In vitro anti-plasmodial activity of new synthetic derivatives of 1-(heteroaryl)-2-((5-nitroheteroaryl)methylene) hydrazine
Azar Tahghighi, Akram Abouie Mehrizi, Sedigheh Zakeri
1Malaria and Vector Research Group, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
2Department of Clinical Research, Pasteur Institute of Iran, Tehran, Iran
ABSTRACT
KEYWORDS: Plasmodium; 5-nitroheteroaryl; Hydrazine;Quinoline; Quinazoline; Acridine
1. Introduction
Malaria remains the most prevalent and deadly tropical disease worldwide. According to the latest World Health Organization malaria report, there were an estimated 219 million cases of malaria in 2017, leading to 435000 deaths in the world[1]. Although malaria elimination and eradication programs have decreased the malaria cases in recent years, in the current situation, due to drugresistant parasites[2]and insecticide-resistant mosquitoes[3], existing eradication tools are likely to become less effective than the past as no significant progress has been achieved in declining malaria cases during 2015-2017[1]. In this light, the malaria Eradication Research Agenda Drugs Consultative Group has suggested that having a successful malaria eradication strategy demands for the development of new anti-malarial drugs in parallel with other malaria control tools[4].
For decades, 4-aminoquinolines such as chloroquine (CQ)have been the most promising drugs against Plasmodium (P.)falciparum (Figure 1). However, it is no longer effective against P.falciparum and P. vivax in many malaria-endemic regions because of drug resistance[5-7]. Furthermore, Plasmodium parasites have indicated resistance to several anti-malarial drugs such as quinine analogs[8-10], anti-folates, and sulfonamides[11-14]. Meanwhile,artemisinin-based combination therapy, as the best available treatment, has already shown prolonged parasite clearance times in Cambodia[15-18]and Thailand[19]. This development of resistance to artemisinin and partner drugs is felt apprehensive and gives rise to untreatable malaria (super-malaria), a threat to malaria elimination.Therefore, owing to the rapid emergence of drug-resistant strains and the lack of access to a fully protected vaccine, developing new anti-malarial drugs that can be effectual against resistant strains is an urgent global health priority.
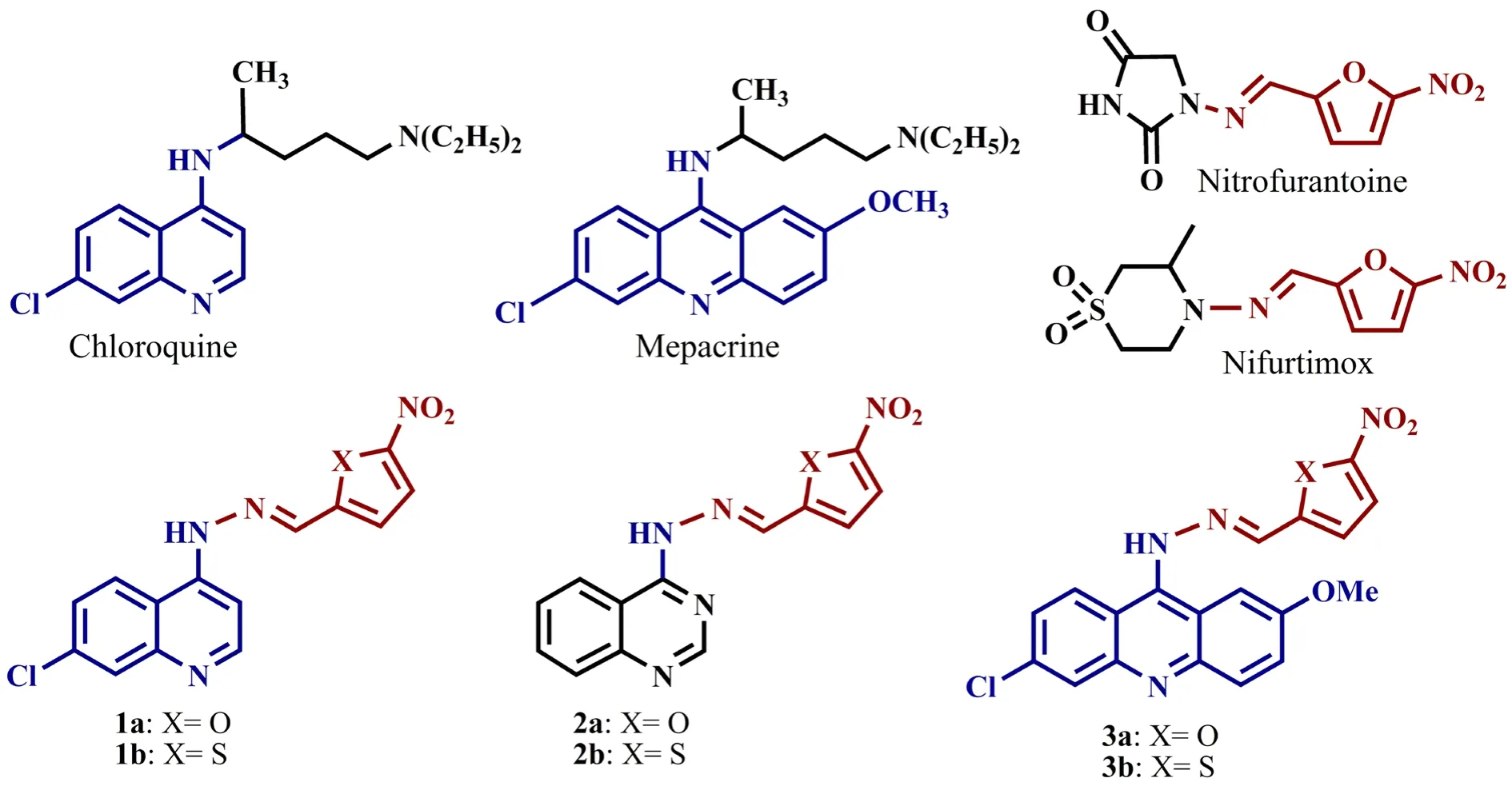
Figure 1. Chemical formula of reference drugs and designed synthetic compounds for evaluating in vitro anti-plasmodial activity. The quinoline,quinazoline, and acridine rings from reference drugs and active moiety of (5-nitroheteroaryl) methylene hydrazine, inspired from nitrofurantoin and nifurtimox, were used to design and synthesize six new derivatives (1a, 1b, 2a, 2b, 3a, and 3b). 1a: 1-(7-chloroquinolin-4-yl)-2-((5-nitrofuran-2-yl)methylene) hydrazine; 1b: 1-(7-chloroquinolin-4-yl)-2-((5-nitrothiophene-2-yl)methylene) hydrazine; 2a: 1-(quinazolin-4-yl)-2-((5-nitrofuran-2-yl)methylene)hydrazine; 2b: 1-(quinazolin-4-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine; 3a: 1-(6-chloro-2-methoxyacridine-9-yl)-2-((5-nitrofuran-2-yl)methylene) hydrazine; 3b: 1-(6-chloro-2-methoxyacridine-9-yl)-2-((5-nitrothiophene-2-yl)methylene) hydrazine.
