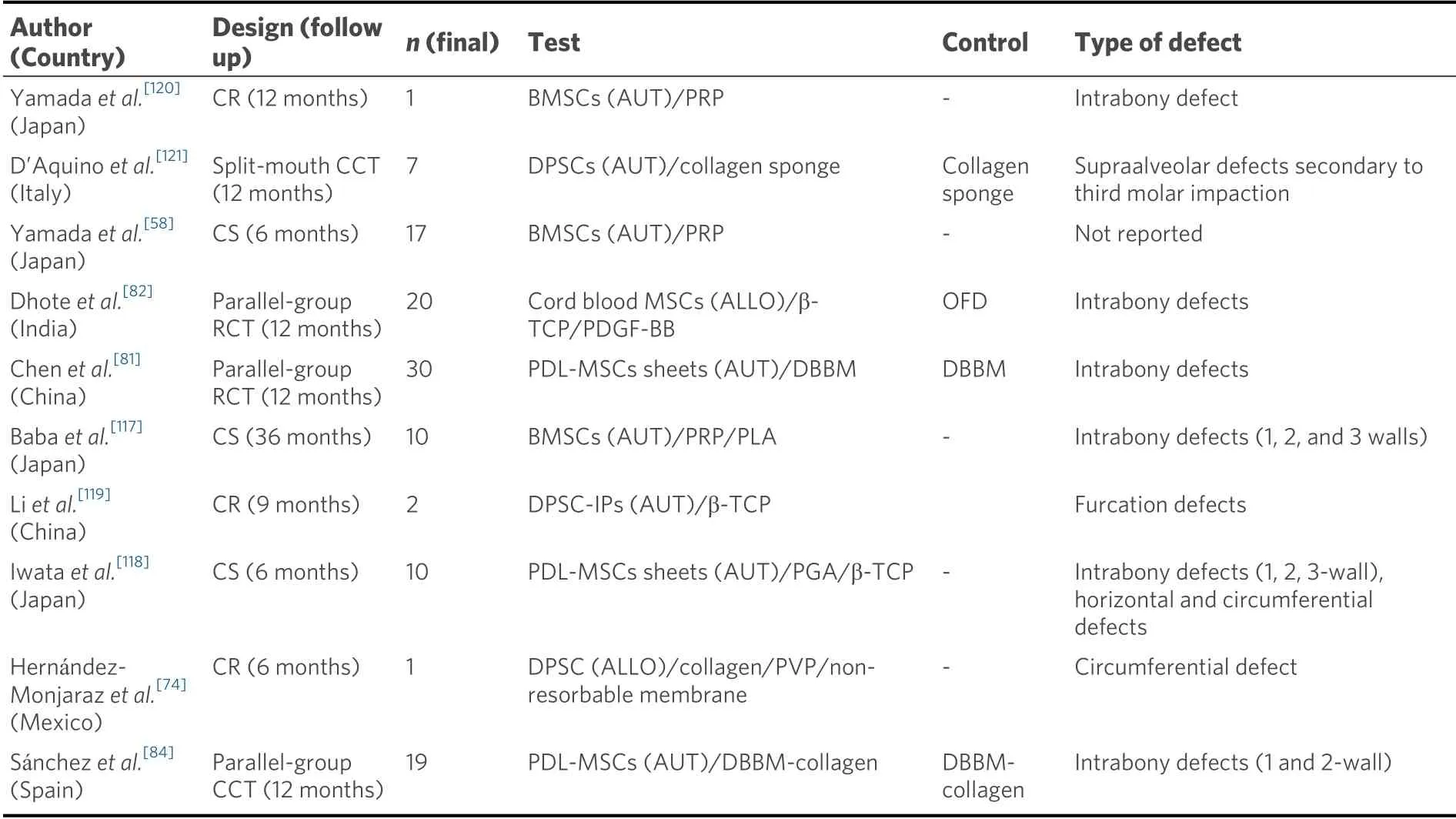
Table 2. Design of clinical studies with MSCs for periodontal regeneration
In terms of its safety, all clinical studies agree that MSCs-based therapies for periodontal regeneration are safe. No serious adverse events, other than the common complications of standard periodontal regenerative surgeries, such as medium-sized pain and swelling, sensitivity, and angular cheilosis, have been reported[74,81,84,117,118,121]. Patients completely and spontaneously recovered in few weeks and no tooth lost was found during the follow up[117].
