Bone marrow-derived mesenchymal stem cell transplantation attenuates overexpression of inflammatory mediators in rat brain after cardiopulmonary resuscitation
Qing-Ming Lin , Xia-Hong Tang Shi-Rong Lin Ben-Dun Chen Feng Chen
1 Institute of Fujian Emergency Medicine, Clinical College of Fujian Medical University, Fuzhou, Fujian Province, China 2 Department of Emergency, Fujian Provincial Hospital, Fujian Provincial Emergency Center, Fuzhou, Fujian Province, China
Abstract
Key Words: antibody array; asphyxia; brain damage; cardiac arrest; cardiopulmonary resuscitation; global cerebral ischemia; inflammatory mediator; mesenchymal stem cell; neurological deficit score; S100B
Introduction
Cardiac arrest is one of the major causes of death in adults,especially among patients with heart disease. Despite progress in cardiopulmonary resuscitation, the average survival rate of patients with cardiac arrest is < 10% (Berdowski et al.,2010). Neurological outcomes are also disappointing in patients with a return of spontaneous circulation after cardiac arrest, as a result of cardiac arrest-induced global cerebral ischemia/reperfusion injury (Caltekin et al., 2016). Only 10-30% of patients with initially successful cardiopulmonary resuscitation have subsequently favorable neurological outcomes (Ragoschke-Schumm et al., 2007). There have been no recent effective pharmacological treatments to improve neuroprotection in patients with cardiac arrest.
Bone marrow (BM)-derived mesenchymal stem cells(MSCs) represent a promising approach for cell therapy. BMMSCs differentiate into fat, bone, and cartilage, and also into neuronal- and glial-lineage cells under certain experimental circumstances (Kassis et al., 2011; Cheng et al., 2019; Zhang et al., 2019). BM-MSCs have proven effective in neurological function in preclinical and clinical studies of brain ischemia(Bang et al., 2005; Pavlichenko et al., 2008; Lee et al., 2010;Lin et al., 2013) and peripheral nerve injury (Fernandes et al., 2018; Seo et al., 2018). The mechanisms of action of BMMSCs transplanted into the damaged brain may include neuroprotection (Jung et al., 2016), regulation of cytokine expression (Gu et al., 2014), and vascular effects (Nam et al.,2015). The neuroprotective effects of transplanted MSCs have been strongly associated with cytokine expression (Li et al.,2010, 2018; Alhazzani et al., 2018; Guan et al., 2018), and Li et al. (2010) also found that MSC treatment increased expression of the anti-inflammatory cytokine interleukin-10 (IL-10)and improved neurological function in Macacafascicularis.However, they focused on observations of one or several cytokines. In the field of cardiopulmonary resuscitation, no studies on the expression profiles of brain cytokines have been found after BM-MSC transplantation. Importantly, several novel cytokines can now be detected using protein chip analysis and their interactions can thus be further examined.
In this study, we simulated human cardiac arrest and explored the possible neuroprotective mechanisms of MSCs in brain resuscitation by establishing an experimental rat model of cardiac arrest-induced global cerebral ischemia, and determining the effects of BM-MSC transplantation on the expression profiles of multiple cytokines in the brain.
Materials and Methods
Animals
Twenty-seven male specific-pathogen-free Sprague-Dawley rats (license No. SCXK (Jing) 2012-0001) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China. Three 5-week-old rats (100-120 g) were used for culture of stem cells and 24 10-week-old rats (350-450 g) were used for in vivo experiments. Animals were housed at 22-25°C under a 12-hour light/dark cycle.The experimental protocol was approved by the Institutional Animal Care and Use Committee of Fujian Medical University, China in January 2016 (approval No. 2016079).
