Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19
,Lifng Yin ,
a Jiangsu Province Engineering Research Center for R&D and Evaluation of Intelligent Drugs and Key Functional Excipients,China Pharmaceutical University,Nanjing 210009,China
b Department of Pharmaceutical Analysis,School of Pharmacy,China Pharmaceutical University,Nanjing 210009,China
c Department of Medicinal Chemistry,School of Pharmacy,China Pharmaceutical University,Nanjing 210009,China
Keywords:COVID-19 Remdesivir Pulmonary delivery Liposomal aerosol Nucleotide triphosphate
ABSTRACT Strong infectivity enables coronavirus disease 2019 (COVID-19) to rage throughout the world.Moreover,the lack of drugs with definite therapeutic effects further aggravates the spread of the pandemic.Remdesivir is one of the most promising anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) drugs.However,the limited clinical effects make its therapeutic effect controversial,which may result from the poor accumulation and activation of remdesivir in the lung.Therefore,we developed lyophilized remdesivir liposomes (Rdv-lips) which can be reconstituted as liposomal aerosol for pulmonary delivery to improve the in vivo behavior of existing remdesivir cyclodextrin conclusion compound (Rdv-cyc) injections.Liposome encapsulation endowed remdesivir with much higher solubility and better biocompatibility.The in vitro liposomal aerosol characterization demonstrated that Rdv-lips possessed a mass median aerodynamic diameter of 4.118 μm and fine particle fraction (< 5 μm) higher than 50%,indicating good pulmonary delivery properties.Compared to the Rdv-cyc intravenous injection group,the Rdv-lips inhalation group displayed a nearly 100-fold increase in the remdesivir-active metabolite nucleotide triphosphate (NTP) concentration and better NTP accumulation in the lung than the Rdv-cyc inhalation group.A faster transition from remdesivir to NTP of Rdv-lips (inhalation) could also be observed due to better cell uptake.Compared to other preparations,the superiority of Rdv-lips was further evidenced by the results of an in vivo safety study,with little possibility of inducing inflammation.In conclusion,Rdv-lips for pulmonary delivery will be a potent formulation to improve the in vivo behavior of remdesivir and exert better therapeutic effects in COVID-19 treatment.
1.Introduction
First appearing in late 2019,the status of the pandemic of coronavirus disease 2019 (COVID-19) is still serious and affecting more than 200 countries and regions [1,2] .The pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with high infectiousness [3–5],leading to various symptoms from asymptomatic disease to pneumonia and life-threatening complications,including acute respiratory distress syndrome,multisystem organ failure,and ultimately death [6–9] .As of August 13,2021,more than 206 million coronavirus cases and 4.3 million deaths were reported [2] .The rapid mutation of the virus made the pandemic situation more severe,and threw out a challenge to vaccine development,which led to a more urgent need for other ways to control the COVID-19.Unfortunately,although extensive clinical trials for COVID-19 have been carried out,few antiviral drugs have been proven to be effective in a strict randomized,double-blind,placebo-controlled study [10] .Therefore,it is an immediate problem to develop drugs with definite therapeutic effects on COVID-19.
Remdesivir (Veklury?) was the first drug approved by the U.S.Food and Drug Administration (FDA) for the treatment of COVID-19 [11] .However,it was originally a nucleotide prodrug developed by Gilead Sciences,Inc.against Ebola virus (EBOV)[12] .Through intracellular metabolic activation,remdesivir converts to active nucleoside triphosphate (NTP),which can incorporate into nascent viral RNA chains and interfere with RNA-dependent RNA polymerase (RdRp),leading to the premature termination of viral RNA transcription and finally the inhibition of virus replication [6,13–16].Further studies demonstrated that remdesivir was a broad-spectrum antiviral agent with satisfactory activity against a wide array of RNA viruses,including coronaviridae (such as SARS-CoV and MERS-CoV),paramyxoviridae (such as Nipah virus,respiratory syncytial virus,and Hendra virus),and filoviridae (such as Ebola virus) [14,15,17–19].SARS-CoV-2 is highly homologous to SARS-CoV [20] in terms of both genome (79.6%) and amino acid sequences of seven conserved nonstructural proteins(94.4%) [21] .Thus,remdesivir was tested as a candidate drug for COVID-19.Thein vitro
antiviral experiments and compassionate use in COVID-19 patients gave encouraging results for remdesivir [15,22],and it was approved for the treatment of COVID-19 requiring hospitalization on October 22,2020 [11] .Unlike EBOV,the leading cause of death among SARSCoV-2-infected people is acute respiratory distress syndrome(ARDS) [23] instead of severe systemic disease [24,25],indicating that the treatment for COVID-19 should focus on the respiratory system,especially the lung.However,remdesivir was not able to exert fully a therapeutic effect in the lung because of poor accumulation after intravenous injection and low expression of the enzymes necessary for activation [26,27] .In fact,a growing body of evidence suggests that remdesivir also does not achieve optimal results in COVID-19 patients [28] .On the other hand,the dose regimen for remdesivir is 200 mg administered intravenously on the first day,followed by 100 mg daily for 4–9 consecutive days [6] .The low water solubility (<
0.03 mg/ml)[29] of remdesivir,which cannot be satisfied with the relatively large administration dosage,will raise difficulties for the preparation process.Gilead Sciences,Inc.used the cyclodextrin inclusion technique to increase the solubility of remdesivir.A 30-fold of sulfobutyl ether-β
-cyclodextrin (SBEβ
-CD) was used to ensure the complete inclusion of remdesivir[30] .The direct entry of a large amount of SBE-β
-CD into the bloodstream limited the use of remdesivir in patients with renal insufficiency [30–32] .In addition,the intravenous route is not commonly used for self-administration of medicine,which would limit its application under the shortage of medical resource.Therefore,an improved delivery method for remdesivir with increased solubility,optimizedin vivo
behavior and convenient administration route is urgently needed.Pulmonary delivery of remdesivir through inhalation administration is an appropriate strategy that can bypass the serious liver first-pass effect for remdesivir and considerably increase the drug concentration in the lung [33–35] .More drugs accumulating in the lung also lead to a reduction in dosage and a decrease in systemic side effects [36–38] .Other benefits of pulmonary delivery include a large absorption area,rapid onset of pharmacological action and good compliance[39,40] .Liposome is an emerging carrier for pulmonary drug delivery [41–43] .It has outstanding biocompatibility with alveolar surfactants,whose main components are also lipids(90%) [44,45] .Through liposome encapsulation and pulmonary administration,the preparation of remdesivir combined the advantages of both liposomes and inhalant.The solubility and stability of remdesivir can be enhanced through incorporation into the lipid bilayer of liposomes.At the same time,liposomes also help to reduce the stimulation of remdesivir in the respiratory tract and lung.Direct delivery of remdesivir into the lung by liposomal aerosols ensures a higher accumulation and longer retention time of the drug in disease lesions,which will be beneficial for the enhanced therapeutic effect of remdesivir against SARS-CoV-2.
