Active tuberculosis in inflammatory bowel disease patients under treatment from an endemic area in Latin America
Flora Maria Lorenzo Fortes, Ney Boa Sorte, Victor D Mariano, Laila D Andrade, Fernanda A Oliveira, Monique CA Santos, Claudia Ivanilda N dos Santos, Catharina A Passos, Mila P Pacheco, Valdiana C Surlo, Neogelia P de Almeida, Jaciane AM Fontes, Andrea M Pimentel, Raquel Rocha, Genoile Oliveira Santana
Abstract
Key Words: Inflammatory bowel disease; Therapy; Tumor necrosis factor alpha; Relative risk; Tuberculosis; Latent tuberculosis
INTRODUCTION
Inflammatory bowel disease (IBD) has a higher incidence and prevalence in developed countries[1]. However, the number of cases is increasing in Latin American countries, including Brazil. A recent systematic review of studies in Latin America and the Caribbean showed an increased incidence in Brazil from 0.68/100000 person-years in 1991-1995 to 5.5/100000 person-years in 2015. The same study showed that the prevalence of Crohn's disease (CD) in Brazil increased from 0.24 per 100000 persons (1986–1990) to 24.1 (2014), and the prevalence of ulcerative colitis (UC) rose from 0.99 to 14.1 during the same period[2]. The prevalence was 12.8/10000 persons in the northeast region of Brazil[3].
Biological therapy emerged with the advent of studies that identified the key presence of pro-inflammatory cytokines in IBD patients[4,5]. The appearance and release of drugs blocking tumor necrosis factor alpha (TNFα) for the treatment of IBD patients changed the course of these diseases and effectively induced clinical remission and mucosal healing[6]. Anti-TNFα therapy for the management of immune-mediated inflammatory diseases improved the prognosis and quality of life of these patients. CD patients using anti-TNFα therapy in Brazil increased from 29.6% (2005-2012) to 43.4% (2013/2014)[2]. However, an increased risk of infections was also observed as a consequence, including tuberculosis (TB)[7,8].
TB is also a very common infectious disease in Brazil, and it is considered a serious public health problem and life-threatening condition[9,10]. Globally, it is estimated that 10 million people develop TB annually, and this number has remained stable according to the United Nations (UN)[11]. Brazil reported 73864 new cases of TB in 2019, with an incidence of 35 cases/100000 person-years and ranging between 11.9/100000 person-years and 104.6/100000 person-years. Contact withMycobacterium tuberculosis(Mbt) leads to cure, pathogen latency or active TB. The airway is a gateway for the bacilli, which translocate to the respiratory tract after inhalation, where it finds the macrophage alveoli[12]. Dendritic cells or inflammatory monocytes transport Mbt to the pulmonary lymph nodes to initiate T cell stimulation of TNFα and interferon gamma secretion, which contribute to granuloma formation[12,13]. Granulomas are characteristic of human TB and are composed of clusters of macrophages and multinucleated giant cells that are surrounded by newly recruited monocyte/macrophage aggregates, neutrophils and lymphocytes[14,15]. These granulomas are essential for the control of Mbt, but they also provide an environment for the survival, multiplication, latency and dissemination of Mbt[16].
Several studies investigated the probable association between anti-TNFα therapy and the development of active TB. Previous studies reported several cases of active TB in patients with immune-mediated inflammatory diseases, such as rheumatoid arthritis and ankylosing spondylitis[8,17]. Ma?osaet al[18]reported an incidence of 1.2% (4/330) active tuberculosis in IBD patients under anti-TNFα therapy in Spain. Leeet al[19]showed an incidence of active tuberculosis of 1.4% (9/661) in patients with CD who were treated with anti-TNFα in Korea. However, studies in Brazil and Latin America on the development of active TB in patients with IBD under treatment are scarce[20].
In this scenario of high active TB incidence, increased IBD cases and improved access to anti-TNFα therapies, the present study evaluated the risk of active TB and possible associated variables in patients with IBD under treatment in an endemic TB area in Latin America.
MATERIALS AND METHODS
Data source and study design
We performed a retrospective cohort study of IBD patients who were followed up at a referral center in Salvador, Bahia, Brazil. The research center is the state's reference center for the treatment of IBD patients and the provision of prescriptions for high-cost drugs. The state health program only released anti-TNFα therapy for CD during the year of the present research. Data from August 2017 to November 2018 were collected. Patients diagnosed with IBD according to the European Crohn’s and Colitis Organization (ECCO) consensus criteria[1]were included.
