Safety and efficacy of intraperitoneal perfusion with tumor vesicle-encapsulated methotrexate for the treatment of cancerous ascites-an open,randomized and controlled clinical trial
He Zhang,Jing-Yi Zhang,Jing-Bo Zhai,Hong-Bo Zhang,Yi Lou,Li-Zhu Shan
1Department of Oncology, Tianjin Nankai Hospital, Tianjin 300100, China.2Tianjin Institute of Clinical Evaluation, Tianjin University of Traditional Chinese Medicine,Tianjin 300193,China.
Abstract
Background: Cancerous ascites is a common and severe complication that occurs in patients with late-stage malignant tumors.The prognosis of cancerous ascites is poor,clinical treatment is difficult and therapeutic outcome is disappointing.In the present study, tumor cell-derived vesicles were used as drug delivery vehicles that encapsulated a chemotherapeutic agent and were perfused into a patients’abdominal cavity to effectively kill the cancer cells in cancerous ascites.Pre-clinical data has demonstrated that tumor vesicles that carry low-dose chemotherapeutics can efficiently eliminate metastatic tumor cells in the abdominal cavity with minimal toxic or adverse effects.When combined,tumor cell-derived vesicles can sensitize tumor cells,which facilitates the entry of chemotherapeutics into tumor cells,thereby enhancing killing of tumor cells and limiting the risk of drug resistance.In this study,we designed a clinical trial to evaluate the safety and efficacy of intraperitoneal perfusion with tumor vesicle-encapsulated methotrexate for the treatment of cancerous ascites.Methods: Sixty patients with cancerous ascites were enrolled in this open, randomized and controlled clinical trial.Participants were randomly assigned a visit number and, according to their visiting order for which a random numerical table was used, were assigned to the trial group or the control group in a 1:1 ratio.The change in ascetic volume was used as the study outcome and adverse events were monitored during the entire length of the study.Conclusion: In this clinical trial,randomization and electronic case report forms were implemented.The trial indicated that tumor vesicle-encapsulated methotrexate was proposed to be a safe and effective method for treating malignant ascites.Our study may provide at the first time evidence for the clinical application of tumor vesicles in tumor therapy.
Keywords:Cancerous ascites,Efficacy,Random and controlled trial,Tumor vesicle,Safety
Background
In patients with late-stage malignant tumor, cancerous ascites(CA)is a common and severe complication that causes symptoms such as abdominal distension,anorexia and dyspnea.In general, the condition is repetitive and gradually worsens over time, which severely affects a patient’s quality of life.A patient diagnosed with CA has a poor prognosis and the average survival time is approximately 20 weeks[1–3].Clinical treatment of CA is a challenging task and the therapeutic outcome is disappointing.
In the present study, tumor cell-derived vesicles that encapsulated a chemotherapeutic agent were used as drug delivery vehicles and perfused into a patients’abdominal cavity to effectively kill cancer cells in CA.Pre-clinical data have demonstrated that tumor vesicles that carry low-dose chemotherapeutics can effectively eliminate metastatic tumor cells in the abdominal cavity with minimal toxic or adverse effects.When tumor cell-derived vesicles sensitize tumor cells, the entry of chemotherapeutics into tumor cells is facilitated, thereby reversing drug resistance and enhancing killing of the tumor[4–6].
A cell is comprised of components that are encapsulated by the cell membrane and consist of a phospholipid bilayer and mosaic proteins.The spherical structure of the cell is maintained by a concentric pulling force exerted by intracellular protein filaments, known as the cytoskeleton.In response to external stimuli, part of the filaments at the connection between the cytoskeleton and the cell membrane may break or lose attachment and the sudden loss of concentric pulling force can cause bulge of the localized cell membrane.The bulging membrane then expands outwards, encapsulates cellular contents and subsequently releases as a vesicle to an extracellular sub-structure with a size ranging from 100 nm to 1,000 nm that is located between cell and molecule.Compared with exogenous vehicles in chemotherapeutic delivery or “cellular vesicle”, the endogenous vehicle used in the current study can enhance drug efficacy and reduce drug resistance in vivo.Because the cellular vesicles that are used to encapsulate chemotherapeutics are derived from tumor cells, these vesicles can easily fuse with membranes of tumor cells in a patients’body.This approach,in which the drug will be efficiently delivered into tumor cells,can overcome two major difficulties in tumor therapy,namely, killing of normal cells by chemotherapeutics and drug resistance of tumor cells.