In this study,we developed remdesivir liposome (Rdv-lips)aerosols,which can be supplied in the form of lyophilized liposome powder and reconstituted to liposomal suspension for pulmonary delivery.The Rdv-lips exhibited not only good drug-loading capacity and aerodynamic properties,but also improvedin vivo
behavior with a much higher concentration of NTP in the lung as illustrated in Scheme 1 .All these results provide promising prospects for Rdv-lips aerosols to be applied in COVID-19 treatment with enhanced therapeutic effects.
Scheme 1 -Schematic illustration of the Rdv-lips aerosol inhaled into lung.The Rdv-lips suspension is atomized and then inhaled into lung to increase the drug accumulation.After depositing at alveoli,the Rdv-lips are taken by the alveolar epithelial cells rapidly due to the better cytocompatibility and high loading rate of Rdv-lips.All these above lead to a sustained higher concentration of active metabolite NTP in lung.
2.Materials and methods
2.1.Materials
Remdesivir with more than 97% purity was furnished from Nanjing Kang Chuan Ji Pharmaceutical Co.,Ltd.(Nanjing,China).Sulfobutyl ether-β
-cyclodextrin (SBEβ
-CD) was purchased from Qianhui Biotechnology Co.,Ltd.(Shandong,China).Both 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) were purchased from Shanghai AVT Pharmaceutical Technology Co.,Ltd.(Shanghai,China).Cholesterol and trehalose were purchased from Sigma-Aldrich Co.,Ltd.(Jiangsu,China).1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR) was purchased from Thermo Fisher Scientific Inc.(Shanghai,China).A remdesivir triphosphate standard (NTP,GS-443902) was purchased from Shanghai Biochempartner Co.,Ltd.(Shanghai,China).Sofosbuvir triphosphate internal standard (PSI-7409)was purchased from MedChemExpress LLC.(Shanghai,China),and interleukin-6 (IL-6),interleukin-10 (IL-10) and tumor necrosis factor-α
(TNF-α
) ELISA kits were purchased from MULTI Science Biotech Co.,Ltd.(Hangzhou,China).A high mobility group box 1 protein (HMGB-1) ELISA kit was purchased from Elabscience Biotechnology Co.,Ltd.(Wuhan,China).A549 cells and fetal bovine serum were purchased from Nanjing KeyGEN Biotech Co.,Ltd.(Nanjing,China).RPMI-1640,trypsin and penicillin-streptomycin solution were purchased from Nanjing FcmacsBiotech Co.,Ltd.(Nanjing,China).2.2.Preparation of Rdv-lips and Rdv-cyc
Rdv-lips were prepared by a film hydration method.Lipids(DPPC:cholesterol:DSPE-PEG2000=7:2:1) and remdesivir were dissolved in chloroform solutions with different remdesivir concentrations and then dried at 45 °C by a rotary evaporator (Nanjing Keer Instrument Equipment Co.,Ltd.,Nanjing,China) for 2 h to form a thin film,followed by the addition of pH 6.5 PBS to hydrate the film for 40 min.The film solution was treated with a micro ultrasonic probe(Scientz Biotechnology Co.,Ltd.,Ningbo,China) at 200 W for 10 min.The unencapsulated drugs were removed through ultrafiltration (MWCO=3500 Da) by centrifuging at 8000 rpm for 10 min.The prepared Rdv-lips were freeze-dried by an LGJ-18C lyophilizer (Sihuan Scientific Instrument Co.Ltd.,Beijing) with the addition of trehalose (5%,w/w) to obtain white solid powder for long-term storage.
Rdv-cyc was prepared by the following steps.SBE-β
-CD was weighed and dissolved in water,and then the solution was acidified by the addition of HCl (1 mol/l).Remdesivir was added and stirred until it completely dissolved,and the pH was adjusted to 4 by sodium hydroxide (1 mol/l).A certain procedure was followed to freeze and freeze-dry the remdesivir solution to obtain a white solid powder [29] .All the Rdv-lips samples and Rdv-cyc samples used in experiments were reconstituted from lyophilized powder unless otherwise indicated.The DiR-cyc and DiR-lips were prepared using the same method with Rdv-cyc and Rdv-lips,respectively,by replacing remdesivir with DiR.
2.3.Characterization
The average particle size,polydispersity index (PDI),andζ
potential of liposomes were measured with a dynamic laser scatter instrument (Brookhaven Instruments,Holtsville,NY)at room temperature based on the operating guidelines of dynamic light scattering (DLS).Morphological observation of the samples was performed by JEM-1230 TEM (Tokyo,Japan) with an accelerating voltage of 200 kV.The Rdv-lips used in TEM examination were fresh prepared liposome suspension to remove the influences of cryoprotectant.Before the TEM examination,a drop of the sample was deposited on the carbon network and stained with 2% (v/v) phosphortungstic acid for 3 min.The excess samples and liquid were removed with filter paper and then dried at 25 °C.
