Gracilaria changii (Rhodophyta) alleviates bisphenol A-induced adverse reproductive abnormalities in mice
Chong Lee Ng, Gim Cheong Tan, Yoon-Yen Yow, Mukesh Kumar Gupta, Phek Jin Kwong?
1Department of Agricultural and Food Science, Universiti Tunku Abdul Rahman, 31900, Malaysia
2Department of Biomedical Science, Universiti Tunku Abdul Rahman, 31900, Malaysia
3Department of Biological Sciences, School of Medical and Life Sciences, Sunway University, 47500, Malaysia
4Department of Biotechnology and Medical Engineering, National Institute of Technology, Rourkela, Odisha, 769008, India
ABSTRACT
KEYWORDS:Red seaweed; Gracilaria changii; Antioxidant;Endocrine disruptor; Bisphenol A; Reproduction
1.Introduction
Seaweeds are marine macroalgae that are commonly consumed by the Asian population, particularly in Japan, Korea, and China.Gracilaria is one of the red macroalgae, abundantly available in Malaysia[1].It is known to be easily cultivable and produces valuable bioactive compounds[2].Gracilaria (G.) changii is commonly used as a source of gelling and thickening agents in the food industry[3]and its nutritional composition has been studied[4].The extract from G.changii was reported to exhibit good antioxidant properties[5]and G.changii possessed an abundance of beneficial fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid(EPA)[4].DHA was known to promote fertility by improving sperm morphology and motility[6], oocyte quality[7]and prolonging the female reproductive lifespan[8].Several studies have also reported that Gracilaria spp.contains various prostaglandins such as prostaglandin E(PGE) and prostaglandin F(PGF)[9].These compounds are important in facilitating ovulation, fertilization,implantation, and parturition[10].
Bisphenol A (BPA) is a plasticizer that is frequently used in plastic manufacturing and is a well-known endocrine disrupter[11].The toxicity of BPA on the reproduction system, prenatal and postnatal development has been well reported in literature[12-14].Studies have also shown that the adverse effects of BPA were often related to the increase in the oxidative damage to reproductive organs[15].Since G.changii possesses interesting pharmacological properties and has been used extensively in the food and pharmaceutical industries,this study intended to evaluate the potential of G.changii extract in ameliorating BPA-induced adverse effects on female reproductive function.
2.Materials and methods
2.1.Collection and extraction of G.changii
Fresh G.changii specimens were identified morphologically and collected from Pantai Morib, Selangor (N 02°45.739’; E101°26.070’)in March 2017.Upon collection, G.changii was processed and extracted as described by Pang et al[16].Briefly, the specimens were cleaned, freeze-dried, and then ground into powder.Next,0.02 g/mL of G.changii extracts were prepared by incubating the seaweed powder either with ultrapure water, methanol, or ethanol,respectively in the shaking incubator for 2 d.After incubation, the extracts were subjected to centrifugation at 3 000 × g for 20 min at 4 ℃ to obtain the supernatant.The pink supernatant from aqueous extract was finally freeze-dried; whereas the green supernatant from either methanol or ethanol extract was concentrated using a vacuum concentrator (ScanSpeed 4.0, LaboGene, Germany) at 8 × g, 35 ℃for 2 d prior to storage at -20 ℃ until further study.The percentage of extraction yield was expressed as the weight ratio of final freezedried extract over the G.changii powder used in extraction.
2.2.2,2-diphenyl-1-pircylhydrazyl radical scavenging activity (DPPH)
DPPH scavenging activity of G.changii extract was determined according to the protocol by Pang et al[16].Triplicate measurements were made for each concentration of G.changii extract and ECfor DPPH assay of each G.changii extract was calculated from the doseresponse curve.ECrepresented the concentration of G.changii extract, which exhibited 50% of DPPH scavenging activities.
2.3.2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) scavenging activity (ABTS) assays
The ABTS scavenging activity of G.changii extract was determined according to the protocol reported by Pang et al[16].Triplicate measurements were conducted for each concentration of G.changii extract and ECfor ABTS of each G.changii extract was calculated from the dose-response curve.
2.4.Determination of total phenolic content (TPC)
TPC of G.changii extracts was measured using Folin-Ciocalteu method described by Pang et al[16].Triplicate measurements were performed for each G.changii extract and calculated as equivalence to milligram gallic acid per gram of G.changii extract.
2.5.Liquid chromatography-mass spectrometry (LC-MS)
The composition of aqueous extract from G.changii was determined using LC-MS as described by Yap et al[17].The mass spectral was analyzed using Agilent MassHunter Qualitative Analysis B.07.00.The data was processed using molecular feature extraction and only include compounds with absolute height of≥ 5 000 counts and relative height ≥ 2.5%.Each compound was then identified by matching with METLIN database based on mass with 5 ppm tolerance.For compounds not found in the database,the chemical formula was calculated using the built-in molecular formula generator (MFG) algorithms[18].
2.6.Animal husbandry and experimental design
Institute of cancer research (ICR) mice were used as the experimental model to coincide with the animal model used in toxicology studies related to BPA that have been reported to affect estrous cycle and uterus weight of ICR mice[19,20].The mice were housed under the controlled environment of (25±2) ℃, relative humidity of (50±15)%, and 12:12 h light-dark cycle.Water and mice pellet from Gold Coin Feedmills Pte.Ltd were provided ad libitum.All procedures were conducted with appropriate measures to minimize discomfort or pain.