In the field of drug discovery, a suitable and an efficient drug needs to be safe with minimal side effects, low costing, and easy manufacturing. In this regard, new anti-malarial drugs are no exception. Various strategies are used to develop novel anti-malarial drugs. High-throughput screening of compound libraries and finding new targets are newly developed strategies for designing a suitable drug[20]. Moreover, many efforts have been directed toward the improvement of existing molecules that have already become ineffective by virtue of drug resistance[20]. To this end,4-aminoquinoline scaffolds are regarded to design anti-malarial drugs owing to high efficacy (against non-resistant Plasmodium parasites), safety, affordability, and easy synthesis. Thus, one of the priorities of researchers in the development of novel anti-malarial drugs is the design of new drugs from quinoline analogs by making chemical changes in their structures[21,22]. Hocart et al.[23]have evidenced that more than half of their designed 4- aminoquinolinebased structures have anti-plasmodial activity against both CQ-resistant and CQ-sensitive parasites. In another investigation conducted by Rajapakse et al.[24], the same result was obtained from drugs designed based on 4-aminoquinoline. The mode of action of CQ and its analogs is related to the interference in the detoxification of free heme or hematin polymerization in the lysosomal digestive vacuole of a parasite, which is essential for its continuous growth and proliferation[25]. The heme detoxification pathways and metabolic functions associated with hemoglobin digestion still display a credible target for the discovery of new anti-malarial drugs[26].
Considering the CQ resistance of Plasmodium parasites in many malaria-endemic areas, to design new alternative drugs based on CQ,it is necessary to integrate important rings, such as quinoline ring or its analogs, with a side chain to create a new drug with the ability to overcome the resistance of CQ-resistant parasites. The selection of a side chain is preferred to be done based on a compound with anti-parasitic efficacy. For instance, compounds that produce reactive oxygen and induce oxidative stress can suppress parasite growth[27-30]; therefore, they can be considered as side chains for integrating with biologically important rings such as quinoline for anti-plasmodial activity. Nitrothiophenes and other nitroaromatic compounds are able to produce the reactive oxygen species, which can have anti-plasmodial properties[27,28]. Nitroaromatic drugs are in use to treat a wide variety of diseases[31-36], especially parasitic diseases such as Trypanosoma infections[37]. Nitrofurantoin and nifurtimox (Figure 1) are nitroheteroaromatic compounds capable of converting to highly reactive electrophilic intermediates[28,38], which could have anti-malarial effect. In addition to this feature, these drugs inhibit the growth of Trypanosoma cruzi via reducing the parasite dehydrogenase activity and negative effect on the mitochondrial membrane[39]. Therefore, in the current investigation, the reactive moiety of two drugs, nitrofurantoin and nifurtimox, were taken into account as side chains for the development of anti-malarial drugs based on quinoline and its related rings, quinazoline and acridine.
This study attempted to design, synthesize and evaluate new synthetic derivatives by integrating biologically important rings of quinoline, quinazoline, and acridine with the active moiety of (5-nitroheteroaryl) methylene hydrazine, inspired from nitrofurantoin and nifurtimox (Figure 1). For the evaluation of new derivatives, synthetic compounds were initially investigated for their cytotoxicity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Subsequently, the anti-plasmodial activity of new synthetic compounds was examined against CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains by parasite lactate dehydrogenase assay (pLDH). Ultimately,based on the structural similarity of synthetic compounds with 4-aminoquinoline, to know whether they have a mode of action the same as CQ or not, we performed β-hematin assay. The data obtained from this study could be utilized in designing and developing anti-malarial derivatives in future.
2. Materials and methods
2.1. Chemistry
Chemical reagents and materials were purchased from Sigma-Aldrich Company (USA), and solvents were acquired from Sumchun Company (South Korea). The main intermediate, 1-(7-chloroquinolin-4-yl) hydrazine (7) and final compounds 1a and 1b (Figure 2) were prepared according to the methods described in the literature with some modifications[40,41]. The IR spectra were obtained on a Shimadzu 470 spectrophotometer (potassium bromide dicks).H-NMR andC-NMR spectra were recorded on a Varian Unity 500 spectrometer, and chemical shifts (δ) were reported in parts per million (ppm) relative to tetramethylsilane, as an internal standard. The mass spectra were run on a Finnigan TSQ-70 spectrometer (Finnigan, USA) at 70 eV. Merck silica gel 60 F254 plates were used for analytical Thin Layer Chromatography (TLC).