Preparation of BM-MSCs
Rat BM-MSCs were isolated and cultured as described previously (Lin et al., 2013). Briefly, BM was obtained from rat femurs and tibias by washing the cavity with Dulbecco’s modified Eagle’s medium-F12 (DMEM-F12; Gibco, Grand Island,NY, USA). The cell suspension was collected, washed with phosphate-buffered saline (PBS), and centrifuged at 310 × g for 5 minutes. The cells were then re-suspended in DMEM-F12 containing 10% fetal bovine serum (Hyclone, South Logan,UT, USA), 100 IU/mL penicillin, and 100 μg/mL streptomycin, plated in plastic flasks, and cultured in an incubator (Sanyo,Osaka, Japan) at 37°C with 5% CO2. The medium was removed after 3 days and adherent cells were then cultured for a further 4 to 6 days until they reached approximately 90% confluence.The cells were then trypsinized with 0.25% trypsin (Gibco)and sub-cultured at a one-to-two ratio. Cells at passage three were used for experiments. BM-MSCs were identified by flow cytometry, as described previously (Wang et al., 2017). Briefly,cells were stained with fluorescein isothiocyanate (FITC) or polyethylene-labeled specific antibodies against CD29, CD45,CD90, and CD11b (BioLegend, San Diego, CA, USA), and surface antigen expression was then determined using a BD FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA).Passage 3 MSCs were labeled with lentiviral green fluorescent protein (GFP; GenePharma, Shanghai, China) prior to transplantation, according to a previous study (Tang et al., 2017).Cardiopulmonary resuscitation models
A rat cardiac arrest/cardiopulmonary resuscitation model was established as described previously (Lin et al., 2013).Successful cardiopulmonary resuscitation was defined as return of spontaneous circulation for more than 5 minutes.Male Sprague-Dawley rats (350-400 g) were anesthetized with intraperitoneal pentobarbital 45 mg/kg and an endotracheal tube (22 gauge intravenous catheter) was inserted into the trachea. A polyethylene-50 catheter No. 23 (Jingtai Biotech, Shanghai) filled with 5 IU/mL heparin saline was then inserted into the left femoral artery to monitor mean arterial blood pressure. Limb lead II electrocardiogram was monitored throughout the experiment. Data were recorded using a BL-420S BioLab System (Techman, Chengdu, China). Rats were ventilated with air at a tidal volume of 0.65 mL/100 g body weight and ventilation rate of 100 breaths/minute.
The rats were then injected with vecuronium (1 mg/kg)via the femoral artery to induce asphyxia and the endotracheal tube was disconnected from the ventilator (Alcott,Shanghai, China). Cardiac arrest was confirmed by loss of an arterial pulse and a mean arterial blood pressure ≤ 20 mmHg. Cardiopulmonary resuscitation was started 6 minutes after cardiac arrest, with chest compressions at a rate of 200/minute and ventilation with 21% O2. Adrenaline (0.1 mg in 1 mL 0.9% saline) was injected slowly into the femoral artery after 2 minutes of compressions. Spontaneous circulation was considered to be restored once a supraventricular rhythm was observed and a mean arterial blood pressure ≥60 mmHg had been achieved for > 5 minutes. If no return of spontaneous circulation was achieved within 4 minutes,cardiopulmonary resuscitation was stopped. Animals were ventilated for 2 hours after return of spontaneous circulation. When the animals were awake, the endotracheal tube and catheter were removed.
MSC treatment
Animals were assigned randomly to a sham group (n = 8),PBS group (n = 8), and MSC group (n = 8). One hour after successful cardiopulmonary resuscitation, animals in the PBS and MSC groups were injected with 0.5 mL PBS and 0.5 mL PBS containing 1 × 106MSCs, respectively, via the tail vein.Rats in the sham group underwent the same surgery without cardiac arrest. Three days after cardiopulmonary resuscitation, the rats were anesthetized by intraperitoneal injection of pentobarbital and then sacrificed. Venous blood was obtained from the inferior vena cava and the brains were harvested.Serum and brains were stored at -80°C for further analysis.Whole brains from three rats in each group were used for antibody array analysis and the remaining five brains from each group were used for immunohistochemistry.