Female ICR mice aged 28-day-old and weighting [(20-25) g]were randomly assigned into four treatment groups (24 mice each).Treatment 1:vehicle control (VC) group, was administrated with 5 mL/kg body weight (bw) of ultrapure water, followed by 5 mL/ kg bw of virgin olive oil (VOO).Treatment 2:BPA group,was administrated with ultrapure water, followed by 60 mg/ kg bw of BPA (Nacalai Tesque) dissolved in VOO.The mice were administered with 60 mg/kg bw of BPA, because this dosage was higher than the dosage at which no adverse effect on reproductive and developmental toxicity in lab animal (50 mg/kg) was reported by the World Health Organization[21].Treatment 3:G.changii (GC)control group, was administrated with 200 mg/kg bw aqueous extract from G.changii, followed by VOO.Treatment 4:GC + BPA group, was treated with 200 mg/kg bw of aqueous extract from G.changii, followed by 60 mg/kg bw of BPA.As previous studies have reported that 200 mg/kg of seaweed such as Spirulina platensis was able to ameliorate oxidative stress and improve reproductive performance, the same dosage of G.changii extract was used in this study[22,23].All treatments were carried out via intragastric route daily for 6 consecutive weeks.Mice weight, food, and water intake were recorded daily.
After the treatments, 8 of the female mice from each treatment group were euthanized at estrus, which was determined based on the vaginal appearance and vaginal smear as recommended by Croy et al[24].The wet weight of the uterus was recorded and uterus index was calculated by dividing the uterine wet weight by body weight(mg uterus/kg bw).The uteri were used for measurement of lipid peroxidation level, histology and immunohistochemistry studies.
2.7.Measurement of lipid peroxidation level in uterus
The lipid peroxidation level in the uterus was measured using QuantiChromTBARS assay kit (BioAssay Systems, USA)with slight modification.Briefly, the uterus was homogenized at 50 mg/ mL in ice-cold phosphate-buffered saline.The uterus homogenates were mixed with an equal volume of thiobarbituric acid (TBA) and incubated in 95 ℃ water bath for 60 min.A similar step was carried out to obtain the malondialdehyde (MDA) standard curve by substituting the uterus homogenates with (0-30) μM of MDA.Finally, the absorbance of supernatant was measured at 535 nm using a 96-wells microplate reader and the lipid peroxidation level was expressed as equivalence to μM MDA per g wet weight of the uterus.
2.8.Histological study
The histology slides of the uterus were prepared in hematoxylin and eosin staining using the protocol described by Woo et al[25].The histopathological changes in the myometrium, endometrial gland, and eosinophil number in the endometrium were observed.Enumeration of endometrial gland and eosinophil numbers were conducted on 10 fields per slide of the uterus at 400× magnification.Less than 7 endometrial glands per field was considered as lower level.This classification was based on previous studies by Spencer et al[26], which have reported that a normal 60 days old rodent should have an average of 7 glands/uterine field.In order to classify the level of eosinophil numbers in the uterus, the eosinophil number of the control group was used as a reference.In this study, the eosinophil number from 8 control mice was determined and a normal distribution curve was constructed.The number of eosinophil at the 25th-75th percentile of the normal distribution curve was used as the reference for the classification mentioned earlier.The analysis showed that the number of eosinophil between the 25th-75th percentiles of the distribution curve was 44-306 eosinophil per field.Hence, mice of treatment groups with uterus section showing less than 44 eosinophils per field were considered as a group with lower eosinophil number.The histology observations were expressed as the percentage of abnormal mice in each treatment group.
2.9.Immunohistochemistry
The uterine expressions of estrogen receptor α (ERα), estrogen receptor β (ERβ), and complement C3 were evaluated as described by Abdel-Salam et al[27]with slight modifications.The tissue sections were incubated with anti-ERα rabbit monoclonal antibody(M00057-2, Boster) or anti-ERβ rabbit polyclonal antibody (A00786,Boster, US) or anti-C3 rabbit polyclonal antibody (A00168-1,Boster, US) at 1:100 dilution, followed by incubation with 1:100 horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L)secondary antibody (BA1054, Boster, US).The expression level of ERα, ERβ and complement C3 were evaluated based on the 3,3′-Diaminobenzidine (DAB) staining intensity in glandular and luminal epitheliums and ranked as “+” for pale/weak staining or“++” for dark/strong DAB staining.The results were expressed as the percentage of mice expressing “++” dark DAB staining of each protein in each treatment group.
2.10.Percentage of pregnancy
The remaining 16 female mice from each treatment were mated with fertile ICR male mice (8-12 weeks old) at 1:1 ratio for 5 d in order to determine the percentage of pregnancy.The percentage of pregnancy was calculated as the number of pregnant female mice over the total number of female mice subjected to mating.
2.11.Statistical analyses
All data were analyzed using SPSS version 25.The normality of data distribution was checked using Shapiro Wilk W test.Data were presented as mean±SD.The mean comparison between parametric variables was analyzed using one-way ANOVA followed by Duncan multiple comparison at a significance level of α=0.05.Percentage of pregnancy was analyzed using Fisher Exact test, followed by Bonferroni multiple comparison.The result for percentage of pregnancy was considered significant when P<0.008 3 to avoid false-positive analysis as 6 comparisons were made in Bonferroni multiple comparisons.
