When the bowel meets the bladder:Optimal management of colorectal pathology with urological involvement
Conor Keady,Daniel Hechtl,Myles Joyce,Department of Colorectal Surgery,Galway University Hospital,Galway H91 YR71,Ireland
Abstract Fistulae between the gastrointestinal and urinary systems are rare but becoming increasingly more common in current surgical practice.They are a heterogeneous group of pathological entities that are uncommon complications of both benign and malignant processes.As the incidence of complicated diverticular disease and colorectal malignancy increases,so too does the extent of fistulous connections between the gastrointestinal and urinary systems.These complex problems will be more common as a factor of an aging population with increased life expectancy.Diverticular disease is the most commonly encountered aetiology,accounting for up to 80% of cases,followed by colorectal malignancy in up to 20%.A high index of suspicion is required in order to make the diagnosis,with ever improving imaging techniques playing an important role in the diagnostic algorithm.Management strategies vary,with most surgeons now advocating for a single-stage approach to enterovesical fistulae,particularly in the elective setting.Concomitant bladder management techniques are also disputed.Traditionally,open techniques were the standard;however,increased experience and advances in surgical technology have contributed to refined and improved laparoscopic management.Unfortunately,due to the relative rarity of these entities,no randomised studies have been performed to ascertain the most appropriate management strategy.Rectourinary fistulae have dramatically increased in incidence with advances in the non-operative management of prostate cancer.With radiotherapy being a major contributing factor in the development of these complex fistulae,optimum surgical approach and exposure has changed accordingly to optimise their management.Conservative management in the form of diversion therapy is effective in temporising the situation and allowing for the diversion of faecal contents if there is associated soiling,macerated tissues or associated co-morbidities.One may plan for definitive surgical intervention at a later stage.Less contaminated cases with no fibrosis may proceed directly to definitive surgery if the appropriate expertise is available.An abdominal approach with direct repair and omentum interposition between the repaired tissues has been well described.In low lying fistulae,a transperineal approach with the patient in a prone-jack knife position provides optimum exposure and allows for the use of interposition muscle grafts.According to recent literature,it offers a high success rate in complex cases.
Key words:Colovesical fistula;Enterovesical fistula;Rectourinary fistula;Intestinal fistula;Diverticular fistula;Diverticular disease;Laparoscopic surgery;Colorectal cancer
INTRODUCTION
An enterovesical fistula (EVF) is an abnormal connection between the mucosal surfaces of the intestine and the bladder.It was first described by Rufus of Ephesus in AD 200 and first reported in modern literature by Cripps in 1888.The exact incidence of these fistulae is unknown;however,it has been reported to be responsible for 1 in every 3000 surgical admissions[1].
They are more common in men,with a male to female ratio of 3:1[2-4].
This discrepancy is believed to be due to the uterus,adnexae and broad ligaments interposed between the bladder and sigmoid colon.A higher incidence has been noted in females who have had a previous hysterectomy[2]and it is easy to conceptualise how the inflammatory process can spread to involve the bladder directly.
Depending on the bowel segment affected,EVF can be divided into four anatomically distinct subgroups,colovesical (70%),rectovesical (11%),ileovesical(16%),and appendicovesical (< 3%).Colovesical fistulae (CVF) are by far the most common,and usually involve the sigmoid colon and the dome of the bladder.
AETIOLOGY
Inflammatory
Aetiological causes of EVF are shown in Table1.Diverticulitis is indisputably the most common cause,accounting for up to 80% of cases,the vast majority of which are colovesical in origin and involving the sigmoid colon[3-8].Although the cause of CVF is predominantly diverticular,it has a relative risk of only 2%-4% in those with diverticular disease[6,7,9].CVF are postulated to formviathe direct extension of an inflamed,ruptured diverticulum,or erosion through the bladder wall by a pericolic abscess/inflammatory process[3,7,10].
Crohn's disease (CD) is the most common cause of small bowel EVF,accounting for up to 10% of all EVF cases.It is the most frequent cause of ileovesical fistulae;however,it is rare,with a reported incidence of just 2%-6% in CD[11,12].They are caused by transmural fissures extending from the diseased bowel segment to the normal bladder[12],and may be preceded by the patient suffering from subacute small bowel obstructive episodes secondary to an associated Crohn's stricture.
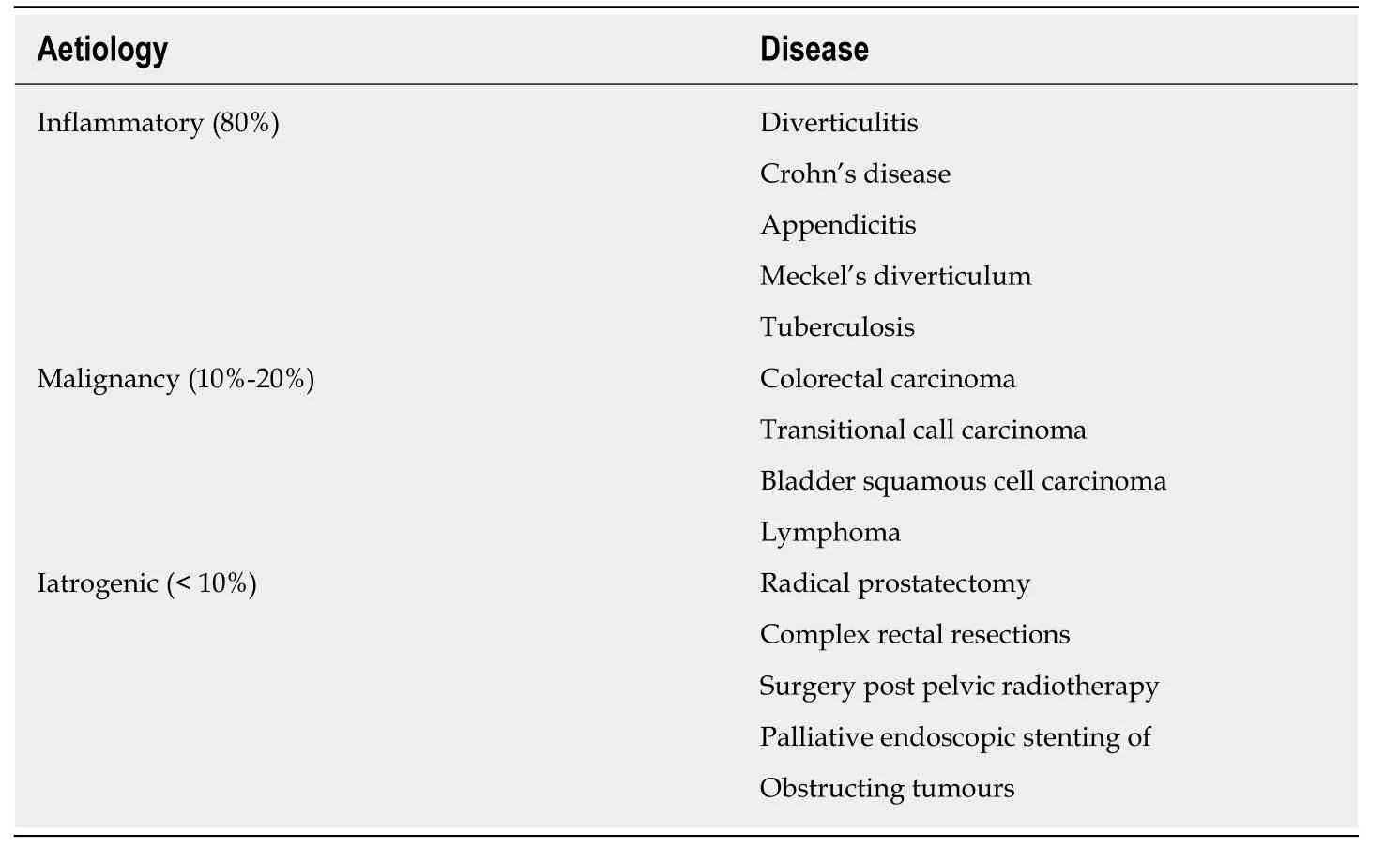
Table1 Aetiological causes of enterovesical fistulae
Other inflammatory causes include appendicitis[13],Meckel's diverticulum[14],genitourinary coccidioidomycosis[15],tuberculosis[16]and syphilis.