The encapsulation efficiency (EE) and drug loading efficiency (DL) of Rdv-lips were determined by ultraviolet(UV) spectrophotometry (Shimadzu,Suzhou,China) at a wavelength of 245 nm.Before measurement,the liposomes were diluted with methyl alcohol to the appropriate concentration.EE and DL were calculated using the following equations:
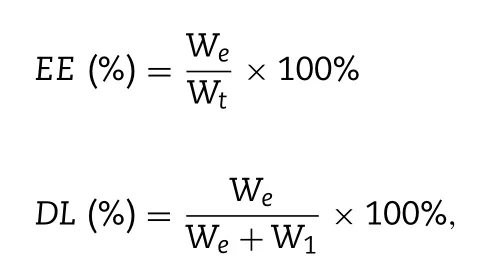
W
t,W
e,andW
l are the weight of the drug fed to the liposomes,the detected weight of the drug encapsulated in the liposomes,and the weight of the lipid added to the system,respectively.2.4.Drug release
Drug release was performed by a dialysis method using an RC806D dissolution tester (TDTF,Tianjin,China).An appropriate amount of different Rdv-lips suspension (Freshly prepared Rdv-lips:Rdv-lips suspension prepared before the dissolution test without lyophilization;Lyophilized Rdvlips:Rdv-lips freeze-dried powder reconstituted by saline;Lyophilized Rdv-lips after atomization:Rdv-lips suspension collected after atomization of reconstituted Rdv-lips freezedried powder.) equivalent to 0.45 mg remdesivir was added into a dialysis tube (MWCO 3500 Da) and incubated with 200 ml simulated lung fluid (SLF) at 37 °C with a stirring speed of 50 RPM.SLF was prepared according to previous reports[46,47] .Aliquots were drawn at predetermined time points(5,10,15,30,60,120,240 and 360 min),and the medium was immediately replenished with fresh SLF.The release of remdesivir at different time points was measured by highperformance liquid chromatography (HPLC) which details were provided in the supplementary information.
2.5.Stability
The stability of Rdv-cyc solution under different temperature was measured by HPLC.Rdv-cyc solution was kept at 10 °C,25 °C and 40 °C separately and aliquots were drawn at predetermined time points (0,2,6 and 24 h).The HPLC analysis method was the same as that described in“2.4.Drug Release”.The total impurities content was calculated by area normalization method.The stability of Rdv-lips evaluation lasted for 10 d.The freeze-dried powder of Rdvlips was reconstituted with RO water,saline,and pH 6.5 PBS,respectively,and stored at 4 °C and 25 °C.At predetermined time points (0,6,12,24,36,48,72,96 and 240 h),the samples were withdrawn and determined size and PDI as described above.
The long-term stability of lyophilized Rdv-lips lasted for 6 months.The freeze-dried powder of Rdv-lips was stored at 4 °C and was reconstituted at predetermined time point to measure the size,PDI,EE and DL as described above.
2.6.In vitro aerosol characterization
The size distribution of aerodynamic particles was measured by Next Generation Impactor (NGI)-120 (Copley Scientific Ltd.,UK).The NGI was operated at a flow rate of 15 l/min using a high capacity pump precooled to 5 °C for 90 min.Four milliliters of Rdv-lips suspension or Rdv-cyc solution was nebulized into the NGI.After 10 min of atomization,the sample plates of stages 0–7 and the filter paper of the microorifice collector (MOC) were washed with methyl alcohol to collect the sample.All the samples were centrifuged at 8000 RPM for 5 min,and the supernatant was collected for HPLC determination.The mass median aerodynamic diameter(MMAD),fine particle fraction (FPF) and geometric standard deviation (GSD) were calculated by Copley Inhaler Testing Data Analysis Software (Version 3.10).
The BPS1100 breath simulator (Copley Scientific Ltd.,UK)and filter device were installed according to the guidelines.Two milliliters of Rdv-lips suspension or Rdv-cyc solution was added to the atomization cup.The breath simulator was set to adult mode (breathing rate:15 cycles/min,tidal volume:500 ml and ratio of inhalation to exhalation time 1:1).The samples of the first stage (the first minute) and the second stage (the remaining time) were collected by washing the filter paper with methyl alcohol.Then,the samples were centrifuged at 8000 RPM for 5 min to collect supernatant for HPLC determination.
2.7.Cell culture
A549 cells were cultured in RPMI 1640 medium containing a combination of 10% fetal bovine serum and 1% penicillin streptomycin at 37 °C with 5% CO.Trypsin was used for cells harvested.
2.8.Laboratory animal care
All experimental animals were purchased from the Sino-British SIPPR/BK Lab.Animal Ltd.(Shanghai,China) and were cared for in accordance with the Principles of Experimental Animal Care and Guide for the Care and Use of Laboratory Animals.All animal experiments were carried out in accordance with the plan approved by the China Pharmaceutical University Institutional Animal Care and Use Committee.
2.9.In vivo pharmacokinetics and tissue distribution
Normal BALB/c mice (male,18–22 g) were treated with Rdv-cyc (intravenous injection),Rdv-cyc (transtracheal injection),or Rdv-lips (transtracheal injection) at a remdesivir dosage of 20 mg/kg per mouse.For the determination of NTP pharmacokinetics in the lung,the mice (n
=3) were ordinally sacrificed to isolate the lung tissue at predetermined time points (1,2,4,8,12 and 24 h).Precold extraction buffer containing 0.1% potassium hydroxide and 67 mM EDTA in 70%methanol [14] and 0.2 μM sofosbuvir triphosphate (internal standard) was added and homogenized.The homogenate was centrifuged at 20 000 g for 20 min,and then the supernatants were collected and dried in a centrifugal vacuum concentrator(Labconco Corporation,USA).The dried samples were reconstituted with 100 μl mobile phase and then centrifuged at 20 000 g for 20 min.The NTP concentration in supernatants was determined by LC/MS/MS experiments,which details were provided in the supplementary information.For the determination of NTP tissue distribution,the mice (n
=6)were ordinally sacrificed to isolate the tissue (the heart,liver,spleen,lung,kidney,brain,and testis) at predetermined time points (4 h and 24 h).The sample treatment method and LC/MS/MS method were the same as above.2.10.In vitro cell uptake
The cell uptake experiment was performed on A549 cells,a cell line derived from a human adenocarcinoma of the lung,which resemble type II alveolar epithelial cell [48] and are widely used in the study of the drug delivery and metabolic at the pulmonary epithelium [49–51] .A549 cells were inoculated in 12-well plates at a density of 1 ×10cells per well and cultured for 24 h.The DiR-cyc and DiR-lips were added into 12-well plates at a fixed concentration of 0.1 μg/ml DiR.After 1 h,2 h and 4 h co-cultured,the cells were washed with cold PBS for 3 times and resuspended in 500 μl of PBS for flow cytometry analysis (Accuri C6,BD,America).