2.12.Animal ethics statement
All animal handling and experiments were conducted as per the guidelines set by the National Institutes of Health (Guide for the Care and Use of Laboratory Animals) which were approved by the Scientific and Ethical Review Committee, Universiti Tunku Abdul Rahman (Approval number:U/SERC/79/2017).
3.Results
3.1.Extraction yield, antioxidant capacity, and total phenolic content of G.changii
Among the different solvent extractions, the aqueous extract gave significantly (P<0.05) higher extraction yield (27.81±3.25)% compared to methanol (11.44±0.19)% and ethanol (6.32±0.20)% extracts.Aqueous extract also showed significantly (P<0.05)higher DPPH scavenging activity with ECof (0.17±0.01) mg/ mL than ethanol [(0.22±0.03) mg/mL]and methanol extracts[(0.65±0.12) mg/mL].Similarly, extraction of G.changii with water and ethanol showed significantly (P<0.05) stronger ABTS scavenging activity with ECof (0.35±0.31) mg/mL and(0.41±0.04) mg/mL, respectively compared to methanol extraction(2.78±0.42) mg/mL.The TPC in the aqueous extract of G.changii[(6.25±0.44) mg GA/g)]was also significantly (P<0.05) higher than methanol [(3.09±0.68) mg GA/g)]extract and ethanol extract[(4.04±0.40) mg GA/g)].Meanwhile, antioxidant capacity of the aqueous extracts of G.changii was stable, maintaining above 80% for 9 months after the initial extraction (Figure 1).Hence, the aqueous extract of G.changii was selected for further animal study.
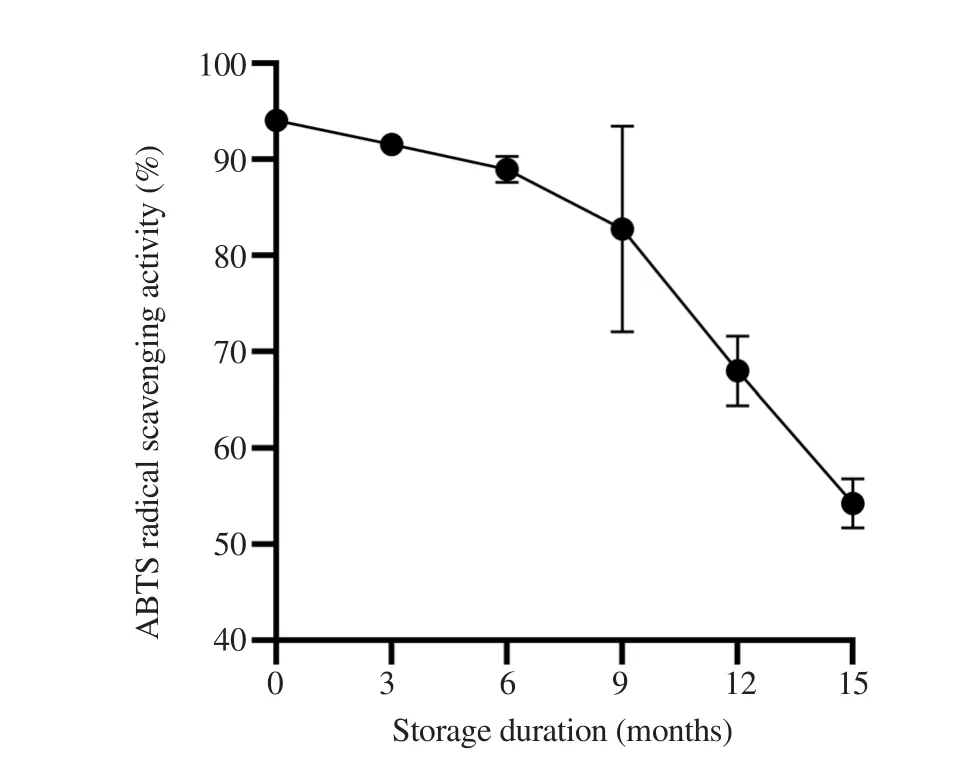
Figure 1.Stability of antioxidant capacity in aqueous extract of Gracilaria changii.Gracilaria changii extract was stored in paraffin film-sealed container at -20 ℃ throughout the duration of study.ABTS was used as the representative overall antioxidant activity in the extract (n=3).Data are expressed as mean±SD.
3.2.LC-MS analysis of aqueous extract from G.changii
LC-MS detected 55 and 17 peaks in the positive and negative ion mass spectra, respectively.Eight positive and one negative ion mass spectra have matched with known compounds in METLIN metabolomics database, with MFG score of > (90±5)%.The identified compounds in the extract included 2-ethyl-5-methylthiophene, homoarginine, hydroxyprolyl-asparagine,agaritine, amino acids, (x)-2-heptanol glucoside, etomidate, vitamin D3 derivative, and diacylglycerol as presented in Supplementary Table S1.Whereas 23 other compounds with MFG score more than 90% that did not match with any compound in METLIN database(Supplementary Table S2).The images of chromatograms and mass spectra were presented in Supplementary Figures S1 and S2.
