Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
Ahme Hssen Shnti, Shruk Khn, Gnesh Tpiy, Ann Chettuplli, Shwet Soo,Moh Syee Shikh, Flk Siiqui, Rmkoteswr Ro Amr
a.College of Science for Women, Babylon University, Hilla, Babil 00964, Iraq
b.MUPs College of Pharmacy (B Pharm), Washim, Maharashtra 444506, India
c.Shreeyash Institute of Pharmaceutical Education and Research, Aurangabad, Maharashtra 431010, India
d.Department of Pharmaceutical Sciences, Center for Nanomedicine, Anurag University, Hyderabad 501301, India
e.Government College of Pharmacy, Karad, Maharashtra 415124, India
f.Department of Pharmacy, Shri Jagdishprasad Jhabarmal Tibrewala University, Vidya Nagari, Rajasthan 333001, India
Keywords Sulfonamides Antimalarials Antifungal Antibacterial Plasmodium cysteine protease falcipain-2 2,3-Diphenylquinoxaline-6-sulfonamide Plasmodium falciparum
ABSTRACT Objective Sulfanilamide, sulfadiazine, and dapsone were the first sulfonamides to be used to treat malaria by disrupting the folate biosynthesis process, which is essential for parasite survival.Therefore, we aimed to synthesize novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives through a rational drug design approach.Methods All compounds were synthesized by the conventional method, and the products were characterized by spectral analysis(1H NMR and mass spectrometry).The progression of the reaction was monitored using thin-layer chromatography (TLC).All the derivatives were analyzed for their effective binding mode in the allosteric site of the plasmodium cysteine protease falcipain-2.Antibacterial and antifungal activities were determined using the broth dilution method.Results S6 (N-(2-thiazol-4yl)-acetyl-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide and S9 (N-(1H-benzo[d]imidazol-2-yl)aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide formed five hydrogen bonds; S8 (N-(2-1H-imidazol-2yl)aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide and S10 (N-(1H-benzo[d]imidazol-5-yl)aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide formed four hydrogen bonds with the allosteric site of the enzyme.Considering the docking scores and formation of hydrogen bonds with the target enzyme, the novel derivatives were processed for wet lab synthesis.All the newly synthesized derivatives were subjected to in vitro antimalarial, antifungal, and antibacterial activities.All the derivatives exhibited sufficient sensitivity to the Plasmodium falciparum strain compared to the standards.Moreover, compounds S9 and S10 showed the most potent dual antimicrobial and antimalarial activities.They also exhibited powerful molecular interactions in molecular docking studies.Conclusion Based on the above results, it was concluded that N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives have excellent biological potential to act as antimalarial, antifungal, and antibacterial agents.
1 Introduction
Malaria poses a critical public health threat and a significant financial burden in malaria-endemic countries[1].There has been increased attention and new affirmation to the suppression of malaria, which has steadily resulted in over a million deaths, and drug safety from each new chemotherapeutic methodology, particularly inPlasmodium falciparum, the parasite responsible for nearly all malaria-related deaths[2].According to the World Malaria Report 2020 statistics, in 2019, there were an estimated 229 million cases of malaria worldwide.The estimated number of malaria deaths was 409 000 in 2019.Children under 5 years of age are the most vulnerable group affected by malaria; they accounted for 67%(274 000) of all malaria deaths worldwide in 2019[3].Plasmodium ovale, Plasmodium vivax, Plasmodium falciparum, andPlasmodium malariaeare the four varieties of human malaria spread by mosquitos[4].The first two are the most common, whereasPlasmodium falciparum, often present in Africa, accumulates in organ failures and brain capillaries, eventually leading to coma and death.There is evidence that the mortality attributable toPlasmodium vivaxis underestimated[5].Falcipain-2, the predominant cysteine protease ofPlasmodium falciparum, the mammalian malaria parasite, is a hemoglobinase and a potential therapeutic target[6].
Modernization and behavioral changes negatively affect people's lives, including their physical and mental well-being.This abnormal human state and numerous toxins attract many pathogens, including infectious, fungal, and plasmodial[7,8].The scientific community has been working to inhibit or eliminate these diseases worldwide.Drug molecules derived from biological sources and synthetic analogs have been used to treat infectious disorders.Most of these natural products are heterocyclic, according to structural analysis[2,4,5].
Millions of humans are now affected by bacterial diseases triggered by pathogenic bacteria, responsible for elevated child mortality rates in developed countries[9].Not all bacteria are pathogenic.For example, there are thousands of bacterial organisms in the human digestive tract, some of which are harmless and even useful.Furthermore, various mechanisms of action on the target site can aid in discovering potential drugs for the development of antibacterial agents[10].However, since bacteria have developed antibiotic tolerance, it has become difficult to find new antibacterial agents.Gram-positive bacteria, such as methicillin-resistantStaphylococcus aureus, Staphylococcus epidermis,vancomycin-resistantEnterococci,and penicillin-resistantStreptococcus pneumoniae, induce most bacterial infections.Fungal infections have become more frequent, with the majority being of low severity[11].Various varieties of fungi cause infections[12], such asCandidaandAspergillus[13].
Sulfonamides are antimalarial drugs that disrupt the folate biosynthesis process, which is essential for parasite survival.The first sulfa medications used to prevent malaria infections were sulfanilamide, sulfadiazine, and dapsone[5].It has been well documented that heterocyclic compounds isolated from natural sources possess diverse antimicrobial[14], antifungal[15], and antimalarial[16]biological activities.Alkaloids are heterocyclic nitrogen-containing chemicals that exhibit a wide range of antibacterial activities.Quinine is a cinchona alkaloid that belongs to the aryl amino alcohol class of medicines.It has a rapid schizonticidal effect against intra-erythrocytic malarial parasites[17].Therefore, to obtain more potent heterocyclic compounds, we investigated the nitrogen, sulfur, and oxygen atoms containing heterocyclic scaffolds.We have designed and synthesized novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives.All the derivatives have been studied for their effective binding mode in the allosteric site of the plasmodium cysteine protease falcipain-2.Considering the docking scores and formation of hydrogen bonds with the target enzyme,the novel derivatives were processed for wet lab synthesis.All the newly synthesized products were subjected toin vitroantimalarial, antifungal, and antibacterial evaluations.
2 Materials and methods
2.1 Design of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides
2,3-Diphenyl quinoxaline and sulfonamides are an imperative class of nitrogen and sulfur-inclosing heterocycles with diverse pharmacological profiles[18–26].In this study, we designed and synthesized novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives.The designed derivatives were screened by molecular docking.A total of 10 derivatives were designed, as shown in Figure 1.Derivatives with excellent binding affinities, followed by the formation of potent hydrogen bonds with an allosteric site of the target enzyme, were selected for the synthesis.
2.2 Molecular docking (MD) simulation
We used Autodock Vina 1.1.2 in PyRx-Virtual Screening Tool 0.8 to execute the MD on a Lenovo Think-Pad T440p[27].The structures of all the derivatives were sketched in ChemDraw Ultra 8.0 and saved in molfile format.Energy minimization (optimization)was executed using a universal force field (UFF)[28].