2.11.In vivo safety study
To evaluate thein vivo
safety of different preparations,thirty normal BALB/c mice (male,18–22 g) were randomly divided into five groups (n
=6) and received control (saline,inh.,once a day),blank lips (blank liposomes 100 mg/kg,inh.,once a day),Rdv-cyc (remdesivir 20 mg/kg,i.v.,once a day),Rdv-cyc (remdesivir 20 mg/kg,inh.,once a day) and Rdv-lips(remdesivir 20 mg/kg,inh.,once a day) treatments.The Rdvlips and Rdv-cyc were prepared as described above,and the two inhalation groups were administrated by intratracheal instillation [52,53] .The body weight was measured every 2 d during the treatment period.Ten days after administration,the mice were sacrificed to isolate the major tissues for hematoxylin-eosin staining (H&E) analysis.Serum,lung tissue homogenate and bronchoalveolar lavage fluid (BALF) were collected for enzyme-linked immunosorbent assay (ELISA).2.12.H&E stain
The histomorphology of different tissues was detected using H&E (Cat:C0105S;Beyotime) kits and performed according to the standard instructions.The isolated tissues were fixed in 4% paraformaldehyde and paraffin-embedded to prepare 5 μm sections.The sections were observed under an optical microscope (Ts2R,Nikon,Japan) to provide a final report in 5 representative fields.
2.13.ELISA
IL-6,IL-10,TNF-α
and HMGB-1 levels in serum,BALF were measured using mouse ELISA Kits according to the manufacturer’s instructions.The levels of these cytokines were measured by Varioskan Flash (POLARstar,Omega,Germany).2.14.Statistical analysis
Data are presented as the mean ± standard deviation (SD).Statistical analysis was conducted by one-way analysis of variance (ANOVA),and aP
value less than 0.05 was considered significant.3.Results and discussion
3.1.Preparation and characterization
In this study,Rdv-lips aerosol was developed to improve thein vivo
behavior of the existing Rdv-cyc injection.Liposomes,which exhibit excellent biocompatibility,biodegradability and drug loading ability [54],were chosen as solubilizer of remdesivir to replace SBE-β
-CD.Half of the lipid that comprises 90% of pulmonary surfactant is DPPC [44,45] .Therefore,DPPC was employed as the main liposome material for better biocompatibility.Rdv-lips were prepared by film hydration followed by the probe supersonic method.A series of Rdv-lips with different drug/lipid ratios were prepared to study the effect of remdesivir on liposomes (Table 1).The encapsulation of remdesivir modestly increased the size of liposomes from 105.4 ±1.7 nm (blank liposomes) to approximately 120 nm and showed a negligible influence on zeta potential (from -5.46 ± 1.42 mV (blank liposomes) to approximately -7 mV).The nanoscale of the liposomes could help it to evade the clearance of macrophages [55,56] .Through liposome encapsulation,the solubility of remdesivir could be increased dramatically with DL up to 11.10% (Table 1).This enabled the reduction of excipients compared to Rdv-cyc which needed as high as 30-fold cyclodextrin to ensure the complete inclusion according to the product description of Gilead Sciences,Inc[30] .The spherical morphology and uniform size of Rdv-lips were confirmed by TEM,and a smaller size of approximately 60 nm of Rdv-lips was observed in Fig.1 B,which might be the result of losing the hydrated layer during sample preparation.
Table 1-Characterization of liposomes with different drug/lipid ratios.
3.2.In vitro stability and drug release
The stability of Rdv-cyc solution was investigated by HPLC method.The total impurities increased quickly over time even at a lower temperature (Table S1),indicating a poor stability of remdesivir in solution consistent with the previous report [57,58] .To maintain the stability of liposomes during long-term storage,the Rdv-lips could be lyophilized in the presence of cryoprotectants,such as trehalose and lactose.Lyophilization also helped to concentrate the liposomes to meet the requirement of administration dosage.After lyophilization,the size of Rdv-lips increased to 238.1 ±3.2 nm with a slight decrease in EE and DL (Table S2).Reconstitution with an appropriate solvent would help to ensure the stability and uniformity of the preparation during ultrasonic atomization and storage.The size and PDI of the liposomes reconstituted with different solvents and stored at room temperature (25 °C) or low temperature (4 °C) were measured at predetermined time points (Fig.1 D and Fig.1 E).While an increase in size was observed in the reconstituted preparation,the liposomes displayed excellent stability in all three kinds of solvents (water,saline and pH 6.5 PBS) with slight variations (<
5%) in size and small PDI values,which provided a basis to avoid aggregation and sedimentation during ultrasonic atomization.On the other hand,the longterm stability of lyophilized Rdv-lips was further evidenced by the stable size,PDI and negligible leakage of remdesivir from liposomes measured after a 6-month storage (Table S3).The good stability of Rdv-lips provides prospects for large-scale industrial production.
Fig.1-Characterization.(A) Size distributions of freshly prepared Rdv-lips.(B) TEM images of freshly prepared Rdv-lips under different scales.(C) In vitro release profile of different Rdv-lips in SLF at 37 °C (mean ±SD,n=3).Freshly prepared Rdv-lips is Rdv-lips suspension prepared before the dissolution test without lyophilization;Lyophilized Rdv-lips is Rdv-lips freeze-dried powder reconstituted by saline;Lyophilized Rdv-lips after atomization is Rdv-lips suspension collected after atomization of reconstituted Rdv-lips freeze-dried powder.In vitro stability of lyophilized Rdv-Lips reconstituted by reverse osmosis water,saline and pH 6.5 PBS at (D) 25 °C or (E) 4 °C (mean ±SD,n=3).