Neurological deficit scores
Neurological function was evaluated 3 days after cardiopulmonary resuscitation using a neurological deficit score, as described previously (Geocadin et al., 2000). The scoring system included measures of arousal, sensory, motor, reflex,and balance responses as follows: general behavioral deficit(total score = 19), brainstem function (total score = 21), motor assessment (total score = 6), sensory assessment (total score = 6), motor behavior (total score = 6), behavior (total score = 12), and seizures (total score = 10), with a total score of 80 indicating normal brain function and a score of 0 indicating brain death.
Serum S100B levels
Serum S-100B is a specific marker for central nervous injuries, can be used to evaluate the extent of brain injuries and prognosis of brain injuries. Serum S100B levels were determined using a rat S100B enzyme-linked immunosorbent assay kit, according to the manufacturer’s directions(CUSABIO Life Science, Wuhan, China). Briefly, the plate well was coated with capture antibody against S100B to form a solid-phase antibody. Diluted specimens were then added to each well and reacted with the capture antibody. The S100B detection antibody, conjugated secondary antibody,and substrate were then added to each well to form a colored product. The optical density was then measured at 450 nm and the serum S100B concentration was calculated from the standard curve.
Detection of GFP-labeled MSCs in vivo
Continuous coronal cryostat slides (10 μm) were produced for immunofluorescence examination. Nonspecific binding sites were blocked by adding 10% bovine serum albumin.The slides were then incubated with a mouse polyclonal antibody (1:100; Abcam, Cambridge, MA, USA) against GFP,followed by a secondary anti-mouse FITC-conjugated secondary antibody. Negative control slides were treated with the same procedures without the first antibody. A total of five non-overlapping microscopic fields (20× magnification,inverted microscope; Carl Zeiss, Jena, Germany) were selected and GFP-positive MSCs in different brain regions were counted.
Antibody array analysis
Brain protein levels were analyzed following cardiac arrest/cardiopulmonary resuscitation-induced global cerebral ischemia. Rat brains were collected and homogenized 3 days after cardiopulmonary resuscitation and the expression levels of 90 proteins were measured using a RayBio?L-Series Rat 90 Antibody Array (RayBiotech, Norcross, GA, USA).The whole brain was isolated from three rats in each group.Total protein was extracted from 250 mg of brain tissue with 1 mL of ice-cold tissue protein extraction reagent containing protein degradation inhibitors (Kangcheng, Shanghai, China). Protein concentrations were determined using a BCA Protein Assay Kit (Kangcheng). The RayBio?L-Series Rat 90 antibody array membrane (RayBiotech) was blocked for 30 minutes by adding blocking buffer, and then incubated with the protein samples at room temperature for 1 to 2 hours. The samples were then discarded, the chip membrane was cleaned with buffer, and then incubated with biotin-labeled antibodies at room temperature for 1 to 2 hours. The membrane was then washed with buffer and incubated with streptavidin (1:1000) coupled with horseradish peroxidase at room temperature for 2 hours. The membrane was thoroughly cleaned and then reacted with chemiluminescence reagent (RayBiotech, Norcross, GA, USA) in the dark and exposed to X-ray film, and images were obtained using a film scanner (i3200, Kodak, Rochester, NY, USA). The original signal intensities of the proteins were determined by densitometry and standardized using positive controls after correction. Relative protein expression levels were obtained by comparing standardized values.
Statistical analysis
Normal and non-normal data are presented as the mean ±SD and median (25th, 75thpercentile), respectively. Statistical analysis was performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Multiple comparisons were analyzed using one-way analysis of variance or Kruskal-Wallis H-test and pairwise comparisons were analyzed using Bonferroni’s post hoc test. P < 0.05 was considered statistically significant.
Results
Baseline parameters
There was no difference among the groups in terms of body weight, heart rate, mean arterial blood pressure, or respiratory rate (Table 1).