The crystal structure ofPlasmodium falciparumcysteine protease falcipain-2(PDB ID:1YVB,https://www.rcsb.org/structure/1YVB) was obtained from the RCSB Protein Data Bank (PDB).No inhibitor/native ligand was present in the 1YVB.With the aid of Discovery Studio Visualizer 2019, the enzyme structure was optimized, purified, and prepared for MD[29].The crystal structures ofPlasmodium falciparumcysteine protease falcipain-2 crystal structure are illustrated in Table 1.
The derivatives and target enzyme file structures were then imported into PyRx software and converted to pdbqt format.The molecules (pdbqt files), both ligands, and targets were selected for MD[30].For the MD simulation, a three-dimensional (3D) grid box(size x =39.101 492 977 7 ?; size y= 43.678 539 274 3 ?;size z = 39.698 857 395 7 ?) was built using Autodock tool 1.5.6, with an exhaustiveness value of 8[31].The active amino acids in the protein were examined using a BIOVIA Discovery Studio Visualizer (version-19.1.0.18287)[27].In the complete MD method, the cavity and active amino acid residues were identified per the procedure described by KHAN et al.[30–35].The purified structure of the enzyme is shown in Figure 2.
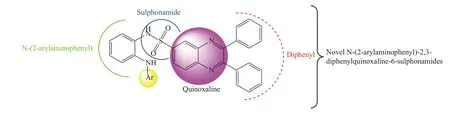
Figure 1 Designing of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives
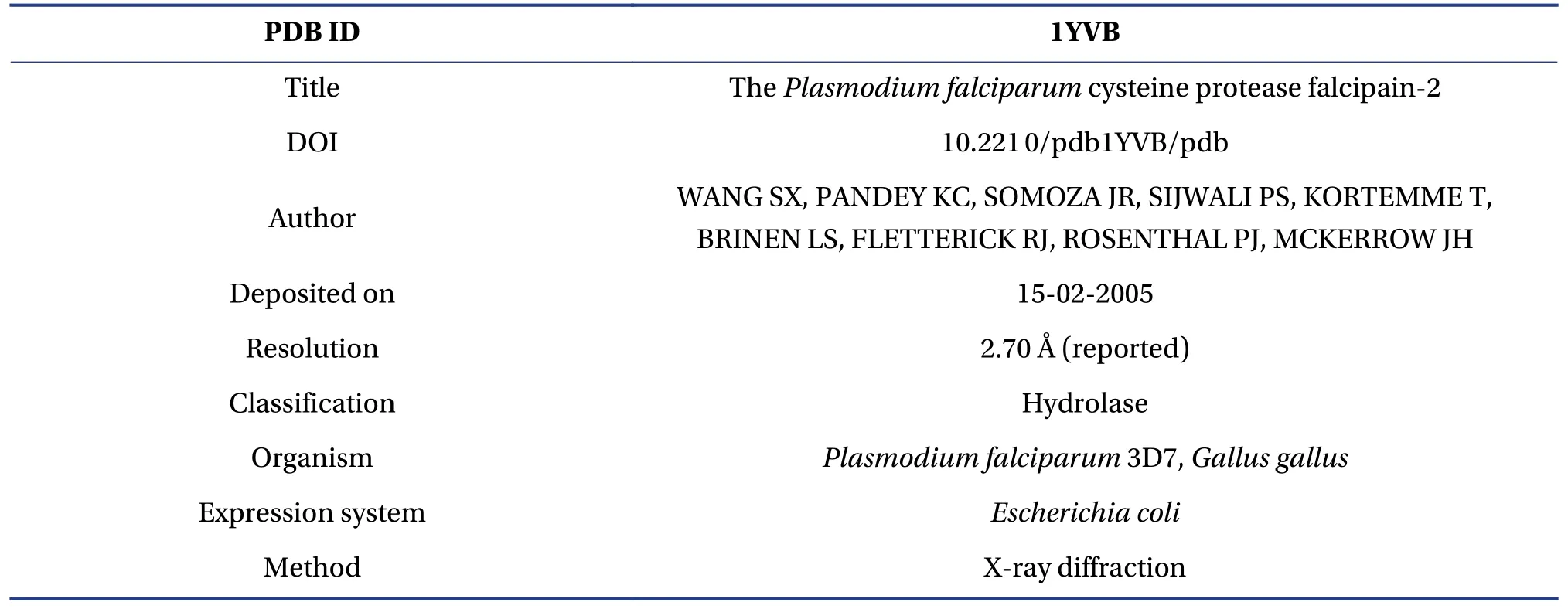
Table 1 The essentials of the crystal structure of the Plasmodium falciparum cysteine protease falcipain-2
Figure 2 A purified structure of the Plasmodium falciparum cysteine protease falcipain-2
2.3 Screening through Lipinski rule of five
The designed derivatives were checked to exceed Lipinski's rule of five, and the ones that passed were further tested for compliance.The properties of the derivatives were measured using the SwissADME online tool (http://www.swissadme.ch/index.php).
2.4 Wet lab synthesis
Synthetic grade chemicals and reagents were purchased from Lab Trading Laboratory, Aurangabad,Maharashtra, India.The melting points were measured using a VEEGO MODEL VMP-D melting point apparatus.Thin-layer chromatography (TLC) was used to validate the completion of reactions using preparative TLC plates (Merck precoated silica GF 254).Qualitative tests were used to validate functional group conversions.On a Varian-VXR-300S at 300 MHz NMR spectrometer,1H and13C NMR spectra were reported with DMSO as the solvent and TMS as the internal standard; chemical shift values were expressed inδppm.The solutions were kept in high performance liquid chromatography(HPLC)grade methanol for mass spectra, and spectra were generated using electron ionization at 70 eV.
2.4.1 Synthesis of parent nucleusIn the first step,chlorosulfonation of 2,3-diphenyl quinoxaline was performed to yield 2,3-diphenylquinoxaline-6-sulfonyl chloride.In an ice-cold fuming cupboard with continuous stirring, 2,3-diphenylquinoxaline (0.01 mol,2.84 gm) was treated with chlorosulfonic acid.The reaction mixture was stirred until the temperature reached room temperature (25 ? 30 °C).The resulting mixture was dissolved in water to produce 2,3-diphenylquinoxaline-6-sulphonyl chloride.In the second step,N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide was synthesized from the product obtained in the first step.O-phenylenediamine (0.01 mmol, Batch No.MCR-19023-01) was dissolved in dichloromethane (20 mL) in a beaker.In another beaker, pyridine (1.6 mL, Batch No.MCRT-20839) was progressively added to a solution of 2,3-diphenylquinoxaline-6-sulfonyl chloride (1.93 gm,10 mmol) in dichloromethane (10 mL, Batch No.MCR-4126) at 0 °C.Both solutions were combined,and the reaction mixture was agitated at room temperature for 1.5 h before being washed with aqueous 1 mol/L HCl (Batch No.MCR-24517) and brine and dried over Na2SO4(Batch No.MCR-24118).The crude product was recrystallized in dichloromethane after evaporating the organic solvent, and N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide was collected as a light-yellow solid[36,37].