3.3.Effect of G.changii and/or BPA on mice weight, uterus index, and lipid peroxidation level
The weight of the mice of all treatment groups from Day 0 to Day 42 followed an increasing trend (Figure 2) but the increments were not significantly (P≥0.05) different among the treatment groups.Upon examination on the uterus of the treated mice,the uterus index of BPA-treated group [(3.45±1.08) mg/ kg]was significantly (P<0.05) lower compared to VC group[(5.27±1.21) mg/kg], GC group [(5.18±1.32) mg/kg]and GC+BPA group [(5.47±1.85) mg/ kg].Although the lipid peroxidation level in the BPA group was relatively lower than VC group, the lipid peroxidation level did not differ significantly (P≥0.05)among BPA group [1 476.88±460.97) μM MDA/g uterus],VC group [(1 946.25±655.15) μM MDA/g uterus], GC group[(2 100.00±715.23) μM MDA/g uterus]and GC+BPA group[(2 013.13±661.66) μM MDA/g uterus].
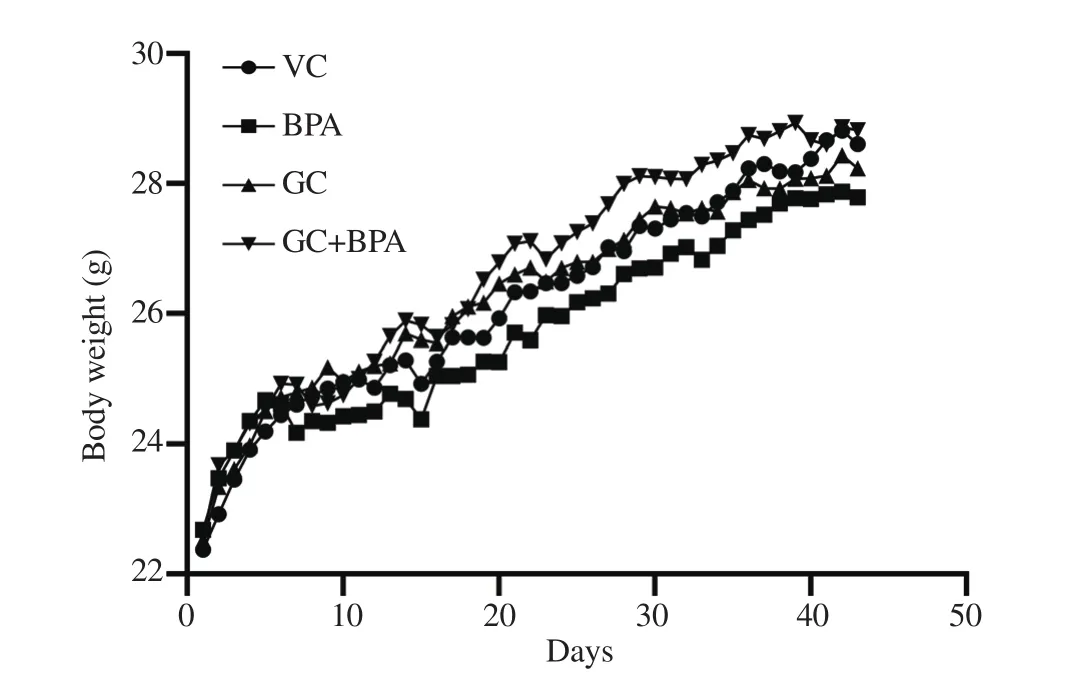
Figure 2.Changes of weight in female mice administered with vehicle control (VC), BPA, aqueous extract of Gracilaria changii (GC), or GC+BPA throughout the treatment period (n=24).BPA:bisphenol A.
3.4.Histological changes in uterus of mice administered with aqueous extract of G.changii and/or BPA
Histological examination on the uterus revealed that the BPA group had the highest percentage of mice with decreased eosin staining in myometrium (37.5%), followed by the GC+BPA group (25.0%), the VC (12.5%), and the GC groups (12.5%).Furthermore, 50.0% of BPA administered mice also showed decreased eosinophil number in the endometrium, which was higher compared with the VC,BPA, and GC+BPA treatment groups (25.0% for each group).The percentage of BPA administered mice (37.5%) showing reduced endometrium gland number was the highest followed by 25.0% in the VC group, 12.5% in the GC, and 12.5% in GC+BPA group,respectively.The photomicrographs also showed decreased eosin staining in myometrium, eosinophil number in endometrium and endometrial gland number (Figures 3, 4 and 5), respectively.
3.5.Effect of G.changii and/or BPA on uterine expression of ERα, ERβ, and complement C3
In this experiment, the percentage of mice from BPA group with high expression of ERα in glandular and luminal epithelium was lower (25.0%) compared to the VC and GC groups with 75.0% of mice, respectively showing high expression (Figure 6).Similarly, in terms of the expression of ERβ, the BPA group showed the lower percentage of mice with high expression of ERβ (25.0%) compared to the VC (62.5%) and the GC (50.0%) groups (Figure 7).The percentage of mice with high expression of ERα (0%) and ERβ(12.5%) in glandular and luminal epithelium was lowest in the GC+BPA group.In terms of complement C3 expression, all mice from the VC group exhibited high expression level of complement C3 in glandular and luminal epithelium.In contrast, only 75.0% of mice of the other treatment groups showed high expression of complement C3 (Figure 8).