Malignancy
Malignancy is the second most common cause of EVF and accounts for 10%-20% of cases[2-8].Colorectal carcinoma,particularly sigmoid tumours,are the most commonly associated neoplasms,and occur due to direct invasion of the tumour into the wall of the urinary bladder[17].Fistulae caused by transitional cell bladder cancer are much rarer;however,certain studies have reported an incidence of 3%-8%[2,6,7].Transitional cell carcinoma is a far more common cause than squamous cell carcinoma of the bladder[18],due to its greater prevalence.There have also been case reports of lymphoma associated fistulae[19,20].
Iatrogenic
EVF are very rarely induced by surgical procedures;with radical prostatectomy accounting for a significant proportion of rectourethral fistulae.Palliative endoscopic stenting of malignant obstruction caused by colorectal tumours is also a welldocumented cause[21,22].Other surgical procedures known to be implicated in EVF formation include complex rectal resections,and cases have been reported of fistulae occurring secondary to laparoscopic inguinal hernia repair,albeit rarely[23,24].
EVF development post pelvic radiation are rare but well described,and are discussed later in this article.They are most often seen years after treatment,with many occurring without primary tumour recurrence[25-28].
Other rare but documented causes of EVF include spilled gallstones during cholecystectomy[29,30],penetrating abdominal trauma from gunshot wounds[31],and the presence of foreign bodies in pelvic organs such as chicken bones[32].
Clinical features
Although the aetiology of EVF is almost exclusively intestinal in origin,symptoms are generally urological in nature (Table2[2-4,6-8]).This is accounted for by the mechanical properties of both the bowel and the bladder.The bladder is a highly compliant organ,with a relatively low intraluminal pressure when compared to the colon,which favours the flow of contents from the bowel to the bladder[10,33].
Terminal pneumaturia (64%-95%) and faecaluria (36%-82.5%)[3,4,7,8]are the most common symptoms,with urine flow from the rectum found in 15% of patients.Pneumaturia,generally occurring at the end of micturition due to the gravitational effect on gas in the bladder,is a non-specific symptom and as such,other causes must be investigated and excluded.Infections with gas-forming bacteria such as Pseudomonas,rare types ofEscherichia coli[34]and yeast infections in diabetic patients with persistent glucosuria[35]must be excluded,along with recent bladder instrumentation and,although exceptionally rare,emphysematous cystitis[4].The vast majority of patients (up to 90%)[6]will describe these pathognomonic features,as well as recurrent Urinary tract infections (45%-87.5%)[3,4,6-8]or episodes of urosepsis,with infections being almost exclusively caused by gut commensals such asEscherichia coli,enterococci and other coliform bacteria[4,6].
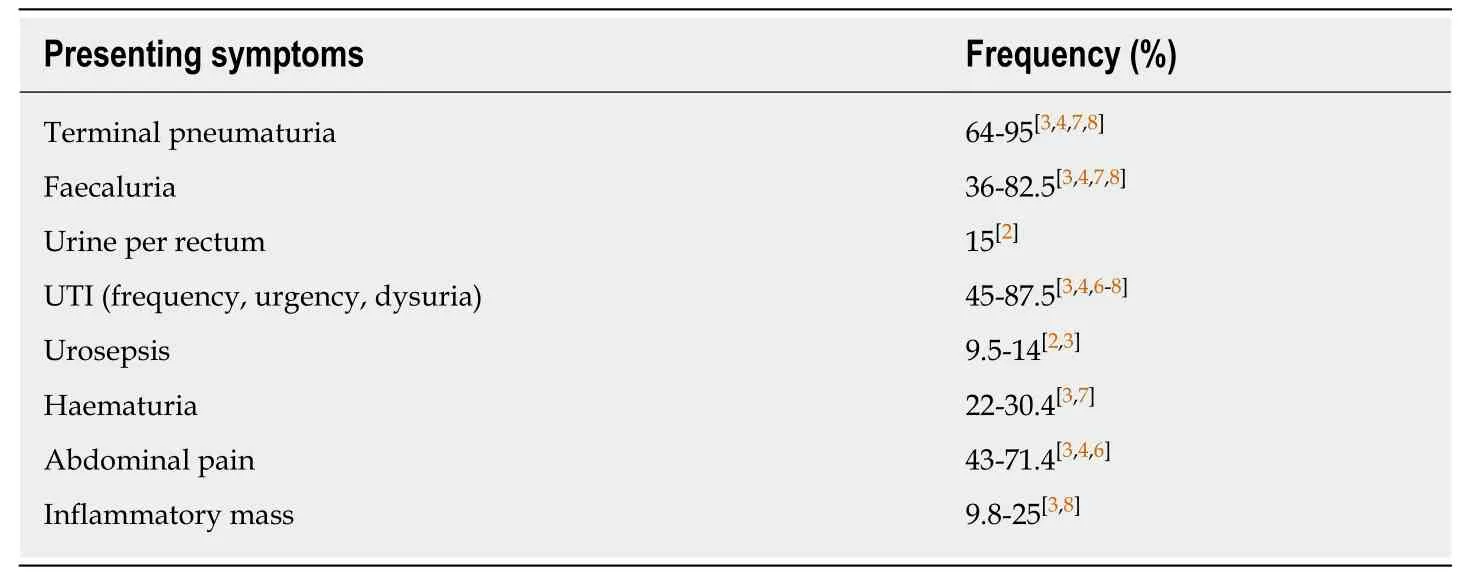
Table2 Clinical presentation of enterovesical fistulae and reported frequencies
Delayed diagnosis can occur due to non-specific symptoms at presentation such as;urinary frequency,urinary urgency,dysuria,altered bowel habit and non-specific abdominal pain[6].
Diagnosis
The diagnosis of an EVF can be challenging,and thus a high index of suspicion is required in non-specific presentations.Investigations aim to confirm its presence firstly,and then to establish the underlying aetiology as well as anatomical location.
Laboratory investigations
Blood investigations are of little value in the diagnostic algorithm of an EVF as they tend to be within normal limits.
Urinalysis and urine culture can be useful in the diagnostic process,with a positivity rate of > 90% being reported,with both coliform and anaerobic bacteria identified[6].Urine centrifuge has also been used to aid in the diagnosis,revealing the urinary presence of faecal and vegetable material in one study[4].
The utilisation of dyes and colouring agents has been described in the attempted diagnosis of EVF,with the aim being to identify these in the urine following rectal instillation,which would be considered diagnostic.The instillation of methylene blue per rectum has been trialled,with the presence of blue coloured urine being considered diagnostic[6].Doubt has been cast over the diagnostic accuracy of this method;however,as methylene blue is absorbed by gastrointestinal mucosa and excreted by the kidneys leading to inaccurate results.The use of orally and rectally administered indocyanine green has also been evaluated and has been shown to be very specific in early studies[36].Most recently,a study assessing indocyanine green as an alternative investigation tool in the assessment of EVF,has demonstrated a sensitivity of 92%[37].
The charcoal test is a bedside test that involves the ingestion of activated charcoal.It has shown to have a sensitivity of 100% in confirming the presence of a fistula if the presence of charcoluria is present within 24 h[38].The poppy seed test has also been well described and involves the consumption of 50 grams of poppy seeds,followed by evaluation of urine for their presence 48 h later.Studies have shown it to have a sensitivity of 95%-100% for the presence of an EVF[39].Both diagnostic investigations are cheap and accurate but do not delineate the aetiology or anatomy of the fistula any further.In addition,modern imaging modalities are extremely accurate,readily available and can be performed in an outpatient setting.