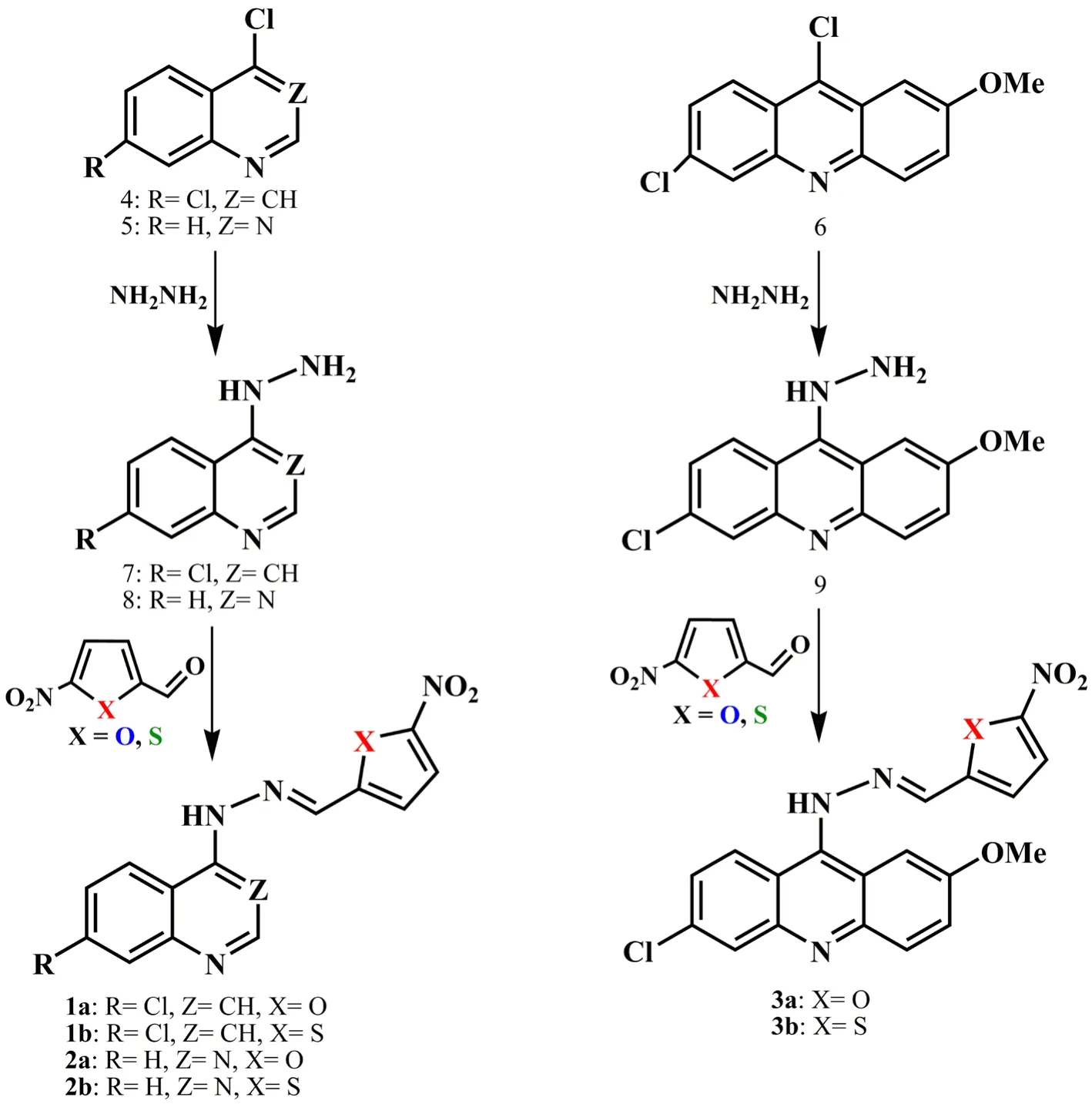
Figure 2. The route of synthesis of designed compounds to evaluate the anti-plasmodial activity.
2.1.1. Synthesis of 1-(7-chloroquinolin-4-yl)hydrazine (7)
4,7-dichloroquinoline (4, Figure 2) (1 g, 5.048 mM) and hydrazine hydrochloride (1.38 g, 20.190 mM) were refluxed in 20 mL of ethanol for (3-4) h. The completion of the reaction was detected by TLC. The solvent was evaporated under reduced pressure to obtain a cream solid, and then, ethyl acetate and water were added. The organic layer was extracted and dried with anhydrous NaSOand evaporated under reduced pressure. The solid recrystallized from ethanol and intermediate compound 7 (Figure 2) was obtained as a white solid.
2.1.2. Synthesis of 1-(quinazolin-4-yl)hydrazine (8)
4-chloroquinazoline (5, Figure 2) (0.2 g, 1.21 mM) and hydrazine hydrate (3 mL) were stirred in 15 mL of methanol at room temperature for (3-4) h. The completion of the reaction was detected by TLC. The solvent was evaporated under reduced pressure, and then, ethyl acetate and water were added. The organic layer was extracted and dried with anhydrous NaSO, and concentrated under reduced pressure to obtain a light yellow solid. The solid recrystallized from 2-propanole and the intermediate compound 8 (Figure 2) was obtained as a yellow solid.
2.1.3. Synthesis of 1-(6-chloro-2-methoxyacridine-9-yl)hydrazine (9)
6,9-Dichloro-2-methoxyacridine (8, Figure 2) (1 g, 3.60 mM) and hydrazine hydrochloride (1.23 g, 25.24 mM) were re fluxed in 20 mL of methanol/HO (4: 1 ratio) for (4-5) h. The completion of the reaction was detected by TLC. The resulting product was extracted with ethyl acetate and washed with water. The organic layer was dried with anhydrous NaSOand evaporated under reduced pressure. The solid recrystallized from ethanol/dichloromethane and the intermediate compound 9 (Figure 2) was obtained as a yellow solid.
2.1.4. Synthesis of 1-(7-chloroquinolin-4-yl)-2-((5-nitrofuran-2-yl)methylene)hydrazine (1a)
A mixture of 1-(7-chloroquinolin-4-yl)hydrazine (7, Figure 2)(0.2 g, 1.03 mM) and 5-nitro-2-furaldehyde (0.146 g, 1.03 mM) was stirred in 15 mL of methanol at room temperature using catalytic amounts of hydrochloric acid for (2-3) h. The completion of the reaction was detected by TLC. Then, the insoluble impurities were separated from the soluble product using filtration. The resulting product was purified using silica gel column chromatography eluting with ethyl acetate/methanol. Compound 1a was obtained as a yellow solid.
2.1.5. Synthesis of 1-(7-chloroquinolin-4-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine (1b)
A mixture of 1-(7-chloroquinolin-4-yl)hydrazine (7, Figure 2)(0.2 g, 1.03 mM) and 5-nitro-2-thiophenecarboxaldehyde (0.16 g,1.03 mM) was stirred in 15 mL of methanol at room temperature using catalytic amounts of hydrochloric acid for (3-4) h. The completion of the reaction was detected by TLC. The solvent was evaporated under reduced pressure, and then, ethyl acetate and water were added. The organic layer was extracted, dried with anhydrous NaSOand evaporated under reduced pressure. The solid recrystallized from acetone/petroleum ether to give compound 1b as a yellow solid.
2.1.6. Synthesis of 1-(quinazolin-4-yl)-2-((5-nitrofuran-2-yl)methylene)hydrazine (2a)
A mixture of 1-(quinazolin-4-yl)hydrazine (8, Figure 2) (0.12 g,0.75 mM) and 5-nitro-2-furaldehyde (0.11 g, 0.78 mM) was stirred in 15 mL of methanol at room temperature using catalytic amounts of hydrochloric acid for 10 min. The completion of the reaction was detected by TLC. The reaction flask was placed in an ice bath. The yellow precipitate was filtrated and washed three times with 20 mL of cold water. The residue was crystallized from acetone/petroleum ether to give compound 2a as a yellow solid.
2.1.7. Synthesis of 1-(quinazolin-4-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine (2b)
A mixture of 1-(quinazolin-4-yl)hydrazine (8, Figure 2) (0.12 g, 0.75 mM)and 5-nitro-2-thiophenecarboxaldehyde (0.12 g, 0.76 mM) was stirred in 15 mL of methanol in room temperature using catalytic amounts of hydrochloric acid for 2 h. The completion of the reaction was detected by TLC. The reaction flask was placed in an ice bath. The orange precipitate was filtrated and washed three times with 20 mL of cold water. The residue was crystallized from acetone/petroleum ether to give compound 2b as an orange solid.
