Extracellular vesicles as mediators of alloimmunity and their therapeutic potential in liver transplantation
Sotiris Mastoridis, Marc Martinez-Llordella, Alberto Sanchez-Fueyo
Sotiris Mastoridis, Department ofSurgery, Oxford University Hospitals, Oxford OX37LE, United Kingdom
Marc Martinez-Llordella, Institute of Liver Studies, King's College Hospital, Medical Research Council (MRC) Centre for Transplantation, London SE59NU, United Kingdom
Alberto Sanchez-Fueyo, Department of Liver Sciences, King's College Hospital, Medical Research Council (MRC) Centre for Transplantation, London SE59NU, United Kingdom
Abstract Extracellular vesicles (EVs) are a heterogenous group of nanosized, membranebound particles which are released by most cell types. They are known to play an essential role in cellular communication by way of their varied cargo which includes selectively enriched proteins, lipids, and nucleic acids. In the last two decades, wide-ranging evidence has established the involvement of EVs in the regulation of immunity, with EVs released by immune and non-immune cells shown to be capable of mediating immune stimulation or suppression and to drive inflammatory, autoimmune, and infectious disease pathology. More recently, studies have demonstrated the involvement of allograft-derived EVs in alloimmune responses following transplantation, with EVs shown to be capable of eliciting allograft rejection as well as promoting tolerance. These insights are necessitating the reassessment of standard paradigms of T cell alloimmunity. In this article, we explore the latest understanding of the impact of EVs on alloresponses following transplantation and we highlight the recent technological advances which have enabled the study of EVs in clinical transplantation. Furthermore, we discuss the rapid progress afoot in the development of EVs as novel therapeutic vehicles in clinical transplantation with particular focus on liver transplantation.
Key Words: Extracellular vesicle; Transplantation; Liver; Alloimmunity; Tolerance; Therapy
INTRODUCTION
The adaptive immune response to an allograft is initiated upon activation of T lymphocytes recognising donor major histocompatibility (MHC) antigens principallyviatwo distinct mechanisms which can occur concurrently but differ in the origin of antigen presenting cell (APC) and in their contribution to the alloresponse over time (Figure 1). The first of these,directallorecognition, occurs without the need for antigen processing by APCs, and involves the interaction of recipient T cells with intact allogeneic MHC-peptide complexes (pMHC) displayed on the surface of transplanted cells. It has been widely accepted, until recently, that ‘passenger leukocytes’, dendritic cells (DCs) in particular, transported within transplanted tissues and trafficking to recipient secondary lymphoid organs (SLOs) are primarily responsible for triggering the recipient immune responseviathe direct pathway[1]. The second,indirectallorecognition, occurs upon recipient T cell recognition of processed donor peptides presented by recipient antigen presenting cells. Given that thymic selection of T cells is not directed either in favour or against any given non-self MHC, the frequency of T cells recognising intact allogeneic MHC can be as high as 10% of the total population and so the direct pathway is considered the driving force behind acute allograft rejection[2,3]. In contrast, the frequency of T cells exhibiting alloreactivity to any given allopeptide which is processed and subsequently presented by APCs is low (< 1/100000) and so, though this indirect pathway is less likely to be pivotal in acute rejection, there is circumstantial evidence of its role in governing alloantibody production and chronic rejection[4].
Recent studies have called into question the centrality of passenger leukocytes in the generation of the direct alloresponse following transplantation. Mounting data from both vascularised and non-vascularised animal models demonstrate that in the early post-transplant period few if any such cells are found in SLOs[5,6]. Rather, within hours of transplantation, a far greater number of recipient APCs carry intact allogeneic MHC on their surface capable of being presented directly, without further antigen processing, to cognate T cells. As we will show, recent work demonstrates that the presence of donor MHC on host-APCs is in large part attributable to extracellular vesicles (EVs) released by the allograft. Here, we review current understanding of the role of EVs in the transfer of donor MHC following transplantation, and we assess the impact on graft rejection and tolerance. Drawing on this, we go on to consider the potential of EVs as therapeutic vehicles in transplantation with reference to the significant progress afoot in this area of novel biotherapeutics.
EV-mediated MHC transfer and its impact on alloresponses

Figure 1 Extracellular vesicle biogenesis and composition.