A standardized questionnaire was used for each patient under direct interviews and a review of medical charts. The cohort baseline was set as the date of the first immunosuppressive or immunobiological therapy prescription, when the TB screening was first performed. The patients were screened for latent TB before starting immunosuppressive or immunobiological therapy. Medical record reviews and interviews included demographic variables (sex, age), self-declared ethnicity, type of IBD, and clinical aspects of IBD disease (time of diagnosis, age at diagnosis, Montreal classification[21], and ongoing treatment).
The Roberto Santos General Hospital Research Ethics Committee approved this research under the opinion number 1935.651/2017. The patients signed the Informed Consent Term before any procedure.
Criteria for TB diagnosis
Interviews and medical record review collected data on the history of active TB during treatment (e.g., time of diagnosis, location, diagnostic criteria, duration of treatment with immunosuppressant and/or anti-TNFα before the diagnosis of active TB), results of tuberculin skin test (TST), and past latent TB infection (LTBI). Patients were classified as positive or negative according to the TST scores, and the following risk factors were considered: Use of immunosuppressive drugs, use of anti-TNFα and chest radiography consistent with past TB. Patients were considered to have a positive TST if the TST result was ≥ 10 mm alone or ≥ 5 mm with at least one of the risk factors listed above[9,22].
The treatment of LTBI during the research followed the guidance of the Brazilian Ministry of Health for isoniazid from 5 to 10 mg/kg/d, with a maximum dose of 300 mg daily for 6 mo. However, the Ministry of Health changed the treatment of latent tuberculosis in March 2020 to rifapentine associated with isoniazid, with a weekly dose for 3 mo[23].
The Brazilian Ministry of Health defines active TB as a person with typical symptoms of active TB, bacterial confirmation (smear and/or rapid molecular test and/or culture) and chest radiography[9].Active TB was included for analysis when the diagnosis of active TB occurred during the interview or IBD treatment.
Statistical analysis
The results are presented as the means ± SD or proportion. The incidence rate was calculated by the ratio of the number of cases of active TB to the total number of cases evaluated. The crude relative risk (RRcrude) of active TB development in patients treated with anti-TNFα, azathioprine and anti-TNFα plus azathioprine compared to other treatments was obtained with the respective 95%CI. Adjusted RR (RRadj) for age, sex, type of IBD and latent TB was calculated using Poisson regression with robust variance (sex–model 1; sex and IBD type–model 2; sex, IBD type, latent TB–model 3; and sex, age, IBD type, latent TB–model 4). Statistical analyses were performed using SPSS software (version 21.0, Chicago, IL) and Stata?, version 13.3. APvalue < 0.05 was considered statistically significant.
RESULTS
A total of 301 patients were evaluated, including 186 (61.8%) patients with UC and 115 (38.2%) patients with CD. The mean ± SD age was 45.8 ± 15.0 years. There was a higher frequency of females (188/301; 62.5%) and patients from urban areas (244/301; 82.7%). The self-declared skin color was mixed race more frequently, with 145 (52.5%), followed by blacks with 109 (36.2%). Demographic and clinical characteristics are summarized in Table 1.
Overall, 131 (43.5%) patients were on immunosuppressive/biological therapy. Twenty-seven (9.0%) patients received anti-TNFα as a monotherapy, 31 (10.3%) patients received anti-TNFα associated with azathioprine, 3 (1.0%) patients received anti-TNFα treatment associated with methotrexate, and 70 (23.3%) patients only used azathioprine (Table 2).
TST was performed in 184 patients, and chest radiography was performed in 142 patients to screen for LTBI. Twenty (10.9%) patients were diagnosed and treated for LTBI. Eight (5.6%) patients had X-rays suggestive of TB sequelae. The TST was greater than or equal to 10 mm in 20 (10.9%) patients.
Eight (2.6%) patients developed active TB during treatment, four (50%) of the patients with UC and four (50%) of the patients with CD. The IBD duration in patients who developed active TB was 111.2 ± 58.9 mo. The age of patients who developed active TB during treatment was 40.3 ± 14.7 years at the time of the interview. Six (75%) patients were male. The mean ± SD time between the start of anti-TNFα therapy and the diagnosis of active TB was 20.0 ± 18.7 mo. One patient developed active TB three months after the start of anti-TNFα therapy, and the other three patients developed TB after more than 3 mo. Two patients received treatment with mesalazine, one patient received mesalazine associated with azathioprine, and one patient received azathioprine only.