The release profile was further investigated in SLF [46,47]based on HPLC assay.Remdesivir was released from freshly prepared liposomes at a moderate speed,and more than 85%of the drugs were released in 6 h (Fig.1 C).Lyophilization was reported to change the size,morphology and bilayer structure of liposomes,and finally affected the release profile [59] .Lyophilized Rdv-lips showed a slower release rate at the early periods and a slightly higher total release amount (>
90%).The changes of release profile maybe caused by the interaction between cryoprotectants and the lipid bilayer [60,61] .3.3.In vitro aerosol characterization
The aerodynamic diameter of an airborne particle is the key property that directly affects drug deposition and clinical efficacy.Particles with a mass median aerodynamic diameter between 1 μm and 5 μm can easily reach the lower respiratory tract and deposit on the surface of the trachea,bronchi and alveoli.Out of this range,larger particles tend to deposit in the oropharynx and upper respiratory tracts,and smaller particles will be eliminated from the lung during exhalation [45,62] .
To determine thein vitro
aerodynamic behavior of the drug particles,the Rdv-lips suspension was nebulized into NGI.Previous studies reported that solutions of cyclodextrin inclusion compound could undergo aerosolization and produce droplets with appropriate size for pulmonary delivery [63] .Therefore,Rdv-cyc solution was chosen as the control group to observe the effect of liposomal vehicles on aerodynamic parameters better.According to Fig.2,compared to Rdv-cyc,the size distribution of Rdv-lips shifted slightly to the left,which meant that liposomes lead to an increase in the aerodynamic diameter of airborne particles [64] .FPF of Rdv-lips exhibited that more than 50% of the aerosol particles were below 5 μm.The MMAD of Rdv-lips was 4.118 ±0.104 μm.GSD was~2,which indicated a narrow distribution for Rdvlips [64] .Although these two formulations were different in MMAD,FPF,and GSD,they both possessed good characteristics for pulmonary delivery (Table 2).
Table 3-Delivery rate and total delivery amount of the Rdv-lips suspension aerosol and Rdv-cyc solution aerosol.
Fig.2-Drug deposition rate of the Rdv-lips suspension aerosol and Rdv-cyc solution aerosol at different stages.Data are expressed as the mean ±SD (n=3).
Table 2-Particle size distribution of Rdv-lips suspension aerosol and Rdv-cyc solution aerosol.Data are expressed as the mean ±SD (n=3).
The study of delivery rate and total delivery amount was conducted by a respiratory simulator and filtration system to further investigate the delivery properties of liposomes.Rdvlips showed a faster delivery rate and higher total delivery amount than Rdv-cyc,indicating better pulmonary delivery properties for Rdv-lips with less loss of drug during ultrasonic atomization (Table 3).
3.4.Atomization stability
The suspension of Rdv-lips after nebulization was collected to determine the influence of ultrasonic atomization (Table S4).Significant decrease in size and PDI of Rdv-lips was observed after atomization,which could attribute to the shearing provided by the nebulizer [65] .The force may lead to the fragmentation of the liposomes and the leakage of the drug.However,there was a little decrease in EE and DL of Rdv-lips after atomization.According to previous studies,hydrophobic drugs kept better physical stability during atomization than hydrophilic drugs,which explained the little leakage of Rdvlips.Notably,the significant change in size after atomization did not affect the release profile of Rdv-lips (Fig.1 C),which meant that the size of liposomes was not the key factor for the release of remdesivir.
3.5.Pharmacokinetics of NTP in the lung
A previous report indicated that plasma exposure to remdesivir and even NTP could not reflect the clinical efficacy well [26] .Remdesivir was designed as a prodrug of nucleoside monophosphate (Nuc-MP) to improve its cell membrane permeability.It was extremely unstable in the presence of plasma esterase (T <
