Off-the-shelf mesenchymal stem cells from human umbilical cord tissue can significantly improve symptoms in COVID-19 patients:An analysis of evidential relations
Phuc Van Pham,Ngoc Bich Vu,Stem Cell Institute,University of Science,Ho Chi Minh 08000,Viet Nam
Phuc Van Pham,Ngoc Bich Vu,Vietnam National University,Ho Chi Minh 08000,Viet Nam
Abstract
Key words:Coronavirus;COVID-19;Mesenchymal stem cells;Umbilical cord-derived mesenchymal stem cells
INTRODUCTION
Coronaviruses express spike glycoprotein on their surfaces,which facilitates binding and entry into host cellsviaangiotensin I-converting enzyme 2 (ACE2) receptor expressed on host cells[1,2].Although this receptor exists in almost all cells of the human body,it is highly expressed in alveolar type II (AT2) cells,the capillary endothelium,endothelial cells,and smooth muscle cells[3].Therefore,all of these cells can be infected by a novel coronavirus (nCoV).
Similar to other viral infectious diseases,virus-infected cells are recognized and killed by the immune system of patients.With multiple affected organs,the host immune system is activated to kill the virus and virus-infected cells through activation of immune cells,such as natural killer (NK) cells,which produce a large number of inflammatory factors.This so-called severe cytokine storm entails the release of cytokines such as interleukin 2 (IL-2),IL-6,IL-7,granulocyte-colony stimulating factor(G-CSF),interferon-inducible protein of 10 kD,monocyte chemoattractant protein-1(MCP-1),macrophage inflammatory protein 1-alpha (MIP-1A),and tumor necrosis factor alpha (TNF-α).In addition to affecting the virus and virus-infected cells,these immune cells and their cytokines have an extreme effect on normal cells,causing edema,dysfunction of air exchange,acute respiratory distress syndrome (ARDS),and acute cardiac injury.As a result,there are multiple injuries in multiple organs and tissues.Moreover,these injured tissues and organs can be infected by other microorganisms.After entry of coronavirus into ACE2 receptor-expressing cells,multiple organs are injured in patients,which subsequently leads to death.Examinations of peripheral blood from coronavirus disease-2019 (COVID-19) patients have revealed increases of inflammatory factors such as IL-6,IL-10,and TNF-α[4].In intensive care unit patients,the plasma levels of IL-2,IL-7,IL-10,G-CSF,IP-10,MCP-1,MIP-1A,and TNF-α also increase[5].
It appears that the death of nCoV-infected patients is related to the injury and failure of multiple organs,which is triggered by the host immune system.The positive feedback regulation of the immune system not only allows attack of the virus and virus-infected cells but can also destroys healthy cells,leading to severe injury in some vital organs such as the lungs,heart,liver,and kidneys.In two reports about the clinical features of COVID-19 patients in Wuhan,China,COVID-19 was found to cause complications such as ARDS,arrhythmia,shock[6],severe kidney injury,acute cardiac injury,liver dysfunction,and secondary infection[7].
Current treatments for COVID-19 are mainly focused on symptomatic and respiratory support.Almost all patients receive oxygen therapy and rescue treatment with convalescent plasma,and in some critical cases,immunoglobulin G is applied.In addition to some anti-viral drugs,chloroquine has emerged as a repurposed drug with a great potential to treat COVID-19[8,9].Indeed,chloroquine inhibits the replication of several viruses[10-12]and has immunomodulatory effects such as suppressing the production and release of TNF-α and IL-6[13].Although the use of chloroquine/hydroxychloroquine may be promising,their use should be restricted to clinical trials.Indeed,to date,there have been 17 clinical trials regarding the use of chloroquine/hydroxychloroquine for COVID-19 worldwide,but none have been completed[14].Thus,there is insufficient evidence to recommend them for routine treatment of COVID-19.