Targeting effect
In the abdominal cavity of cancer patients, the chemotherapeutics-encapsulating vesicles are specifically taken up by tumor cells only and thereby highly desired targeted treatment of tumor cells is achieved.Compared with normal cells, tumor cells are known to display a higher rate of endocytosis of tumor cell-derived vesicles.In this study, we have compared the endocytosis capacity in a variety of human cell types and found that tumor cells had the strongest capacity of endocytosing tumor cell-derived vesicles,which was 3–5 fold higher compared to that in normal cells.Moreover, in contrast to the microvascular permeable size in normal tissues, vascularization of tumor tissues is deregulated and the microvascular permeable size is 100–780 nm.The average size of tumor cell-derived vesicles is 500 nm.Therefore,vesicles that are unable to enter normal tissues can easily enter tumor tissues, which ensures that the toxic effect to normal tissues is reduced.
Focusing effect
In conventional chemotherapy a given dose of chemotherapeutics is very high, however the drug is distributed through the entire body.This results in dilution of the drug and therefore the actual working concentration in the tumor may not reach the concentration that is required to achieve a therapeutic effect.Moreover, chemotherapeutics that travel in a regular solution can be pumped out of the tumor cell through the pump mechanism that is present on the cell membrane, which will dampen the killing effect of chemotherapeutics.In this study, tumor cell-derived vesicles were used to encapsulate a chemotherapeutic agent and, during the preparation process, the maximum dose was used that almost saturated every single vesicle.When entering the body, even though the vesicle concentration declines, the dose of drug in each vesicle was identical to when the vesicles were prepared.Once a vesicle reaches the tumor, it will release the entire drug content to the tumor cells.By using this drug delivery mechanism, the amount of drug acting on the tumor cells is far higher compared to that in conventional chemotherapy, therefore the drug can easily kill the tumor cells.This feature may be considered a focusing effect.
Pump-off effect
The efflux pump system that is present on the cell membrane contains a porous structure.When a vesicle is derived from the cell membrane, fragments present in the vesicle can fuse with the tumor cell membrane and, after a vesicle enters the cell, interfere with the pump system on the tumor cell membrane.This will result in a decrease of the pumped-out drug, and maintains the effective drug concentration at a relatively high level in the tumor cells.Compared with exogenous vehicles, apoptotic tumor cell-derived vesicles can enhance the therapeutic effect of encapsulated chemotherapeutics and reduce drug resistance in the body.
Immune activation effect
For malignant tumors, neither surgery nor chemoradiotherapy is able to fully eradicate the tumor and killing of residual tumor cells totally relies on body ’ s own immune system.Although the body’s immune cells can attack tumor cells, tumor cells develop an immune escape mechanism during tumor formation and progression.During tumor development,macrophages do not kill tumor cells, but tumor cells educate them to become an important partner in helping tumor cells evade immune surveillance.Moreover,macrophages can release angiogenic growth factor in tumor tissues and promote tumor angiogenesis.By taking advantage of the phagocytotic features of macrophages, vesicle-encapsulated chemotherapeutics can efficiently enter and eliminate macrophages in the tumor and thereby augment anti-tumor immunity.The drug-encapsulating vesicle alters the immunogenicity of tumor cells and help the immune system recognize tumor antigens.On the other hand, it stimulates and reinforces the activity of immune cells and decreases the number of immune-suppressive cells.Meanwhile, it reduces the killing of normal immune cells.Overall, the drug-encapsulating vesicle achieves an immune activation effect.
The aim of this clinical trial was to evaluate the safety and efficacy of intraperitoneal perfusion of tumor vesicle-encapsulated methotrexate compared to cisplatin,for the treatment of CA.
Methods
Study design
The total duration of this open, randomized and controlled clinical trial was eight weeks.The design of this trial complied with Helsinki Declaration and Guidelines as well as Clinical Trial Guidelines (Figure 1) and was conducted in strict accordance with the scientific methods of a clinical study.
Medical ethics
This clinical trial was approved by the ethics committee of Tianjin Nan Kai Hospital (2015-040-01).Both the study plan and the informed consent form complied with scientific and ethical guidelines.The informed consent forms were obtained from all participants or their authorized representatives before enrollment in the study.
Sample size
Based on the experts’ advice, the sample size of this pilot clinical trial was determined to be 60.During the review process, the drop-out rate was estimated below 20%.In both the test group and the control group, an equal number of participants was enrolled (n = 30 per group).
Inclusion criteria
(1) The diagnosis of a malignant tumor by means of pathology or be confired by the cell biology of ascites by abdominal B-ultrasonography or computer tomography.
(2) Ⅴital signs are stable,Karnofsky score ≥60.
(3) Age between 18 and 70 years old.
(4) Normal bone marrow hematopoietic function, no hemorrhagic tendency (international normalized ratio<1.5); blood routine: hemoglobin (HGB) ≥90g/L,white blood cell (WBC) > 4.0 × 109/L (neutrophil(NEU)≥1.5×109/L),platelet(PLT)≥100×109/L.