5 min).After entering the blood stream,remdesivir distributed into the peripheral blood mononuclear cells (PBMCs) rapidly and converted to NTP [14,17] .However,due to the negative charge and the polarity,NTP and the intermediate metabolites would be trapped inside the cell.Hence,both remdesivir and NTP were hard to be determined in plasma.The entrapment of NTP inside PBMCs also limited its tissue permeation and distribution,making it hard to correlate the plasma exposure with tissue concentration [26] .When in the tissue,remdesivir converted to NTP rapidly and became the predominant intracellular metabolite with a much longer half-life [14,17] .Therefore,the concentration of pharmacological active NTP in tissue,especially in lung which primarily affected by virus,would be a better indicator for the comparison of different preparations of remdesivir.To evaluate the improvements of Rdv-lips for pulmonary delivery,we determined the pharmacokinetics of NTP in the lung of different preparations,which had a direct link with thein vivo
efficacy.Rdv-cyc solution given by intravenous injection was chose as a control group to show the enhancement of Rdv-lips inhalation group (Rdv-lips inh.) compared to the commercial product of Gilead Sciences,Inc.Additionally,cyclodextrins including SBE-β
-CD have been widely studied as solubilizer for pulmonary delivery [66–68] .Therefore,Rdv-cyc solution was also used for inhalation as a positive control to deliver the insoluble remdesivir to lung.In line with our expectations,two aerosols exhibited remarkable pulmonary accumulation because of the direct delivery of remdesivir to the lung (Fig.3 A and 3 B).Compared to that of the Rdv-cyc intravenous injection group (Rdv-cyc i.v.),a nearly 100-fold increase in peak NTP concentration in the Rdvlips inh.and a 77-fold increase in the Rdv-cyc inhalation group(Rdv-cyc inh.) were detected,indicating the incomparable superiority of the pury delivery system.In the two inhalation groups,differences in dosage form caused differences in their pharmacokinetic profiles (Fig.3 A and 3 B).Liposomes group displayed much quicker transition from remdesivir to NTP as its peak concentration appeared at the first time point (1 h after administration) compared to cyclodextrin group,whoseT
was at 4 h after the administration.This result could be explained by the high drug loading rate and cell membrane biocompatibility of liposomes,leading to quicker and higher cell uptake of remdesivir.The cell uptake ability of Rdv-lips was reinforced by the results in A549 cells (Fig.S1).DiR was used as a substitute of remdesivir due to its hydrophobicity.Compared to DiR-cyc,DiR-lips displayed a much better cell uptake in both rate and amount,which was consistent with the rapid intracellular NTP conversion of Rdv-lipsin vivo
.It was unexpected that direct pulmonary delivery did not change theT max
in lung of Rdv-cyc compared to intravenous injection (Fig.3 B),which meant that the administration site of Rdv-cyc was not the determinant of the NTP conversion speed in lung.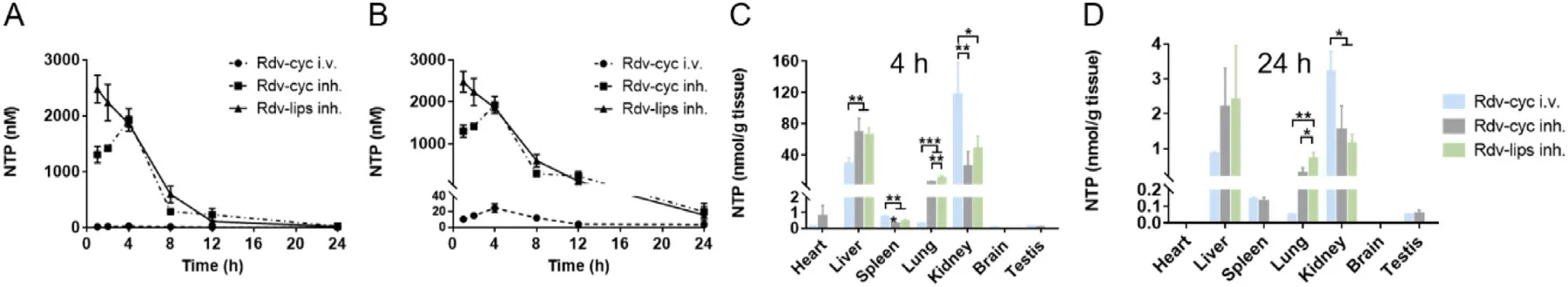
Fig.3-Pharmacokinetics and tissue distribution of NTP in vivo .(A) Concentration-time curves of NTP in lung homogenate measured by LC/MS/MS after different treatments in mice at a remdesivir dose of 20 mg/kg (n=3).(B) The larger version of(A).The concentration of NTP measured by LC/MS/MS in major organs collected from the mice at 4 h (C) and 24 h (D) after administration (n=6).Data are expressed as the mean ±SD.?P < 0.05,??P < 0.01,???P < 0.001.
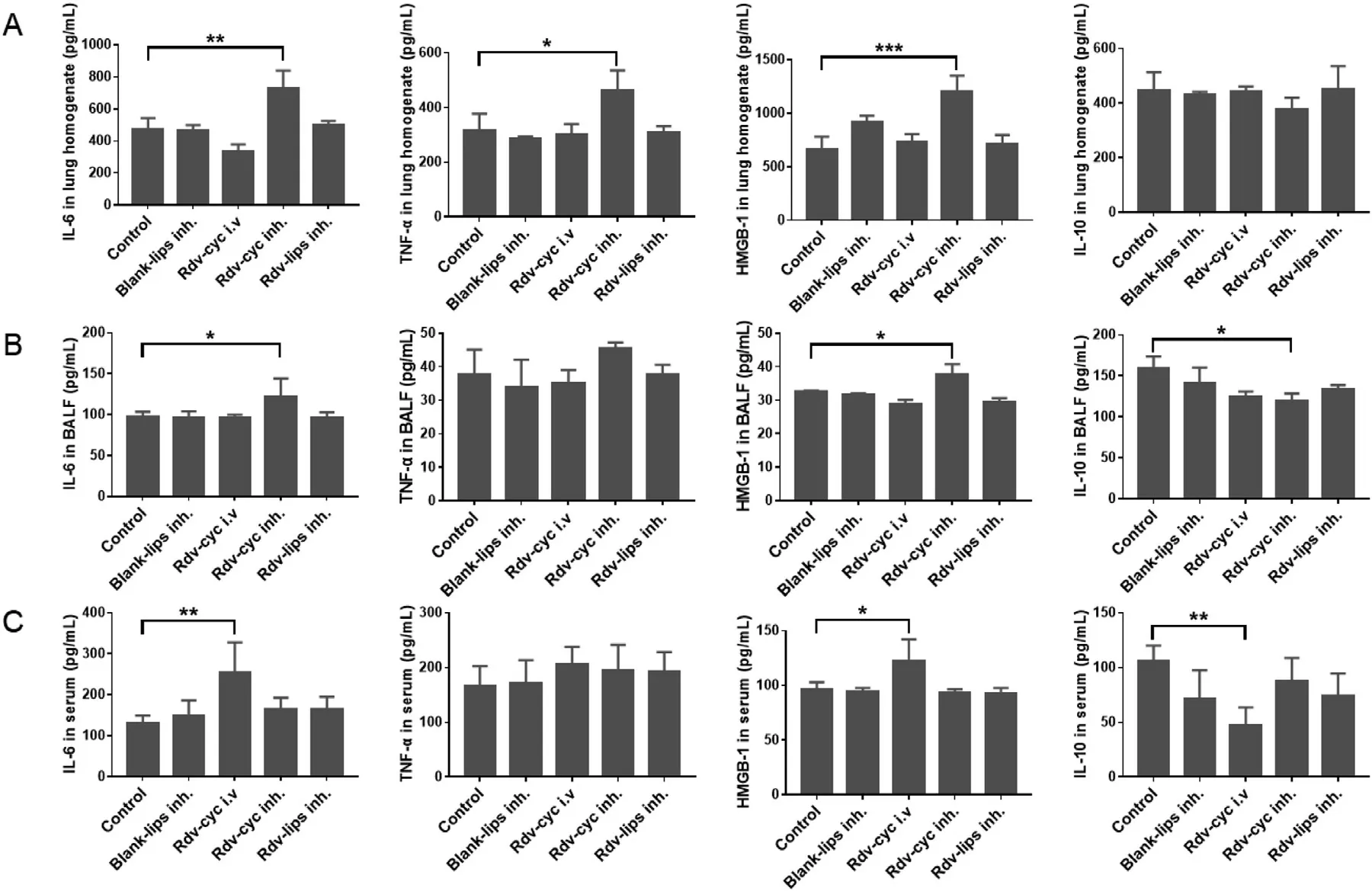
Fig.4-Inflammation-linked cytokines.Quantitative analysis of IL-6,TNF-α,IL-10 and HMGB-1 in (A) lung homogenate(n=3),(B) BALF (n=3) and (C) serum (n=6).Serum,lung tissue homogenate and BALF were collected 11 d after administration.Cytokines were measured by ELISA (mean ±SD,?P < 0.05,??P < 0.01,???P < 0.001).