HOW MESENCHYMAL STEM CELLS CAN BE USED IN COVID-19 TREATMENT
Mesenchymal stem cells (MSCs) are the most common stem cells in the human body.They exist in almost all tissues and organs,especially bone marrow and adipose tissue[15,16].These cells have been isolated from umbilical cord tissue[17-19]and umbilical cord blood[20,21].They are characterized by their shape when adhered onto the surface of a tissue culture vessel and the expression profile of markers (positive for cluster of differentiation 44 [CD44],CD73,CD90,and CD105,while negative for CD14,CD34,CD45,and human leukocyte antigen DR [HLA-DR]).They are also unique in their potential to differentiate into mesodermal cells including adipocytes,osteoblasts,and chondrocytes[22,23].
MSCs have been used clinically to treat more than 10 different diseases[24,25].Importantly,MSC transplantations have been approved in some countries to treat various inflammatory diseases[26,27].In Canada,since 2012,bone marrow-derived MSCs have been approved for graft-versus-host disease (GVHD) treatment (Prochymal)[28,29].Also in that year,umbilical cord blood-derived MSCs (UC-MSCs) were used in South Korea to treat knee osteoarthritis (Cartistem)[30].Recently,the Japanese government has approved HS Temcell,which contains allogeneic MSCs from bone marrow for GVHD treatment[31,32].In Europe,adipose-derived MSCs have been approved for Crohn’s disease[33].Indeed,there have been many products related to clinical MSC transplantation,which have been used or are being investigated in clinical trials in several countries[34].
MSC transplantation for pulmonary diseases has also been carried out with positive outcomes[35,36].In our recent publication,we showed that transplantation of UC-MSCs significantly improved symptoms of chronic obstructive pulmonary disease (COPD) in late-stage patients[37].
Lastly,a clinical trial by Lenget al[38](2020) showed that transplantation of MSCs was a safe and effective treatment for patients with COVID-19,especially critically severe patients.Seven patients in the study were cured or showed significant improvement of pulmonary functions with symptoms of COVID-19,but without observed adverse effects.Interestingly,C-reactive protein (CRP) and TNF-α were decreased,and cytokine-secreting immune cells,such as CXCR3+CD4+T cells,CXCR3+CD8+T cells,and CXCR3+NK cells,disappeared after 3-6 d of treatment[38].
POSSIBLE MECHANISMS OF MSCS IN COVID-19 TREATMENT
Direct effect on nCoV
Although the role of MSCs in killing nCoV has not been reported,some published studies have shown that MSCs attack bacteria and viruses[39-44].In 2009,Gonzalezet al[39]reported that MSC transplantation reduces the number of bacterial colony-forming units in the blood,liver,spleen,and peritoneal fluid of septic mice.The mechanism of the anti-bacterial activity of MSCs remains unclear.Some reports have suggested that MSCs enhance phagocytosis of monocytes[40,41].An increase of C5a complement was found by Krasnodembskayaet al[41](2012).The anti-bacterial activity is also enhanced by triggering the phagocytic index of macrophages by transferring MSC-derived mitochondria to macrophages[42,43].
The patent WO2010053350A1 from Erasmus University Medical Center in Rotterdam,Netherlands,describes methods to produce concentrated MSCconditioned medium (C-MSC-CM) that retains potent anti-viral activity.C-MSC-CM inhibits hepatitis C virus (HCV) and hepatitis B virus replication.Recent studies have suggested that MSCs also display anti-viral activities.For example,the study by Qianet al[44](2016) was the first to demonstrate that MSCs inhibit HCV infectionviatheir exosomes.The authors showed that exosomes from MSCs contained functional miRNAs,mainly let-7f,miR-145,miR-199a,and miR-221.These microRNAs possess binding sites for HCV RNA.
Effects on the immune system of patients
The primary mechanism by which MSCs improve the symptoms of COVID-19 is related to their immunomodulation potential (Figure1).MSCs efficiently regulate inflammation by suppression of effector immune cells,upregulation of regulatory T cells,and inhibition of T-cell activation[45-47].Therefore,MSCs have great potential to treat immune diseases,especially acute and chronic inflammation[48-51].