2.1.8. Synthesis of 1-(6-chloro-2-methoxyacridine-9-yl)-2-((5-nitrofuran-2-yl)methylene)hydrazine (3a)
A mixture of 1-(6-chloro-2-methoxyacridine-9-yl)hydrazine (9, Figure 2)(0.21 g, 0.76 mM) and 5-nitro-2-furaldehyde (0.11 g, 0.8 mM) was stirred in ethanol at 50 ℃ using catalytic amounts of acetic acid for (6-7) h. The completion of the reaction was detected by TLC.The solvent was evaporated under reduced pressure, and then, ethyl acetate and water were added. The organic layer was extracted and dried with anhydrous NaSO. After evaporation of ethyl acetate, the final product was purified using silica gel column chromatography and eluted with ethyl acetate/petroleum ether. Compound 3a was obtained as a brown solid.
2.1.9. 1-(6-chloro-2-methoxyacridine-9-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine (3b)
A mixture of 1-(6-chloro-2-methoxyacridine-9-yl)hydrazine (9, Figure 2) (0.21 g, 0.76 mM) and 5-nitro-2-thiophenecarboxaldehyde (0.13 g, 0.8 mM) was was stirred in ethanol at 50 ℃ using catalytic amounts of acetic acid for (7-8) h.The completion of the reaction was detected by TLC. The solvent was evaporated under reduced pressure, and then, ethyl acetate and water were added. The organic layer was extracted and dried with anhydrous NaSO. After evaporation of ethyl acetate, the final product was purified using silica gel column chromatography and eluted with ethyl acetate/petroleum ether. Compound 3b was obtained as a brown solid.
2.2. Biology
2.2.1. Cytotoxicity effects of synthetic compounds on the cell viability of COS-7 cell line
Green monkey kidney cells (COS-7, American Type Culture Collection, and Manassas, VA, USA) were maintained in the complete culture medium containing RPMI 1640 medium (Gibco-BRL, Paisley, UK) supplemented with 10% fetal calf serum (FCS,Sigma, USA), penicillin-streptomycin [(78.5-100.0) U/mL], and 10 mM of HEPES (pH 6.8-8.2). The cells were incubated in humid atmosphere of 5% COat 37 ℃. Toxicity of synthetic compounds was measured using MTT assay. The samples were dissolved in DMSO/isopropanol (10 mM) and were then serially diluted in the complete culture medium to obtain the desired concentration. The final concentration of DMSO/isopropanol did not exceed 2%. This amount of DMSO/isopropanol without drug was used as a negative control, which is suggestive of its harmless effect on cells.
To perform the experiment, cells were seeded in a flat-bottom 96-well tissue culture plate (Orange Scientific, EU, Belgium) at 2×10cells/well (100 μL/well) and kept in humid atmosphere of 5% COat 37 ℃ for 24 h. Afterward, the medium was removed, and the cells were washed with PBS 1× (pH: 7.2). The cells were then incubated with 100 μL of the complete culture medium containing different concentrations of compounds (150.00, 75.00, 37.50, 18.75, 9.38,4.69, 2.34, 1.17, 0.58, and 0.29 μM) in humid atmosphere of 5% COat 37 ℃. The concentrations of compounds for test were selected after optimization experiments. The medium alone and medium containing solvent (DMSO/isopropanol; 2%) were used as controls.After 24 h, the medium was removed, and 100 μL of PBS 1×(pH 7.2) containing 0.5 mg/mL of MTT was added to the wells, and the plate was maintained in humid atmosphere with 5% COat 37 ℃for 4 h. Following the removal of the MTT solution, the formazan crystals were dissolved in isopropanol, and the absorbance was read at OD. The absorbance of untreated wells was considered as 100% viability, and the impact of different concentrations of samples were compared to the untreated wells to obtain the IC. The impact of toxicity was determined by analyzing the selectivity index (SI) as the ratio of cytotoxicity for COS-7 to the anti-plasmodial activity of each tested compound and SI>1 indicates minimal cytotoxicity.
2.2.2. In vitro culture of P. falciparum
Laboratory-adapted P. falciparum K1 (CQ-resistant) and 3D7 (CQ-sensitive) strains were used for in vitro anti-malarial bioassay. P.falciparum parasites were continuously cultured based on a modified method described previously[42]. Brie fly, P. falciparum parasites were cultured on human erythrocytes (blood group O+) in the presence of RPMI 1640 medium (Gibco, Invitrogen, Scotland, UK) supplemented with 25 mM of NaHCO, 0.2% AlbuMAX ∏ (Gibco, Invitrogen,Scotland, UK), 1.96 gr/L of glucose (Sigma, USA), 12% of human AB+serum, 25 mM of HEPES, and 60 μg/mL of gentamicin sulfate.The cultures were kept in an atmosphere of 91% nitrogen, 6% CO, and 3% oxygen, and the medium was changed daily. The culture was synchronized two times in 96-h intervals using 5% D-sorbitol (Sigma, St Louis, MO, USA). For synchronization,RBCs of a P. falciparum culture flask with 5%-10% parasitemia and ring dominance were precipitated for 5 min at 250 ×g at room temperature. The pellet was washed twice with pre-warmed RPMI and centrifuging for 5 minutes at 250 ×g at room temperature. Next,the RBCs were suspended in 9 volumes (~5 mL) of pre-warmed sorbitol solution (5%) and incubated at 37 ℃ for 20 min. Later,the cells were pelleted by centrifugation for 5 minutes at 250 ×g at room temperature and the pellet was washed twice with pre-warmed RPMI. Subsequently, the pellet was used to culture by adding complete culture medium and fresh RBC to maintain hematocrit value at 10%.
2.2.3. In vitro anti-plasmodial activity of synthetic derivatives
Synthesized compounds in the current study were assessed for in vitro anti-plasmodial activity using pLDH method as described before[43,44]. For this experiment, at first, the compounds were dissolved in DMSO/isopropanol (1:1 ratio) at the concentration of 10 mg/mL. To measure the activity of synthetic compounds, a wide range of concentrations [(75.000-0.146) μM]of each sample was used in four repeats. The final concentration of solvents exposed to parasites was <2%. Therefore, in control wells, the parasites were maintained in the culture medium containing 2% DMSO/isopropanol. CQ diphosphate (Sigma, USA) was used as a control and a reference drug in the final concentration of 1.5 μM in all experiments. Synchronized P. falciparum parasites (3D7 and K1 strains) in ring stage with parasitemia of 2% and a final hematocrit of 1.3% were employed for this test. Besides, un-infected RBCs were applied in each plate in 1.3% hematocrit as negative controls. The test plates were incubated at 37 ℃ in the presence of 91% nitrogen,6% CO, and 3% oxygen. After 48 h, the thin blood smears were prepared from one of the repeats for microscopy assessment, and the other triplicates were applied for pLDH assay. In this assay, the RBCs were hemolyzed three times by freezing and thawing thermal shock , and then, their resuspended aliquots (20 μL) were transferred to 100 μL of the Malstat reagent (0.11% v/v Triton-100, 115.7 mM of lithium L-lactate, 30.27 mM of Tris, 0.62 mM of 3-Acetylpyridine adenine dinucleotide (APAD; pH 9; Sigma-Aldrich, USA)[43,44]and 25 μL of a solution of 1.9 μM of nitro blue tetrazolium and 0.24 μM of phenazine ethosulphate in a 96-well microtiter plate. The plate was then incubated at 37 ℃ for 30 min to allow color development,and absorbance was measured at ODusing an ELISA reader(BioTek, USA). The anti-plasmodial activity of synthetic compounds was expressed as IC(mean±SD) of three separate experiments performed in triplicate. The OD values from control wells without any compounds or drug were considered as having 100% pLDH activity.