Extrapulmonary TB was diagnosed in two patients (25%). Five patients (62.5%) developed active TB, despite the negative screening for LTBI. Three (37.5%) patients underwent treatment for LTBI (Table 3).
Latent tuberculosis was a risk factor for active tuberculosis (RRcrude= 8.28; 95%CI: 2.13-32.18, Table 4).
The frequencies of active TB in patients undergoing anti-TNFα therapy and with infliximab and adalimumab were 6.5% (4/61), 7.1% (2/28) and 6.1% (2/33),respectively. The four patients received combination therapy with azathioprine. Therapy with immunosuppressants, specifically azathioprine, anti-TNFα and the combination of these two drugs, were associated with a higher risk of active tuberculosis, with RRcrudevalues of 5.85 (1.20-28.48); 3.93 (1.01-15.29) and 9.03 (2.38-34.28), respectively (Table 4).
Multivariate analysis consistently reinforced that therapy with TNFα blockers significantly increased the relative risk of developing active TB compared to other treatments. Four multivariable models were evaluated, and the use of TNFα blockers alone or in combination with azathioprine was an important risk factor for the incidence of active TB in all models. When adjusted for sex, age, type of IBD and latent TB, anti-TNFα combined with azathioprine consistently increased the relative risk to 17.8 times more than conventional treatment (95%CI: 5.91-53.67;P< 0.001, Table 5).
Latent TB was an independent risk factor for the incidence of new cases of active tuberculosis with the use of isolated TNFα blockers and azathioprine-associated use.
DISCUSSION
According to the WHO[11], Brazil is one of the 30 countries with the highest TB burden. Therefore, the risk of active TB is near high levels in Brazil. However, despite being endemic for TB, little is known about the development of active TB in IBD patients under treatment. Our study observed an increased risk of active TB consistent with the use of immunosuppressants, especially after adjusting for age, sex, type of IBD and latent TB. Specifically, the combination therapy with anti-TNFα and azathioprine increased the risk of active TB by nearly 18-fold compared to conventional treatment. To our knowledge, this report is the first study performed in northeastern Brazil, which is an endemic region of TB in Latin America that is characterized by low development indicators, to consistently demonstrate this association.
Our results of the treatment of LTBI were similar to several previous studies. This finding is similar to countries with lower rates of TB, such as Spain, where Taxoneraet al[24]reported that the occurrence of positive TST was 11.5% in IBD patients undergoing screening for LTBI. Another Spanish study found that 30 (7.0%) patients with IBD had LTBI prior to treatment with anti-TNFα[25]. A 2015 Korean study[6]assessed the risk of active TB in patients with IBD using anti-TNFα therapy and found a frequency of LTBI of 10.6%. Kimet al[26], used chest radiography, IGRA and TST asscreening measures and confirmed LTBI in 30 patients (8.0%). A similar rate of LTBI is observed in countries with intermediate and high TB burden, but the treatment of this condition does not exclude the risk of IBD patients developing active TB. Instead, a history of latent TB increased the risk of active TB during immunosuppressive therapy.

Table 4 Univariate analysis assessing the relative risk (95%CI) of inflammatory bowel disease patients under treatment who developed active tuberculosis from a referral center
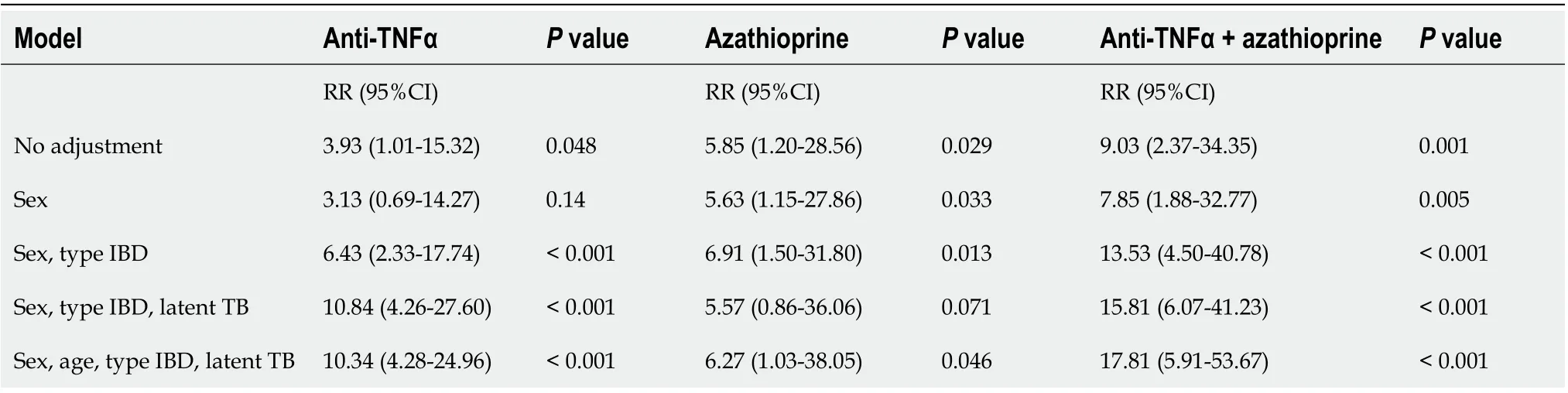
Table 5 Multivariate analysis of developing active tuberculosis by Poisson regression in patients with inflammatory bowel disease under treatment from a referral center