(5) Liver function: total bilirubin (TBIL) ≤1.5 fold of the upper limit of the normal range, aspartate aminotransferase (AST) and alanine aminotransferase(ALT)≤2.5 fold of the upper limit of the normal range(≤5 fold of the upper limit of the normal range if abnormal liver function was caused by tumor infiltration), alkaline phosphatase (ALP) ≤1.5 fold of the upper limit of the normal range.
(6) Renal function: blood urea nitrogen (BUN) and creatinine (Cr) ≤1.5 fold of the upper limit of the normal range,Cr clearance rate ≥60 mL/min.
(7) Normal electrocardiogram (ECG) and a normal blood glucose level.
(8) The patient or his/her family consented to participate in this study and signed the informed consent form.
(9) No signs of serious cardiopulmonary diseases.
Exclusion criteria
(1) Women who were breastfeeding, pregnant or trying to conceive.
(2) Allergy-prone or multiple-drug allergies.
(3) Severe cardiac, pulmonary, hepatic or renal dysfunction, such as decompensated dysfunction or failure of heart, lung, liver, kidney or other major organs; poor control of blood glucose, chemotherapy intolerance.
(4) Concurrent severe infections.
(5) Cognitive dysfunction, or judgement by investigators as poor compliance of the patient with chemotherapy.
(6) Participation in other clinical trials during the last three months.
(7) Not suitable for the clinical trial as assessed by the investigators.
Rejection criteria
The enrolled subjects who did not meet the inclusion criteria were rejected based on the following: (1)misdiagnosis; (2) missed enrollment; (3) patients who had never taken the drug; and (4) patients without any evaluation record.
The reason for a rejected case should be provided,and the medical record should be kept for further inspection.Subjects who had received at least one treatment and had at least one safety record could take part in the safety analysis.
Drop-out(withdrawal)criteria
(1) Adverse events; (2) lack of efficacy; (3) violation of trial plan (including poor compliance); (4) it was suggested by the investigator that the subject should suspend the trial from a medical point of view; (5) the patient asked for withdrawal.
In case of a drop-out, the investigators should write down the reason for drop-out in the case report form(CRF), contact the patient to complete as many assessments and related examinations as possible and fill in the CRF about the last contact with the patient,including the time and efficacy of the last treatment.In case of drop-outs due to adverse events, the adverse events should be documented in the CRF no matter whether they are related to the trial drug or not.
Suspension criteria
Suspension of the clinical trial means ending of the trial ahead of schedule and suspension of all tests.The purpose of trial suspension is to protect the participants’rights, to ensure the quality of the trial and to avoid any unnecessary economic loss.
(1) A serious safety problem occurs during the trial and the trial should be suspended immediately.
(2) A major mistake found in the trial plan makes it challenging to evaluate the efficacy of the drug; or a significant deviation occurs during the implementation of a well-designed trial plan and continuation of the trial makes it difficult to evaluate the efficacy of the drug.
(3) The sponsor asks for suspension(due to funding or management issues).
(4) The government authority suspends the trial.
Randomization
Subjects were assigned to the test group or the control group according to a first-come-first-serve basis using a random numerical table.
Intervention of trial group
Treatment schedule.Intra-peritoneal perfusion with vesicle-encapsulated methotrexate solution.Intraperitoneal perfusion from the first day of enrollment and on every other day for a total of four weeks(one course).
Dosage.When a substantial amount of ascites was observed, an abdominal puncture was given and a central venous catheter was placed to slowly drain the ascites.After completion of the drainage,intraperitoneal perfusion was performed through the central venous catheter.When absence or a limited amount of ascites was confirmed by B-ultrasonography,250 ml vesicle-encapsulated methotrexate solution was perfused into the abdominal cavity every time.This solution contained 6 units of vesicles (Hubei Soundny Bio-Tech Co., LTD, China; 1 unit contains 1×107vesicles and 5 μg methotrexate).
Chemotherapeutic agent.Methotrexate (Jiang Su Heng Rui Medicine Co.,LTD,China;0.1 g/ampule).
Intervention of parallel control group
Treatment schedule.Intraperitoneal perfusion with cisplatin for four weeks(one course).
Dosage.When B-ultrasonography ascites was absent or limited, 50 mL saline + 60 mg cisplatin + 10 mg dexamethasone was perfused into the abdominal cavity every time.To reduce the toxic effect of cisplatin, a large amount of fluids and a diuretic were administrated on the day of the intraperitoneal perfusion.
Chemotherapeutic agent.Cisplatin (Qilu Pharmaceutical Co.,LTD,China;10 mg/ampule).