3.6.Effect of G.changii and/or BPA on percentage of pregnancy
Female mice in the BPA group showed lower percentage of pregnancy (43.75%) than the VC group (68.75%), whereas GC treated-mice showed significantly (P<0.05) higher percentage of pregnancy (93.75%) than the BPA group.Mice from the GC+BPA group showed improvement in the percentage of pregnancy (75.00%)compared to the VC group.
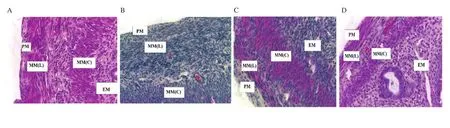
Figure 3.Hematoxylin and eosin-stained tissue section of myometrium at 400× magnification.Mice were administered with (A) vehicle control (VC), (B) BPA,(C) Gracilaria changii (GC) and (D) GC + BPA.The different areas of uterus in the image are labeled with PM as perimetrium, MM(L) as longitudinal myometrium, MM(C) as circular myometrium, and EM as endometrium (n=8).
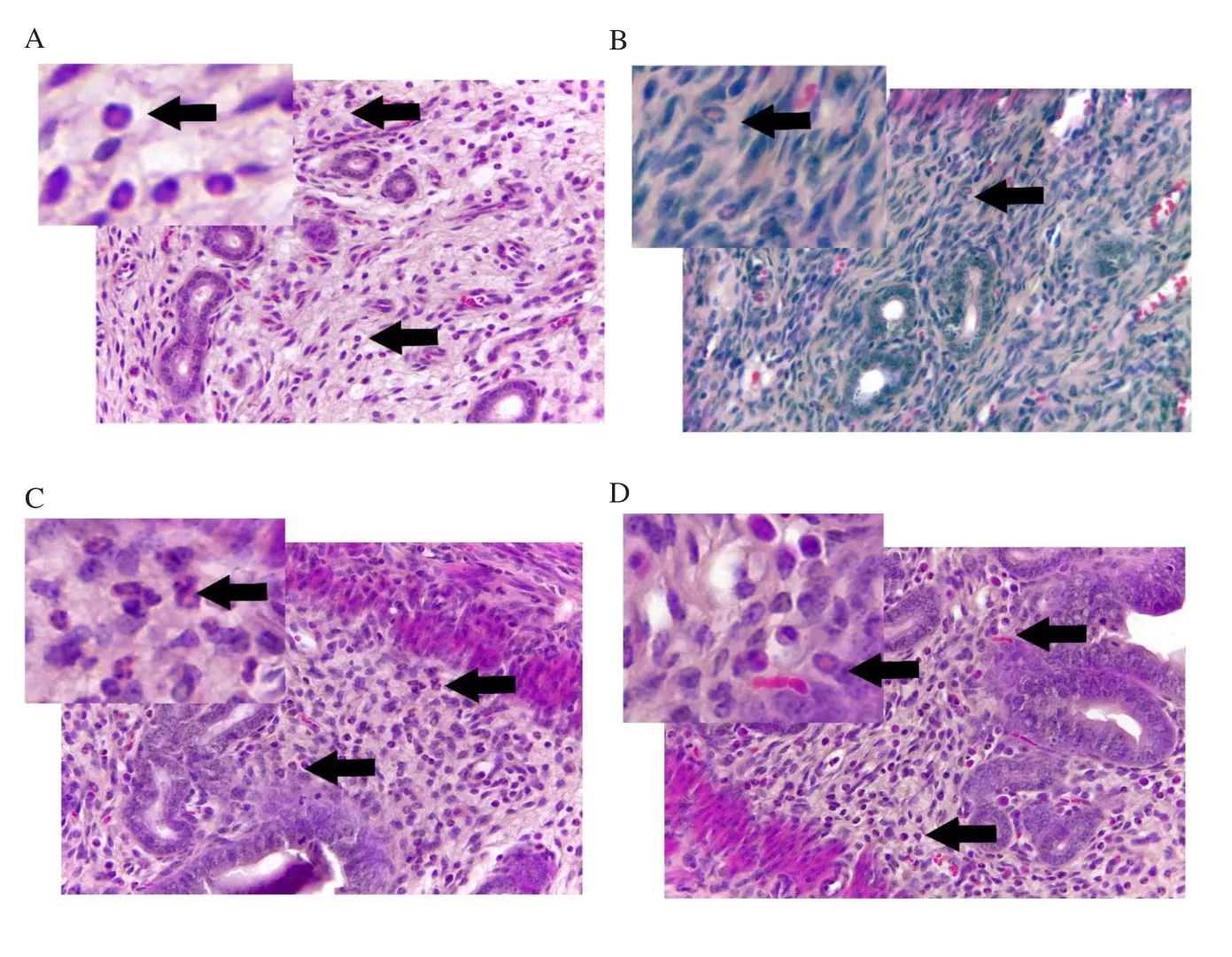
Figure 4.Representative image of eosinophils in the endometrium at 400× magnification.Mice were administered with (A) vehicle control (VC), (B) BPA,(C) Gracilaria changii (GC), and (D) GC + BPA.Hematoxylin and eosin stain.Black arrows indicate eosinophil in the endometrium (n=8).