To measure ICvalues, the inhibition of each compound concentration was compared to the untreated control (as 100% activity of pLDH). These values were then expressed as a percentage of 100% growth value and plotted against the corresponding concentrations of the compounds, using Gene5 micrroplate data collection and analysis software (BioTek, USA) to generate log dose-response curves.
2.2.4.β
-hematin assayTo evaluate the mechanism of action of synthetic compounds,β-hematin assay was performed to measure the hemozoin formation as described previously[45]. In this experiment, synchronized P.falciparum parasites in ring stage with parasitemia of 2% and a final hematocrit of 5% in each well were utilized. Synthetic compounds were dissolved in DMSO/isopropanol as mentioned above and then were diluted in the culture medium to obtain the working concentrations (20-0.156 μM). To determine the activity of synthetic compounds, different concentrations of each sample was used in duplicate in 24-well microplates. CQ diphosphate (1.5 μM) was employed as a reference drug in all the experiments. Besides, uninfected RBCs were applied in each plate in 5% hematocrit as negative controls and infected RBCs without any drug or test samples were used as positive controls. Hemozoin formation in the positive control wells was considered as 100%. The test plates were incubated at 37 ℃in the presence of 91% nitrogen, 6% CO, and 3% oxygen. After 48 h,hemozoin formation was measured as follows: at first, the experiment was optimized with the known concentration of ferriprotoporphyrin IX chloride (hemin; Sigma, USA). To perform the β-hematin assay,the content of each well was mixed with 2% Triton X-100 solution in 1:1 (v:v) ratio and pelleted at 4 ℃ at 11000 ×g for 15 min. The supernatant was discarded, and the pellet was resuspended in 1 mL of distilled water and centrifuged again at 4 ℃ at 11000 ×g for 15 min. Subsequently, 130 μL of distilled water was added to the hemozoin pellet, followed by adding 15.5 μL of 1 N of NaOH and 31 μL of pyridine and mixing by vigorous shaking. After the addition of this mixture to two wells of a 96-well microplate,5 μL of 2.5 mM potassium ferricyanide and “a pinch” of sodium hydrosulfite were added to the first and the second wells to oxidize and to reduce heme, respectively. Next, the ODwas measured by an ELISA reader (BioTek, USA). The ODof the oxidized sample was subtracted from that of the reduced sample. To assess the anti-malarial activity, the inhibition of synthetic compounds was compared relative to the untreated culture.
2.3. Statistical analysis
Data were analyzed using SPSS 20.0 software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY,USA). Comparisons of the mean levels of IC(pLDH, β-hematin,or MTT tests) among the tested groups were performed by one-way ANOVA followed by Tukey’s test to compare between two groups.In addition, independent sample t-test was conducted to compare the mean ICvalues with treated 3D7 and K1 P. falciparum parasites for each tested compound. P<0.05 was considered as statistically significant.
2.4. Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board of Pasteur Institute of Iran (No. IR.PII.REC.1394.41).
3. Results
3.1. Chemistry
3.1.1. 1-(quinazolin-4-yl)hydrazine (8)
The intermediate compound (8) was successfully synthesized with a yield of 80%. TheHNMR spectra data of this compound are listed below:HNMR (DMSO-d6, 500 MHz)δ: 9.64 (brs, 1H, -NH-), 8.48(brs, 1H, Ar), 8.17 (brs, 1H, Ar), 7.75 (brs, 1H, Ar), 7.67 (brs, 1H,Ar), 7.47 (brs, 1H, Ar), 4.73 (brs, 2H, -NH).
3.1.2. 1-(6-chloro-2-methoxyacridine-9-yl)hydrazine (9)
The intermediate compound (9) was successfully synthesized with a yield of 73%. All of the spectra data of this compound are listed below: IR (KBr, cm): 3435.49, 3106.77, 1558.58;HNMR(DMSO-d6, 500 MHz)δ: 11.96 (brs, 1H, -NH-), 8.22 (d, 1H, J=8.7 Hz, Ar), 7.61 (d, 1H, J=3.0 Hz, Ar), 7.57 (brs, 1H, Ar), 7.54 (d, 1H,J=9.0 Hz, Ar), 7.44 (dd, 1H, J=9.0 & 2.8 Hz, Ar), 7.25 (dd, 1H,J=8.7 & 1.8 Hz, Ar), 3.86 (s, 3H, -CH).
3.1.3. 1-(7-chloroquinolin-4-yl)-2-((5-nitrofuran-2-yl)methylene) hydrazin (1a)
The final compound (1a) was successfully synthesized with a yield of 47%. All of the spectra data of this compound are listed below:IR (KBr, cm): 3566.00, 3154.99, 1652.38, 1565.86, 1318.92.HNMR (DMSO-d6, 500 MHz)δ: 11.68 (brs, 1H,-NH-), 8.41 (d, 1H,J=8.7 Hz, Ar), 8.31 (s, 1H, =CH-), 7.82 (d, 1H, J=4.2 Hz, Furan),7.65 (t, 1H, J=5.9 Hz, Ar), 7.50 (brs, 1H, Ar), 7.39 (d, 1H, J=8.3 Hz,Ar), 7.25 (brs, 1H, Furan), 6.90 (d, 1H, J=7.2 Hz, Ar). MS (m/z, %):318.1 (M, 12.6), 316.1 (M, 38.9), 292.1 (10.5), 270.1 (4.2), 242.1(7.4), 216.1 (12.6), 178.0 (8.4), 150.0 (29.4), 123.0 (12.6), 99.0(13.7), 69.1 (17.9), 44.0 (100).