Imaging
The aim of imaging in the assessment of EVF is to determine and visualise the anatomical location of the fistulous tract.Historically,the use of plain abdominal xray,barium enema and intravenous urography have all been used to establish the anatomical location of fistulae or assess for secondary signs suggestive of a fistula.However,with advances in both computed tomography (CT) and magnetic resonance imaging (MRI),as well as improved accessibility of both modalities,conventional radiology is no longer advocated.
CT is considered the imaging modality of choice for diagnosing EVF,having a vastly superior sensitivity when compared to conventional radiology.It is widely available,non-invasive and can be performed and interpreted quickly and accurately.With a reported diagnostic accuracy of 61%-100%[2,33,40]it is recommended by the American College of Radiology as the first-line imaging modality in cases of suspected EVF.CT findings which suggest the presence of an EVF include air in the bladder without a history of recent bladder instrumentation,perivesical colonic thickening adjacent to a locally thickened bladder suggesting adherence,and the presence of oral contrast medium in the bladder on a scan without the use of intravenous contrast medium[7,41].Unfortunately,these are indirect signs,and CT still remains suboptimal in the delineation of the fistulous tract,with the pathognomonic sign of intravesical air contributing massively to the diagnostic accuracy of the test[17].CT has been reported to delineate the fistula directly in only 64% of cases[42].In order to opacify the fistulous tract on CT,a hydrosoluble enteral contrast agent(Gastrografin) must be given,which can become more dilute and therefore less opaque as it transitions through the bowel.This can lead to false-negative examinations due to this dilutional effect,and in cases where the tract has been occluded due to inflammatory oedema.This is essential in determining the presence of a fistula,as the presence of contrast in the bladder must come directly from its passage through the fistula.Intravenous contrast can render the scan obsolete as it is excretedviathe kidneys and thus will be present in the bladder,obscuring results.
As the pathological processes that result in EVF are generally extraluminal(peridiverticular inflammation,pericolic abscess development),CT is advantageous in that it assesses for the presence of extraluminal pathology[4,7,41],and also allows diagnosis and staging if a malignant process is suspected,by evaluating all visceral abdominal organs.Visualisation of extraluminal pathology also aids in surgical decision making pre-operatively as the presence of extensive metastatic disease or a pelvic abscess will alter the surgical plan[3].Three-dimensional CT imaging,when compared to traditional axial CT imaging provides a more detailed assessment of the anatomical relationship between the bladder and bowel in EVF,which can further aid in diagnosis,provide more detailed evaluation of anatomy and ultimately improve surgical planning[43,44].Most modern CT scanning software contains algorithms to reconstruct raw data into three-dimensional images without additional cost[17].Thus,CT scanning with the aforementioned symptoms is often sufficient to establish the diagnosis.
MRI can also be used in the assessment of EVF,and its use is well established.MRI has superior soft-tissue resolution when compared with CT and can also provide multiplanar imaging sequences[45].MRI is advantageous as the fluid within the fistulous tract acts as a natural contrast agent.Multiple imaging sequences are generally performed as visualisation of the tract depends on its content,i.e.,fluidvsair[17].T2-weighted imaging shows high signal fluid within the fistulous tract,as well as extraintestinal fluid collections and localised inflammation within the muscle wall of the bladder[46,47].T1-weighted imaging can characterise the fistulous tract with regard to adjacent hollow viscera and also demonstrate inflammatory changes in surrounding fat planes[17,48].
MRI has a sensitivity and specificity of up to 100% in detecting EVF and has similar accuracy in identifying the underlying aetiology[47,48].
Endoscopy
Direct visualisation of a fistulous tract at endoscopy can be difficult;however,an endoscopic examination allows for the determination of the aetiology of the fistula.
Cystoscopy can suggest the presence of a fistula by evaluating for secondary changes within the bladder.It is beneficial in excluding urological malignancy as the causative factor of the fistula[3].A localised area of erythema,oedema and congestion within the bladder mucosa are suggestive of the presence of a fistula.Other reported findings include cystitis glandularis,localised polypoid lesions,ulceration and the presence of faeces[4].The sensitivity of cystoscopy in directly visualising a fistulous tract is reported at 46%-60%[3,49],with some studies advocating cystoscopy as the firstline investigation in suspected EVF[4].
Colonoscopy is poor at visualising the fistula;however,it is essential in diagnosing the causative bowel pathology,and thus an integral part of the diagnostic algorithm.It has been reported that colonoscopy can have a sensitivity of over 50% in directly visualising the fistula tract[2].Colonoscopy is recommended as the first-line investigation if malignancy is the suspected cause of the fistula following CT evaluation[7].In addition to excluding a neoplasm,colonoscopy will allow assessment of the extent of the diverticular process,the area affected by hypertrophy,determine if there is an associated stricture and it will help to determine the extent of colonic resection required if surgery is deemed suitable.
Table3[2,3,49,6,33,36,38-40,47,48]summarises the sensitivities of the various diagnostic test used in the diagnosis of an EVF.
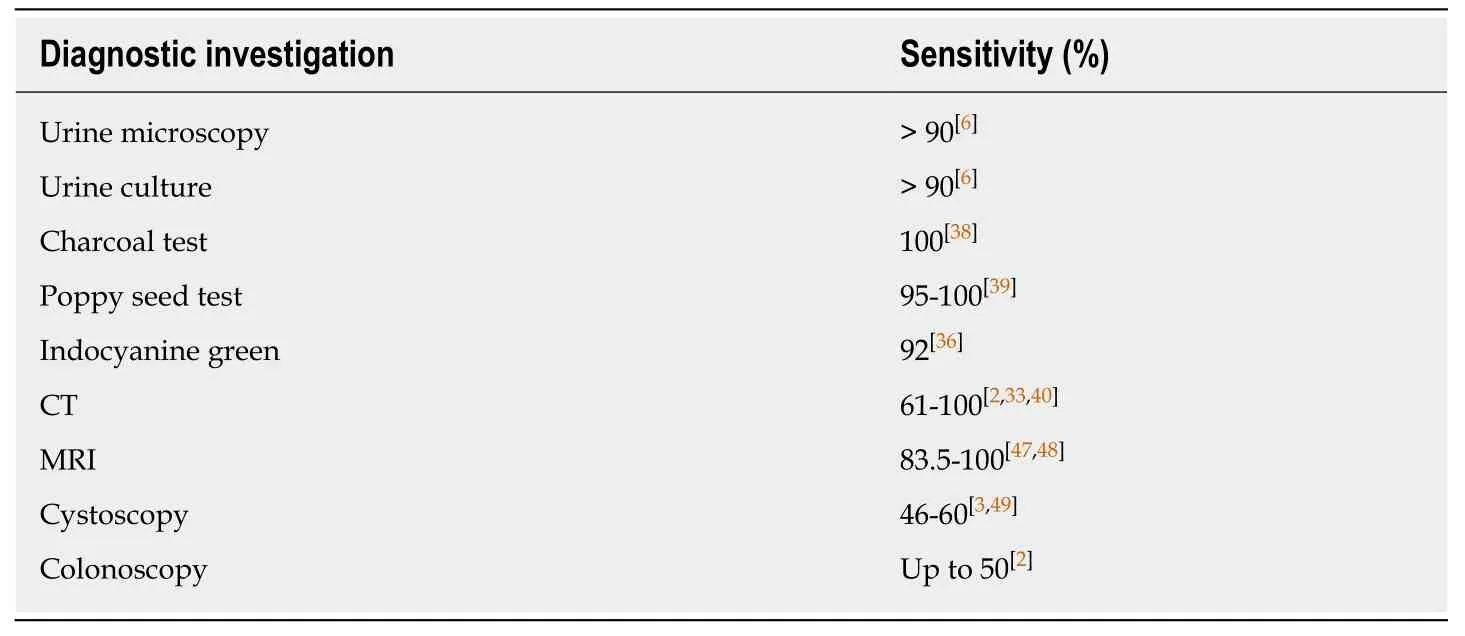
Table3 Diagnostic tests for investigating enterovesical fistulae and reported sensitivities
MANAGEMENT
Conservative
Conservative management is generally reserved for those deemed unfit for surgery,those with minimal symptoms and those who do not want surgical intervention despite associated symptoms.Some authors advocate for a conservative approach in confirmed cases of benign fistulae,and that surgery in this cohort should be carried out based on a quality of life basis[6].In a 50 patient series,there was found to be no significant difference in disease-specific mortality between the group who underwent surgical intervention and the group managed conservatively.It was also found that amongst their cohort,untreated CVF were present for a cumulative total of 3254 wk,with patients symptomatic of urinary tract symptoms but none developing overt urosepsis.Furthermore,in this study,32 patients had an untreated CVF for a sixmonth period without significant evidence of a decline in renal function during that period[6].