Figure1 Possible mechanisms of mesenchymal stem cells in coronavirus disease-2019 treatment.Mesenchymal stem cells directly affect novel coronavirus,affect the immune system of patients,and stimulate wound healing.
In the report by Lenget al[38](2020),the levels of CRP and TNF-α were both reduced in treated COVID-19 patients.These observations are similar to those previously published on MSC transplantation for immunological diseases[52,53].MSCs also inhibit the maturation of T cells and prevent them from becoming too active[54,55].This may be why some cytokine-secreting immune cells decrease significantly in treated COVID-19 patients.
Indeed,underin vitroconditions,MSCs have been observed to inhibit the production of TNF-α by immune cells.Zhenget al[56](2008) reported that allogenic MSCs inhibit both CD4+ and CD8+ T cells from producing interferon gamma (IFN-γ)and TNF-α.Another study showed that MSCs inhibit macrophages from producing proinflammatory cytokines such as IFN-γ,IL-6,and TNF-α[57].
Similar to sepsis and septic shock,patients infected with COVID-19 experience a severe cytokine storm that is harmful and ultimately leads to death.Preclinical trials in rodents showed that MSC transplantation improves sepsis and septic shock[58-61].In a meta-analysis,Laluet al[62](2016) analyzed 20 controlled comparison experiments (980 animals from 18 publications) ofin vivosepsis models and found that MSC transplantation significantly reduced mortality under a range of experimental conditions.Some current clinical trials have reported that MSC transplantation is safe in severe sepsis patients[63].
Stimulation of wound healing
Several tissues and organs affected by activated immune cells and severe cytokine storms can be injured.MSCs contribute to stimulating the wound healing process.In preclinical and clinical trials,MSC administration facilitated healing a range of injuries.Some publications have shown that MSC transplantation triggers lung injury healing.In models of radiation-induced lung injury in rats and mice,MSC transplantation significantly reduced pulmonary radiation fibrosis[64],reduced serum levels of IL-1,IL-6,and TNF-α[65],and inhibited fibrosis[66].
The roles of MSCs in wound healing appear to be related to the growth factors that they produce and secrete into the medium.These factors include epidermal growth factor,fibroblast growth factor,and insulin-like growth factor.All of these growth factors strongly stimulate wound healing.Moreover,MSCs promote wound healingviatheir exosomes (extracellular vesicles) that are secreted and targeted to injured tissues.These growth factors and exosomes produced by MSCs enable MSCs to exert their anti-apoptosis effects,rescuing apoptotic cells from traumatic exposure to hypoxia,chemicals/acidity,and mechanical damage or radiation[67-69].
Although MSCs provide benefits for COVID-19 patientsviathese possible mechanisms,the treatment efficacy of COVID-19 by MSC transplantation is variable among patients because of the complexity of COVID-19.The stage of COVID-19 may be the main issue affecting treatment efficacy.MSCs appear to be effective to control inflammation and the cytokine storm by immunomodulation.This effect also reduces the immune response to virus-infected cells and bacteria.Therefore,the risk of bacterial infection can be increased in MSC-transplanted COVID-19 patients.The rejection response of recipientsversustransplanted allogenic MSCs should also be considered and monitored.Theoretically,MSCs do not express HLA.Hence,they can be used in allogenic transplantation.However,expression of HLA can be upregulated after MSCs are transplanted into patients and differentiate into specialized cells.Indeed,chronic rejection of transplanted allogenic MSCs decreases the treatment efficacy and causes slight fever for a long time,even after successful treatment of COVID-19.