Precaution
(1) During perfusion, signs of dizziness, paleness,sweating, palpitation and abdominal pain were closely watched for.Occurrence of dizziness, palpitation,sweating, paleness, weak pulse and chill may indicate the possibility of a peritoneal reaction in the patient and aspiration should be stopped immediately.The patient should be laid on his/her back, blood pressure and pulse closely should be watch and attention should be paid to the patient’s feelings.Dexamethasone may be used when necessary.Aspiration should not be too fast or too much, in order to avoid a sudden drop of intraperitoneal pressure that may cause pulmonary edema, circulatory disturbance, mediastinal shift and other complications.The volume of the first aspiration should not exceed 1,000 mL, and the maximal volume of the subsequent aspirations should not exceed 3,000 mL per perfusion.Once drainage of ascites was confirmed by B-ultrasonography, instilling the vesicle-encapsulated chemotherapeutic solution via the catheter could be started.
(2) After the drug was administrated, the drainage tube was switched off.Patients were asked to lie on their back,stomach,left side and then on the right side.Each position was taken twice allow exposure of the drug to the entire peritoneum.
(3) Catheterization procedures.The puncture site was determined by B-ultrasonography when patients were on their back, and abdominocentesis was performed following a routine procedure.The needle was positioned using a negative suction pressure until ascites flushed into the syringe.The metal guidewire was pushed along the needle into the abdominal cavity(approximately 6–8 cm was left inside the abdomen)and the needle and syringe were retracted.The guidewire will be covered with an expender, which will be pushed inside the abdomen along the guidewire till a sense of suspending(approximately 3–5 cm).The skin will be cut and the expender taken out.Subsequently, the catheter will be pushed along the guidewire into the abdomen (8–10 cm deep)and at the same time, the guidewire will be retracted.The catheter will be sutured and fixed at approximately 2 cm from the puncture site and the catheter will be connected with the drainage tube and vacuum bag.The drainage switch will be adjusted to control the flow rate of ascites and allow slow drainage for 12–24 h.The ascites will be sent for biochemical and cell biological analysis and evaluated for tumor markers.
Combined medication
(1) When additional hormone therapy is required under special circumstances (such as allergy, fever,infusion reaction and other severe adverse reactions during the intervention period),describe the case in the CRF regarding illness, drug, dose, and administration method.
(2) Antibiotics can be used in cases of a confirmed infection, or when core body temperature reaches ≧38.0℃and infectious fever cannot be excluded.The case will be recorded in the CRF.Any use of antifungal or antiviral agents should also be recorded in the CRF.(3) The use of traditional Chinese medical or herbal medicine during the study should be described in detail.
Determination of sample size and randomization
In this pilot phase II clinical trial we aimed to provide scientific evidence for promoting the therapeutic scheme into a phase III clinical trial.The sample size was determined to be 60, with 30 subjects in each group.Patients were assigned to a trial or control group in a 1:1 ratio according to their visiting order using a random numerical table.