Conservative management is generally associated with high morbidity and mortality,particularly in younger patients with a longer life expectancy,and thus potential to be affected by related symptoms.In a review series of 90 cases of EVF,18 patients were managed conservatively,with one-year follow-up revealing seven deaths,2 of which were attributed to urosepsis[3].Morbidity in the literature ranges from 4%-45%,with mortality ranging from 0%-60%[2,3,50]in certain series.Patients treated conservatively tend towards higher mortality rates,which may have an associated bias as this patient cohort are often in poor physical condition and have associated co-morbidities.There is also the risk of an undiagnosed malignancy carrying the potential for progression.Patients electing to follow a conservative pathway should be warned about the potential for urosepsis or development of significant intra-abdominal sepsis[2].
In another study,8 out of 12 patients managed conservatively either due to patient preference or medical co-morbidities died from sepsis during the subsequent followup period[51].Thus,the option of conservative management depends on a myriad of factors including patient co-morbidities,patient preferences and associated symptoms.It is also important to avoid the patient re-presenting in the acute setting which may then necessitate a Hartmann's procedure.
Due to the high morbidity and mortality associated with EVF,the presence of this diagnosis should generally be considered an indication for surgery in all patients deemed fit for it and whom understand the associated surgical risks.The reported rate of spontaneous closure of these fistulae is about 2%[4,51].This,coupled with the risk of sepsis and ongoing quality of life issues,favours surgical management where possible.Many patients are initially abhorrent to the concept of surgery with the associated risk of stoma formation,but ensuing symptoms and poor quality of life often results in a change in perspective.
Operative management
The surgery performed generally depends on the underlying pathological process,the anatomical location of the lesion and the patient's premorbid condition.Treatment can involve a single-stage or multi-stage approaches.
Initial surgical approaches to benign EVF advocated for the formation of a proximal defunctioning colostomy as the only intervention,or prior to definitive pelvic dissection[52,53].This afforded patients symptomatic relief and minimised surgical trauma,but did little to treat the causative problem,as the rate of spontaneous fistula closure is reported at only 2%[4,51].These patients are also still prone to subsequent attacks of urosepsis.As surgical practice has evolved,it is now standard practice to resect the involved segment of bowel and associated fistula in order to prevent a recurrence,and subsequently re-anastomose both ends to achieve best results.Current surgical options include one-stage,two-stage and three-stage procedures,depending on patient age,comorbid state,disease factors,and whether the patient presents as an emergency or electively[54].
Single-stage repair
A single-stage procedure is advocated for the majority of cases of EVF[3,5,40,50,55].Evidence shows that resection of the involved bowel segment and primary anastomosis without a protective ostomy is possible in most instances.A review carried out by Woodset al[50]from 1960-1986 looking at 92 diverticular fistulae found that resection and primary anastomosis was possible in 62%,resection with primary anastomosis and covering ostomy in 24%,Hartmann's procedure in 7% and a threestaged procedure in 7%.There was a trend towards more single-stage procedures in the latter stages of the study,and they found no significant difference in complication rates between single and multi-stage procedures.They also found a prolonged overall hospital stay in patients undergoing staged procedures[50].Another review series of 90 patients with EVF established that resection and primary anastomosis was achieved in 92% of patients undergoing left-sided procedures.The use of a covering loop ileostomy was required just once.In the same series,the anastomotic leak rate was reported at 2.8% with no mortalities[3].These figures are consistent with those from other studies which have found no significant difference between single-stage and multi-stage procedures with regard to complications[49,56],and report a primary anastomosis in 82% to 95% of patients[57].Length of hospital stay has also been shown to be less in patients undergoing single-stage procedures[55].Table4[3,5,40,50,55]summarises the adoption of a single-stage approach over time,with associated anastomotic leak rate.
Staged procedures are recommended in the setting of gross faecal contamination,large pelvic abscesses,advanced malignancy,previous surgical procedures,intraoperative complications such as ureteric injury,post-radiation changes or in patients who would not survive an anastomotic leakage due to co-morbidities[5,55].
Two-stage repair
A two-stage procedure involves resection of the involved segment and primary anastomosis with an upstream,protective/diverting loop ileostomy.A two-stage procedure is recommended if there is a question about the integrity of the anastomosis[2].This is endorsed instead of a Hartmann's procedure as it is more readily and easily reversed.In most institutions,the reversal of a loop ileostomy is done through the ileostomy site once anastomotic healing is confirmedviaa Gastrografin enema.In addition,some centres perform early closure of the ileostomy.Certain surgeons favour a defunctioning colostomy,but in the authors experience we wish to avoid any potential problems with the marginal blood supply at time of reversal.In contrast,in order to restore intestinal continuity post-Hartmann's procedure,a further major surgical intervention is required and in a percentage of cases,a laparotomy may be necessary[9].
Three-stage repair
A three-stage strategy is generally not recommended unless the risks of surgery are very high,the patient has extensive co-morbidities,associated faecal incontinence or the patient presents emergently with associated sepsis[55].In addition if the patient presents emergently under the care of a general surgeon with limited colorectal experience,a Hartmann's type procedure would be reasonable and safe.Subsequent restoration of intestinal continuity could be performed under the direction of a colorectal surgeon.Nowadays,significant advances in post-operative intensive care are an important adjunct to patient care[50,58].Three-stage procedures involve an initial defunctioning surgery,followed by resection and eventual restoration of intestinal continuity.Defunctioning of the fistula containing bowel segment;however,rarely leads to closure of the fistula,and there is a high chance of fistula recurrence should it lead to closure initially[4,51].These patients are vulnerable to episodes of urosepsis as the connection between bowel and bladder remains patent[3].Nevertheless,it allows stabilization of the process,thus allowing definitive surgical resection of the fistulized segment at a later stage under more optimal conditions.
Management of the bladder
Management of the bladder is not yet standardised,with variations in intra-operativeevaluation of the bladder opening,its repair and post-operative care.Multiple methods have been trialled and published including suprapubic cystostomy,excision of the fistula and primary closure,simple closure alone,omental interposition and bladder drainage with a urinary catheter[50].