The treatment efficacy also depends on several factors included the MSC type,quality of MSCs,and dose of MSCs.MSCs can be obtained from various tissues for clinical treatment,but MSCs from different sources usually exhibit different biological characteristics,especially immunomodulation,as well as angiogenic characteristics.These characteristics play important roles in COVID-19 treatment.The therapeutic characteristics can also be affected by the biological status,especially the senescent status,tissue donation,and culture conditions forin vitroexpansion.Indeed,some studies have shown that immunomodulation of MSCs is reduced significantly in the senescent phase and in MSCs derived from older individuals.In vitroexpansion conditions significantly affect MSC immunomodulation.Chenet al[70](2017) showed that MSCs expanded in a 3D-stirred tank bioreactor/microcarrier culture system display better immunomodulation than those expanded in flasks.Some recent studies have shown MSCs expanded in a platelet lysate have a superior capacity to suppress the proliferation of allogeneic lymphocytes than those expanded in fetal bovine serum[71,72].Kabatet al[73](2020) analyzed 914 MSC trials reported through 2018,concerning the MSC dose for transplantation.They found that the minimal effective dose (MED) of MSCs ranged from 70 to 190 million MSCs/patient/dose.Lower or higher intravenous doses of MSC transplantation were less effective.Indeed,the dose of MSCs directly affected the immunomodulation response in recipients.At a lower dose of MSCs than the MED,the immunomodulation response was insufficient to induce therapeutic effects in patients.However,at higher doses,MSCs caused too much immunomodulation that increased side effects.Therefore,determining the optimal dose of MSCs for transplantation is important for effective treatment with the lowest side effects.
OFF-THE-SHELF MSCs FROM UMBILICAL CORD TISSUE ARE A SUITABLE SOURCE FOR COVID-19 TREATMENT
Although MSCs can be obtained from several tissue sources,it appears that UC-MSCs are optimal to treat COVID-19.The first reason is that transplantation of UC-MSCs is safe and effective in humans for sepsis and chronic inflammation.Heet al[63](2018)reported no serious adverse events associated with infusion of UC-MSCs in 15 patients.At a high dose of UC-MSCs (3 × 106cells/kg),the allogenic UC-MSCs were also found to be safe and well tolerated in the 15 patients with severe sepsis.Additionally,in chronic inflammation such as COPD,we confirmed that infusion of allogenic UC-MSCs is safe[37].
UC-MSCs have great potential and suitability for COVID-19 treatment because of their useful properties.A publication in 2019 demonstrated that MSCs from Wharton’s Jelly (a part of the UC) improve bacterial clearance and survival in sepsis mouse models,whereas bone marrow-derived MSCs did not have these effects[74].Several studies have suggested that UC-MSCs perform their immunomodulation better than bone marrow-derived MSCs and adipose-derived MSCs[75,76].This therapeutic effect is obtained by the activatable status of UC-MSCs.Selichet al[77](2019) demonstrated that UC-MSCs are activatable for immunomodulation,whereas bone marrow-derived MSCs[78,79]and adipose-tissue derived MSCs[80]are activated by TNF-α[80],IFN-γ[79],and lymphocyte extracts[78]to trigger immunomodulation.UC-MSCs also express higher levels of immunomodulatory surface proteins,such as CD200,CD273,and CD274,and cytokines such as IL-1β,IL-8,leukemia inhibitory factor,and TGF-β2 compared with bone marrow-derived MSCs[81],which makes them the strongest immunomodulatory MSCs.
UC-MSCs are suitable sources of allogenic MSCs with low immunogenicity and high yield manufacturing.In 2018,Kimet al[76]compared the immunological characteristics of MSCs derived from the periodontal ligament,umbilical cord,and adipose tissue.They found that UC-MSCs expressed minimal levels of HLA-DR and HLA-ABC after activation by IFN-γ[76]compared with adipose tissue- and periodontal ligament-derived MSCs.Liet al[82](2018) also obtained similar findings in which UCMSCs displayed the strongest immunomodulatory ability compared with MSCs from exfoliated deciduous teeth,bone marrow,and gingival tissues.After treatment with IFN-γ,UC-MSCs expressed HLA-DR at a low level compared with those from other sources,whereas bone marrow-derived MSCs expressed HLA-DR at the highest level compared with those from other sources[82].High expression of HLA-DR or HLA-ABC is a major obstacle for allogenic transplantation.