3.1.4. 1-(7-chloroquinolin-4-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine (1b)
The final compound (1b) was successfully synthesized with a yield of 56%. All of the spectra data of this compound are listed below: IR (KBr, cm): 3444.85, 3106.61, 1651.98, 1521.32,1323.93.HNMR (DMSO-d6, 500 MHz)δ: 11.91 (brs, 1H, -NH-),8.58 (d, 1H, J=8.5 Hz, Ar), 8.25 (s, 1H, =CH-), 8.12 (d, 1H, J=5.0 Hz, Thiophen), 7.76 (t, 1H, J=5.5 Hz, Ar), 7.56-7.51 (m, 3H, Ar &Thiophen), 7.00 (d, 1H, J=8.5 Hz, Ar); MS (m/z, %): 334.1 (M,37.31), 332.1 (M, 100), 285.1 (5.97), 253.1 (7.46), 204.0 (8.95),163.0 (79.10), 128.1 (31.34), 95.0 (32.84), 69.1 (41.79), 43.1(89.55).
3.1.5. 1-(quinazolin-4-yl)-2-((5-nitrofuran-2-yl)methylene)hydrazine (2a)
The final compound (2a) was successfully synthesized with a yield of 72%. All of the spectra data of this compound are listed below:IR (KBr, cm): 3436.19, 3147.87, 1623.08, 1576.22, 1344.03;HNMR (DMSO-d6, 500 MHz)δ: 11.83 (s, 1H, -NH-), 8.44 (s, 1H,=CH-), 8.24 (d, 1H, J=7.7 Hz, Ar), 7.97 (s, 1H, Ar), 7.85 (d, 1H,J=3.3 Hz, Furan), 7.73 (t, 1H, J=7.4 Hz, Ar), 7.56 (d, 1H, J=7.8 Hz, Ar), 7.48 (t, 1H, J=7.4 Hz, Ar), 7.40 (brs, 1H, Furan);CNMR(DMSO-d6, 500 MHz) δ: 153.78, 152.33, 147.51, 144.73, 144.142.37, 133.98, 127.54, 127.50, 124.81, 120.02, 115.58, 115.32; MS(m/z, %): 283.1 (M, 71.43), 256.1 (6.70), 237.1 (13.39), 198.1(6.70),171.1 (26.79), 149.0 (8.93), 145.0 (26.79), 129.0 (51.79), 103.0(76.79), 69.1 (51.79), 57.1 (78.57), 43.1(100).
3.1.6. 1-(quinazolin-4-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine (2b)
The final compound (2b) was successfully synthesized with a yield of 74%. All of the spectra data of this compound are as follows:IR (KBr, cm): 3272.23, 3055.85, 1615.68, 1532.39, 1316.89;HNMR (DMSO-d6, 500 MHz)δ: 12.34 (brs, 1H, -NH-), 8.43(d, 1H, J=7.9 Hz, Ar), 8.37 (s, 1H, =CH-), 8.14 (d, 1H, J=4.4 Hz,Thiophen), 7.96 (s, 1H, Ar), 7.80 (t, 1H, J=7.7 Hz, Ar), 7.67-7.62(m, 3H, Ar & Thiophen);CNMR (DMSO-d6, 500 MHz) δ: 153.78,147.28, 147.00, 144.53, 144.10, 137.97, 134.39, 131.42, 129.44,128.03, 127.78, 125.41, 119.83; MS (m/z, %): 299.1 (M, 100), 253.1(12.12), 171.1 (84.85), 145.1 (63.64), 129.1 (34.85), 118.1 (34.85),103.1 (81.82), 95.1 (37.88), 76.1 (33.33), 45.1 (15.15).
3.1.7. 1-(6-chloro-2-methoxyacridine-9-yl)-2-((5-nitrofuran-2-yl)methylene)hydrazine (3a)
The final compound (3a) was successfully synthesized with a yield of 9.5%. All of the spectra data of this compound are listed below:IR (KBr, cm): 3442.30, 3101.65, 1652.49, 1652.33, 1521.00,1337.9;HNMR (DMSO-d6, 500 MHz)δ: 11.35 (brs, 1H, -NH-),9.11 (s, 1H, =CH-), 8.50 (d, 1H, J=8.8 Hz, Ar), 7.91-7.84 (m, 3H,Ar & Furan), 7.70 (m, 1H, Ar), 7.33-7.28 (m, 2H, Ar & Furan),7.15 (d, 1H, J=8.8 Hz, Ar), 3.89 (s, 3H, -CH); MS (m/z, %): 398.2(M, 2.1), 396.2 (M, 5.2), 368.4 (2.7), 313.3 (6.5), 284.1 (5), 258.1(20), 243.1 (25.5), 200.0 (16), 149.0 (12.5), 97.1 (20.5), 69.1 (36),43.1(100).
3.1.8. 1-(6-chloro-2-methoxyacridine-9-yl)-2-((5-nitrothiophen-2-yl)methylene)hydrazine (3b)
The final compound (3b) was successfully synthesized with a yield of 7%. All of the spectra data of this compound are listed below:IR (KBr, cm): 3436.40, 1652.33, 1521.00, 1337.44.HNMR(DMSO-d6, 500 MHz)δ: 11.85 (brs, 1H, -NH-), 8.91 (s, 1H, =CH-),8.23-8.21 (m, 2H, Ar & Thiophen), 7.67 (brs, 1H, Thiophen), 7.61(d, 1H, J=3.0, Ar), 7.56-7.52 (m, 2H, Ar), 7.44 (dd, 1H, J=9.0 & 2.85 Hz, Ar), 7.23 (dd, 1H, J=8.7 & 1.9 Hz, Ar), 3.92 (s, 3H, -CH); MS(m/z, %): 414.1 (M, 2.3), 412.1 (M, 4.6), 368.4 (3.8), 310.0 (7.7),259.1 (100), 216.0 (15.4), 188.0 (32.3), 153.1 (18.5), 126.0 (10.8),98.1 (16.9), 69.1 (26.1), 43.1 (49.2).
3.2. Biology
3.2.1. Cytotoxicity of synthetic compounds against mammalian cells (COS-7)
To evaluate the toxicity of synthetic compounds, it is important to note that the compounds should not be cytotoxic in the effective doses against P. falciparum strains. The viability of COS-7 cells was evaluated in the presence of different concentrations of synthetic compounds (0.29-150.00) μM. The results showed that all the compounds were non-cytotoxic at the effective dose (ICvalues)against CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains. Compounds 3a [IC: (49.775±8.193) μM, SI: 6.426-9.078]and 3b [IC: (118.444±2.578) μM, SI: 16.906-31.977]were safer than each of other tested compounds (P<0.05, Tukey’s test; Table 1).