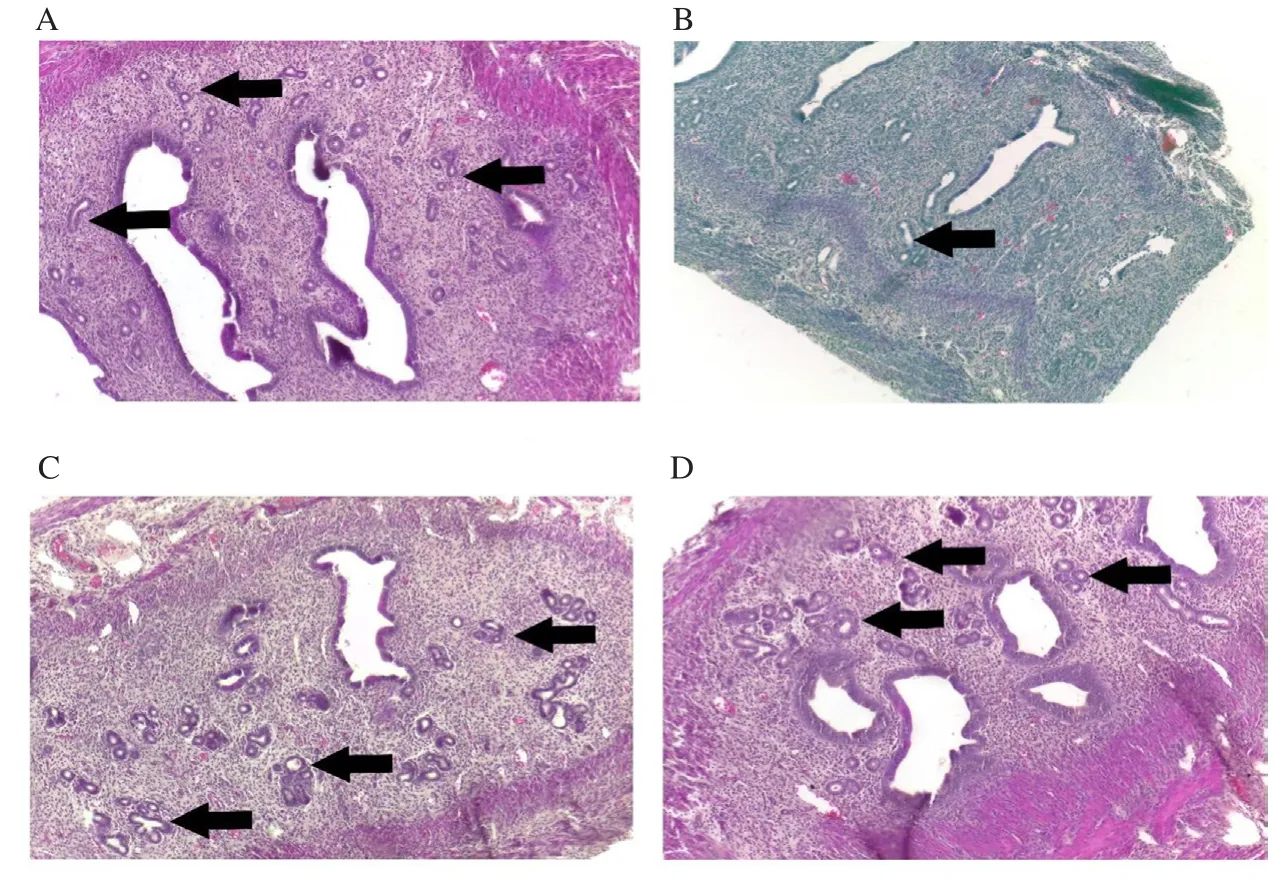
Figure 5.Representative image of of endometrial glands in the uterus at 100× magnification.Mice were administered with (A) vehicle control (VC),(B) BPA, (C) Gracilaria changii (GC) and (D)GC + BPA.Hematoxylin and eosin stain.The endometrial glands are indicated by black arrows(n=8).
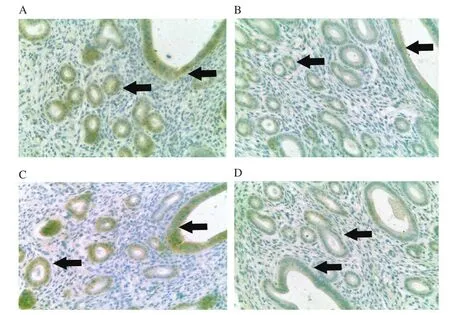
Figure 6.Expression of ERα in the luminal epithelium and glandular epithelium from different treatment groups at 400× magnification.Mice were administered with (A) vehicle control(VC), (B) BPA, (C) Gracilaria changii (GC) and(D) GC + BPA (n=8).The expression of ERα in the luminal and glandular epithelia are indicated by black arrows.
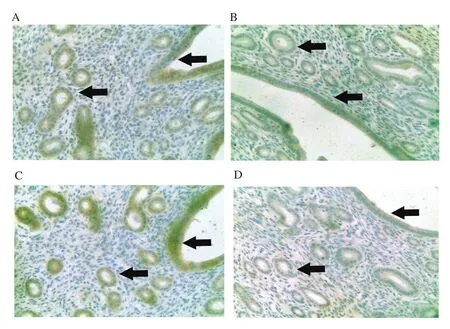
Figure 7.Expression of ERβ in the luminal epithelium and glandular epithelium from different treatment groups at 400× magnification.Mice were administered with (A) vehicle control(VC), (B) BPA, (C) Gracilaria changii (GC) and(D) GC + BPA (n=8).The expression of ERβ in the luminal and glandular epithelia are indicated by black arrows.