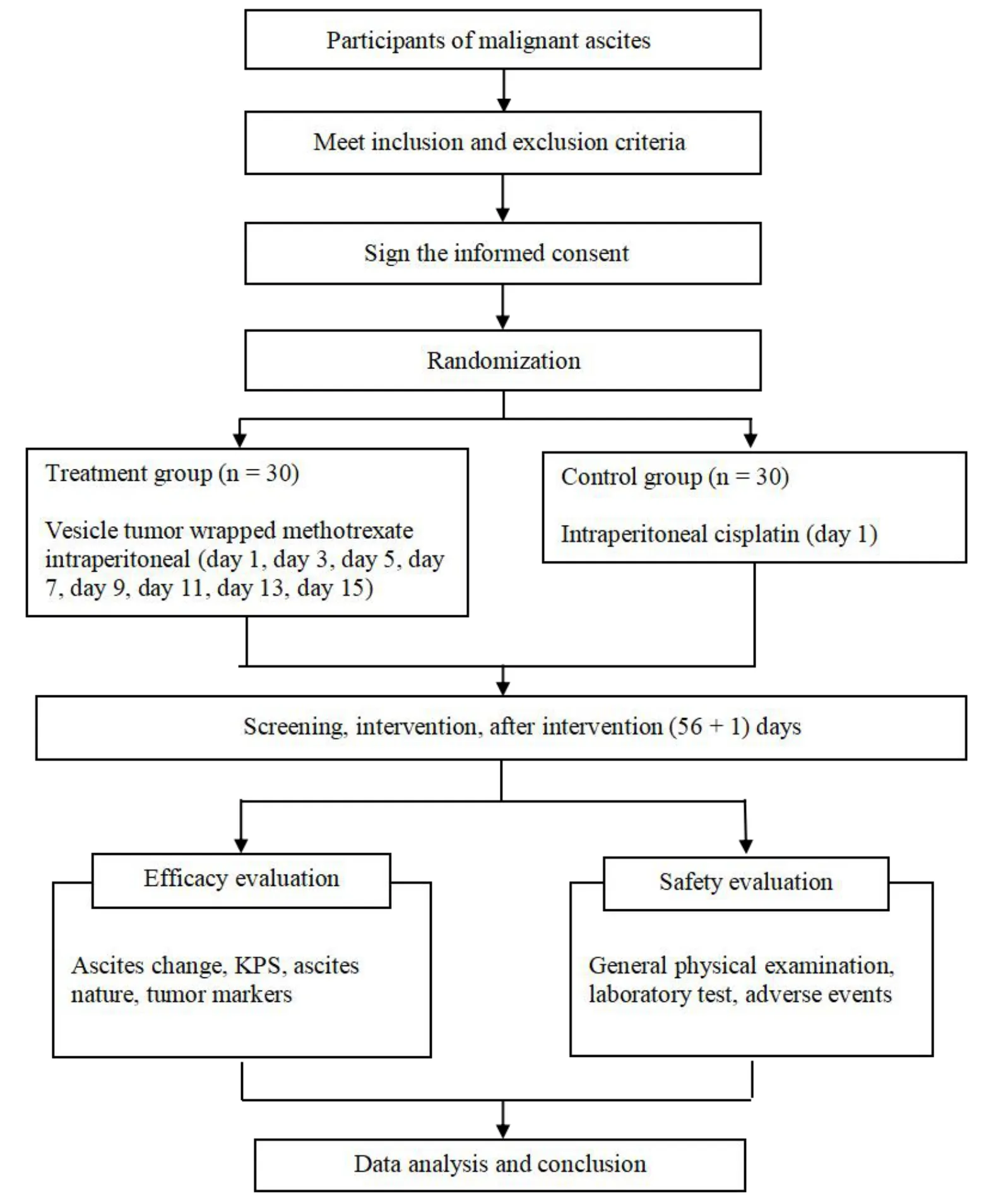
Figure 1 Study flow chart
Activities and data collection time-points
Activities and intra-trial data collection requirements were as follows:
(1) Screening period(day 0):24 h prior to enrollment.
(2) Intervention period(day 1–15):daily follow-ups.
(3) Post-intervention period (after intervention, until day 56): on day 28 and day 56 of continuous follow-up.
Ⅴarious items were measured according to the data collection time table(Table 1).
Primary outcome measures
The change in volume of the ascites was determined by B-ultrasonography and the efficacy was evaluated as follows.The efficacy was evaluated according to the standards of the World Health Organization [7] (Table 2).
Secondary outcome measures
(1) Dynamic monitoring on vital signs, quality of life and survival time before and after intervention.If,after intervention, the patient’s vital signs were stable and the KPS score was elevated by 10 or more compared with the pre-intervention KPS score, the intervention was considered effective.
(2) Change in nature of the ascites.If, based on routine examination of the appearance of pathological indicators,such as properties,protein content,cell type and count of the ascites were improved, the intervention was considered effective.
(3) Dynamic monitoring on tumor markers and related indicators.The effect of vesicle therapy on cancerous ascites is evaluated in an objective fashion, by examining tumor markers in the blood and ascites before and after intervention.
Evaluation of safety
Drug safety was evaluated by subjective symptoms such as nausea,vomiting,loss of appetite and alopecia,routine examinations on blood, urine and stool, ECG and physiological index during the treatment, as well as liver and renal function prior to and post intervention.
Report on adverse events
In case of an adverse event during the course of the trial such as sudden death or a life-threatening event,disability or a prolonged hospital stay, emergency measures or treatments need to be taken immediately no matter whether or not the adverse event was related to the trial or the trial drug.The goal is to keep the patient safe.The case needs to be reported immediately to the chief investigator and the organizer needs to be informed within 24h after the adverse event occurred.
Statistical analysis
Statistical analysis was performed by the Tianjin Clinical Assessment Institute.All data was analyzed using the Statistical Analysis System (version 9.3).Data and efficacy assessments were analyzed according to the intention-to-treat principle.Ⅴalues are expressed as the mean ± standard deviation and were analyzed using a Student’s t test.The categorical variables are presented in percentages and were analyzed using a Chisquared test.
Quality control of data
Quality control of the research data will be achieved by rigorous monitoring throughout the trial process.Trial monitors will pay regular visits to each site,where they will recheck CRF, inspect the storage of the investigational drugs and review investigators’records.After verification of the content of the written CRF,data will be collected and inputted into a database by two full-time research staff independently.The standard operating procedures will be invariably followed.