2.4.2 Synthesis of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivativesIn a 250 mL RBF, an appropriate chloro-substituted heterocyclic compound (0.01 mol) and K2CO3(0.02 mol,2.76 gm, Batch No.MCR-24331) were agitated at room temperature in dimethylformamide (DMF, 20 mL,Batch No.MCR-22588) for 30 min; KI (Batch No.MCR-24 288) pinch was added to the solution.N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide (0.01 mol) was added to the reaction mixture.The reaction was refluxed for 12 h until TLC showed reaction accomplishment.The reaction mixture was poured into water (20 mL), and the mixture was extracted with ethyl acetate (3 times and 20 mL for each) using a separating funnel.The organic extracts were washed with water, dried over anhydrous sodium sulfate, and condensed to produce a crude product.To obtain a pure compound, the residue was recrystallized from diethyl ether[38].The chemical reactions are shown in Figure 3.
2.5 In vitro biological evaluation
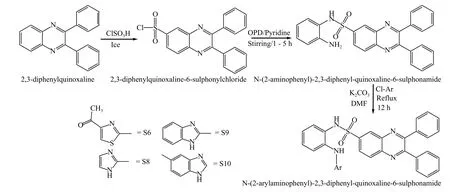
Figure 3 The proposed reaction scheme for the synthesis of novel derivatives
Various concentrations of the derivatives were prepared in DMSO (Batch No.MCR-18421-01) to assess their antibacterial and antifungal activities against standard strains using broth dilution.Bacteria were maintained, and the drugs were diluted in Mueller-Hinton broth.The broth was inoculated with 108colony-forming units (cfu) per milliliter of test strains(Institute of Microbial Technology, Chandigarh, India) determined by turbidity.Stock solutions of the synthesized derivatives (2 mg/mL) were serially diluted for primary and secondary screening.The primary screen included 1 000, 500, and 250 μg/mL of synthesized derivatives, and those with activity were further screened at 200, 100, 50, 25, 12.5, and 6.250 μg/mL.A control without antibiotic was subcultured (before inoculation) by spreading one loopful evenly over a quarter of a plate of medium suitable for growing test organisms and incubated at 37°C overnight.The lowest concentrations of derivatives that inhibited bacterial or fungal growth were considered minimal inhibitory concentrations(MICs).These were compared with the amount of control growth before incubation (original inoculum)to determine the MIC accuracy.The standards for antibacterial activity were gentamycin,ampicillin,chloramphenicol,ciprofloxacin,and norfloxacin served, and those for antifungal activity were nystatin and griseofulvin[33].The antimalarial behavior was tested usingPlasmodium falciparum, with quinine and chloroquine as standards[39].Both experiments were conducted at the Microcare Laboratory and Tuberculosis Research Centre (TRC) in Surat, Gujarat.
3 Results
3.1 MD Simulation
The molecular formula, ligand energy (kcal/mol),binding affinities of conformers (kcal/mol) withPlasmodium falciparumcysteine protease falcipain-2,and root mean square deviation (rmsd)/upper bound(ub) and lower bound (lb) of the designed derivatives are tabulated in Table 2.The active amino acid residues, active atoms from ligands, bond length (?),bond type, and bond category of molecules with falcipain-2 are shown in Table 3.The 3D- and 2D-docking poses of the molecules in the allosteric site of falcipain-2 are shown in Table 4.Calculated druglikeness properties (rule of five) of the molecules are shown in Table 5.
3.2 Characterization data of the synthesized molecules
From virtual screening, it was concluded that molecules S6, S8, S9, and S10 exhibited high binding affinity for the formation of more than two hydrogen bonds with falcipain-2.Considering this, we proceeded with synthesizing these four molecules, followed by their characterization and biological activity.1H NMR and mass spectroscopy confirmed the synthesis of the compounds.The spectra are provided in the supplementary file.
N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide(Parent Nucleus)
King Cloverleaf and Queen Frivola had but one child, and this Princess had from her very babyhood been so beautiful, that by the time she was four years old the Queen was desperately2 jealous of her, and so fearful that when she was grown up she would be more admired than herself, that she resolved to keep her hidden away out of sight
Yield: 48%; color: pale yellow solid; solubility: ethanol, acetone, benzene; melting point: 100 – 101 °C;Rfvalue: 0.68; elemental analysis (calc.): C, 69.01; H,4.45; N, 12.38; O, 7.07; S, 7.09.1H NMR (DMSO-d6300 MHz)δppm: 4.0 (s, -NH2, -NH), 6.23, 6.39 (s, 4H,Ar-H), 7.39 ? 7.69 (d, diphenyl Ar-H), 8.16, 8.44 (d,2H, Ar-H).MS: m/z 451.76, 455.01 (m +1), 456.20(m +2).
S6(N-(2-thiazol-4yl)-acetyl-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide
Yield: 63%; color: light orange; solubility: ethanol,benzene, slightly soluble in hot water; melting point:111 ? 113 °C; Rfvalue: 0.78; elemental analysis (calc.):C, 64.45; H, 4.01; N, 12.12; O, 8.31; S, 11.10.1H NMR(DMSO-d6300 MHz)δppm: 2.4 (s, -CH3), 4.0 (s, -NH,-NH), 6.24, 6.36 (s, 4H, Ar-H), 7.32 ? 7.91 (d, diphenyl Ar-H), 8.45, 8.43, 8.62 (d, 2H, Ar-H).MS: m/z 576.36,578.23 (m+1), 579.45 (m+2).
S8(N-(2-1H-imidazol-2yl)aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide
Yield: 81%; color: light orange; solubility: ethanol,benzene, water; melting point: 120 ? 122 °C; Rfvalue:0.63; elemental analysis (calc.): C, 67.17; H, 4.28; N,16.21; O, 6.17; S, 6.18.1H NMR (DMSO-d6300 MHz)δppm: 4.0 (s, -NH, -NH), 6.23, 6.37 (s, 4H, Ar-H), 7.23(d, 2H, Imidazole), 7.34 ? 7.49 (d, diphenyl Ar-H),8.15, 8.16, 8.42, 8.7 (d, 2H, Ar-H).MS: m/z 513.32,518.20 (m +1), 520.45 (m +2).