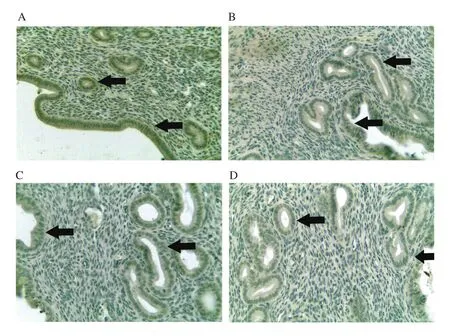
Figure 8.Expression of complement C3 in the luminal epithelium and glandular epithelium from different treatment groups at 400× magnification.Mice were administered with (A) vehicle control(VC), (B) BPA, (C) Gracilaria changii (GC)and (D) GC + BPA (n=8).The expression of complement C3 in the luminal and glandular epitheliums are indicated by black arrows.
4.Discussion
This study investigated the antioxidant properties of G.changii extract and its potentials in ameliorating BPA-induced adverse reproductive effects.It was observed that the aqueous extract of G.changii contained higher TPC and had antioxidant properties with lower ECfor DPPH and ABTS scavenging activities than those of methanol and ethanol extracts.However, these results were in contrast to the findings reported by Chan et al[5], as the methanol and ethanol extracts of G.changii in their study exhibited better antioxidant properties and higher TPC than its aqueous extract.The reason that led to these contradicting results might be due to the differences in the methods and incubation time used in extracting G.changii.Chan et al[5]extracted G.changii using hot water and shorter incubation periods, while in this study, G.changii was exposed to an incubation period of 2 d in the ultrapure water.Since, the aqueous extract of G.changii in this study showed the highest TPC and antioxidant properties, this extract was used in subsequent animal study.
LC-MS analysis of the aqueous extract from G.changii detected 72 peaks in chromatogram with 9 of the compounds matching with known compounds in METLIN database.One of the identified compounds is diacylglycerol, which contains arachidonic acid and EPA side chains and could be used for prostaglandin synthesis.Similarly, Norziah and Ching[4]also reported that G.changii contained as high as 74% of unsaturated fatty acid with 33.1% being the EPA.G.changii extract upon ingestion could provide additional EPA and arachidonic acid for prostaglandin synthesis in the uterus.Other Gracilaria spp.have also been reported to contain prostaglandins[9].Prostaglandin such as PGEand prostacyclin has been shown to facilitate ovulation and involves cyclooxygenase-2 dependent embryo implantation and decidualization,respectively[10,28].Besides, compounds such as 2-ethyl-5-methyl thiophene, (x)-2-heptanol glucoside, and vitamin D3 derivative with the compound formula of CHOwere also found in G.changii extract.These compounds were also known to exhibit antioxidant properties[29,30].Compounds such as homoarginine and vitamin D3 derivative detected in the extract have also been reported to show various biological activity on reproduction function.Homoarginine could act as the substrate for nitric oxide synthase to promote invasion of cytotrophoblast during initial pregnancy[31], while vitamin D3 derivative has also been known for its importance in supporting follicular development, progesterone biosynthesis, and luteinization of granulosa cells[32].
It was observed that BPA treatment reduced the lipid peroxidation in the uteri during estrus.The uterine lipid peroxidation during estrus could partly be generated from the peroxidase activity induced by endogenous estrogen[33-35].The lower uterine lipid peroxidation level in the BPA group might be due to the antagonistic effect of BPA against the action of endogenous estrogen in the uterus of the treated mice during estrus as reported by Gould et al[36].Besides that, literature has also suggested that lipid peroxidation could be important in hyperactivation and acrosome reaction of sperm prior fertilization[37,38].The lower uterine lipid peroxidation in the BPA group in this study could potentially reduce the hyperactivation and acrosome reaction of sperm upon entry into the female reproductive tract, which could then result in decreased percentage of oocyte fertilization by the sperm.
Besides, the uterus index of the BPA group was also significantly lower than the VC group.This suggested that the uterine proliferation during estrus was reduced upon BPA administration.This was in agreement with the histology findings, which showed that uteri from the BPA group were not properly developed and had higher incidence of decreased eosin staining in myometrium, decreased endometrial glands, and eosinophils in endometrium.Similarly,reduction in uterine gland number has also been reported following reduced ER level in diethylstilbestrol-treated rat[39].Furthermore,eosinophil was known to migrate cyclically into the non-pregnant uterus of rodent and human in response to serum estrogen, with the peak eosinophil number during the estrus stage[33- 35,40].The lower endometrial glands and eosinophil number present in the uteri from BPA-treated mice during estrus in this study might indicate that the uterine tissue was not well prepared for the embryo implantation.In a previous study, CD-1 mice treated with 600 μg/kg BPA have spent significantly less time in proestrus and estrus stages than metestrus and diestrus stages over 2 weeks compared with those without exposure to BPA[14].This supported that BPA could reduce proliferation of the uterus and result in longer duration in less proliferative stages such as metestrus and diestrus stages.