Moreover,UC-MSCs are easily collected and expandedin vitrowith minimal ethical concerns.UC-MSCs also grow faster than adipose tissue- and periodontal ligamentderived MSCs[76].UC-MSCs can be obtained easily from the umbilical cord,according to GMP-compliant conditions for clinical usage[19,37]with established high yield manufacturing[83](Figure2).These characteristics suggest that UC-MSCs are suitable MSC therapeutic candidates for COVID-19.
OFF-THE-SHELF UC-MSCS FOR COVID-19 TREATMENT
Clinically,patients with COVID-19 have two phases of immunoresponses after nCoV infection.The first phase is the incubation and non-severe stages in which the immunoresponse of patients is triggered to eliminate the virus.The second phase occurs when the immune system fails to eliminate the severe acute respiratory syndrome coronavirus 2,which will cause the severe stage because of severely damaged lungs.These differences in the immunoresponse suggest that different approaches are needed to treat COVID-19 at incubation and severe stages.
In the first stage,some strategies related to boosting the patient’s immune system may provide good outcomes,whereas lung inflammation control is important for severe-stage patients.Xuet al[84](2020) reported that lung inflammation is the main cause of life-threatening respiratory disorders.UC-MSC transplantation that suppresses inflammation and manages symptoms appears to be effective to treat severe-stage COVID-19 patients.
COVID-19 patients should be confirmed by a real-time reverse transcription polymerase chain reaction assay and classified as the severe stage of COVID-19 that released by National Health Commission of China included respiratory distress,RR ≥30/min;oxygen saturation ≤ 93% at rest;arterial partial pressure of oxygen/fraction of inspiration O2≤ 300 mmHg,1 mmHg = 0.133 kPa[38].
Based on the above analyses,severe-stage COVID-19 patients would be transfused intravenously with 1 million thawed off-the-shelf UC-MSCs per kilogram of body weight.Inflammatory markers,including cytokines (CRP and TNF-α) and cytokinesecreting immune cells (CXCR3+CD4+T cells,CXCR3+CD8+T cells,and CXCR3+NK cells) should be monitored everyday post-stem cell transplantation.The UC-MSC transplantation should be repeated with the same dose of off-the-shelf UC-MSCs after 2 wk.Decreases of inflammatory cytokine concentrations in peripheral blood are a good indicator of reduced inflammation in combination with other improved symptoms.Other therapies should be maintained during UC-MSC transplantation until COVID-19 symptoms clearly improve.
CONCLUSION
COVID-19 has spread rapidly across the globe.The World Health Organization officially declared COVID-19 as a public health emergency of international concern.In addition to anti-viral drugs and oxygen therapy,MSC transplantation is a therapeutic option for the treatment of COVID-19.Based on the related evidence of COVID-19,the biology of UC-MSCs,and the initial results of a clinical trial using MSCs for COVID-19 treatment,we believe that off-the-shelf UC-MSC transplantation may be an additional therapy to improve treatment efficacy,especially in critically ill COVID-19 patients.