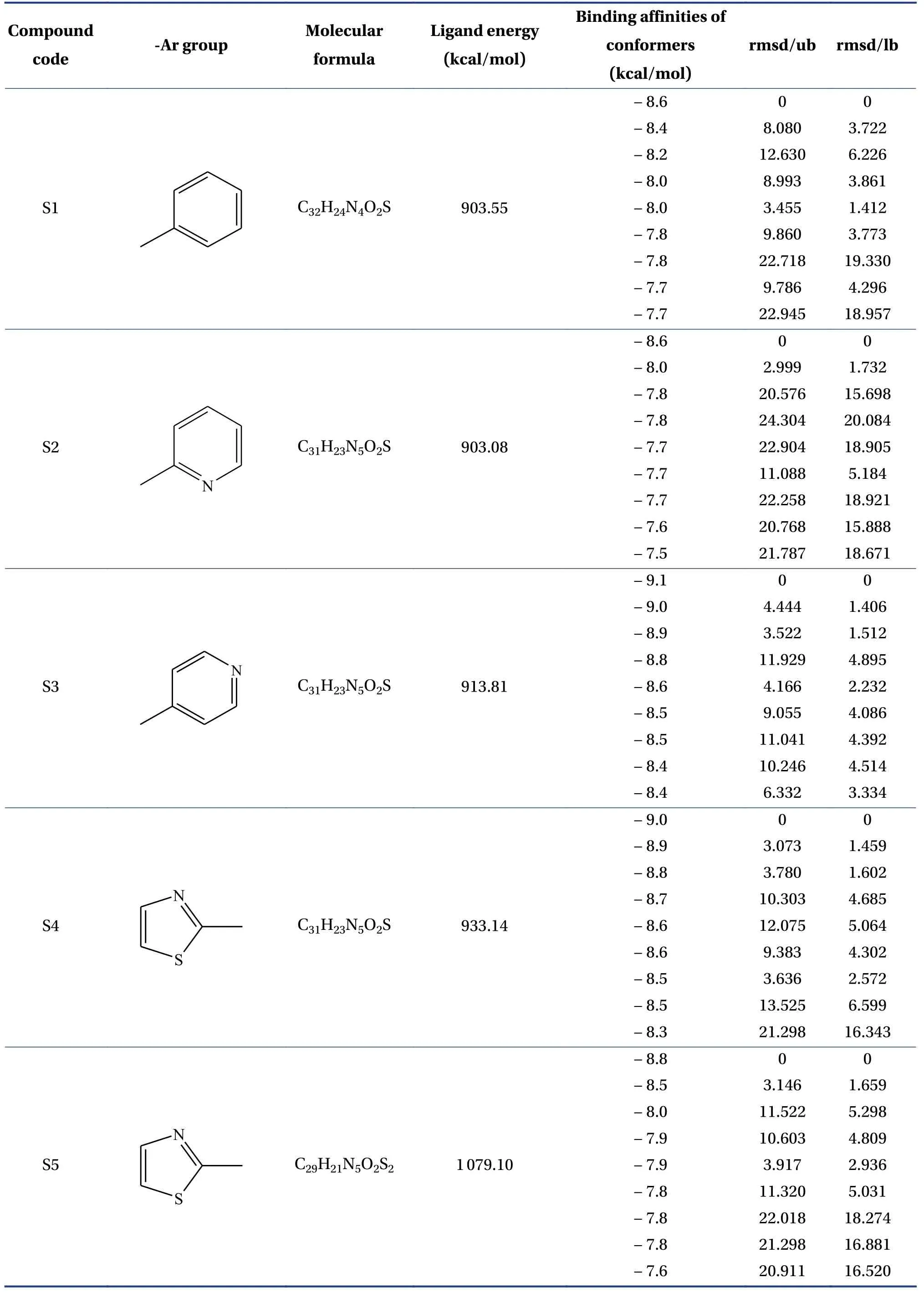
Table 2 The -Ar group, molecular formula, ligand energy (kcal/mol), binding affinities of conformers (kcal/mol)rmsd/ub, and rmsd/lb of designed derivatives
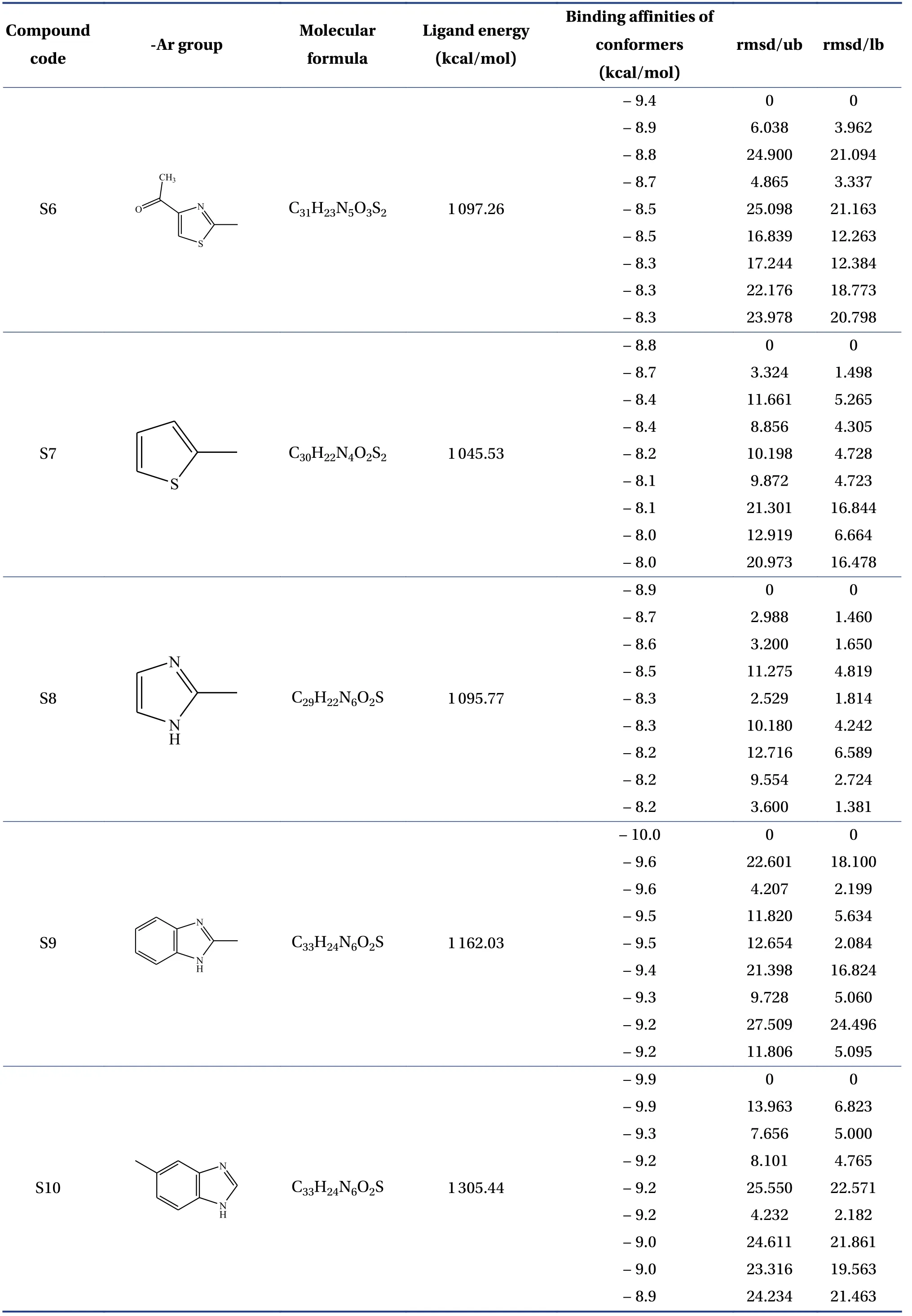
Table 2 Continued
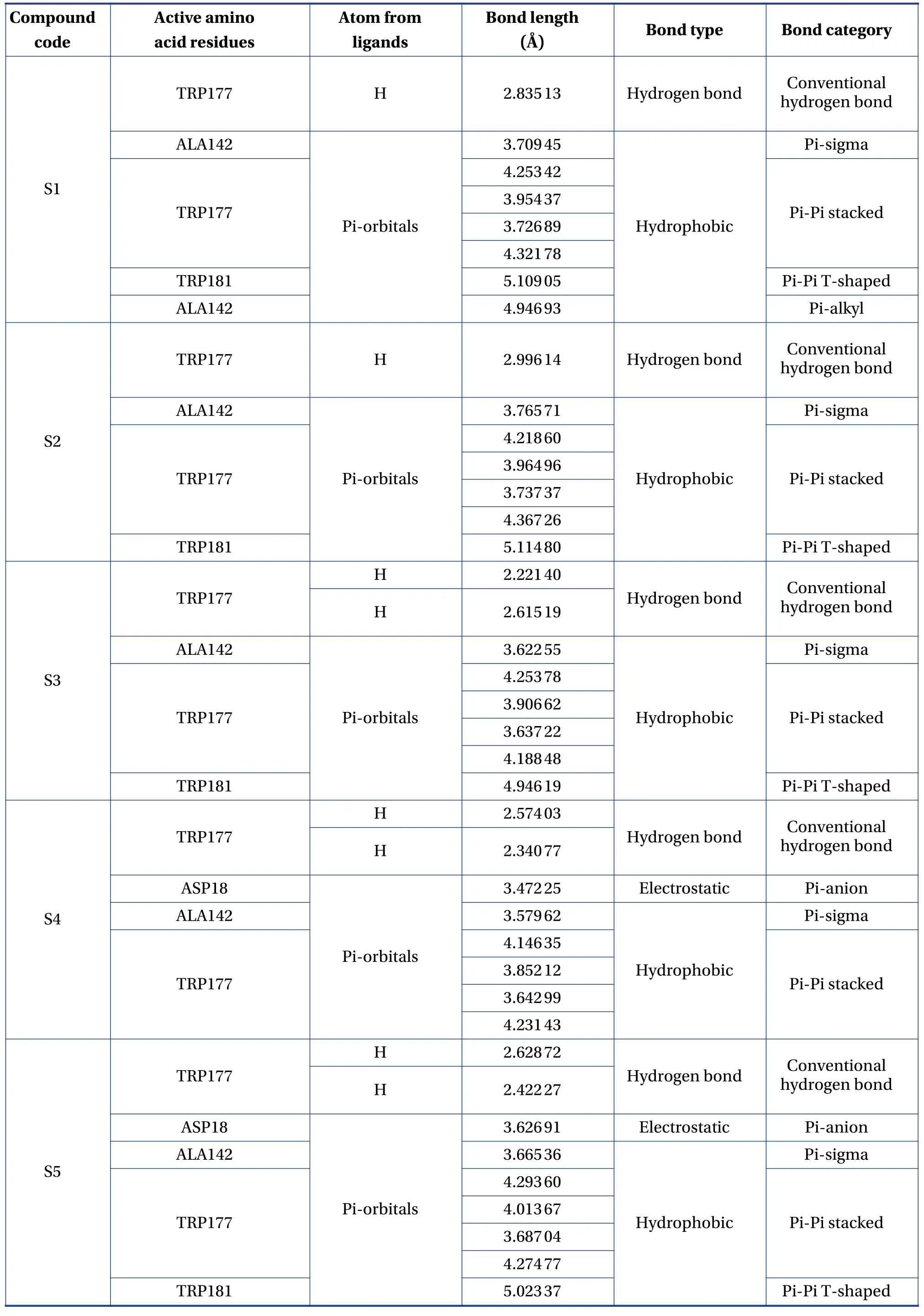
Table 3 The active amino acid residues, active atom from ligands, bond