Analyses of only the group using anti-TNFα therapy revealed an increased frequency of 6.6%. Korea has a high prevalence of TB, and one study found a frequency of 2.0% of active TB in IBD patients using anti-TNFα therapy[26]. Another Korean study by Byunet al[6]showed a TB rate of 1.1% (6/525) in patients with IBD, with 3.1% (5/160) using anti-TNFα. These results show that the prevalence of active TB in Korean IBD patients was lower than the present study. A Spanish group identified that 1.2% (4/329) of IBD patients using anti-TNF α developed active TB[25], and a cohort of 765 patients in Portugal reported 25 cases (3.3%) of active TB while receiving anti-TNFα therapy[27]. These studies showed a low prevalence of active TB in patients using anti-TNFα, which was very likely due to the low prevalence of active TB in the general population.
Research in Fortaleza/Brazil of mostly rheumatological patients and a small group with psoriasis and Crohn's disease diagnosed active TB in 5 (6.3%) of the 79 patients treated with immunobiological agents and in 1 (4.6%) of the 22 patients treated with other immunomodulators/immunosuppressants[28]. Another study in Campo Grande/Brazil evaluated active TB cases in patients using Adalimumab and found a prevalence of 3.9% (3/77) in one year of follow-up[20]. Salvador had a TB incidence of 49.4 cases/100,000 person-years. Fortaleza had a similar high incidence of 54.9/100000 person-years, and Campo Grande had an intermediate incidence of 23.3/100000 person-years, which may explain the similar rate of active TB in immunosuppressive patients[10].
Our group of patients with active TB who received anti-TNFα therapy showed that the association with azathioprine increased the crude relative risk to 9.03 times greater. Byunet al[6]reported that 97.5% of patients with active TB who used anti-TNFα therapy were exposed to azathioprine/6-mercaptopurine. Meta-analysis evaluating the risk of reactivation of TB when anti-TNFα therapy was combined with immunosuppressive agents showed a 13-fold increased risk of TB reactivation[29]. As seen in the present study and the meta-analysis cited, the risk for active TB increased with this association, so care must be redoubled. Until now, there has been no uniform program to prevent the development of active TB in IBD patients undergoing combination therapy. Each country adopts national guidelines to care for this risk according to the local prevalence and populational risk of active TB.
As seen in Table 5, azathioprine increased the risk of developing TB almost 6 times. Few studies relating the risk of active TB in patients under treatment with azathioprine are reported. Generally, a description of the risk is an association of anti-TNF with azathioprine[29]. A Spanish group evaluated the risk of developing active TB in patients after lung transplantation and showed that azathioprine increased the risk 10.6 times[30].
The assessment made by multivariate analysis showed the presence of a high risk of developing active TB in patients with IBD treated with azathioprine alone. However, such data need to be confirmed by prospectives studies in patients with IBD from countries with low endemicity for TB.
Of the total active TB cases using anti-TNFα, 2 (50%) patients had pleural TB. The usual presentation of active TB described previously in patients under anti-TNFα therapy is extrapulmonary and disseminated. Abitbolet al[31]found that 91% of active TB in patients under anti-TNFα therapy had at least one extrapulmonary involvement. A Portuguese study reported that 15 (60.0%) of the 25 patients who developed active TB in their study had extrapulmonary TB, nine of which were disseminated[26].The pathophysiology of TB, the host’s defense mechanism and granuloma formation explain why patients using anti-TNFα therapy are more prone to extrapulmonary TB. The use of anti-TNF drugs prevents the formation of granuloma[12,32]. The low frequency of extrapulmonary/disseminated TB in the present sample was likely due to the probability of acquiring a new infection and not a reactivation.