Most of the published studies are case reports/series, with follow-ups ranging from 6 to 36 months, in which, the use of allogeneic or autologous MSCs for the treatment of intrabony, circumferential, and furcation defects, seems to significantly improve clinical and radiographic parameters[58,74,81,82,117-120].
Only three parallel-group controlled clinical trials, evaluating the effect of MSCs therapy on the functional regeneration of lost periodontal tissues, have been published to date[81,82,84]. They are all 12-month controlled clinical trials, including 20-30 patients initially, suffering from periodontitis, with the presence of at least one tooth with a deep intrabony defect. Dhoteet al.[82]reported significantly greater probing pocket depth reduction and CAL gain after treating the defects allocated to the test group with allogeneic cord blood MSCs cultured in a β-TCP scaffold with PDGF-BB, (4.50 ± 1.08 mm and 3.91 ± 1.37 mm, respectively), when compared with a control treatment, consisting of an OFD alone (3.50 ± 0.90 mm and 2.08 ± 0.90 mm, respectively;P< 0.05). However, when in addition to the OFD, a scaffold was utilized as control treatment, the differences between the groups lost the statistical significance[81,84]. Chenet al.[81]transplanted PDL-MSCs sheets together with demineralized bovine bone mineral (DBBM) in 21 intrabony periodontal defects, and the scaffold alone in 20 defects. Twelve months later, the results showed significant improvements in both groups, in terms of alveolar bone fill, the primary outcome variable in terms of efficacy, but differences between the cell and the control group could not be detected[81]. Similarly, Sánchezet al.[84]did not find a significant added beneficial effect of transplanting autologous PDL-MSCs embedded in a DBBM/collagen scaffold when grafted in 1 and 2-wall intrabony defects, compared with the use of the same scaffold without the cells. However, in this study a clear trend favoring the cell group was reported in terms of mean probing pocket depth reduction and CAL gain. Unlike the two previous randomized controlled clinical trials (RCT), the latter study could not use a randomized allocation, as not all patients provided teeth with PDL samples exhibiting appropriate cell proliferation, thus the assignment of patients to the test group was based on the ability of cell growth from the periodontal ligament remnants from their extracted teeth[84].In another controlled clinical trials, using a split-mouth design, DPSCs/collagen sponges constructs were transplanted into defects located in the distal aspect of lower second molars after the extraction of the impacted third molars[121]. The results derived from the evaluation of the seven patients that completed the 12-month follow-up revealed that all the defects from the cell group attained ≥ 70% bone regeneration from their initial defect size whilst more than half of the control defects exhibited no regeneration or 30% regeneration of the original defect dimension[121].
Although the most common cell source in the MSCs-based clinical studies for periodontal regeneration has been the periodontal ligament[81,84,118], other intraoral (dental pulp) and extraoral (bone marrow from the iliac crest) sites have been chosen for cell isolation with optimal cell counts after expansion[58,117,119,121]. In addition, most studies have employed cell populations from autologous tissues[58,81,84,117-121]; however, allogeneic sources are becoming an interesting alternative to autologous cells, due to their better costeffectiveness[74,82].
In regards to the surgical technique, the method for cell transplantation into the defect in all the human studies was the OFD, with or without papilla preservation techniques, followed by the graft placement[81,82,84][Table 2].
Whole tissue fractions without ex vivo culture
This strategy is based on transplanting samples of autologous tissue, mainly from autologous periodontal ligament[80,122,123]and dental pulp of permanent teeth[83,124-126], with minimal manipulation and withoutex vivoexpansion[76]. Two different approaches of whole tissue fractions containing stem cells should be distinguished. In one, the tissue sample, immediately after its harvesting and isolation, is directly introduced into the periodontal defect without any manipulation[80,122,123]. On the other hand, the tissue samples once isolated, are mechanically disaggregated with special devices to attain a so-called “micrografts containing different cell fractions, with MSCs among them”[83,124-126].