length (?), bond type and bond category of molecules with falcipain-2
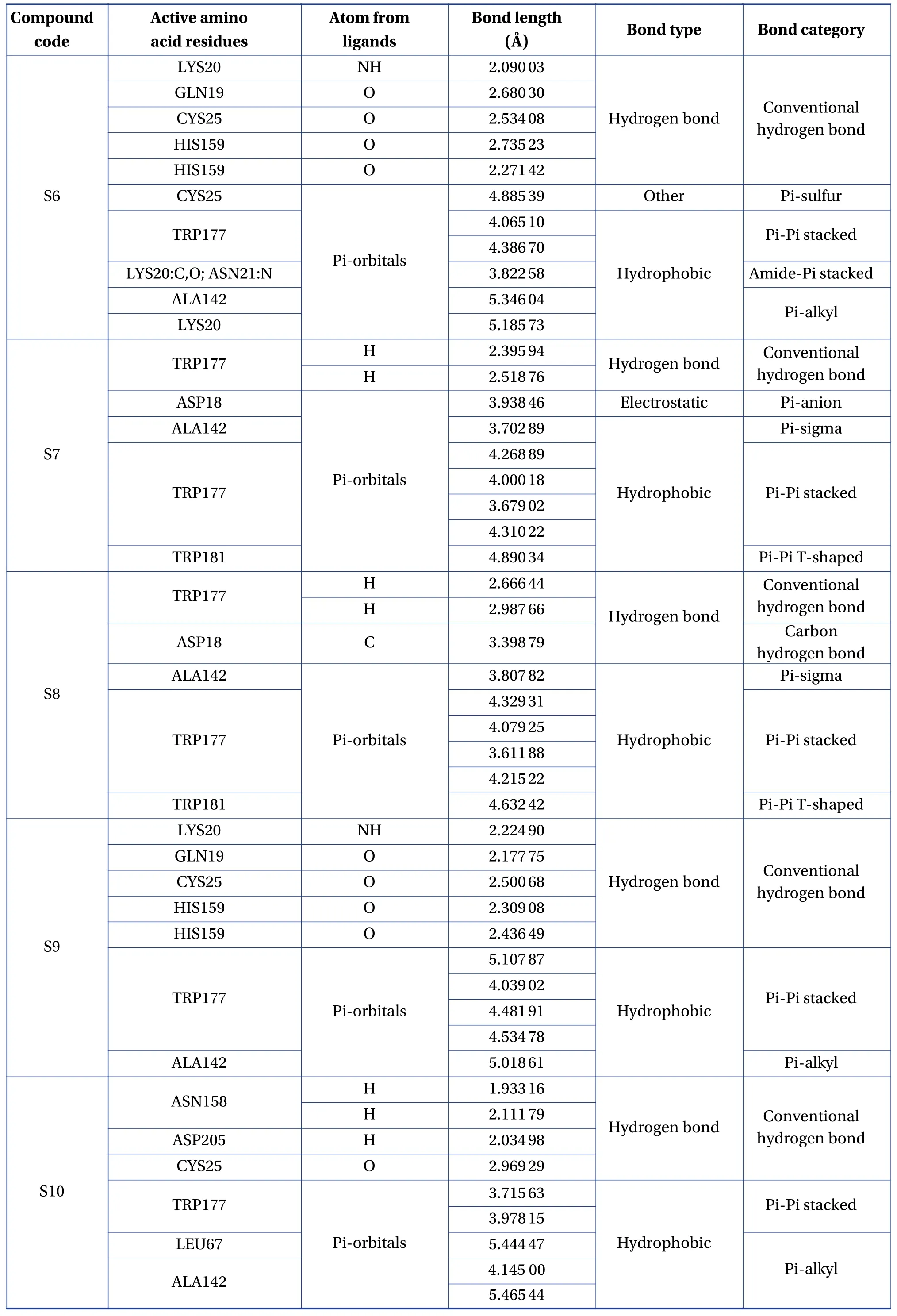
Table 3 Continued
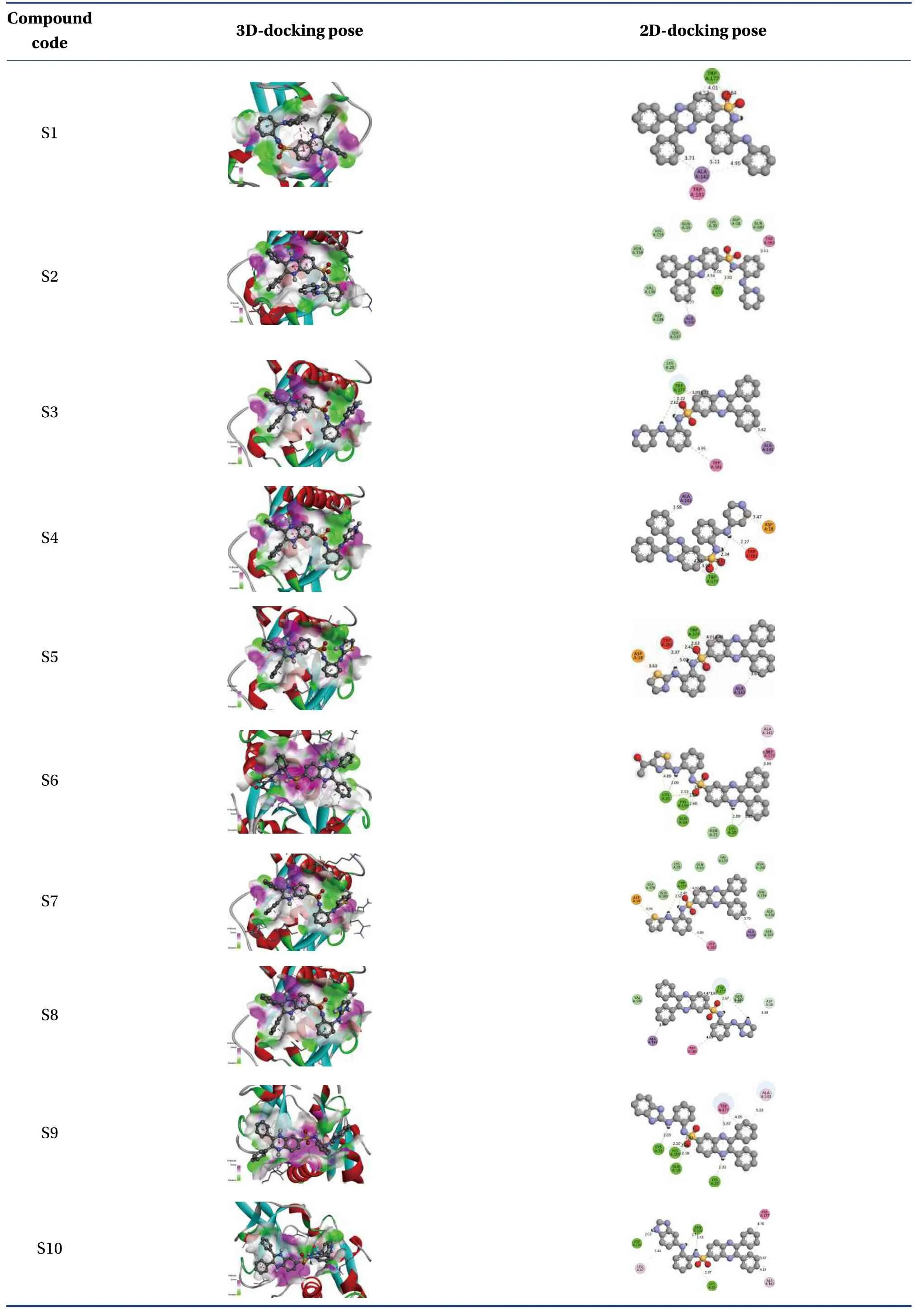
Table 4 3D- and 2D-docking poses of the molecules in allosteric site of falcipain-2
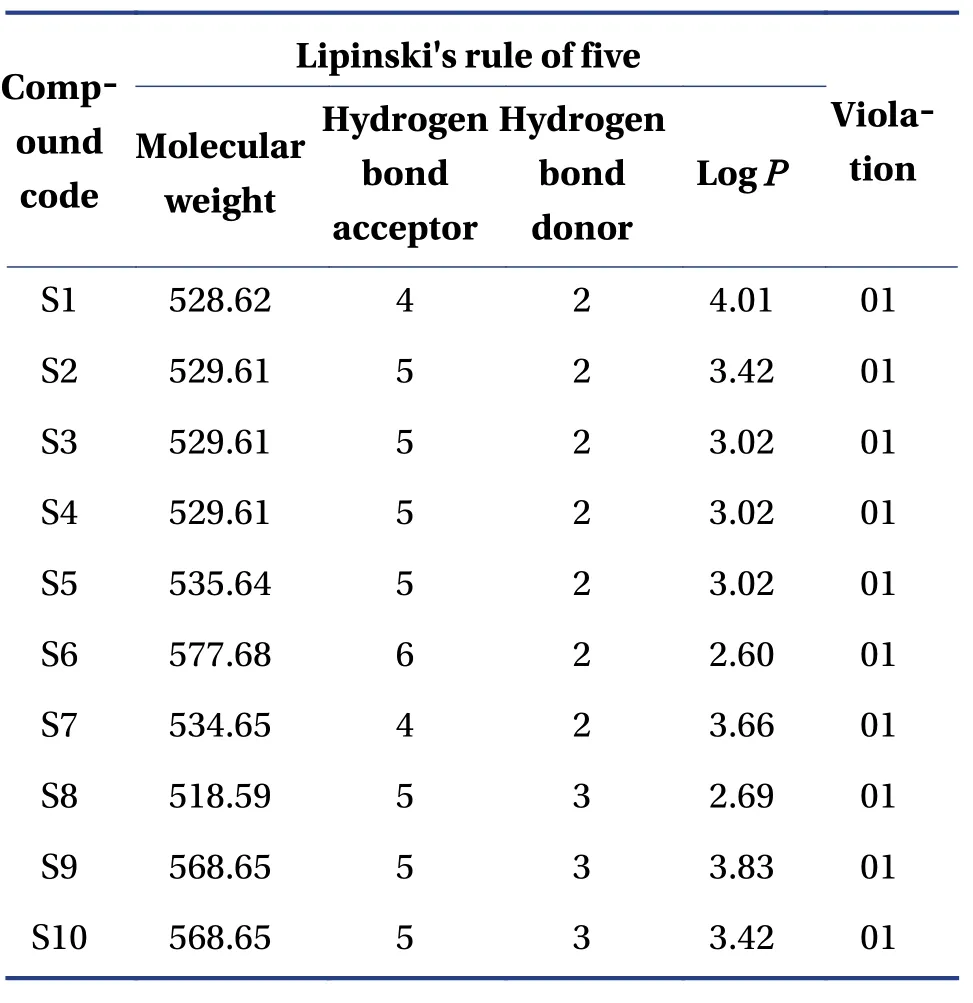
Table 5 Calculated druglikeness properties (rule of five) of the molecules
Yield: 52%; color: light yellow; solubility: ethanol,benzene; melting point: 123 ? 125 °C; Rf value: 0.73;elemental analysis (calc.): C, 69.70; H, 4.25; N, 14.78;O, 5.63; S, 5.64.1H NMR (DMSO-d6300 MHz)δppm:4.4 (s, -NH, -NH), 5.0 (s, -NH imidazole), 6.23, 6.38 (s,4H, Ar-H), 7.48 ? 7.59 (d, diphenyl Ar-H), 8.01, 8.37(d, 2H, Ar-H).MS: m/z 564.30, 567.83 (m+1), 569.34(m+2).