Table 1 Body weight, heart rate, mean arterial blood pressure, and respiratory rate in experimental rats
Characteristics of passage 3 rat MSCs
Passage 3 rat MSCs were plastic-adherent and appeared spindle-shaped and flattened under inverted phase-contrast microscopy (Figure 1A). Flow cytometric analysis revealed that > 99% of MSCs expressed CD29 and CD90 markers (99.73% and 99.90%, respectively), and < 10% expressed CD45 and CD11b (7.55% and 10.63%, respectively) (Figure 1B).
Neurological deficit scores
The neurological deficit scores were significantly lower in PBS control rats compared with the sham rats (P < 0.001);however, scores were significantly higher in MSC-treated rats compared with PBS-treated rats (P = 0.001) (Figure 2).
Serum S100B levels
Serum S100B levels were significantly higher in PBS rats compared with sham rats (P < 0.001); however, MSC treatment significantly reduced S100B levels (P < 0.001) (Figure 3).
Distribution of GFP-labeled MSCs in vivo
Three days after transplantation of GFP-labeled MSCs,GFP-expressing (green) cells were mainly observed in the cerebral cortex in cardiac arrest rats, with only a small proportion of cells in the hippocampus and other brain regions(Figure 4).
Protein levels
The raw data obtained from a ntibody array analysis of all proteins in sham, PBS, and MSC rats 3 days after cardiopulmonary resuscitation are shown in Additional Table 1. Only proteins with a fold change ≥ 1.5 or ≤ 0.5 and which were significantly different (P < 0.05) were included. Levels of the pro-inflammatory mediators tumor necrosis factor (TNF)-α, interferon (IFN)-γ, macrophage inflammatory protein(MIP)-1α, MIP-2, MIP-3α, macrophage-derived chemokine(MDC), and matrix metalloproteinase (MMP)-2 were significantly higher in PBS rats compared with sham rats (P =0.018, 0.009, 0.024, 0.018, 0.013, 0.028, 0.024, respectively).However, anti-inflammatory factor IL-10 levels were significantly reduced in PBS rats compared with sham rats (P =0.017). MSC treatment significantly inhibited the increases in pro-inflammatory mediators and increased IL-10 levels(P = 0.037, 0.028, 0.012, 0.027, 0.006, 0.001, 0.037, 0.008, respectively) (Figure 5).
Discussion
This study found that intravenous BM-MSC injection remarkably ameliorated brain damage and improved the recovery of neurological function at 3 days after cardiac arrest and cardiopulmonary resuscitation in rats. BM-MSC injection also up-regulated expression of the anti-inflammatory factor IL-10 and down-regulated the expression levels of the inflammatory mediators IFN-γ, TNF-α, MIP-1α, MIP-2, MIP-3α, MDC, and MMP-2 in the brain. These results support the hypothesis that inhibition of inflammatory mediators by IL-10 may be responsible for the beneficial effects of transplanted BM-MSCs; however, whether the decrease in inflammatory mediators is regulated by IL-10 remains unclear. In addition, the increased levels of IL-10 in the brain might be due to secretion by the transplanted BM-MSCs or by parenchymal cells. The demonstration of a possible internal relationship between these cytokines represents a novel discovery in this study.
Inflammatory responses characterized by an increase in levels of inflammatory mediators play a critical role in the pathogenesis of ischemic brain damage (Lin et al., 2013;Ernst et al., 2019; Xian et al., 2019), and inappropriate inflammatory responses may contribute to the expansion of brain damage (Denes et al., 2010; Zan et al., 2018; He et al.,2019). Global cerebral ischemia was recently shown to trigger the rapid infiltration of pro-inflammatory T-lymphocytes into the brain, and these cells in turn produce a variety of inflammatory mediators that contribute to ischemic brain damage (Deng et al., 2014). Among these inflammatory mediators, pro-inflammatory cytokines such as TNF-α and IFN-γ, and chemokines such as MIP-1α, MIP-2, MIP-3α,and MDC, play important roles in inflammatory responses(Xiang et al., 2016).