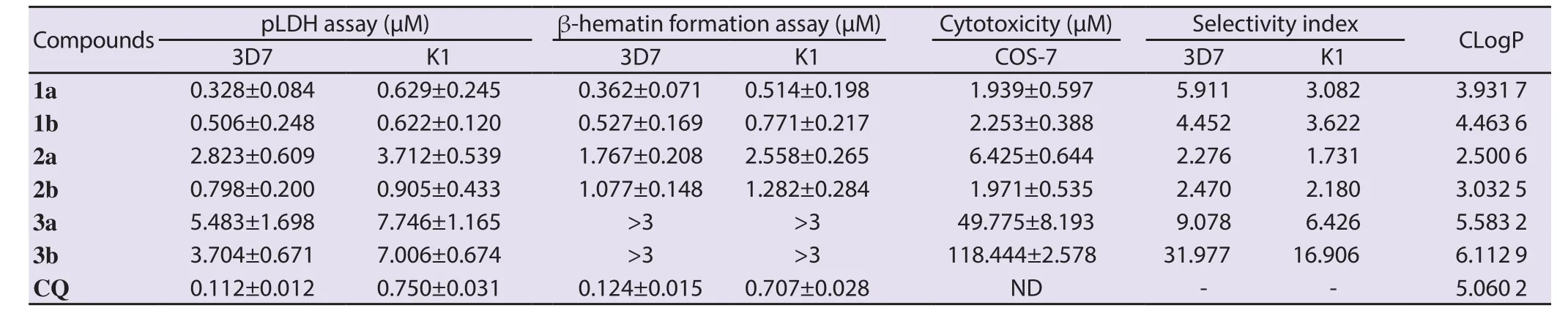
Table 1. IC50 values of in vitro anti-plasmodial activity and cytotoxicity of examined synthetic compounds against CQ-sensitive (3D7) and CQ-resistant (K1)Plasmodium falciparum strains.
3.2.2. In vitro anti-plasmodial activity of synthetic compounds
Anti-plasmodial activities of six synthetic compounds against CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains are shown in Table 1. Based on pLDH assay, compounds 1a and 1b were the most efficient synthetic derivatives against CQ-sensitive [3D7, IC: (0.328±0.084) μM]and CQ-resistant [K1, IC:(0.622±0.120) μM]strains of P. falciparum, respectively. (P<0.001,one-way ANOVA). Regarding CQ-sensitive (3D7) P. falciparum,no significant difference was determined in the anti-plasmodial activity of the most effective compounds (1a, 1b, and 2b) (P>0.05,Tukey’s test; Table 1). However, the effective dose of compounds 3a [IC: (5.483±1.698) μM]and 3b [IC: 3.704±0.671) μM]against CQ-sensitive (3D7) P. falciparum strains was higher than all the other compounds (P<0.05, Tukey’s test). Concerning CQ-resistant (K1) strain, there was no significant difference in the effective dose of the compounds 1a, 1b, and 2b (P>0.05, Tukey’s test; Table 1). However, compounds 3a [IC: (7.746±1.165) μM]and 3b [IC: (7.006±0.674) μM]were effective at much higher doses than other compounds (P<0.05, Tukey’s test). In pLDH assay, CQ as a reference drug showed an ICof (0.112±0.012) μM and (0.750±0.031) μM against 3D7 and K1 strains, respectively.Interestingly, compounds 1a and 1b with 7-chloro quinoline scaffold were more effective than CQ against resistant strain (K1) with ICvalues of 0.629 and 0.622 μM, respectively.
Comparison of anti-plasmodial efficacy of synthetic compounds against CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains revealed that each of compounds 1b, 2a, and 2b did not have statistically significant difference against 3D7 and K1 P. falciparum strains (P>0.05, independent sample t-test; Table 1). However,compounds 1a, 3a, and 3b had significantly higher activity against CQ-sensitive (3D7) P. falciparum strain as compared to CQ-resistant(K1) (P<0.05, independent sample t-test; Table 1). Moreover, CQ,as a reference drug, showed a significantly higher efficacy against CQ-sensitive (3D7) relative to CQ-resistant (K1) P. falciparum strain(P<0.05, independent sample t-test).
The results from anti-plasmodial activity of compounds in relation to lipophilicity revealed that compounds 3a and 3b (CLogP=5.58 and 6.11, respectively) with lipophilicity greater than CQ (CLogP=5.06)had much lower anti-plasmodial activity compared to the reference drug. However, compounds 1a, 1b, and 2b (CLogP=2.93, 4.46, and 3.03, respectively) with lipophilicity lower than compounds 3a and 3b had opposite result, i.e. higher anti-plasmodial activity.
3.2.3. Inhibitory effect of synthetic compounds on hemozoin formation
To confirm the mode of action of synthesized compounds, as the inhibitory compounds of hemozoin formation, β-hematin assay was performed in CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains in the presence of synthetic compounds (Table 1). Based on this experiment, compound 1a was the most efficient derivative for the inhibition of hemozoin formation against both CQ-sensitive [3D7, IC: (0.362±0.071) μM]and CQ-resistant[K1, IC: (0.514±0.198) μM]P. falciparum strains (P=0.000, oneway ANOVA). Multiple comparison analyses showed no statistical difference in the efficacy of compounds 1a and 1b against CQ-sensitive (3D7) strain (P>0.05, Tukey’s test).
Regarding CQ-resistant (K1) strain, no significant difference was found in the effective dose of compounds 1a, 1b, and 2b for the inhibition of hemozoin formation (P>0.05, Tukey’s test).Unfortunately, the ICwas not detected for compounds 3a and 3b due to color interference at concentrations ≥3 μM (Table 1).
4. Discussion
Given the lack of an effective vaccine, unsatisfactory success in mosquito vector control and the spread of anti-malarial drugresistant Plasmodium parasites, malaria still remains a main problem in global health. Drug discovery in this filed plays a vital role in the development of new therapeutically important agents and will help to achieve the elimination and eradication of malaria worldwide.In this study, the novel analogs of 4-aminoquinoline family containing (nitroheteroaryl)methylene hydrazine moiety and heterocyclic ring were synthesized similar to anti-malarial drugs.In fact, an important part of these new compounds was inspired by the anti-microbial and anti-parasitic drugs like nitrofurantoin and nifurtimox with (nitrofuran-2-yl)methylene hydrazine moiety,respectively (Figure 1). Based on the results obtained in this study, all newly synthesized compounds had the anti-plasmodial activity ranging from IC: 0.328 to 5.483 μM against CQ-sensitive(3D7) and from 0.622 to 7.746 μM against CQ-resistant (K1)P. falciparum strains. The pLDH assay demonstrated that the most efficient compound against P. falciparum was compound 1a with a (5-nitrofuran-2-yl)methylene hydrazine side chain and 7-chloroquinoline ring (ICvalues of 0.328 and 0.629 μM against 3D7 and K1 strains, respectively). However, compound 3a with a (5-nitrofuran-2-yl)methylene hydrazine side chain and 6-chloro-2-methoxyacridine ring had the highest ICvalues (5.483 and 7.746 μM against 3D7 and K1 strains, respectively) among the tested compounds. It seems that the heteroaromatic ring is the most critical factor in anti-plasmodial activity compared to (nitroheteroaryl)methylene hydrazine moiety,as indicated by the higher activity of compounds 1a and 1b with quinoline ring relative to compounds 2a-2b and 3a-3b with quinazoline and acridine rings, respectively. Meanwhile, compounds 1a and 1b were more effective than CQ against CQ-resistant parasites, implying the role of a side chain in anti-plasmodial activity against CQ-resistant strain. A possible explanation for this observation may be related to different side chains of compounds 1a and 1b. i.e. (5-nitrofuran-2-yl)methylene hydrazine and (5-nitrothiophen-2-yl)methylene hydrazine, respectively, in comparison to CQ with diethylpentane-1,4-diamine as a side chain.Alteration in side chains may enhance the ability of a molecule to enter the food vacuole. If the compounds tested in this study have the capability of entering food vacuole, the nitro groups (side chains)may generate toxic radical species, leading to parasite’s death, which remains to be studied in the future. Therefore, it could be concluded that among the tested compounds, a quinoline ring with side chains containing nitro groups is likely to be the most efficient compound,which needs to be considered in designing anti-malarial drugs in the future.