S10(N-(1H-benzo[d]imidazol-5-yl)aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide
Yield: 67%, color: light yellow; solubility: ethanol,benzene; melting point: 118 ? 120 °C; Rf value: 0.60;elemental analysis: C =69.70; H =4.25; N =14.78; O =5.63; S =5.64.1H NMR (DMSO-d6300 MHz)δppm:4.2 (s, -NH, -NH), 4.9 (s, -NH imidazole), 6.23, 6.38 (s,4H, Ar-H), 7.1 (d, 1H, Ar-H of benzimidazole), 7.51 ?7.89 (d, diphenyl Ar-H), 8.11 (d, 1H, imidazole), 8.29 ?8.37 (d, 2H, Ar-H).MS: m/z 571.34, 574.75 (m+1),575.45 (m+2).
3.3 In vitro biological activity
The results of antimicrobial, antifungal, and antimalarial activities of the synthesized derivatives are tabulated in Table 6 shows the MICs, minimum fungicidalconcentrations (MFCs), and Half-maximal inhibitory concentration (IC50) values, respectively.
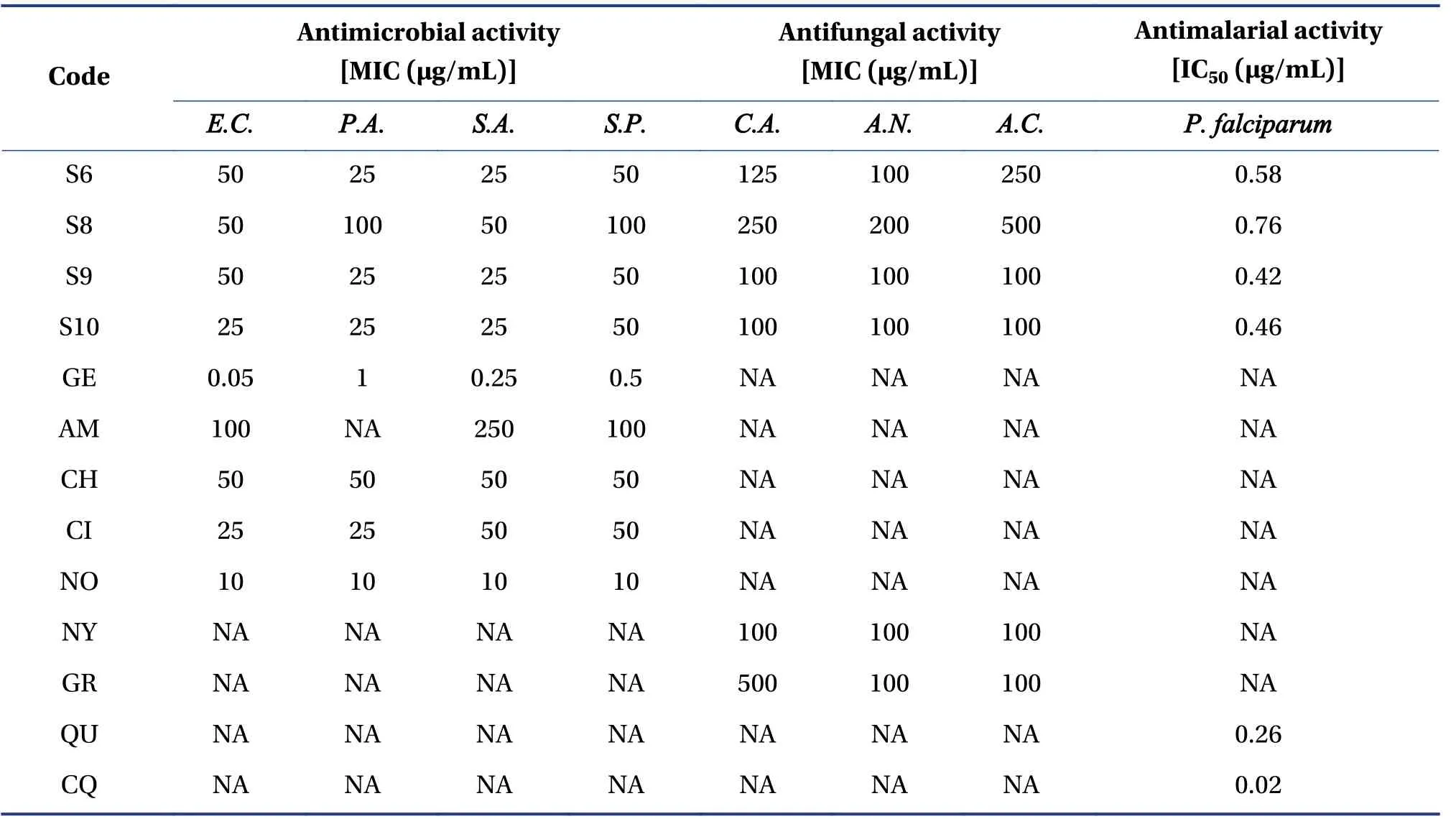
Table 6 Antimicrobial, antifungal, and antimalarial activities of the synthesized derivatives
4 Discussion
For many years, quinine has been the most effective antimalarial medicine; however, since the 1930s, synthetic drugs such as 8-aminoquinolines (e.g., primaquine), 4-aminoquinolines (e.g., chloroquine, amodiaquine), sulfonamides (e.g., sulfadoxine), and sulfones and folic acid biosynthesis inhibitors have essentially supplanted it (e.g., proguanil and pyrimethamine).Malaria was widely anticipated to be eliminated globally by the mid-1950s; however, in the mid-1960s, this confidence had been shattered due to resistance issues[40].As an alternative to chloroquine,some researchers have focused their efforts on the discovery of novel active molecules, particularly artemisinin.At present, no single medicine is helpful for treating multidrug-resistant malaria; therefore,successful combination treatment includes artemis inin derivatives such as artesunate or combinations of earlier drugs such as atovaquone and proguanil.There have been cases of drug resistance to artemisinin derivatives and pharmacological combination therapy.As a result, attempts to develop novel antimalarial medications are urgently required because of the lack of a viable, safe, and widely accessible malaria vaccine[41].Therefore, novel 2,3-diphenylquinoxaline-6-sulfonamides derivatives were designed by considering their potential biological activities.A parent nucleus was synthesized as N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide, from which 10 novel derivatives were designed using different chloro-substituted hetero/homocyclic compounds.These 10 derivatives were studied for their binding affinity towards the allosteric site of the cysteine protease falcipain-2 ofPlasmodium falciparum(PDB ID: 1YVB).It was observed that compounds S6, S8, S9, and S10 possess excellent binding affinity and formed sufficient hydrogen bonds with the amino acids of the target enzyme.Therefore, we decided to synthesize these four derivatives, followed by their characterization andin vitrobiological evaluation.