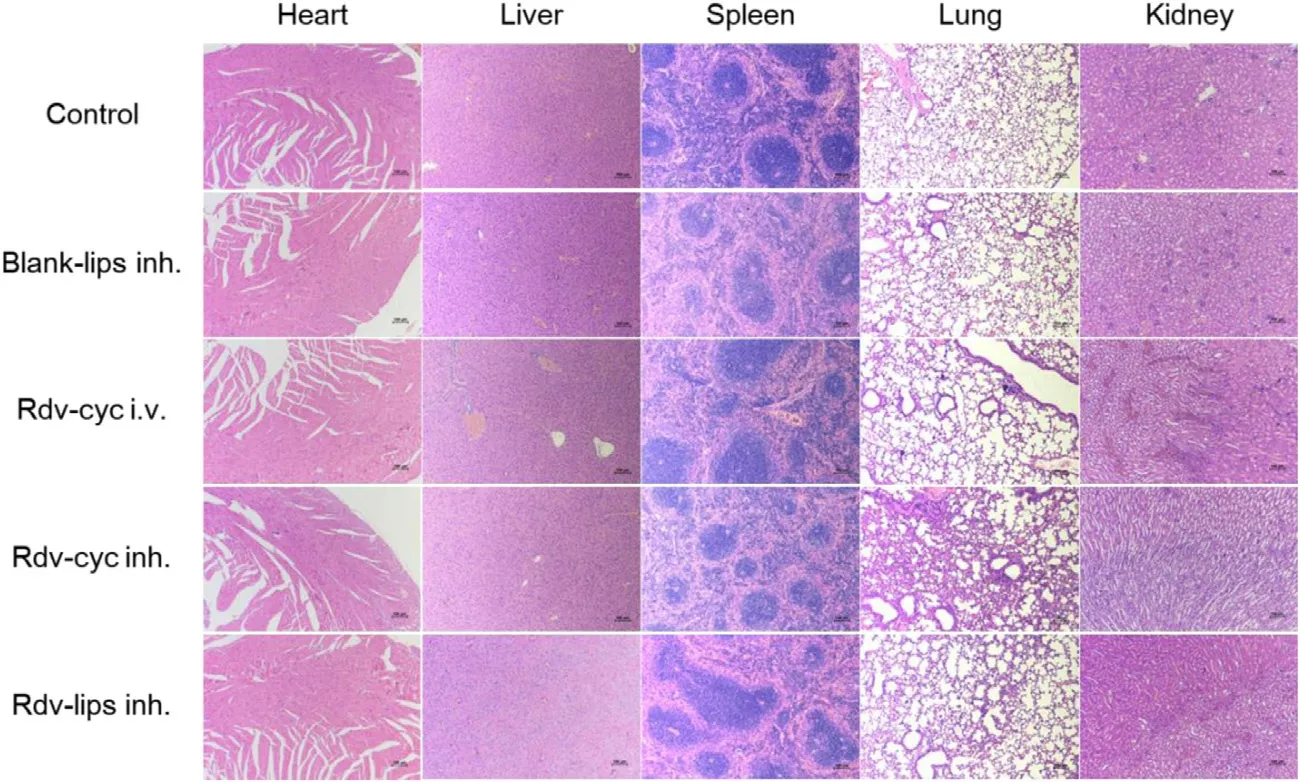
Fig.5-H&E-stained organ slices from normal BALB/c mice treated with saline (control,inh.),Blank-lips (inh.),Rdv-cyc (i.v.),Rdv-cyc inh.and Rdv-lips inh.(n=3).The scale bar is 100 μm.
3.6.Tissue distribution of NTP
The tissue distribution of NTP was further investigated to provide an overall description of the behavior of NTPin vivo
(Fig.3 C and Fig.3 D).4 h later after administration,Rdv-lips inh.displayed a much higher NTP content in the lung than that of Rdv-cyc i.v.,with a 35-fold increase in NTP concentration.This ratio remained 13-fold even at 24 h after administration.Rdv-cyc inh.showed a relatively high NTP content resembling Rdv-lips inh.However,Rdv-lips inh.always displayed a significantly higher NTP concentration than Rdv-cyc inh.at 4 h or 24 h after administration,indicating better pulmonary accumulation of Rdv-lips.This could be explained by the retention capability and rapid cell uptake of liposomes,leading to the entrapment of more remdesivir in the lung and their conversion to NTP.However,liver possessed a much higher NTP concentration than lung in all the groups.Because the enzymes involving in the bioactivation of remdesivir,such as carboxylesterases(CES1),cathepsin A (CTSA) and phosphoramidases (histidine triad nucleotide binding proteins,HINTs),all had high expression in the liver [27,69–71].Thus,a larger quantity of remdesivir could be quicker conversed to NTP in liver,reducing the amount of remdesivir activated in lung,especially in intravenous group.The high NTP concentration in kidney was in line with the fact that kidney is the main excretory organ of remdesivir [30],and higher NTP concentration indicating a quicker clearance of the drug.For other organ like heart,spleen,brain and testis,Rdv-lips inh.always exhibited a significant decrease in NTP accumulation compared to the other two groups,implying less possibility for Rdv-lips to cause systemic toxicity.This phenomenon could be explained by the excellent characteristic of liposomes to accumulate at the administration site.According to the previous report,a high concentration of remdesivir was detected in testis and epididymis at 4 h after administration[17] .And in brain,although the remdesivir concentration was not as higher as other tissue,it remained detectable above the drug plasma levels 168 h after injection [17] .Nevertheless,our results of NTP tissue distribution did not show the same tendency.In the one hand,even though the high fat content made lipophilic remdesivir tend to distribute in these tissues [72],the enzymes for the bioactivation of remdesivir were low expressed in these tissues.In the other hand,the administration route of inhalation and the preparation form of liposome also reduced the drug accumulation in these tissues.