One advantage of these strategies is the preservation of the extracellular matrix together with other cell fractions that are usually discarded when cultivating MSCsex vivo. The hypothesis is that these cells and tissue fractions may have relevant roles in the maintenance of the niche/microenvironment where the stem cells exert their biological activity[123,125]. However, whole tissue fractions contain, not only MSCs, but also other progenitors and cell populations that although not having the differential cell inductive potential for regeneration, as monocytes and other hematopoietic cells, may release growth factors and signalling molecules, which in their physiologic ratios, may promote the regenerative process[127,128]. Another advantage of whole tissue fractions withoutex vivoexpansion is that all the procedures are performed in the dental chair at the same session, so that all regulatory issues when manipulating stem cells are avoided and consequently, their use is much more cost effective. In spite of the absence of preclinical evidence in animal models that confirms the regeneration of the periodontal tissues from a histological and histometric point of view[129], this cell therapy modality has been tested in various controlled clinical studies in which significantly better clinical outcomes have been shown by the “whole tissue fractions” group in comparison to the control group consisting of an OFD or the scaffold alone[80,83,123][Table 3].
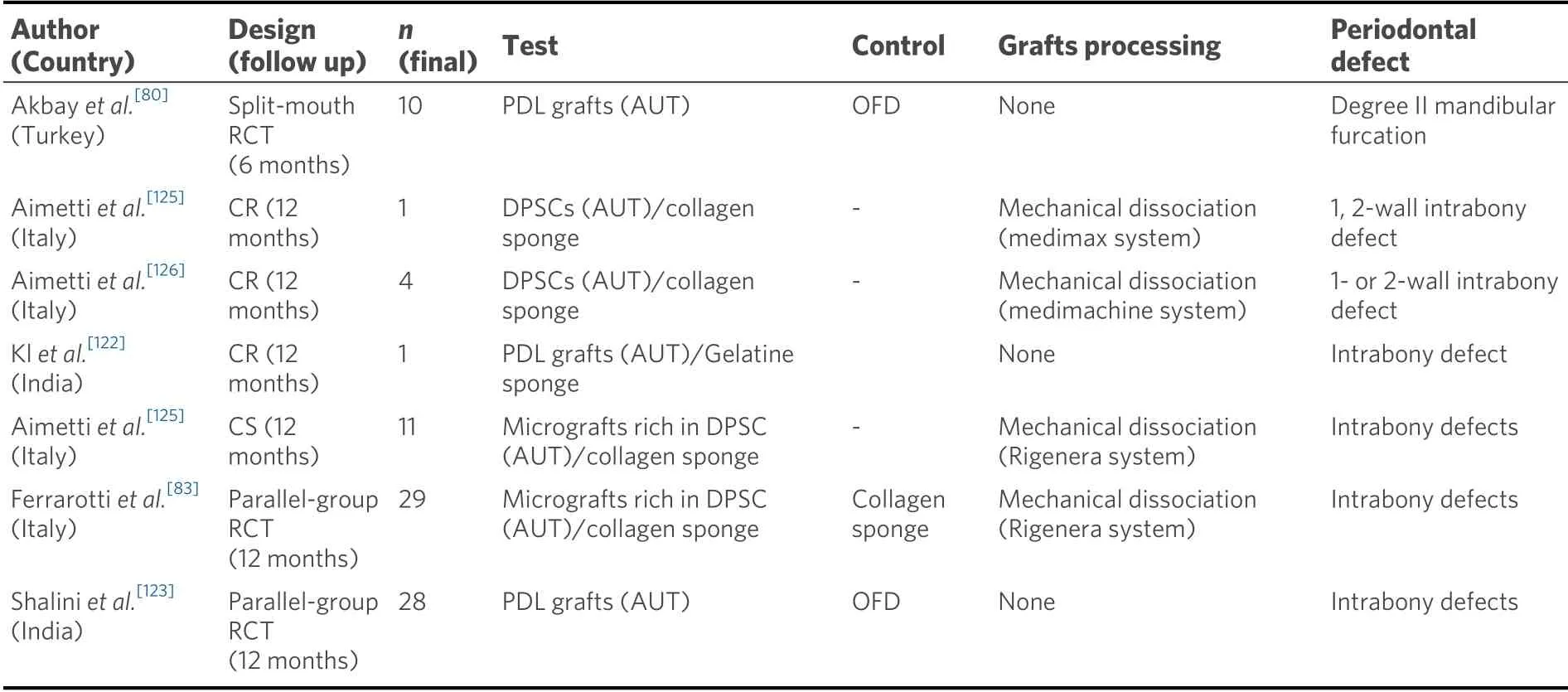
Table 3. Design of clinical trials on periodontal regeneration with whole tissue fractions
In the first clinical report using non-manipulated tissue samples, periodontal ligament grafts, obtained by scraping the mid-third of the root of extracted third molars with a healthy periodontium, were immediate transplanted into class-II furcation lesions in mandibular molars[80]. When compared at six months with the control group using only coronally advanced flaps, the test group exhibited a greater CAL gain and probing pocket depth reduction (P< 0.05). A similar protocol, but, combining PDL scraped from the root surface and the socket walls and mixed a gelatin sponge, the so-called “Autologous Stem Cell Assistance in Periodontal Regeneration (SAI-PRT)” was tested in a 12-month RCT, using as control group OFD in the treatment of intrabony defects[122,123]. This study also reported significant probing pocket depth reductions and CAL gains in the test group[123].