Nine conformers of each molecule were generated through MD.A confirmer with zero rmsd/ub and rmsd/lb was considered the best binding model for the molecule.Compound S1 exhibited – 8.4 a binding affinity and formed one conventional hydrogen bond with TRP177 (2.835 13 ?).Many hydrophobic interactions have been developed through the Pi-orbital system with ALA142 [Pi-sigma(bond type):3.709 45 ?, Pi-alkyl: 4.946 93 ?], TRP177 (Pi-Pi stacked; 4.253 42 ?, 3.954 37 ?, 3.726 89 ?, 4.321 78 ?),and TRP181 (Pi-Pi T-shaped; 5.109 05 ?).Compound S2 demonstrated – 8.6 kcal/mol binding affinity and formed one conventional hydrogen bond with TRP177 (2.996 14 ?) and exhibited hydrophobic interactions with ALA142(Pi-sigma; 3.765 71 ?),TRP177 (Pi-Pi stacked; 4.218 6 ?, 3.964 96 ?, 3.737 37 ?,4.367 26 ?), and TRP181 (Pi-Pi T-shaped; 5.114 8 ?).Compound S3 formed two conventional hydrogen bonds with TRP177 (2.221 4 ?, 2.615 19 ?) through the hydrogen atoms of both -NH and exhibited – 9.1 a binding affinity.It developed hydrophobic interactions in the same way as compound S2.Compound S4 displayed – 9 kcal/mol binding affinity and formed two conventional hydrogen bonds with TRP177(2.574 03 ?, 2.340 77 ?) through the hydrogen atoms of both -NH groups.One electrostatic bond was formed with ASP18 (Pi-anion; 3.472 25 ?) and established hydrophobic interactions with ALA142 (Pisigma; 3.579 62 ?) and TRP177 (Pi-Pi stacked;4.146 35 ?, 3.852 12 ?, 3.642 99 ?, 4.231 43 ?).Compound S5 showed a – 8.8 kcal/mol docking score and exhibited the same binding interaction as shown by compound S5.Compound S6 exhibited – 9.4 kcal/mol binding affinity, and formed five conventional hydrogen bonds with LYS20 (2.090 03 ?) through the hydrogen of -NH, GLN19 (2.680 3 ?) through sulfonyl oxygen, CYS25 (2.534 08 ?) through sulfonyl oxygen,and HIS159 (2.735 23 ?, 2.271 42 ?) through a sulfonyl oxygen atom.A Pi-sulfur bond was formed with CYS25 (4.885 39 ?) through the Pi-orbitals of the aromatic ring system.It developed very potent hydrophobic interactions with TRP177 (Pi-Pi stacked;4.065 1 ?, 4.386 7 ?), LYS20:C, O; ASN21:N (Amide-Pi stacked, 3.822 58 ?), ALA142 (Pi-alkyl; 5.346 04 ?),and LYS20 (Pi-alkyl; 5.185 73 ?).Compound S7 exhibited ? 8.8 kcal/mol binding affinity and formed two hydrogen bonds with TRP177 (2.395 94 ?,2.518 76 ?) through hydrogen atoms of both -NH groups.It developed electrostatic interactions and many hydrophobic interactions with the target.Compound S8 formed two conventional hydrogen bonds with TRP177 (2.666 44 ?, 2.987 66 ?) through the hydrogen of free -NH and -NH of the imidazole ring and one carbon-hydrogen bond with ASP18 (3.398 79 ?)with – 8.9 a binding affinity.It exhibited hydrophobic interactions similar to those exhibited by S2.Compound S9 exhibited a binding free energy of– 10 kcal/mol, formed five conventional hydrogen bonds, and developed many hydrophobic interactions with the same amino acids formed by compound S6.Compound S10 showed – 9.9 kcal/mol binding affinity and formed four conventional hydrogen bonds with ASN158 (1.933 16 ?, 2.111 79 ?)through hydrogen atoms of both -NH, ASP205(2.034 98 ?) through hydrogen atom of -NH of benzimidazole, CYS25 (2.969 29 ?) through a sulfonyl oxygen atom.It has developed hydrophobic interactions with TRP177 (Pi-Pi stacked; 3.715 63 ?, 3.978 15 ?),LEU67 (Pi-alkyl; 5.444 47 ?), and ALA142 (Pi-alkyl;4.145 ?, 5.465 44 ?).Based on the above results, it was decided to perform the wet lab synthesis of compounds S6, S8, S9, and S10.The druglikeness properties (rule 5) were calculated for all the designed derivatives.The derivatives showed one violation due to a molecular weight higher than 500 Da.It was observed that LYS20, GLN19, CYS25, HIS159, TRP177,ASN158, and ASP205 play a crucial role in binding with the ligands by forming conventional hydrogen bonds.
The antimicrobial activity of the synthesized compounds was tested against gram-positive (Staphylococcus aureusandStaphylococcus pyogenes) and gram-negative (Escherichia coliandPseudomonas aeruginosa) bacteria.All the compounds demonstrated more potent activity than ampicillin against gram-positive and gram-negative bacteria.Compounds S6, S9, and S10, were sensitive at 25 μg/mL,which was equipotent to ciprofloxacin againstPseudomonas aeruginosa.Compound S6 was sensitive toStaphylococcus aureusat 25 μg/mL, S8 at 50 μg/mL, S9 at 25 μg/mL, and S10 at 25 μg/mL.All the compounds exhibited more potency than ampicillin, chloramphenicol, and ciprofloxacin.All compounds were sensitive toStaphylococcus pyogenes.Antifungal activity was performed onCandida albicans,Aspergillus niger, andAspergillus clavatusstrains using nystatin and Greseofulvin as standards.Compounds S9 and S10 demonstrated equipotent activity(100 μg/mL) as standards against all fungal strains.All the compounds showed more potent activity againstC.albicansthan Greseofulvin.
Antimalarial activity was assessed usingPlasmodium falciparum.As the derivatives were screened by MD studies on cysteine protease falcipain-2 ofPlasmodium falciparum(PDB ID: 1YVB),the results ofin vitrobiological evaluation support the results obtained from MD.Quinine and chloroquine were used as the standards.All the synthesized compounds were sensitive toPlasmodium falciparum.Compound S6 showed IC50value of 0.58 μg/mL, compound S8 0.76 μg/mL, compound S9 0.42 μg/mL, and compound S10 showed 0.46 μg/mL againstPlasmodium falciparum.Many synthetic compounds have been reported as cysteine protease falcipain-2 inhibitors[42–45]for the treatment of malaria; however, the present investigation provided a novel lead nucleus for further optimization to generate more potent derivatives.
5 Conclusion
Sulfonamides are antimalarial drugs that disrupt the folate biosynthesis process, which is essential for parasite survival.The first sulfa medications to prevent malaria infections were sulfanilamide, sulfadiazine, and dapsone.We have designed and synthesized novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives.Considering the docking scores and formation of hydrogen bonds with the target enzyme, novel derivatives were used for wet lab synthesis.All the newly synthesized products were subjected toin vitroantimalarial, antifungal, and antibacterial evaluations.A parent nucleus was synthesized as N-(2-aminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide, from which 10 novel derivatives were designed using different chloro-substituted hetero/homocyclic compounds.Based on the above results, it was concluded that N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamide derivatives have excellent biological potential to act as antimalarial, antifungal, and antibacterial agents.It can be treated as a lead nucleus to further novel therapeutically active compounds.
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年4期
Digital Chinese Medicine2021年4期
- Digital Chinese Medicine的其它文章
- Digital Chinese Medicine
- Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
- Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
- Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
- Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
- Systematic review of robust experimental models of rheumatoid arthritis for basic research