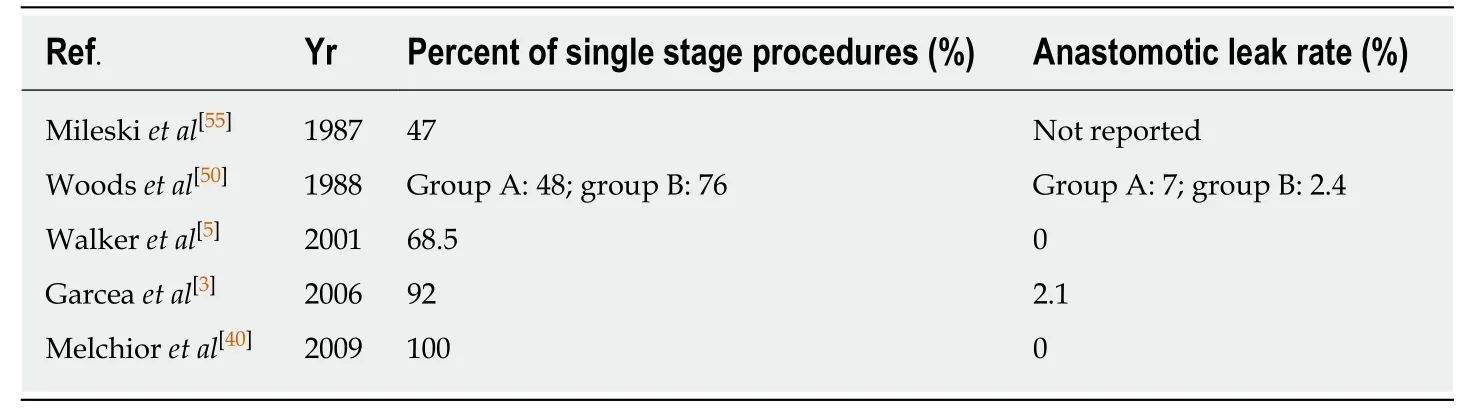
Table4 Reported percent of single stage procedures performed and anastomotic leak rate per series
There is considerable variation in the management of the fistulous tract because it is often not visible or is associated with concurrent abscess.Depending on the underlying aetiology of the fistula;urinary drainage,simple closure or partial/total cystectomy can be performed[59].Visualisation of the fistulous tract can be performed by distending the tract with methylene blue[60,61];however,it is argued that if the tract is not visualised directly then repair is not necessary[62].
A study by Walkeret al[5]following 19 patients found that a standardised protocol of catheter removal 10 d post-operatively revealed no urine leaks in patients who were managed with urinary decompression alone,and one urine leak in a patient who underwent a partial cystectomy for locally invasive colon cancer.At five year followup,there were no fistula recurrences;however,they did find that a significant proportion of their cohort died from non-related causes in the follow-up period[5].
More recently,Fergusonet al[62]carried out a retrospective review of 74 patients with benign fistulae who underwent a procedure for EVF,focussing on the management of the bladder specifically.They found that as most cases were attributable to diverticulitis or CD,resection of the bowel was adequate without the need for concurrent bladder repair or resection.If no bladder defect was visible or palpable by finger,then no repair was necessary.They proposed that an intensive search for bladder defects was not necessary and that bladder decompression with a Foley catheter for 7 d,without the need for cystogram prior to removal,was adequate for bladder healing.Large,full-thickness bladder defects were closed in two layers with Vicryl sutures.All defects healed within one week[62].
Partial cystectomy is necessary in cases of malignancy with an adequate healthy resection margin to lessen the risk of local recurrence[5,63].If there is intra-operative concern about a potential positive marginvsinflammatory changes,a frozen section can be sent.If a malignant colovesical fistula involves the trigone of the bladder,then a complete cystectomy may be required with ileal conduit formation,or the creation of a neobladder.However,pre-operative staging generally will identify the type and extent of resection required.In those patients requiring a partial cystectomy,the bladder is often closed in two layers,as the defect can be directly visualised[62,63].An omental flap,very much favoured by the authors,can be used for interposition between the bowel and bladder in cases of large bladder defects to prevent a recurrence,and provide an autologous buffer between the bowel anastomosis and bladder repair;however,there is minimal convincing evidence for its use[64,65].Most large centres dealing with these complex cases will have Urology services available,who should be involved in the case or be available in scenarios where the fistula is more complicated,such as involving the bladder trigone,with the potential for damage or ligation of the ureteric orifices.
Guidance on post-operative Foley catheter removal is scarce,with no standard approach adopted in the literature[66].Prolonged Foley catheterisation can lead to complications such as urinary tract infections,urinary retention and bladder atony[67],and thus an expedited catheter removal pathway would be favourable if definitively proven safe to do so.In a review performed by de Moyaet al[66]looking at early (< 7 d)and late (> 7 d) Foley catheter removal in 45 patients,they concluded that in patients with CVF secondary to diverticulitis,the catheter can be safely removed early without any increased risk of complications[66].Patients with their catheterin situfor > 7 d were more likely to encounter catheter-related complications and thus an increased length of hospital stay,however,this was not statistically significant.
A recent study by Dolejset al[68]also concluded that catheter removal should occur by day seven at the latest,with an argument being made for removal on postoperative day five as they found that all patients with bladder leaks in their study were identified by day seven,and suspicion was raised by day five with the presence of cloudy,dark urine drainage or high output abdominal drain drainage[68].de Moyaet al[66]also suggested that in cases of simple bladder repair or bladder drainage only post fistula surgery,there is no necessity for routine cystogram;however,they still advocate for cystogram post complex (repair of trigone) bladder repair.There is limited supporting literature for the use of routine cystogram outside of trauma[69].
Dolejset al[68],looking at the role of using an intra-operative bladder leak test in patients with diverticular CVF concluded that all patients undergoing repair should have an intra-operative leak test as,for all of those who had a negative leak test,none went on to develop a urine leak post operatively,and thus theoretically,none would require the need for a closed suction drain or cystogram post-operatively.They found that a positive leak test led to a change in patient management as it allowed for the repair of the bladder defect intra-operatively and thus spared patients the morbidity of prolonged Foley catheter drainage and closed suction drainage.They also determined that there may be benefit for the use of closed suction drainage in patients deemed high risk for urine leak post-operatively,as it allows for the assessment of the drain creatinine to serum creatinine ratio,which has a positive predictive value of 100% and negative predictive value of 93% in identifying bladder leaks.This may also reduce the requirement of post-operative cystograms in these patients[68].Table5[5,62,66,68]summarises the current evidence base for bladder management in the resection of EVF.
URETERIC STENTING
Prophylactic ureteric stenting is performed to potentially reduce the rates of iatrogenic ureteric injury and increase intra-operative detection rates during colorectal procedures[70].However,there is a persistent lack of consensus as to the benefit of this practice[71].
Obliterated tissue planes due to severe inflammation or an infiltrative neoplastic process causing EVF can make tissue dissection difficult[72].The distorted anatomy often faced during these procedures leaves the ureter vulnerable to ligation,crush injury,thermal injury,transection or devascularisation[73].The consequences of an unrecognised ureteric injury can be devastating for patients and may include intraabdominal sepsis,renal failure and loss of the involved renal unit[74].A recent systematic review assessing the benefit of using prophylactic ureteric stents to prevent and identify ureteric injury in colorectal surgery revealed a higher rate of ureteric injury in patients with prophylactic stentsin situ.It is the opinion of the author,however,that there was a strong selection bias,with stents being used at the discretion of the surgeons in anticipation of a difficult dissection[71].The placement of ureteric stents also exposes the patient to further stent-related complications such as urinary tract infections/urosepsis,haematuria,oliguria,reflex anuria and urinary retention,vesicoureteric reflux and ureteric trauma related to stent placement[75].When used,stents are placed at the beginning of the procedure using rigid cystoscopy and fluoroscopic guidance and can be removed at the end of the operation or at an interval of 6-8 wk after placement.The authors favour stent insertion in more complex cases,recurrent surgeries and in patients in whom one anticipates a difficult pelvic dissection due to preceding pelvic sepsis,radiotherapyetc.In these more difficult cases,ureteric stenting,in addition to being beneficial in the identification of the ureters,paradoxically reduces the length of the procedure,as the stent is palpable and thus the ureter is more quickly identified,and therefore avoids excessive and unnecessary dissection.Identification of the ureter at the pelvic brim helps avoid the major pelvic vessels which are often closely adherent to the inflammatory phlegmon,and can be transacted or torn,resulting in torrential haemorrhage.In addition,the ureter is often adherent to the phlegmon and can be easily transected.If any damage to the ureter occurs it can be easily be repaired intra-operatively by a Urologist.If no intra-operative ureteric problems occur the stents can be removed at the end of the case.It is important to examine them and ensure they are intact,as a tear in the stent or a missing segment is a clue that an inadvertent,undetected injury has occurred.