Most cells, including graft parenchymal, endothelial, and immune cells, release nanosized particles delimited by a lipid bilayer membrane which have come to be known collectively as EVs. Owing to their small size, durability, and capacity to transport a variety of biomolecules, EVs function as important mediators of intercellular communication, across a spectrum of tissues and biofluids. EV subtypes, have been categorised variably according to their particular mode of biogenesis, size, morphological characteristics, and/or cell of origin. With the expansion of tools and assays for their isolation, characterisation, and functional assessment, their classification and nomenclature continues to evolve[7-9]. Exosomes are the smallest of described EV subtype, with a diameter of 30-150 nm, and are formed within the lumens of multivesicular bodies (MVBs). The mechanisms responsible for their formation are now well understood and involve the Endosomal Sorting Complex Required for Transport (ESCRT), as well as ESCRT-independent mechanisms such as the tetraspanin family of proteins. The precise complement of these and other proteins likely affects the final composition of released exosomes (Figure 1). Microvesicles are larger, between 100-1000 nm in diameter, and form by pinching off directly from the plasma membrane. This outward budding is heavily dependent on the molecular composition of the plasma membrane. Apoptotic bodies, which tend to be larger still (up to 2000 nm in diameter), are also formed directly from the plasma membrane, however this occurs specifically at the time of apoptosis of the parental cell. Differences in their mode of biogenesis govern to a certain extent the size, cargo repertoire, and morphological features of EV subtypes. The repertoire of cargo of microvesicles is thought to reflect the parental cell of origin more closely than exosomes which undergo more selective enrichment. Though exosome and microvesicle biogenesis occurs at distinct sites within the cell and by different modes, in broad terms there is substantial overlap in the sorting machineries involved as well as in basic morphologic features such as their size and buoyant density. This can make isolation and distinction between them technically challenging[10-13]. In recent years, ‘omics’ analyses have revealed the diversity of the molecular composition of different EV subsets, of EVs released by different cells, and indeed of EVs release by single cells exposed to different environmental stimuli. Thus, the extensive repertoire of EV proteins, nucleic acids, and lipids is as much a reflection of the parental cell and its particular activation state as it is of the particular mode of EV biogenesis[14].
The exchange of molecules such as antigens and surface immunoglobulins between immune cells was first observed over four decades ago and, following this, the transfer of MHC complexes between leukocytes was described in 1974[15]. In the early 2000s, the acquisition of intact donor-derived allogeneic MHC by recipient APCs, DCs in particular, was described in the context of transplantation[16,17]. These ‘cross-dressed’ APCs,i.e.those host APCs noted to have acquired allogeneic MHC, were demonstrated to have the capacity to activate alloreactive T cellsin vitroas well asin vivo, in what represented a novel, third pathway for alloantigen presentation which came to be known as the semi-direct pathway (Figure 2). Cross-dressing was at first understood to be dependent on cell-cell contact, occurring by a process of cell nibbling or trogocytosis. In pivotal work from groups including that of Raposo, it was however noted that among their surface protein cargo, EVs also carry intact MHC class I and class II as well as pMHC[18]. Though it was later established that this conferred to EVs the capacity to activate T cells directly, two seminal studies from 2016 also demonstrated EVs to be responsible for the transfer of intact allogeneic pMHC from the allograft to recipient APCs, and laid bare the biological relevance of this mode of cross-dressing in the generation of alloresponses[5,6].
In first of these studies, Benichou and colleagues revisited the passenger leukocyte hypothesis in skin-grafted mice. Using highly sensitive cytometric, microscopic, and genotypic approaches, they confirmed the absence of donor leukocytes in recipient SLOs[6]. Considering that it typically takes 5 d or more for the neolymphangiogenesis required for passenger leukocyte trafficking to occur, the authors argue that it would be counterintuitive to expect this to be the mechanism responsible for the triggering of T cell alloresponses–often detectable within 48 h of transplantation. Rather than finding donor MHC present on passenger leukocytes, what the group observed upon examining recipient SLOs were large numbers of host APCs cross-dressed with donor MHC molecules. Using advanced imaging flow cytometry, a technique which permits the microscopic visualisation of fluorescently labelled flow-sorted single cells (Figure 3), the group were also able to determine that trafficking EVs were the likely source of graft-derived donor MHC. In the second of these reports from the same year, using a murine model of cardiac transplantation, Morelli and colleagues corroborated the paucity of passenger leukocytes in the period after transplantation, but also went a step further in affirming the ultra-structural mechanism of MHC transfer through their use of immuno-electron microscopy. This clearly demonstrated the way in which recipient APCs acquire donor MHC by capturing clusters of EVs bearing the characteristic marker CD63[5].