Comparing anti-plasmodial activity of synthetic compounds against CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains revealed that compounds 1b, 2a, and 2b did not have statistically significant difference against 3D7 and K1 P. falciparum strains(P>0.05), suggestingthat these compounds are promising in antimalarial drug development. Nonetheless, a significantly higher efficacy of CQ was observed against CQ-sensitive (3D7) compared to CQ-resistant (K1) P. falciparum strain (P<0.05). Looking at the structures of these compounds, we found that compounds 2a and 2b have a common core “quinazoline ring”. This result may lead to this conclusion that among the tested compounds, a quinazoline ring has possibly the most important function against both resistant and sensitive strains with the same efficiency. On the other hand, unlike compound 1a, compound 1b with a similar ring demonstrated the same efficacy against CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains, reflecting the role of the side chain (oxygen replacement in the side chain) in the inhibition of resistance. Regarding the CQ-resistance due to point mutations in PfCRT encoding gene[46,47]and the inhibitory effects of the tested compounds on hemozoin formation, it is proposed that the new structure of mentioned compounds, i.e. 1b, 2a, and 2b, may facilitate their entering to food vacuole in CQ-resistant (K1) P. falciparum strain that requires to be investigated in future research.
Previous studies have disclosed that the lipophilic feature of neutral lipids in food vacuoles is a help for the formation of hemozoin at the lipid/aqueous interface[48], which is feasible through the interaction of inhibitory drugs with free heme at both lipid and aqueous interface[49]. Consequently, lipophilicity of the examined compounds may influence their anti-plasmodial activity. Therefore the observed discrepancy in drug efficacy of synthetic compounds could be related to their lipophilicity feature[48,49]. Therefore, it can be postulated that among the tested samples, compounds 1a, 1b, and 2b have easy accumulation in the food vacuole of parasite and possess the best structure of lipid/aqueous interface for interaction with heme.
Anti-malarial drugs like quinoline and its related derivatives act by interfering with heme metabolism of parasite. These new derivatives containing the active rings of quinoline and its analogs (quinazoline and acridines), which incorporate with (5-nitroheteroaryl) methylene hydrazine moiety in the side chain, are structurally related to CQ and mepacrine (Figure 1). Therefore, their mechanism of action may be similar to that of 4-aminoquinoline anti-malarial drugs.The compound 1a with the favorable anti-plasmodial activity had higher inhibitory action against hemozoin synthesis with ICvalues of 0.362 and 0.514 μM against 3D7 and K1 P. falciparum strains,respectively. In fact, the results of pLDH and hemozoin formation assays confirmed each other, indicating that the main mechanism of inhibitory effect of these compounds is related to the inhibition of β-hematin formation.
Cytotoxic effects of the tested compounds on fibroblast-like cell lines derived from monkey kidney tissue (COS-7) were evaluated with regard to the reactivity of MTT. Based on the obtained results,none of the synthetic compounds had any cytotoxicity in the active dose against 3D7 and K1 P. falciparum strains. This observation motivates researchers to further evaluate these compounds as antiplasmodial compounds with no cytotoxicity at the active dose.
In conclusion, to achieve new, more affordable, accessible, and potent anti-malarial analogs against sensitive and resistant strains of P. falciparum, with minimal potential undesirable side effects,different chemical modifications were performed for the development of potent anti-malarial agents based on 4-aminoquinoline scaffold.In this light, the novel CQ analogs were synthesized for the first time with (nitroheteroaryl)methylene hydrazine moiety. Interestingly,compounds 1b, 2a, and 2b had anti-plasmodial activity against both CQ-sensitive (3D7) and CQ-resistant (K1) P. falciparum strains with no statistically significant difference, suggesting that these compounds could be promising anti-malarial agents. However,further research is needed to investigate the enhancement of the antiplasmodial activities of the tested compounds, such as the production of the compounds in salt form, application of drug delivery systems,the use of other formulation strategies, and co-formulation with other anti-plasmodial drugs.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
This study was supported by a grant from Pasteur Institute of Iran.The authors would like to express their appreciation to the staff of Iranian Blood Transfusion Organization for providing human blood and serum samples. The authors also thank to Mrs. M. Saffari for the English editing of the manuscript.
Funding
It was supported by the grant (No. 852) from Pasteur Institute of Iran to A. A. Mehrizi.
Authors’ contributions
AT synthesized all compounds, designed the study and drafted the manuscript. AAM designed the study, supervised all the experiments,performed all biological experiments (cytotoxicity test, continuous P. falciparum culture, pLDH, and β-hematin assays), analyzed the data and helped write and finalize the manuscript. SZ; contributed in data analysis and helped in the optimization of the biological experiments and critical reading of the manuscript. All authors have read and approved the final version of the manuscript.
 Asian Pacific Journal of Tropical Medicine2021年3期
Asian Pacific Journal of Tropical Medicine2021年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Genotype 4 reassortant Eurasian avian-like H1N1 swine flu virus: An emerging public health challenge
- SARS-CoV-2 may impair pancreatic function via basigin: A single-cell transcriptomic study of the pancreas
- Burkholderia pseudomallei infection manifests as mediastinal/hilar lymphadenopathy:A case report
- How knowledge of hepatitis B disease and vaccine influences vaccination practices among parents in Ho Chi Minh City, Vietnam
- Prevalence and intensity of soil-transmitted helminth infections among school-age children in the Cagayan Valley, the Philippines
- Prevalence of cryptosporidiosis in animals in Iran: A systematic review and metaanalysis