LAPAROSCOPIC SURGERY
Historically,a laparoscopic approach was deemed to be contraindicated in the presence of diverticular fistulae[50]and complicated diverticulitis,due to the intense inflammatory nature of the disease process[76].Longer operating times and highconversion rates further undermined the use of laparoscopic techniques in complicated diverticular disease[77,78].However,some series reported a laparoscopic approach to be a safe surgical option in certain complex cases.Fineet al[79]deemed laparoscopic management of complex diverticulitis safe and feasible,except in cases of CVF where conversion to open was reported in all cases[79].Fibrosis and chronic inflammation in the vicinity of the fistula,as well as adjacent,thickened and scarred colon represents the main technical challenges posed to the laparoscopic surgeon[80].Bleeding,failure to progress and difficulty with the division of the fistula account for conversion rates of up to 60% in certain series[76].
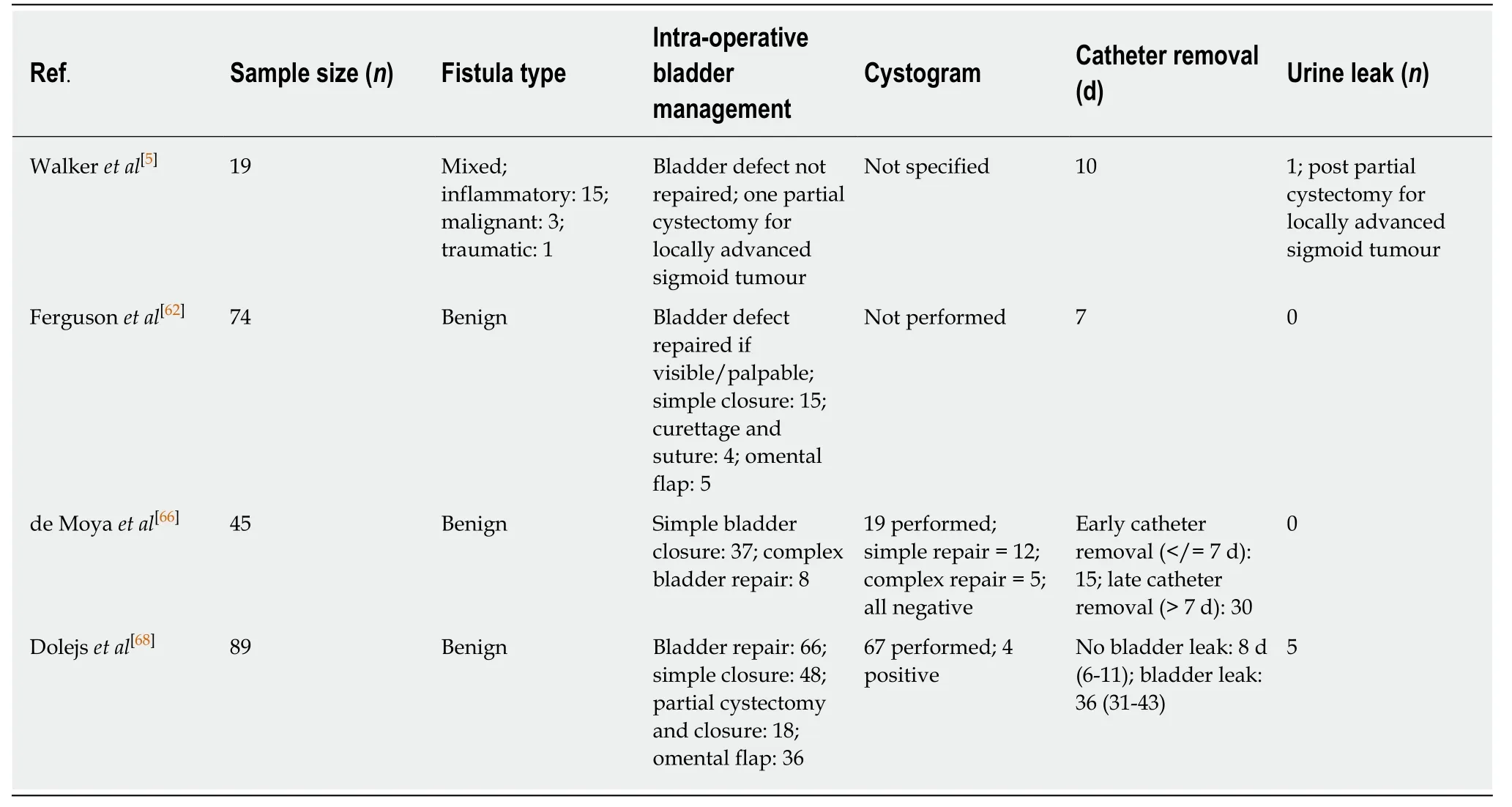
Table5 Summary of current evidences for bladder management in enterovesical fistulae resection
Kockerlinget al[81]found laparoscopic resection in attacks of acute,complicated diverticulitis,and diverticular disease with associated fistulae to be associated with higher complication and conversion rates and concluded that this technique should only be carried out by experienced laparoscopic surgeons.Other series from the same time period reported more success,with Franklinet al[82]reporting successful laparoscopic management of complicated diverticular disease in 90% of cases,including 6 cases of diverticular fistulae[82].However,as the incidence of diverticular disease increases worldwide[83],laparoscopic management of complicated diverticular disease has become more commonplace and feasible.Refinement in technique,increased experience and advances in surgical technology have all contributed to improved laparoscopic management.Engledowet al[84]have noted that their conversion rates have not been uniform over a ten year period,with initial rates at 64%,falling to an overall series conversion rate of 29%.This would suggest a steep learning curve when performing these technically challenging resections.
Laparoscopic sigmoid resection and primary anastomosis is the current standard of care in treating recurrent and complicated sigmoid diverticulitis,leading to a decreased incidence of major complications,a shorter hospital stay and reduced postoperative pain,as well as improved subjective quality of life at 6 mo[80,85].A metaanalysis of openvslaparoscopic resection for diverticular disease has demonstrated that laparoscopic procedures have a lower morbidity and complication rate than open surgery[85].As laparoscopic experience continues to improve,evidence suggests that its use in CVF secondary to complicated diverticular disease is both feasible and safe,with comparable outcomes to those of open surgery.Unfortunately,certain trials do not distinguish outcomes in CVF from those of colovaginal and ileocolic fistulae,and so definitive evidence as to whether laparoscopy is the best treatment strategy in CVF remains to be determined[86].
Variable rates of morbidity have been reported,with rates of 12.5%-15% in patients with mixed diverticular fistulae[60,84,87].However,in a study of 43 patients by Pokalaet al[88],overall morbidity of 30.2% was reported,but morbidity of just 11% was reported in the CVF subgroup[88].A study focusing solely on diverticular CVF demonstrated a morbidity rate of 20%,with no major laparoscopy related complications[89].Another study by Smeenket al[90]studied 40 patients with diverticular fistulae,35 of which were colovesical.They reported a 48% morbidity,primarily due to an anastomotic leakage rate of 28% and thus tentatively recommended the use of a routine defunctioning ileostomy.This recommendation would not be accepted by most authors,however,who would state that a tension-free primary anastomosis in the absence of gross abdominal contamination and distal obstruction should suffice[91].
In general,surgery for complicated diverticulitis carries with it a substantial mortality rate of up to 17%,with much of the risk related to patient factors[92].Studies examining fistulae of mixed aetiology have shown a mortality rate of 4%-17.4%[42,93]and a mortality rate of 6%-8% in patients with diverticular fistulae[84,90].Although studies examining laparoscopic CVF resections alone are sparse,outcomes are good,with Marneyet al[89]reporting a 0% mortality from 15 cases performed,albeit with low numbers.