Having confirmed the route of allo-pMHC transfer to recipient SLOs, the researchers went on to demonstrate the centrality of cross-dressed APCs in initiating the alloresponses leading to acute allograft rejection. Flow-sorted conventional DCs cross-dressed by donor EVs were isolated and shown to be capable of the semi-direct priming of alloreactive CD8 T cells, as well as the indirect activation of na?ve CD4 T cellsin vitro(mixed lymphocyte reactions) andin vivoin mice[5]. These observations are in keeping with the ‘three-cell’ model proposed by Lechler and colleagues in 2004[16]. Adaptive CD8 T cell immunity is the principle arm of the cellular alloimmune response, but its development requires help. This can be provided by CD4 T cells that recognise alloantigen indirectly. According to the three-cell model, cross-dressed APC can indirectly prime an allospecific CD4 T cell which in turn can provide help for the semi-direct activation of CD8 T cells by the same APC (Figure 4A)[1,16]. Corroboration of the salience of crossed-dressed APCs as the main initiators of direct T cell allorecognition was provided whenin vivodepletion of recipient DCs was shown to dramatically reduce alloreactive T cell priming and to delay acute rejection in murine heart transplantation[5,19]. Similarly, in skin-grafted mice, Smyth and colleagues show the acquisition of MHC by DCs to be the main source of alloantigen driving cytotoxic responses and alloimmunity[20].
Taken together, these studies in experimental animal models of vascularised and non-vascularised solid organ transplantation support the view that the release of EVs bearing donor MHC and its subsequent presentation by cross-dressed APCs triggers the T-cell alloresponses involved in acute rejection.
EV-mediated MHC transfer in clinical transplantation
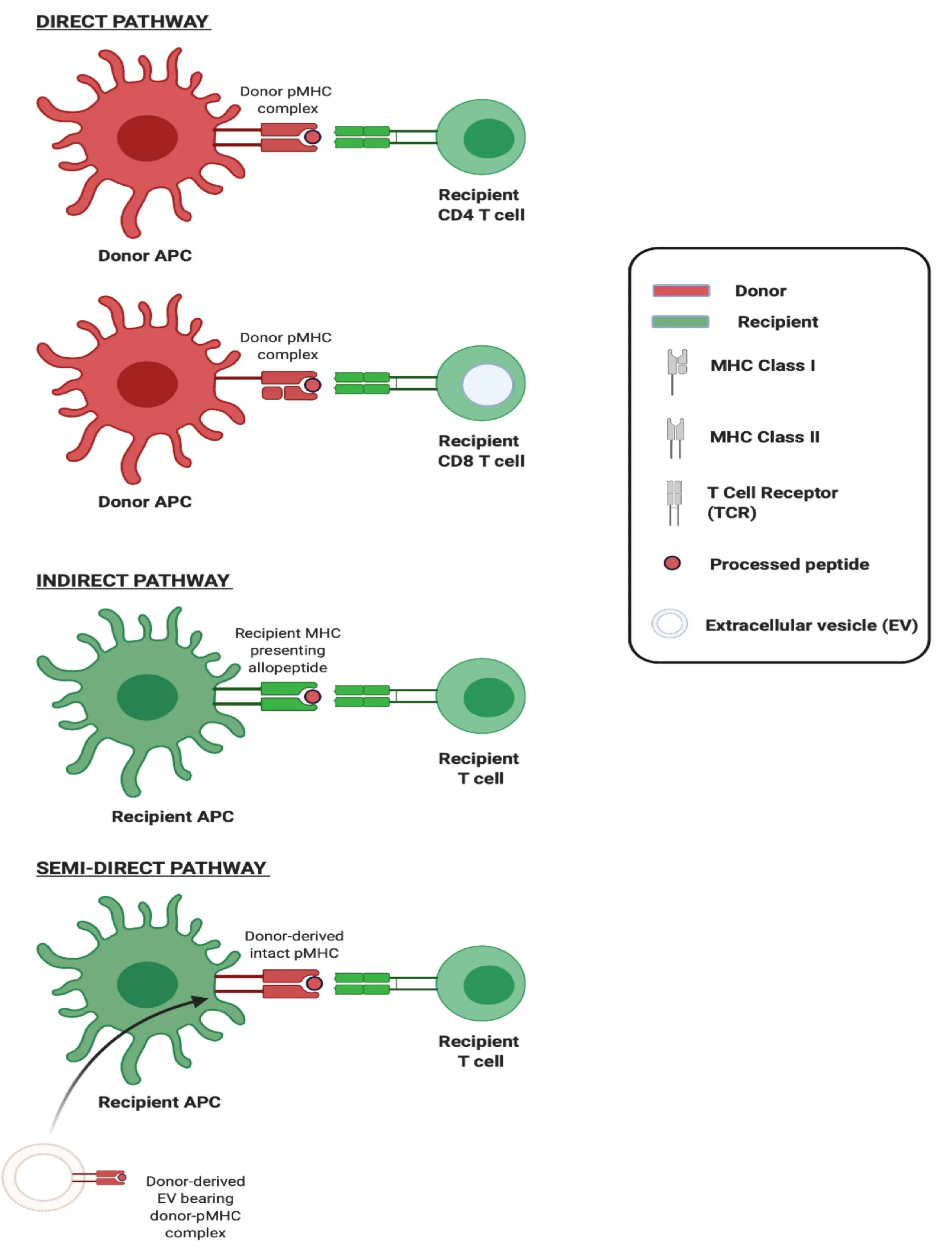
Figure 2 Three pathways of allorecognition.
The pursuit of non-invasive biomarkers of allograft rejection led to the investigation of EVs from a range of biofluids, employing bulk analyses of their varied cargo, and yielding markers of varying specificity, sensitivity, and utility[21-23]. More recently, in order to achieve allograft-specificity, a number of researchers have turned to investigate EVs bearing donor-human lymphocyte antigen (HLA) in particular as biomarkers of allograft function. In 2016, Gunasekaran and colleagues demonstrated the presence of donor-derived EVs bearing donor HLA in the serum of two transplant recipients undergoing bronchiolitis obliterans syndrome; however, their presence was neither reported nor discussed among the control or acute rejection cohorts studied[24]. The following year, Kimet al[25]investigated the presence of donor-specific EVs bearing donor HLA in a single patient having undergone hand-transplantation[25]. Their data suggested that