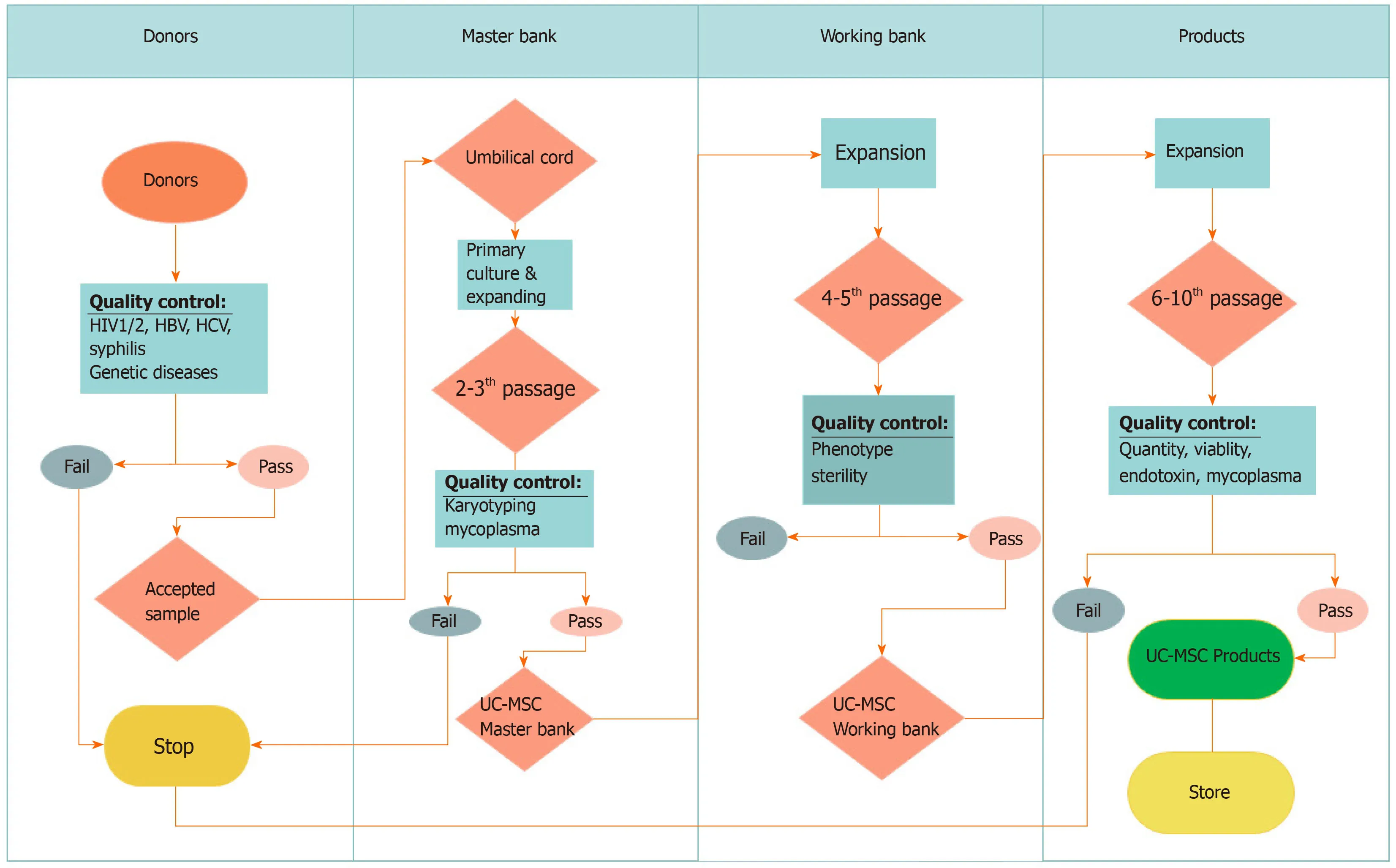
Figure2 Workflow of umbilical cord-derived mesenchymal stem cell manufacturing.Donors are screened for some viruses and genetic disorders.Then,accepted umbilical cords are used to isolate umbilical cord-derived mesenchymal stem cells (UC-MSCs) for harvest at passage 2–3 to produce the master cell bank.A working bank of UC-MSCs is produced from the master bank before expanding UC-MSCs to obtain enough cells for transplantation.HIV:Human immunodeficiency virus;HBV:Hepatitis B virus;HCV:Hepatitis C virus.
 World Journal of Stem Cells2020年8期
World Journal of Stem Cells2020年8期
- World Journal of Stem Cells的其它文章
- Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection
- Practical choice for robust and efficient differentiation of human pluripotent stem cells
- Stem cell therapy for Alzheimer's disease
- Exosomes derived from stem cells as an emerging therapeutic strategy for intervertebral disc degeneration
- Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes
- Autophagy in fate determination of mesenchymal stem cells and bone remodeling