Overall,laparoscopic surgery carries a conversion rate of 5%-36%;however,these studies included all diverticular fistulae (72,87,96).Pokalaet al[88]found that the conversion rate for CVF was 15.4%,vs32.6% when compared to fistulae off all aetiologies.Marneyet al[89]demonstrated a conversion rate of 33.3% in patients undergoing CVF resection,which is comparable to other studies of mixed aetiology.The relationship between surgeon experience and conversion rate has been well documented[60,84,89],and has shown a consistent downward trend in conversion rates with more cases performed.However,it is important to stress that timely conversion in the appropriate situation can reduce both post-operative complications and morbidity[77,94].Table6[81,82,84,88-90]summarises reported outcomes for laparoscopic management of EVF.
RECTOURINARY FISTULA
The development of rectourinary fistulae (RUF) results from an abnormal opening between the rectum and the bladder or urethra,the occurrence of which is relatively rare[95].Its presentation is very similar to that of EVF,with the majority of patients complaining of pneumaturia,faecaluria or the passage of urine per rectum[96].Patients may also present with locally confined sepsis or peritonitis.
RUF can be classified as congenitalvsacquired,with acquired fistulae generally being divided into benign or malignancy-related fistulae.Benign fistulae are as a result of CD,trauma,chronic perirectal sepsis or iatrogenic injury.Malignancy related fistulae occur as a result of a neoplasm,radiation,surgery or a combination of tumour and treatment effects[97].
Most commonly,RUF can follow intervention for prostate cancer,be it surgical,radiation or ablative therapy[98].As the treatment of prostate cancer has developed,and non-operative management has become more sophisticated,there has been a significant increase in the incidence of more complex and difficult to manage fistulae[99].Complex fistulae are defined as those occurring after prior radiation,after failed repair attempts and those > 2 cm in diameter[100].
With advances in the detection and treatment of prostate cancer in recent years,the majority of RUFs are seen in patients who have received multimodal treatment(external beam radiotherapy,brachytherapy,cryotherapy or combination therapy)[98].It is reported that > 50% of RUF occur in the presence of previous pelvic radiation[101].0.1%-3% of patients undergoing these forms of treatment will develop fistulae[99],with the incidence of RUF post brachytherapy being 0.3%-3% and an incidence of 0%-0.6%post EBRT[100].
The use of radiation prior to radical prostatectomy is a known risk factor for the development of fistulae,as it induces tissue fibrosis and destroys tissue planes,increasing the likelihood of rectal injury.The underlying pathophysiology is believed to be microvascular injury in the rectal wall leading to mucosal injury and fibrosis,and subsequently to the development of ulcers,perforation and fistulae[102].The incidence of RUF post salvage prostatectomy ranges from 2%-15%[103].
Due to the poor healing properties of previously irradiated tissues,elective anorectal procedures should be avoided as this substantially increases the risk of fistula development.In a study looking at 6 cases of rectourethral fistulae in patients previously treated with brachytherapy and external beam radiotherapy for localised prostate cancer,all 6 developed fistulae after subsequent rectal biopsy or elective haemorrhoidectomy[104].A subset of patients who have brachytherapy may develop an ulcer related to an implantation site.In inexperienced hands at proctoscopy this may look malignant and result in unnecessary biopsies and subsequent fistula formation.Thus,if one observes an anteriorly located ulcer in a patient who has had previous brachytherapy,if should be referred to an experienced colorectal surgeon todetermine further management,depending on the macroscopic characteristics of the ulcer.
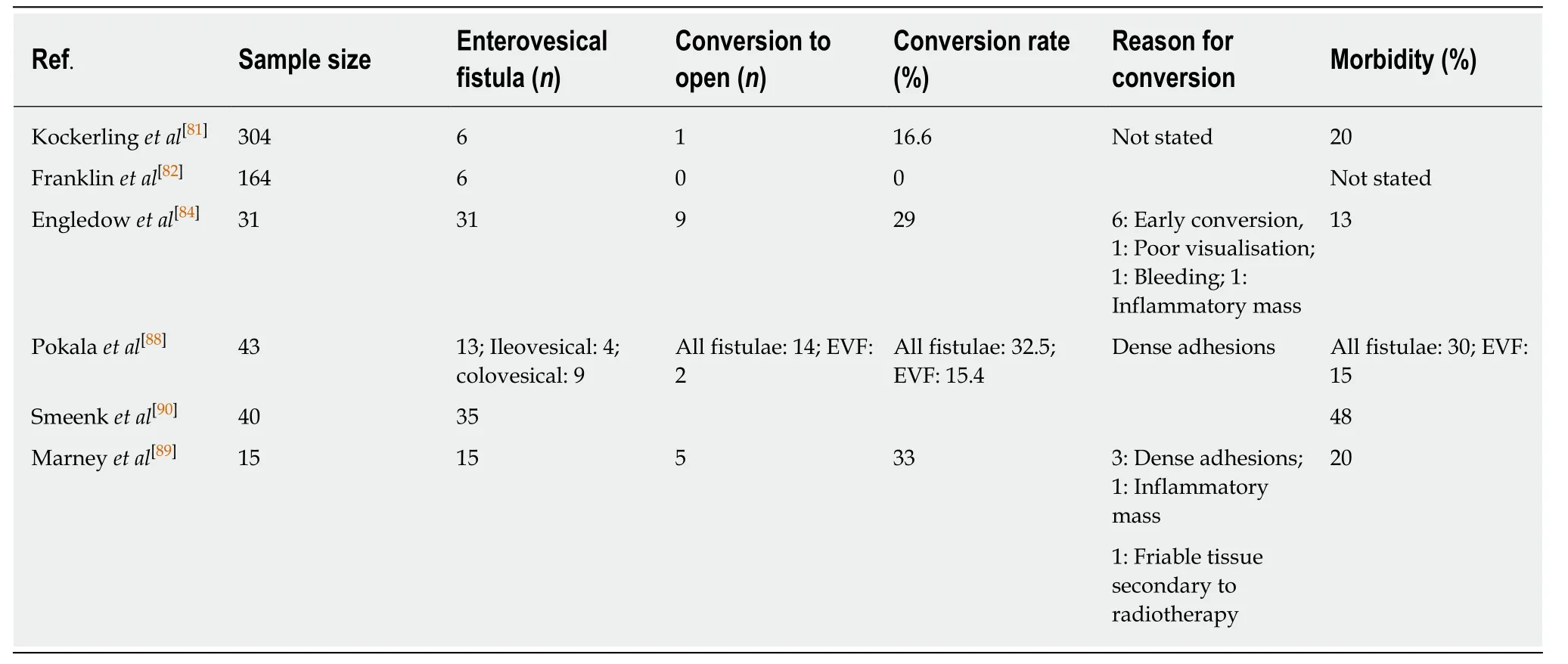
Table6 Summary of outcomes of enterovesical fistulae managed by laparoscopic resection
With regard to the development of fistulae in non-irradiated patients,the most common causes are inflammatory bowel disease (6%),trauma (22%) and surgery(65%).Of those who develop fistulae post-operatively,85% had undergone a previous prostatectomy[99].Radiological evaluation includes CT and MRI as well as diagnostic procedures including voiding cystourethrogram,retrograde urethrography and cystoscopy[99,105].Biopsies should be performed if concern for malignancy,and continence should be assessed as it will impact on treatment options.