donor-EVs increased in the serum with worsening clinical rejection. However, this study was significantly limited in its small sample size, the lack of a control group, and its reliance on conventional flow cytometry–a method known to be incapable of detecting EVs less than 200 nm in size, which make up the bulk of EVs. In the same year, Vallabhajosyula and colleagues provided the first comprehensive demonstration of circulating EVs bearing donor HLA in patients having undergone islet transplantation[26]. Allograft-specific EVs bearing donor HLA class I were noted among all of the 5 study participants analysed at a single post-operative time-point. Though the impact of rejection on donor-derived EVs was demonstrated by the group in a murine model of islet transplantation, such analyses were not undertaken in their clinical cohort. EV characterisation was performed using nanoparticle tracking analysis (NTA) by NanoSight which, whilst enabling small EV detection well below the limits of cFCM, achieves only semi-quantitative enumeration of donor-HLA EVs.

Figure 3 Advanced imaging flow cytometry by ImageStreamx.
These studies, which are among the first attempts to characterise circulating donorspecific EVs, demonstrate the major challenge in the field to find sensitive and robust technological platforms by which to study EVs on a vesicle-by-vesicle basis. This is particularly true for small EVs (sEVs) including exosomes and smaller microvesicles which are less than 200 nm in diameter. Techniques which permit sEV visualization, such as electron microscopy or atomic force microscopy, preclude the analysis of sEVs in large numbers and, in so doing, limit robust statistical assessments. Western blotting, lipidomics, proteomics, and flow cytometry of bead-captured vesicles are useful methods in the analysis of bulk isolates but are unable to distinguish variations in the number of vesicles from changes in molecular composition, and are incapable of multiparametric analysis of single sEVs[27]. Pioneering work, in particular by groups such as that of Lannigan and Erdbrügger, established the potential of imaging flow cytometry (iFCM) using ImageStreamx (ISx) (EMD Millipore) in the characterisation of sEVs. ISx has all the advantages of traditional flow cytometry, including highthroughput and multiparametric analysis, with the added value of providing a microscopic image of individual cells/particles upon which fluorescence can be overlayed (Figure 3)[28-31]. This is achieved using spatially registered charged camera coupled (CCD) which, unlike photomultiplier tubes found on cFCMs, exhibit the larger dynamic range and lower ‘noise’ required for accurate detection of small EVs. Furthermore, the advanced ISx fluidics enable the slower flow rates required for the avoidance of coincident detection of multiple sEVs.