Discussion
Tumor immune therapy relies on the recognition of tumor specific or related antigens by the activated immune system.In late 2013, Science journal awarded tumor immune therapy the first place of top 10 scientific and technological progresses in 2013 [8].In addition, Nature journal released an issue on the prospects of tumor immune therapy [9].Although many advances have been made in the field of tumor immune therapy, the current treatment outcome is still unsatisfactory as many lives are lost.Therefore,identifying novel strategies and techniques for tumor immune therapy are clearly warranted.
Conventional chemotherapy was simply considered as direct killing of tumor cells by chemotherapeutics.However, recent findings suggest that chemotherapy can support tumor immune therapy at multiple steps through various processes [10, 11].For example, (1)chemotherapeutics induces upregulation of tumor-associated antigen in tumor cells, thus increasing recognition and killing by T cells; (2) by killing tumor cells, chemotherapeutics promote the capture of tumor antigens by antigen presenting cells;(3) dead tumor cells induce maturation of dendritic cells by releasing high mobility group box-1, heat shock protein and other inflammatory stimuli; (4)chemotherapeutics can disrupt the immune-suppressive microenvironment by killing tumor-related immune cells; (5) by killing tumor cells, chemotherapeutics make the solid tumor tissue become “l(fā)oose”, which facilitates infiltration of cytotoxic T cells into the tumor.Therefore, a combination of tumor immune therapy with chemotherapy has been emerging and shows advantages over traditional chemotherapy treatment alone.
Recent studies have indicated that, during cell activation or apoptosis, changes in the cytoskeletonmay lead to the release of vesicles with a diameter of 100 nm to 1 μm.These vesicles, known as microparticles, consist of a plasma membrane and encapsulated cell contents [12].Microparticles, of a size between molecules and cells, can store and transport cellular information [13].The size of microparticles is far beyond the pores of normal vessels (5–8 nm), however they are able to pass through the pores of tumor vessels (100–780 nm) [14].Therefore,microparticles represent an ideal vehicle for tumor-targeted drugs.In our previous studies we demonstrated that platelet-derived microparticles could transport enzyme molecules to mast cells and thereby promoted the formation of anti-inflammatory lipoxins[15].This finding implicates the potential of microparticles as promising drug vehicles.