In another protocol, the isolated tissues (in this case fresh pulpal tissue obtained immediately after tooth extraction), were mechanically disaggregated with a special device to obtain the so-called “micrografts”, which were subsequently filtered and transplanted into the defects embedded in collagen sponges[124]. Clinical reports using this approach have shown beneficial clinical and radiographic effects when utilized for the treatment of non-contained intrabony defects[124-126]. Recently, a RCT compared the MIST to treat deep intrabony periodontal defects using either dental pulp micrografts placed in a collagen sponge biocomplex (test) or the same technique with the collagen sponge alone (control)[83]. The results after 12 months showed that the application of the cell-based therapy significantly improved the clinical and radiographic parameters, suggesting that this strategy could represent a promising and easy method for periodontal regeneration[83].
CONCLUSION
The scientific evidence from preclinical and clinical research has proven that the application of MSCs used in periodontal regenerative interventions is safe. These cell-based treatments can use eitherex vivoexpanded MSCs or minimally manipulated whole tissue fractions. The transplantation ofex vivoexpanded MCSs into preclinical models of experimental periodontal defects have resulted in significant attainment of histological periodontal regeneration. However, the clinical evidence is still limited. When compared to OFD, this strategy has shown significant benefits in both clinical and radiographic outcomes. However, when the control group is the same biomaterial used as cell carrier without MSCs, controversial results have been published, although the number of RCTs is very limited. The implantation of whole tissue fractions (mainly dental pulp and periodontal ligament) has also reported significant clinical benefits when compared with a negative control group. However, the histological outcomes of this cell therapy strategy have not yet been reported.
The present evidence of the efficacy and efficiency of these technologies based on cell therapies is too preliminary, with only a handful of clinical trials published. There is, therefore, a clear need of further clinical research, mainly in the form of RCT with adequate sample sizes, longer follow-ups, and robust designs. These designs should use patient-based analysis and the control group should be identical to the test group, but without cells or tissue extracts. Besides efficacy, other issues such as cost-effectiveness, patient morbidity, and patient-reported outcomes should be added.
DECLARATIONS
Authors’ contributions
Wrote the manuscript and designed the tables summarizing the results: Sanchez N, Sanz M
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2021.
 Plastic and Aesthetic Research2021年10期
Plastic and Aesthetic Research2021年10期
- Plastic and Aesthetic Research的其它文章
- GENERAL INFORMATION
- EDITORIAL BOARD
- AUTHOR INSTRUCTIONS
- A personalized nutrition plan based on genetic profile improves outcomes of facial regeneration with Platelet-Rich Fibrin liquid matrices
- The role of free tissue transfer in reconstruction of full thickness scalp defects