In 2018, our group demonstrated the use of ISx in the multiparametric analysis of circulating small EV subtypes, including exosomes[27]. Furthermore, we set out to explore the utility of the approach in the detection and characterisation of circulating tissue/organ-specific sEVs. The EVs of 3 Liver allograft recipients’ circulating EVs were labelled with a pan-EV marker, a bona fide marker of exosomes (CD63), and probes for donor and recipient HLA. Donor-specific allograft-derived sEVs were confirmed to be detectable in circulation after liver transplantation. Further multiparametric analyses were employed to interrogate gated donor-sEVs for costimulatory/inhibitory molecules, thereby providing additional support for the application’s potential for characterisation and functional insights. In a study from 2020, we applied this approach to the detection of allograft-derived EVs in a larger cohort of liver or kidney transplant recipients[32]. Analyses of circulating cross-dressed cells and passenger leukocytes were also performed. We showed, for the first time, that cross-dressed recipient leukocytes can be found in the circulation following liver transplantation and that their numbers far exceed those of passenger leukocytes in keeping with the experimental animal models. The presence of circulating crossdressed cells coincided with a rise in circulating allograft-derived sEVs in the early post-transplant period. This was a transient phenomenon, with numbers of both circulating donor-sEVs and cross-dressed cells rapidly waning and becoming undetectable by day 30 post-transplant. We speculate that, as shown in murine models, following clinical organ transplantation recipient APC cross-dressing continues to occur in the allograft and/or secondary lymphoid tissues for prolonged periods of time, and detection in circulation wanes[5,6,20,26,33]. For obvious reasons, corroboration of this in clinical contexts presents a challenge given limited availability of such tissues to perform detailed cross-dressing analyses upon. Employingin vitrofunctional analyses using human cells, we determined that DCs which had undergone EV-mediate MHC cross-dressing acquired the capacity to elicit the proliferation of syngeneic CD8 T cells.

Figure 4 Three-cell model of semi-direct allorecognition.
In summary, developments in EV analytic approaches have, in recent years, enabled the description of the kinetics of donor-specific allograft-derived EV release following clinical transplantation, and evidenced the capacity for these to cross-dress recipient APCs through the transfer of donor MHC. Given the pre-eminence of cross-dressed cells in experimental and clinical transplantation and bearing in mind the recognised impact of these on alloresponse generation, it is likely important these pathways be considered when designing tolerance-promoting protocols.
The role of EVs and cross-dressing in liver transplant tolerance
In models of transplantation cross-dressing of APCs with allo-MHC is a highly immunogenic phenomenon. Several factors can govern the nature and magnitude of the immune response induced by any given antigen. The dose, the proximity of other signals, and the state of the presenting cell are among just a few factors which might influence whether the response is directed towards immunity or tolerance. The same might be expected of a given alloantigen transported upon EVs. Whether the alloresponse is directed towards rejection or tolerance might therefore depend on the quantity of EVs released from a given organ, cell of origin, vesicle subtype, other cotransported EV cargo, the state of the APC which acquires it, and the wider context within which the APC presents the antigen. One related consideration is the site at which cross-dressing occurs. While cross-dressed APCs have principally been observed within SLOs, cross-dressing has also been described within allografts themselves. Thus, in rodent models of islet and kidney transplantation, engagement of effector T cells with cross-dressed graft-infiltrating recipient DCs preceded rejection[34]. However, in a mouse model of spontaneous tolerance following MHC-mismatched liver transplantation, recipient DCs cross-dressed with donor EVs markedly suppressed host alloreactive responses[33]. In this model, crossed-dressed DCs constituted approximately 60% of the intrahepatic DC population, expressed high levels of Programmed Death-Ligand 1 (PD-L1), and induced an exhausted phenotype among donor-reactive CD8 T cells.