Furthermore, we found that the stable tumor cell-derived microparticles could encapsulate chemotherapeutics and also efficiently deliver the encapsulated drug into the targeted tumor cells, thus mediating selective killing of tumor cells and reducing toxic effects of initial chemotherapeutics [16–19].Moreover, tumor cell-derived microparticles could be used as a vaccine that inhibited tumor growth in mice.The above results have been published in Nature Communication[19].
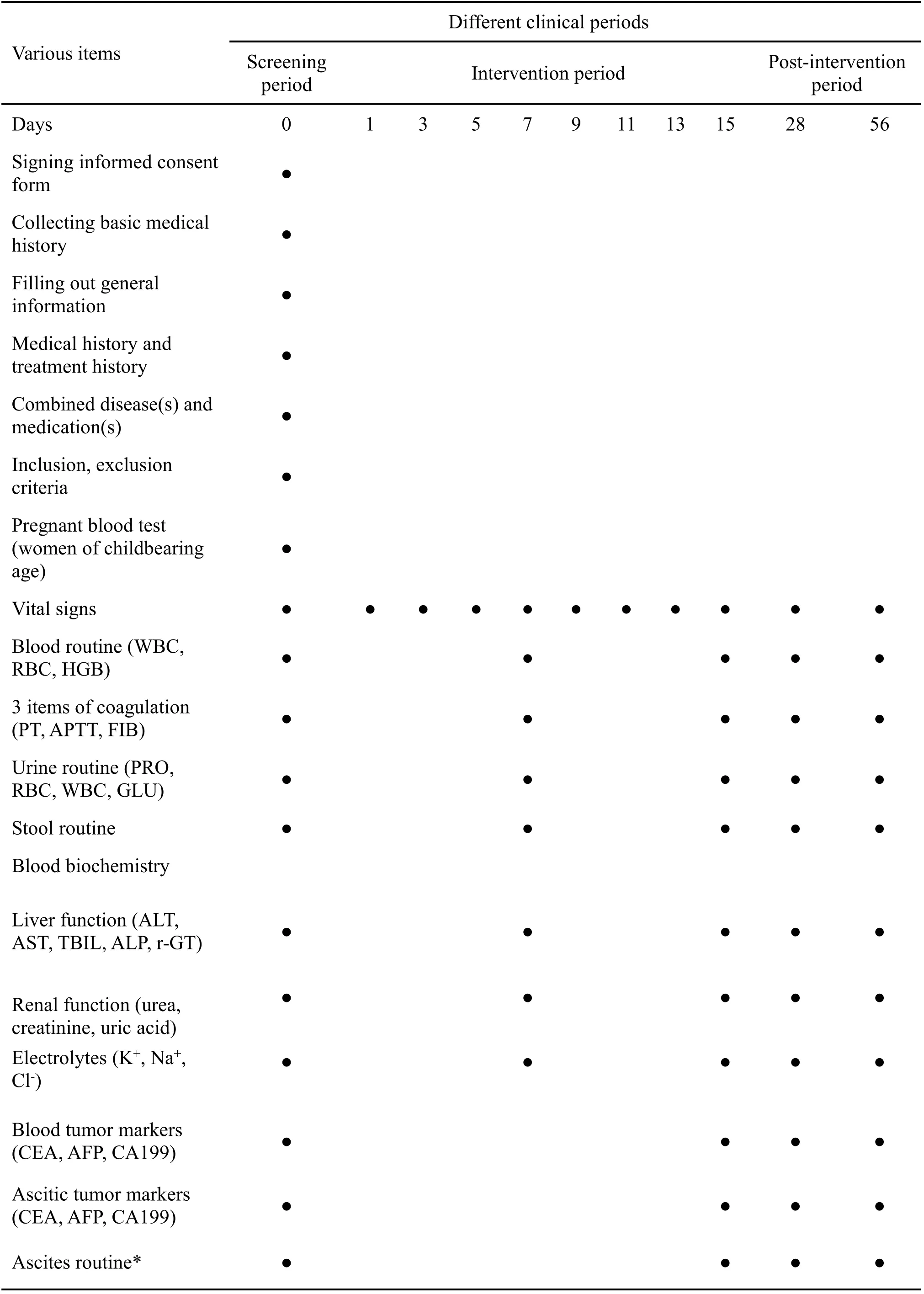
Table 1 Trial workflow
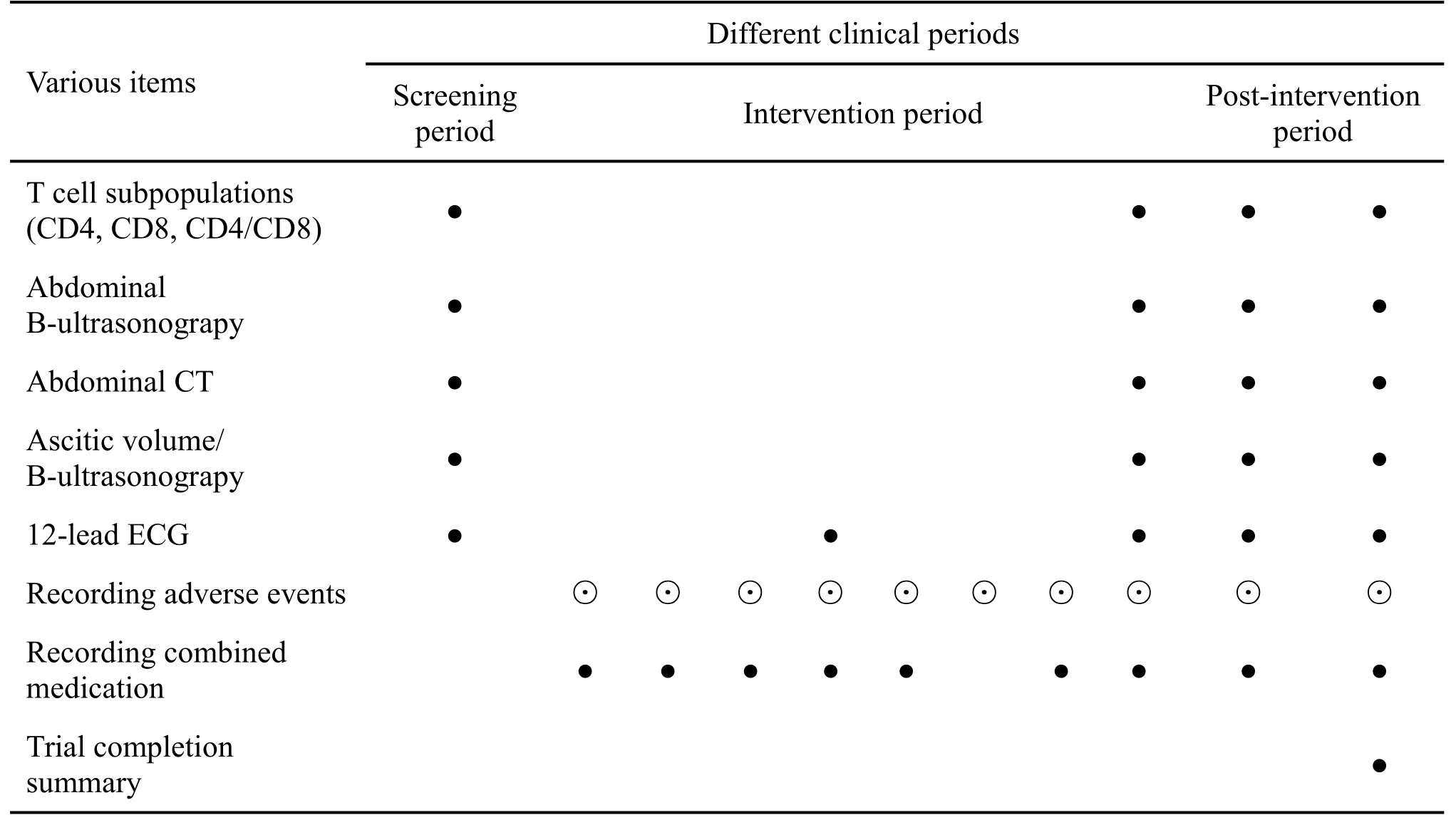
Table 1 Trial workflow
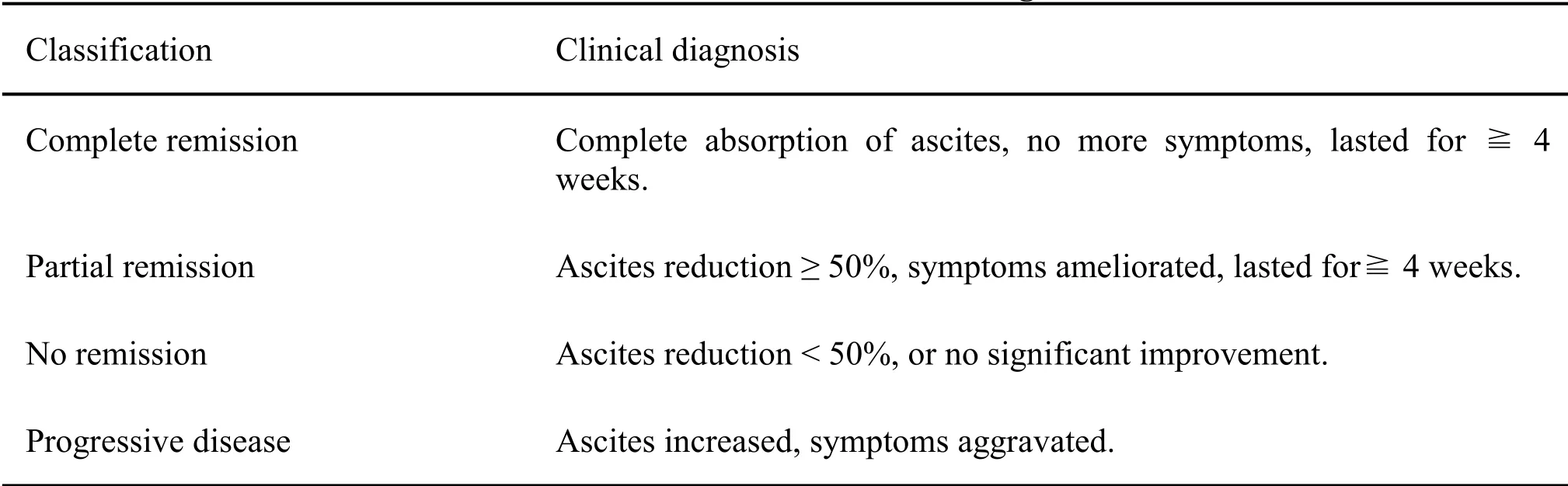
Table 2 Standards of the World Health Organization
Trial status
The trial was initiated in February 2016.Now, the study is in the stage of database testing and is ready to data input and statistics.The completion date of trial is estimated at May 2017.
- TMR Non-Drug Therapy的其它文章
- Progress research on non-drug therapy in cancer-related fatigue
- Clinical observation of acupoint injection combined with nerve electrical stimulation in the treatment of post-stroke dysphagia
- Study on the psychological reaction and coping strategies of nursing students under the spread of COVID-19
- Effect of “Tongji” electroacupuncture on pain and inflammatory factors in patients with lumbar disc herniation in remission stage
- A randomized placebo-controlled trial of Chinese medicine acupoint application on gastrointestinal dysfunction after appendectomy