IFN-γ and TNF-α are well-known pro-inflammatory factors associated with the exacerbation of ischemic brain damage in pre-clinical studies (Offner et al., 2006; Liesz et al., 2009; Silva et al., 2015), and were upregulated within hours in ischemic brain lesions of rats (Zhou et al., 2013;Jafarinaveh et al., 2014). Our study revealed that MSC transplantation inhibited the increases in concentrations of IFN-γ and TNF-α in the brain in rats following cardiac arrest, suggesting that MSCs might attenuate inflammatory responses by inhibiting IFN-γ secretion (Gelderblom et al.,2012). Chemokines are a class of cytokines that signal leukocytes, such as neutrophils and macrophages, to traffic on the ischemic cerebral endothelium (Kong et al., 2014). MIP-1α, MIP-2, MIP-3α, and MDC expression were shown to be upregulated following cerebral ischemia in rats (Ohta et al., 2007), and inhibition of chemokines has been associated with reduced brain damage in mice (Victoria et al., 2017).The current results demonstrated that cardiac arrest-induced global cerebral ischemia noticeably upregulated the expression of the chemokines MIP-1α, MIP-2, MIP-3α, and MDC in the brain, while MSC transplantation markedly reduced the levels of these chemokines. The mechanism of action might be associated with reduced TNF-α expression (Shukla et al., 2017). Cytokines interact with each other. TNF-α canstimulate production and release of chemokines. Therefore,we speculate that the decrease of chemokines may be related to the decrease of TNF-α. In addition, MMP-2 levels in the brain were increased 4 days after cerebral ischemia. MMP-2 has previously been implicated in cerebral ischemia (Planas et al., 2001), and disrupted the integrity of the blood brain barrier and caused neuronal cell death in rats (Amantea et al., 2007). We previously showed that cardiac arrest caused brain edema and ischemic brain damage in rats, while MSC transplantation attenuated these pathological changes (Leong et al., 2016). These effects might thus be achieved via a mechanism associated with MMP-2 (Amantea et al., 2014).The current results also showed that MSC treatment inhibited MMP-2 production in the ischemic brain following cardi
ac arrest.
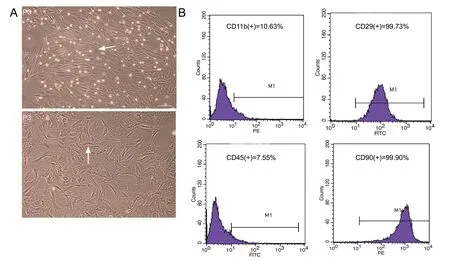
Figure 1 Characteristics of passage 3 (P3) rat bone marrow-derived mesenchymal stem cells in culture.

Figure 2 Neurological deficit scores among sham-, PBS-, and MSC-treated rats 3 days after cardiopulmonary resuscitation.

Figure 3 Serum S100B levels among sham-, PBS-, and MSC-treated rats.

Figure 4 Detection of GFP-labeled MSCs and numbers of labeled MSCs in damaged brain in rats 3 days after cardiopulmonary resuscitation.

Figure 5 Protein levels in the brain in sham-, PBS-, and MSC-treated rats 3 days after cardiopulmonary resuscitation.
In addition to inflammatory responses, proinflammatory cytokines are also involved in apoptosis and pyroptosis after cerebral injury. Liu et al. (2013) revealed that the chemokine CCL2 induced neuronal apoptosis after brain damage, while Poh et al. (2019) reported that apoptosis and pyroptosis in microglial cells were mediated by the inflammasome after cerebral injury. All these processes ultimately cause nerve cell death resulting in decreased neuronal density (Figure 6) and consequently reduced neurological function. In the current study, cardiac arrest caused high levels of proinflammatory cytokines, while MSC treatment inhibited the arrest-induced production of these cytokines. After MSCs transplantation,stem cells in the environment of high levels of proinflammatory cytokines in the brain may reduce proinflammatory cytokines through feedback under the stimulation of inflammation, leading to improved neurological outcomes after cardiac arrest.