These studies also highlight the potential for different organs to produce qualitatively different EVs. The PD-1: PD-L1 axis has emerged as a critical inhibitory signalling pathway involved in the regulation of T cell responses and in the maintenance of peripheral tolerance[35]. PD-L1 is particularly highly expressed among liver parenchymal and non-parenchymal cells. It contributes to local protolerogenic pathways essential to the liver-which is seated at the crossroads between the portal venous system and the systemic circulation-to prevent the induction of immunity against innocuous antigens such as intestinal bacterial degradation products and neoantigens arising from metabolic processing[36]. Intrahepatic PD-L1 expression is upregulated following liver transplantation in both mice and humans and has been implicated in the establishment of liver allograft toleranceviainhibition of alloreactive T cell activation and induction of regulatory cell subtypes[33,37,38]. In our analysis of circulating sEVs following clinical liver transplantation, but not kidney transplantation, we observed that donor-derived sEVs carried significantly more PDL1 than did sEVs of recipient origin. Furthermore, recipient cells which became crossdressed also exhibited higher levels of PD-L1 than did recipient cells which had not been cross-dressed. PD-L1 was noted to co-localise on the APC surface with donor-HLA, which would be in support of their tandem transport on EVs though other groups have reported global upregulation of PD-L1 (potentially due to EV-miRNA transfer) following cross-dressing[39].
Work from the Burlingham laboratory expands further on the tolerogenic potential of EVsviathe upregulation of PD-L1 on DCs. Their work focuses primarily on maternal microchimerism, whereby a tiny population of immune cells are transferred from mother to offspring during pregnancy and breastfeeding and result in the persistent detection of maternal cells throughout adult life[40]. These maternal cells contribute to the induction and maintenance of tolerance against non-inherited maternal antigens (NIMAs) which they bear, including MHC. For example, kidney grafts expressing NIMA-MHC will exhibit longer survival than grafts expressing unrelated MHC. The group demonstrate that the effects of such a small population of maternal cells are mediated and amplified by their avid production of EVs bearing NIMAs which subsequently are taken up by host DCs. The resultant cross-dressed DCs are noted to globally upregulate PD-L1, which the researchers suggest is due to co-transported EV-miRNA, and in doing so inducing NIMA-specific T cell anergy[39,40]. This is of added relevance to our discussion since the establishment of donor chimerism following liver transplantation in particular has long been recognised. Though its beneficial effects on outcome are widely acknowledged, the mechanisms underlying the pro-tolerogenic effect have remained uncertain[41,42].
It would appear then, that under certain circumstances allo-EVs promote tolerance while in others they drive rejection. The three-cell model described above offers a mechanistic framework by which to understand this apparent dichotomy. While allo-MHC transferred intact to an APC will activate CD8 effector T cellsviathe semi-direct pathway, the fate of processed peptides presented indirectly by the same APCs can result in the recruitment either of CD4 cells which will assist in the activation of the effector cell and drive rejection (Figure 4A), or of CD4 regulatory T cells (Tregs) which will inhibit effector cell activation and so promote tolerance (Figure 4B, upper panel)[43]. Proponents of this model would hold that the propensity towards Treg associations is determined by, for instance, the wider setting in which APC crossdressing has occurred. In the liver, where there is high expression of molecules such as PD-L1 and anti-inflammatory cytokines such as interleukin (IL)-10, one might expect Treg recruitment to be more likely.
An alternative is that particular EVs are enriched in cargo capable, once transported to APCs, of contributing to the inhibition of T cells. As discussed, this could take the form of intact molecules transported in tandem or of nucleic acids which induce expression of regulatory molecules in recipient cells. Thus, Burlingham et. al. outline a scenario in which certain EVs (they suggest of maternal cell or of liver allograft origin) induce global PD-L1 expression in APCsviathe co-transfer of miRNAs. This PD-L1 induces anergy of indirect pathway CD4 T cells, which then fail to help direct pathway CD8 T cells (Figure 4B, middle panel)[39]. In our analyses, we demonstrated that EVs derived from liver transplant recipients were able to transiently inhibit CD8 effector responses following uptake by DCs. Given that we observed allograft-derived EVs to be particularly enriched in PD-L1, and PD-L1 to colocalise with allo-MHC on the cross-dressed APC, it could be the case that effector cell inhibition was due to the proximity of intact, co-transported inhibitory signalling (Figure 4B, lower panel)[32]. These are not, it must be emphasized, mutually exclusive scenarios, and future work should delineate the contribution of both. An understanding of the factors that can tip the balance toward tolerance will likely be critical in the advancement of EV-based immunotherapeutics.