Generally, a short period of time between the start of anti-TNFα therapy and the development of active TB is described, which suggests reactivation of LTBI. The range between the onset of anti-TNFα therapy until active TB infection was 20.2 (3-45) mo in our study, with 2 patients showing TB after 24 and 45 mo, which is more suggestive of a new infection. A European study showed an average interval of 14.5 mo between the first injection of anti-TNFα drugs and the diagnosis of active TB, which also suggests that only a small proportion was due to reactivation of TB[31]. Keaneet al[33]demonstrated a median interval of 3 mo between the development of active TB after the initiation of anti-TNFα, which indicates reactivation. A survey in a Korean country found longer intervals, similar to our results, and showed an average time between the beginning of anti-TNF therapy and active TB diagnosis of 23 (2-76) mo, which suggests a new infection[6].The screening for LTBI is only performed before the start of anti-TNF. However, numerous articles showed a later average time for the onset of active TB. A meta-analysis showed that the average duration for the development of active TB was 7 mo from the start of anti-TNFα therapy, and the risk increased even more that after 15 mo[34]. A study in Turkey evaluated patients with past treatment for LTBI and showed the development of active TB at 37.5 ± 27.0 (range: 18-84) mo after starting anti-TNFα therapy[4]. These results raise concerns about how to follow the screening of these patients using biological methods. Perhaps an annual screening of patients who are at risk of developing active TB should be performed in countries with a high TB burden.
Our study has some limitations. It was performed in a single center with a small sample of patients using anti-TNFα therapy. A prospective assessment of these patients would provide better data on risk factors and the development of active TB. Better knowledge about risk factors for active TB, such as smoking history, nutritional status, and occupational or family exposure to tuberculosis, is lacking.
The frequency of active TB in patients with IBD under treatment varies between countries, and one possible explanation for this difference in results is an effect of the epidemiological characteristics of each locality. Trials with anti-TNFα are rigorous, with strict inclusion criteria, and adverse events, such as active TB, are best studied in real-world situations. Most studies that reported the occurrence of active TB in patients undergoing anti-TNFα treatment were performed in countries with a low or intermediate frequency of tuberculosis. There is a knowledge gap in high endemic countries.
CONCLUSION
In conclusion, treatment with anti-TNFα significantly increased the risk of active TB in patients with IBD from an endemic area in Latin America, which is a region with a high TB burden. This risk is present when the IBD patient is under immunosuppressive and anti-TNFα therapy, and it increases when anti-TNFα therapy is combined with azathioprine. Late active TB, which is diagnosed 3 mo after the start of anti-TNFα therapy, was the most common, which suggests a new infection. This finding provides an important alert for the need to maintain care and evaluate when to screen for active TB risk in patients under biological therapy.
ARTICLE HIGHLIGHTS

Research results
Immunosuppressive therapy, specifically azathioprine, anti-TNFα and the combination of these two drugs, were associated with a higher risk of active tuberculosis, with RRs of 5.85 (95%CI: 1.20-28.48), 3.93 (95%CI: 1.01-15.29) and 9.03 (95%CI: 2.38-34.28), respectively. When adjusted for sex, age, type of IBD and latent TB, anti-TNFα combined with azathioprine consistently increased the relative risk to 17.8 times more than conventional treatment (95%CI: 5.91-53.67;P< 0.001). Azathioprine was not affected by other variables, but infliximab presented a higher risk when adjusted for age, gender, latent tuberculosis and the type of inflammatory bowel disease.
Research conclusions
Azathioprine and anti-TNF agents as monotherapy or in combination increased the risk of developing tuberculosis in inflammatory bowel disease patients. We reinforce that screening for latent tuberculosis should also be performed routinely in patients who start azathioprine.
Research perspectives
A prospective study that monitors the evolution of IBD patients under treatment should be performed to identify possible variables that reduce the risk of developing active tuberculosis during treatment.
ACKNOWLEDGEMENTS
We would like to acknowledge all the patients and the CNPq, FAPESB and PICIN/UNEB.
 World Journal of Gastroenterology2020年44期
World Journal of Gastroenterology2020年44期
- World Journal of Gastroenterology的其它文章
- Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer
- Hepatocellular carcinoma with tumor thrombus in bile duct: A proposal of new classification according to resectability of primary lesion
- Fatty liver is an independent risk factor for gallbladder polyps
- Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery
- Emerging use of artificial intelligence in inflammatory bowel disease
- Endoscopic pancreaticobiliary drainage with overlength stents to prevent delayed perforation after endoscopic papillectomy: A pilot study