MANAGEMENT
Management of malignancy-related fistulae requires aggressive surgical intervention,with permanent diversion often being required.Benign fistulae have better outcomes,and there is a chance of spontaneous closure with faecal and urinary diversion.Urinary diversion in the form of a transurethral or suprapubic catheter and faecal diversion in the form of defunctioning ostomy are rarely successful;however,and duration of diversion necessary is unknown[106].These procedures now often act as a bridge to definitive management due to low rates of spontaneous closure and recurrent urinary tract infections in this cohort[107,108].There is currently limited evidence as to the value of a diverting stoma in the management of these fistulae,but in the presence of systemic sepsis,they are necessary[109].
Conservative management
Conservative management encompasses all of the management strategies that do not involve direct surgical intervention of the fistula and generally involves urinary diversion +/- faecal diversion.Conservative management is indicated if there is a fistula < 2 cm,no previous radiation,no malignancy and no concomitant sepsis[99].
Transurethral or suprapubic catheterisation is the first step and should be used very selectively in patients with small,surgically induced fistulae[110].Should the fistula not resolve after 3 mo then faecal diversion should be considered.In a systematic review of various surgical techniques for acquired rectourethral fistulae,the indications for faecal diversion varied considerably from series to series and included;ongoing infection,previous radiation/ablation,previous failed repair,trial of spontaneous resolution,large complex fistulae > 2 cm,persistent symptoms affecting quality of life and patients with multiple comorbidities[99].
In a study carried out in the Mayo Clinic,colostomy formation was performed in all patients with RUF as an initial management step;however,all patients subsequently required definitive fistula repair due to lack of spontaneous closure[111].In another series containing 2447 patients who had undergone radical prostatectomy by Thomaset al[112],13 developed RUF,9 of which were managed with diverting colostomy and transurethral catheterisation,with 33% having spontaneous closure of their fistula[112].
In patients who undergo diversion therapy prior to definitive surgical management,the stoma is kept in place after surgery and healing confirmed with a Gastrografin enema or proctoscopy prior to reversal[107,113,114].Temporary urinary diversion is generally removed following confirmation of closure with retrograde cystourethrogram,retrograde urethrography or cystoscopy[99].Due to the lack of reported success with conservative management,most patients will require definitive surgical management[107].Should the fistula fail to heal with faecal diversion,definitive surgical intervention should be planned[112].This allows the adjacent tissues adequate time to heal prior to definitive repair.
Surgical management
Numerous different surgical approaches have been described for the repair of RUF,indicating the general lack of consensus around the best surgical approach.
Because of the rarity of the condition,there is limited surgical expertise on its management and approaches vary significantly,with an optimal approach still unclear.Approaches described include transperineal,transanal,transsphincteric,transabdominal,transvesical and posterior pararectal,as well as combinations of the above[105].Today,most are carried out by anterior transperineal approach as it provides the best exposure and allows for muscle flap interposition between the rectum and urethra.These complex procedures are often performed as a collaboration between a urologist and colorectal surgeon,and may require a plastic surgeon with experience in flaps[107,113].
TRANSPERINEAL APPROACH
The transperineal approach is the most commonly adopted approach in the management of complex RUF[99].It is recommended in cases of complex RUF secondary to radiation or ablative therapy,as well as fistulae > 2 cm and those requiring urethral reconstruction[115].It is the preferred approach as it offers excellent exposure of both the urethra and rectum,enabling the use of interposition flaps and also allowing for urethral reconstruction.Many different interposition flaps have been described including dartos muscle,gluteus maximus,omentum and dartos pedicle flaps;however,the gracilis flap is the most widely used,owing to its excellent mobility,ease of access and minimal donor site morbidity[115].
The gracilis muscle is the most superficial muscle in the medial compartment of the thigh,originating from the symphysis pubis and inferior pubic ramus[107].After dissection and closure of the fistula,the gracilis muscle is dissected,mobilised,rotated and tunnelled under a skin and subcutaneous tissue bridge to the anterior perineal space.It is then fixed above the RUF site to the apex of the prostate and the proximal rectal wall[107].Care should be taken when rotating the flap to ensure that there is not any tension on the neurovascular bundle,which can lead to flap ischaemia and necrosis[116].Due to the proximity of the urethral sphincter to the operating field in these procedures,stress urinary incontinence is not an uncommon complication,with reported incidences as high as 50%-70% in some studies[100,113].
The transperineal approach has demonstrated excellent fistula closure rates approaching 90%,irrespective of the complexity of the fistula or whether the patient had previous radiotherapy[99].Vanniet al[101]retrospectively reviewed 74 patients who underwent fistula closureviaa transperineal approach with muscle graft interposition and found that 100% of non-radiation induced fistulae and 84% of radiation-induced fistulae were closed by a single procedure at 20 mo[101].Ghoniemet al[107]reported a closure rate of 100% in complex RUF treatedviatransperineal approach at 28 mo follow up;however they reported a 48% urinary incontinence rate,with 16% having a“devastated” urinary outlet requiring permanent urinary diversion[107].Most recently,a multicentre,retrospective review of 201 patients who underwent RUF repair post prostate cancer treatment found an overall success rate of 92%,with a higher urinary incontinence rate (35%) in patients who had previous radiotherapy or ablative procedures,as compared to the radical prostatectomy group (16%)[117].
TRANSSPHINCTERIC APPROACH
The transsphincteric approach (York-Mason Technique) or York-Mason procedure,is a well-described approach to the repair of RUF.It involves a parasacrococcygeal incision,division of the internal and external anal sphincters and the posterior rectal wall to gain access to the fistula.A wide incision is then made around the fistula,and the entire fistulous tract is excised with subsequent repair of the urethra and rectal wall[105].This approach allows for the preservation of urinary continence as well as rectal innervation.However,it limits the ability to adequately mobilise urethral and rectal tissue which is often required to close large defects,and it provides poor access to the urethra,therefore preventing urethral and bladder neck repair[99].It also prevents the use of muscle interposition flaps in the case of complicated radiationinduced fistulae,limiting flap repair in these cases to local rectal tissue advancement flaps.Due to this,it is not recommended in the case of complex fistulae secondary to radiation or ablative procedures due to a fistula recurrence rate of up to 50%[118].The greatest risk associated with this approach,however,is that of faecal incontinence due to the division of both the internal and external anal sphincters.The sphincters are divided in a layered manner,with each layer marked with a coloured suture to aid in the meticulous reconstruction necessary to prevent faecal incontinence[105].The reported risk is < 1%;however,a 40-year review of the technique for RUF by Hadleyet al[119]has reported no incidence of faecal incontinence and this has been supported by Dal Moroet al[105]in 7 patients in a 15-year review.
Transanal (5.9%) and transabdominal (12.5%) (open,laparoscopic or robot) have been described but are much less commonly performed[99].
CONCLUSION
Fistulae between the gastrointestinal and urinary systems are rare but increasing in incidence.They are a heterogeneous group of pathological entities that are uncommon complications of both benign and malignant processes.They generally present in a similar manner,with pneumaturia,faecaluria and recurrent urinary tract infection,and a high index of suspicion is necessary in order to diagnose these conditions appropriately.
With increasing experience in the management of these complex processes,many are now managed in a single-stage process,with laparoscopic management becoming more common as equipment and experience improve.Unfortunately,due to the infrequency of these fistulae,exact management algorithms have not been established,with much of the current evidence of being derived from single centre retrospective and prospective studies.
 World Journal of Gastrointestinal Surgery2020年5期
World Journal of Gastrointestinal Surgery2020年5期
- World Journal of Gastrointestinal Surgery的其它文章
- Management of synchronous lateral pelvic nodal metastasis in rectal cancer in the era of neoadjuvant chemoradiation:A systemic review
- Software improvement for evaluation of laryngopharyngeal pH testing (Restech) - a comparison between DataView 3 and 4
- Effect of cholesterol on in vitro cultured interstitial Cajal-like cells isolated from guinea pig gallbladders
- COVlD-19 outbreak and endoscopy:Considerations in patients encountered in a foregut surgery practice
- lntroduction of new techniques and technologies in surgery:Where is transanal total mesorectal excision today?