Immunohistochemistry examination of the uterus in this study also revealed that percentage of mice with strong uterine expression of ERα and ERβ were reduced in the BPA group compared with the VC and GC groups during the estrus stage.This could be due to the desensitization and downregulation of ER upon continuous activation by BPA, similarly as demonstrated by other studies using different estrogenic compounds such as diethylstilbestrol and estrogen[41-43].Likewise, ERα expression was reduced in vagina of Sprague-Dawley rats during the estrus stage upon exposure in utero to 50 mg/kg BPA[43].In addition, lower uterine ERα expression was reported upon treatment with a higher dose of BPA compared to the lower dose of BPA on mice[44].The endogenous serum estrogen during estrus and the administration of exogenous BPA may exert combinatorial activation of ER and eventually led to desensitization of the uterine ER during the estrus stage while this effect was not exerted during the diestrus stage, where the serum estrogen was lower[36,43].Decrease in ERα and ERβ expressions may have then led to a reduced uterine proliferation in response to endogenous estrogen stimulation.As demonstrated in complement C3 expression in this study, which is one of the ER downstream targets, decreased percentage of mice with high complement C3 expression was observed in the BPA group.This supported that BPA treatment reduced the uterine expression of ERα and ERβ in this study.
Decreased uterine response to estrogen could decrease the uterine proliferation during estrus, which in turn led to lower uterus weight/body weight ratio and less developed uterus morphology as portrayed by the decreased eosin staining in myometrium, reduced endometrial gland number, and eosinophil number in endometrium in BPA-treated mice compared with those in VC and GC group.All these findings could be explained by the reduced response of the uterus to estrogen due to the lower expression of ERs.In addition, BPA could induce proteosomal degradation of ERα, which led to decreased estrogen-induced peroxidase activity and eosinophil migration[33-35].The impacts on the uterine response mentioned earlier might disrupt the preparation of uterus lining for implantation and fertilization of oocyte, which eventually led to the lower percentage of pregnancy observed in the BPA group compared with the control group in this study.
Nonetheless, co-administration of BPA with aqueous extract of G.changii ameliorated the adverse effect of BPA as the mice from the GC+BPA group showed a higher uterus index, uterine lipid peroxidation level, and the percentage of pregnancy.In addition,the percentage of mice with reduced eosin staining in myometrium,eosinophil and endometrial gland number in the uterus was also lower in the GC+BPA group compared with the BPA group.However, ERα and ERβ expressions in luminal and glandular epitheliums in the GC+BPA treatment group were not increased compared with the BPA group.This suggested that the G.changii extract was able to alleviate the adverse effects of BPA in the uterus not through the restoration of the ERα and ERβ but rather through other means.According to Winuthayanon et al[45], the expression of ERα was essential for full extend of uterine proliferation.The lower percentage of mice with high ERα and ERβ expression might lead to a lower percentage of pregnancy in the GC+BPA group compared with the GC group.
In conclusion, the aqueous extract of G.changii showed better antioxidant properties than methanol and ethanol extracts.Although the aqueous extract of G.changii (200 mg/kg/bw) was unable to restore the uterine ER expressions, it has improved the uterine development in mice at the estrus stage after exposure to BPA(60 mg/kg/bw) as evidenced by increase in uterus index, improving eosin staining in myometrium, increase in endometrial gland and eosinophil number within endometrium.Aqueous extract of G.changii was also found to improve the percentage of pregnancy in mice after co-administration with BPA.These suggested that the potential protective effect of G.changii against BPA-induced female reproductive abnormalities was not mediated merely through restoring expression of uterine estrogen receptors and it may be through the modulation of eosinophil migration, endometrial glands formation, and protein expressions in myometrium.Further isolation and characterization of other unknown bioactive compounds from this aqueous extract of G.changii could be conducted in the future.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
Sincere appreciation is extended to the laboratory staff of Faculty of Science, UTAR and research assistant in Sunway University,Malaysia.Appreciation is also extended to the Universiti Tunku Abdul Rahman Research Fund for funding this research (IPSR/RMC/UTARRF 2017-C1/K04).Besides, the authors would like to acknowledge Dr.Devendra Pathak, an independent histologist from GADVASU University, India for examining and verifying the histopathological sections.
Funding
This research work is supported by the Universiti Tunku Abdul Rahman (UTAR) Research Fund (IPSR/RMC/UTARRF 2017-C1/K04).
Authors’contributions
N.C.L.conducted the experiment, analyzed the acquired data and prepared the manuscript.K.P.J.was the principal investigator of the grant, involved in designing the study, analyzing all the acquired data, revised and finalized the manuscript.T.G.C supported in designing the study and assisted in analyzing the histology sections.Y.Y.Y was involved in the collection of G.changii and assisted in designing the extraction of G.changii protocol as well as data analysis on the antioxidant properties of G.changii extract.M.K.G.was involved in reviewing the experimental design, data analysis,examined the histology of uterus and revising the manuscript.All authors read and approved the final manuscript.
 Asian Pacific Journal of Tropical Medicine2021年1期
Asian Pacific Journal of Tropical Medicine2021年1期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Gender disparity in COVID-19:Role of sex steroid hormones
- Association between Guillain-Barré syndrome and hepatitis E infection:A datadriven ecological study in Hong Kong
- Total spinal involvement due to delayed diagnosis and treatment of noncontiguous brucellar spondylitis
- Anopheles gambiae larvicidal and adulticidal potential of Phyllanthus amarus(Schumach and Thonn, 1827) obtained from different localities of Nigeria
- Intestinal parasitic infections and risk factors among Myanmar migrant workers in northeast Thailand
- Seroprevalence of SARS-CoV-2 in Mazandaran province, Iran