EVs as novel therapeutics in transplantation
By virtue of their varied bioactive cargo, stability, capacity for tissue-specific targeting, ability to cross biological barriers, and safety profile, EVs have been identified as having significant therapeutic potential. There are currently over ten clinical trials in progress assessing the efficacy and safety of EV therapies[44]. Therapeutic EVs can broadly be subdivided into those derived from unmodified cellular subsets, and those which have been bioengineered.
Unmodified cell-derived EVs
EV-based therapeutics have, for the most part, turned to the utilisation of EVs derived from stem cell and regulatory cell subsets. Mesenchymal stem cells (MSCs) are among the earliest and most widely employed examples. MSCs were at first believed to mediate protective propertiesviatheir capacity to differentiate into and to replace injured tissue. For instance, following cardiac injury, delivered MSCs were understood to ameliorate damage by differentiate into healthy myocardium. However, it has recently been noted that the effects of MSCs are in large part due to their paracrine effects on surrounding tissues which, in part, are mediated by secreted EVs[45-48]. Since this discovery, the capacity for MSC-EVs to attenuate inflammation and to promote tissue regeneration has been demonstrated in pre-clinical models of respiratory, pancreatic, renal, musculoskeletal, neurological, and of liver diseases (reviewed elsewhere[49,50]). The use of MSC-EVs as an alternative to MSCs confers a number of potential advantages including the ability to cross biological barriers, target-specificity, avoidance of entrapment in microvascular beds, stability in storage, reduced potential for phenotypic alteration upon delivery, relatively lower immunogenicity and tumorigenicity, and improved safety profiles on repeated dosing.
Several experimental studies have demonstrated MSC-EVs to play a therapeutic role in liver ischaemia-reperfusion injury (IRI) through regenerative, autophagic, and immunomodulatory processes[51-54]. These rodent models employ variations ofin vivo, in situ, vascular occlusion to replicate IRI. It remains to be seen what the impact of such therapies would be on the prolongation of allograft survival in models of liver transplantation. In the clinical context, ex-vivo machine perfusion of organs prior to transplantation under either normothermic (NMP) or hypothermic (HMP) conditions has improved assessment of organ viability, enabled the reconditioning of organs which might otherwise have been discarded, but also provided a platform upon which novel therapeutics can be developed and trialled. Very few studies have investigated the application of EVs in this context; though interest is growing rapidly. While studies have demonstrated beneficial effects of MSC-EVs in rodent models of lung and kidney perfusion, the first such demonstration in liver was by Rigo and colleagues in 2018[55-57]. Using a murine model of ex-vivo NMP, the group demonstrated the favourable outcomes in organs treated with human liver stem cell-derived EVs (HLSCEVs), in terms of a reduction in histological damage and of enzyme markers of cytolysis. Several limitations are inherent in these studies including not performing onward transplantation to determine the effects on allograft outcomes, providing little mechanistic evidence of the mode by which EVs exert their effect or whether EVs of alternative origin would differ, and the lack of comprehensive uptake and doseresponse analyses. Further investigation is warranted in experimental animal models, but it is also anticipated that trials will arise in perfused human organs with onward progression into phase I/II studies[58].
In addition to stem cell derived EVs, it is important to also mention Treg-derived EVs. Progress has been made in the implementation of adoptive Treg cell therapy in a number of scenarios which include type 1 diabetes, rheumatoid arthritis, inflammatory bowel disease, graft-versus-host disease (GvHD) following bone marrow transplantation (BMT), and organ transplant rejection[59,60]. Similar to MSCs, considerable barriers have been faced in the ex-vivo expansion of Treg, in maintaining their phenotypic characteristics once delivered, in delivering sufficient numbers particularly in the context of concomitant immunosuppressive therapies, in their oncogenic potential, and in their immunogenicity[61]. In their seminal paper, Okoye and colleagues showed Tregs to release large quantities of EVs carrying a distinct cargo of miRNA, and went on to demonstrate that blocking the release of these EVs abrogated the Tregs’ ability to suppress Th1 cell proliferation and thereby their immunoregulatory capacity[62]. These findings were independently reasserted by Aiello and colleagues, who also went on to demonstrate the capacity of Treg-EVs to prolong kidney allograft survivalin vivo[63]. In recent months, Smyth and colleagues have shown the capacity for Treg-EVs to inhibit T effector cell responses, to affect changes in effector cell cytokine productionviacargo miRNAs, and to protect against rejection in a humanised mouse skin transplant model[64].