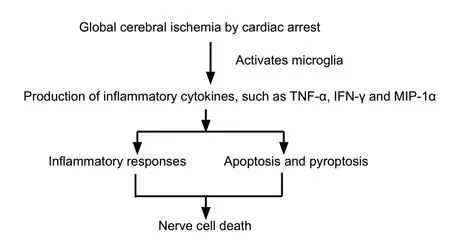
Figure 6 Inflammatory cytokines mediate cell death after global cerebral ischemia induced by cardiac arrest.
IL-10, as an anti-inflammatory cytokine, has been shown to inhibit the pro-inflammatory factors IL-1β and TNF-α and to suppress cytokine receptor expression and activation (Garcia et al., 2017). The neuroprotective role of IL-10 has been confirmed in animal models of cerebral ischemia(Spera et al., 1998; Liang et al., 2015; Zhang et al., 2016),and intracerebroventricular injection of IL-10 injection had neuroprotective effects in an experimental mouse model of stroke (Liesz et al., 2014). In line with these results, the current study demonstrated that MSC transplantation increased brain production of IL-10 in cardiac arrest-induced global cerebral ischemia in rats. The beneficial effect of MSC treatment might thus be attributed to a reduction in pro-inflammatory mediators modulated by IL-10.
The current pathological results demonstrated that transplanted MSCs were mainly located in the cerebral cortex and rarely in the hippocampus or other brain regions. It is likely that the MSCs somehow ‘sense’ and home to the more-damaged cortex; however, the mechanisms responsible for MSC homing to the damaged tissue are not clear. Stromal cell-derived factor 1 expression was upregulated in ischemic tissues and CXC chemokine receptor 4 was shown to be present on the stem cell surface in rats (Li et al., 2017), suggesting that a molecular signal associated with stromal cell-derived factor 1/CXC chemokine receptor 4 may be associated with homing and engraftment of stem cells to the damaged tissues (Shyu et al., 2006). In view of the proposed mechanisms of stem cell homing, we speculated that the ischemic cortex might produce high concentrations of stromal cell-derived factor 1, which could in turn attract more MSCs. However,further studies are needed to prove this hypothesis.
This study had some limitations. First, we did not determine if the microglia and astrocytes in the brain were activated, and their role in the inflammatory responses is thus poorly understood. Second, it remains unknown if IL-10 was secreted by the transplanted MSCs or by resident parenchymal cells in the brain. Finally, we did not quantify the degree of MSC engrafted in the brain. Further investigation of the mechanisms involved in the inhibitory modulation of inflammatory mediators by MSCs is therefore warranted.
In conclusion, MSC transplantation improved neurological outcomes after cardiac arrest and cardiopulmonary resuscitation in rats, possibly by reducing levels of inflammatory mediators and increasing levels of the anti-inflammatory cytokine IL-10.
Acknowledgments:We are very grateful to Wei-Te Zhuang from Fujian Provincial Hospital for providing English-language editing service.
Author contributions:Study design: QML, BDC, FC; experimental implementation: QML, XHT, SRL; data analysis: QML, XHT; paper writing:QML, XHT. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the Natural Science Foundation of Fujian Province of China, No. 2015J01375 (to QML); and Fujian Provincial Hospital Foundation of China, No. 2014070 (to QML).The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This experimental protocol was approved by the Institutional Animal Care and Use Committee of Fujian Medical University, China in January 2016 (approval No. 2016079). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Elham H A Ali, Ain Shams University, Egypt;Elizabeth J. Sandquist, Weber State University, USA.
Additional files:
Additional file 1:Open peer review reports 1 and 2.
Additional Table 1:Cytokines profile in the brain at 3 days after cardiopulmonary resuscitation.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
- Endothelin increases the proliferation of rat olfactory mucosa cells
- Differential neuronal reprogramming induced by NeuroD1 from astrocytes in grey matter versus white matter
- Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia
- Different protein expression patterns in rat spinal nerves during wallerian degeneration assessed using isobaric tags for relative and absolute quantitation proteomics profiling
- Is there a relationship between dopamine and rhegmatogenous retinal detachment?