3.7.In vivo safety evaluation
One of the advantages of aerosols is the lower possibility of inducing systemic side effects because a large amount of drugs are directly delivered to the lesion and exert efficacy there [73] .For Rdv-lips,it also has the better biocompatibility and less excipients dosage to improve its safety compared to Rdv-cyc.IL-6,TNF-α
,and HMGB-1 were measured as proinflammatory cytokine indicators,and IL-10 was measured as an anti-inflammatory cytokine indicator to evaluate inflammation after treatment (Fig.4)[74–76] .Interestingly,in lung-associated samples,such as lung homogenate and BALF,only Rdv-cyc inh.showed an increase in proinflammatory cytokines and a decrease in antiinflammatory cytokines,indicating slight lung inflammation that was induced by the successive administration of Rdv-cyc in the lung (Fig.4 A and Fig.4 B).In regard to plasma samples that reflected systemic inflammation,Rdv-cyc i.v.exhibited a tendency toward inflammation with the upregulation of IL-6 and HMGB-1 and the downregulation of IL-10 (Fig.4 C).In contrast,both the blank-lips (inh.) and Rdv-lips inh.had good safety performance with no difference between them and the control group in ELISA results (Fig.4 A–Fig.4 C).H&E analysis in the main organs did not show any change in histology or morphology (Fig.5).The body weights of mice during administration were not significantly different between groups.These results showed that none of the preparations had obvious organ toxicity or systemic toxicity(Fig.6).
Fig.6-Body weight changes of treated mice (n=6,mean ±SD).
Many studies have implied that intravenous injection might not be the proper mode for the administration of remdesivir due to the following reasons:(1) remdesivir had poor stability in plasma,leading to a concentration lower than EC 50 when it reached lung;(2) the quick distribution in PBMCs of remdesivir limited its tissue penetration,because remdesivir efficiently converted to NTP with low membrane permeability in cells;(3) the abundant enzymes necessary for the bioactivation of remdesivir were highly expressed in liver and kidney,which further reduced the amount of drug reaching lung.
Pulmonary delivery of remdesivir can solve these problems by direct administration to lung [77,78] .There have been some studies on the other preparations for pulmonary delivery of remdesivir,including dry powder inhalant and even liposomal aerosols [57,79] .These preparations also possessed high drug content and good lung deposition efficiency.However,Rdv-lips inh.still have some special advantages.Compared to other preparations for pulmonary delivery,the biocompatibility and size of liposomes endowed Rdv-lips with faster cell uptake and the ability to evade macrophage clearance [55,56],leading to efficient NTP conversion in the targeted cells (alveolar epithelial cells).Rdv-lips inh.are more convenient for selfadministration using ultrasonic nebulizer.The easy operation makes it have better compliance in some special populations like severe patients,old man and children [80,81] .Compared to other liposomal aerosols,Rdv-lips were provided as the form of lyophilized powder to improve the stability of remdesivir and prolong the shelf-life.DPPC,the main material of Rdv-lips,is one of the few carrier materials for inhalation approved by the FDA,which makes it easier for industrial production.
In this study,the great increase of NTP concentration and accumulation in lung validated that Rdv-lips inh.is a potent strategy to improve thein vivo
behavior of remdesivir to exert better therapeutic effects.However,this result could not fully reflect the real situation in human body because of the different enzymes activity and conversion rates of NTP between species.The actual benefit brought by the Rdv-lips aerosol needs further exploration through more preclinical and clinical trials.4.Conclusion
In summary,we developed lyophilized Rdv-lips administered by pulmonary inhalation for COVID-19 treatment.The Rdv-lips were uniform and stable with good aerodynamic properties.Compared to the existing Rdv-cyc injection,even Rdv-cyc aerosol,Rdv-lips aerosol demonstrated significantly increased NTP concentration and improved accumulation in the lung as well as had better safety.The optimization ofin vivo
behavior by Rdv-lips aerosol will be beneficial for remdesivir to overcome the existing limitations and to exert better therapeutic effects.Additionally,based on the broad antiviral ability of remdesivir,we hope that remdesivir liposome aerosols can play a role in more viral diseases,such as Severe Acute Respiratory Syndrome (SARS) and Middle East respiratory syndrome (MERS).Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation (81871477,81603051,and 81673377),Natural Science Foundation of Jiangsu Province (BK20160760 and BK20170748),The Anti-COVID-19 Emergency Research Project of China Pharmaceutical University (2632020ZX007),The Creation of Major New Drugs National Major Projects(2017ZX09101001–004),Fundamental Research Funds for the Central Universities (2016ZPY015,2632017PY18).The authors thank CHIATAI TIANQING Pharmaceutical Group Co.,Ltd.for the assistance with Next Generation Impactor (NGI).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2021.09.002 .
 Asian Journal of Pharmacentical Sciences2021年6期
Asian Journal of Pharmacentical Sciences2021年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- Immunometabolism and potential targets in severe COVID-19 peripheral immune responses
- Naringenin nanocrystals for improving antirheumatoid arthritis activity
- Rapid and effective treatment of traumatic spinal cord injury using stem cell derived exosomes
- Multifunctional self-delivery micelles targeting the invasion-metastasis cascade for enhanced chemotherapy against melanoma and the lung metastasis
- Iron-doxorubicin prodrug loaded liposomenanogenerator programs multimodal ferroptosis for efficient cancer therapy
- Carrier-free prodrug nanoparticles based on dasatinib and cisplatin for efficient antitumor in vivo