Studies are lacking which aim specifically to investigate the tolerogenic potential in transplantation of therapeutically delivered EVs which serve to mediate APC crossdressing. The recent work of Patelet al[65]. serves to demonstrate the potential of such an approach. Donor bone marrow derived EVs bearing allo-MHC were delivered in a non-human primate model of heart and kidney co-transplantation with prior conditioning by thymic irradiation, antithymocyte globulin, and immunosuppression. While design and sample size limit interpretations of functional outcomes, their data shows that delivered EVs are capable of generating stable cross-dressing. They suggest that such EVs might be used in place of whole bone marrow as a tolerance induction strategy and perhaps reduce the need for recipient conditioning[65]. We anticipate that similar approaches might prove more practicable through the development of engineered EVs enriched in specific desired molecules and alloantigens.
Engineered EVs
Broadly, there are two distinct approaches to selective EV cargo loading: (1) Exogenous, after EV isolation from the parent cell; and (2) Endogenous, during EV biogenesis[66]. Methods to achieve the former include techniques such as electroporation and sonication. Methods towards the latter involve exploiting the parent cell’s EV sorting machinery. Desired cargo can be directly transfected into the parent cell or can be engineered to be stably expressed. Fusion of the therapeutic of interest with molecules enriched in EVs will optimise its loading onto them. While examples of engineering approaches to endogenous EV loading and optimisation of delivery have been comprehensively outlined elsewhere[44], one particularly elegant example is that from Sutaria and colleagues who achieved the 65-fold increase of miRNA-199a-3p by associating its production to Lamp2a within the membrane of EVs produced by a HEK293T cell line[67]. Though no applications of engineered EVs have been reported in the literature with regards to liver IRI or tolerance induction, their recent implementation in diverse inflammatory, autoimmune, and oncological conditions, both in experimental models and in limited clinical trials (Table 1), demonstrate their potential.
Engineered EVs offer significant advantages over alternative synthetic drug delivery systems such as liposomes, nanocapsules, and micelles, which have often proven inefficient, poorly targeted, cytotoxic, and/or immunogenic. Nevertheless, widespread clinical utilisation of engineered EVs also faces a number of obstacles. Among these are: (1) The need for GMP-compliant up-scaling of production and isolation processes; (2) The better understanding of uptake kinetics, targeting, bioavailability, and dosing; and (3) The selection ofappropriate assays and biomarkers for the purpose of monitoring function. The significant progress underway in each of these areas has been reviewed elsewhere[44,68-71].

Table 1 Clinical trials of engineered extracellular vesicle-based therapies
CONCLUSION
EVs have emerged as key contributors to T cell alloimmunity. Progress in the accurate identification and analysis of these nano-sized vesicles has confirmed their capacity to transport graft-derived alloantigen to recipient APCs in both experimental models of transplantation and in the clinical setting. While the consequence can be the initiation of strong inflammatory responses leading to acute graft rejection, it is possible in certain settings that tolerogenic responses are mediated and allograft injury allayed. EVs are emerging as potent therapeutic entities with innate potential for use as vehicles for the targeted delivery of small-molecule drugs, nucleic acid species, and therapeutic proteins including alloantigen. Improved understanding of their role in immune homeostasis, tolerance, and rejection, and optimised methods of production make it likely that EVs will serve diverse roles a future platform for biopharmaceuticals in transplantation and beyond.
 World Journal of Transplantation2020年11期
World Journal of Transplantation2020年11期
- World Journal of Transplantation的其它文章
- COVID-19 infection in a kidney transplant recipient—special emphasis on pharmacokinetic interactions: A case report
- Exploring the safety and efficacy of adding ketoconazole to tacrolimus in pediatric renal transplant immunosuppression
- Intraoperative thromboelastography as a tool to predict postoperative thrombosis during liver transplantation
- Obstetrical and gynecologic challenges in the liver transplant patient
- Donor-specific cell-free DNA as a biomarker in liver transplantation: A review
- Lenvatinib as first-line therapy for recurrent hepatocellular carcinoma after liver transplantation: Is the current evidence applicable to these patients?
