Semen collection methods and cooling rates affect post-thaw sperm motility and kinematic parameters of Saanen goat
Kambulu Lukusa, John Kabuba
1Department of Animal & Wildlife Sciences, University of Pretoria, Private Bag X20, Hatfield, 0028, South Africa
2Department of Chemical Engineering, Vaal University of Technology, Private Bag X021, Vanderbijlpark, 1911, South Africa
ABSTRACT
KEYWORDS: Artificial vagina; Electro-ejaculator; Sperm characteristics; Cryopreservation
1.Introduction
Semen cryopreservation protocol consists of common stages including cooling, freezing, storage, and thawing.However, semen quality could be affected at each stage and there are noticeable variations even between individuals.This leads to the need of assessment of specific sperm motility and velocity modifications at each step[1].Motility is among the most important parameters for evaluating the fertilization capacity and fertility itself.Studies that employed a computer-assisted sperm analyzer showed that the semen samples with higher values of curvilinear velocity (VCL),straight-line velocity (VSL), and average-path velocity (VAP)parameters provided higher fertility and pregnancy rates[2].Several studies have demonstrated that high sperm velocity and kinematic parameters are landmarks of fertility[3-5].
The success of artificial insemination in goats depends on the ability to properly collect and cryopreserve spermatozoa efficiently from proven bucks[6,7].Buck semen is commonly collected by artificial vagina and electro-ejaculator.The collection of semen by artificial vagina is more preferable method as it resembles natural mating; however, it is more time consuming as it requires training of animals for more than four weeks prior to semen collection[8].On the other hand, the electro-ejaculator can be an alternative method in cases where males are not or impossible to train and could be a viable procedure of collecting semen from a large number of animals[9].However, differences on semen quality between semen collected with artificial vagina and electro-ejaculator have been reported in goats[10].Moreover, significant differences exist in seminal plasma composition between semen collected by artificial vagina and electro-ejaculator[10,11].These differences may have substantial effect on the response of sperm to different procedures of cryopreservation.
Post-thaw sperm quality also differs according to the collection method applied.Jiménez-Rabadán et al[10,11]observed higher postthaw sperm quality when collected by artificial vagina as evidenced by increased percentages of all sperm parameters.Consequently, it is also reasonable to speculate that cooling rates for semen obtained by artificial vagina and electro-ejaculator may influence sperm motility and velocity parameters for cooled and frozen-thawed buck sperm.Cooling rate of semen from temperature of dilution (37 ℃) to 4 ℃-5 ℃ determines the success of freezing protocols in terms of sperm survival[12].The cooling period is necessary to decrease the effect of temperature changes and to allow equilibration of the spermatozoa with the diluents before freezing.Improper cooling rate instigates temperature shock and causes membrane disruption due to structural disorganization of proteins, disturbance of ion channels,production of reactive oxygen species and loss of mitochondrial membrane potential[12,13].
These detrimental effects can be reduced by optimizing cooling rates before freezing[14].Hence, different rates (slow and fast) have been studied in goats with variable results[12,15].Despite few studies done in goats regarding cooling rates, no optimal cooling rate has been established.However, it is essential that an optimum cooling rate with prolonged sperm viability before and after cryopreservation should be investigated.No attempts have yet been made to associate cooling rates with methods of semen collection in Saanen bucks.Therefore, the rate of cooling and its influence on both cooled and post-thaw semen quality obtained by artificial vagina and electroejaculator methods require further investigation.
Therefore, it is hypothesized that cooling rate may be determined through semen collection method, to carry out successful cryopreservation.The aim of this study was to evaluate the effects of semen collection methods and different cooling rates on cooled and frozen-thawed Saanen goat semen.
2.Materials and methods
2.1.Study area
This study was conducted at University of Pretoria experimental farm, Hatfield, Pretoria (latitude 25o45? South, longitude 28o16? East).The experimental farm is situated in the Highveld region of South Africa, at an altitude of 1 327 m above mean sea level.
2.2.Animals and experimental design
The study was carried out during breeding season over a threemonth period from autumn to winter (April 2019 to June 2019) in South Africa.A total number of 20 Saanen bucks aged 17-18 months,with (54.10±2.65) kg average body weight were used.Bucks were divided into two groups: the artificial vagina collection method group (n=10) and the electro-ejaculator collection method group(n=10).Samples from each collection method were pooled and divided into two cooling methods: slow and fast.The experimental animals were maintained together in the same pen and fed daily with approximately 1.65 kg per animal of locally available milled lucerne containing 207 g/kg dry matter of crude protein and 1.9 Mcal metabolize energy (ME) (ME/kg dry matter), without providing formulated diet and had no access to fresh growing forages.Water was provided ad libitum throughout the experiment.
2.3.Semen collection
The collection was performed by using artificial vagina and electro-ejaculator.Ejaculates were collected once weekly throughout the experiment.
2.3.1.Artificial vagina
For artificial vagina method, bucks were trained for more than six weeks prior to semen collection by using a doe on heat.On the day of semen collection, the artificial vagina was prepared by filling the space between the casing and liner with approximately 55 ℃ warm water[8].The lubricant (K-Y*Lubricating Jelly, Johnson & Johnson Medical, South Africa) was applied to the inner liner at the open end of the artificial vagina before collection.At the other end of the artificial vagina, a graduated glass tube (Ramsem, South Africa) was inserted for semen collection.Before collection, the prepuce of the buck was wiped clean with distilled water to reduce contamination.The oestrous doe was restrained in collection pen.The semen collector held the artificial vagina alongside the doe flank with the open end facing towards the buck and downwards at an angle of 45 ℃.When the buck mounted the doe, the erect penis was diverted into the artificial vagina.After ejaculation, the graduated glass tube was separated from the artificial vagina, and then semen volume was recorded and transferred into the conical tube (Minitube, South Africa).
2.3.2.Electro-ejaculator
For electro-ejaculator method, an electro-ejaculator (Ramsem,South Africa) with a rectal probe 28 cm long, 2 cm in diameter,standardized for small ruminants was used.The animal was physically restrained in a lateral position on the floor.The rectum was cleaned of faeces and the prepuce area was shaved and washed with distilled water and wiped with paper towel (Kimberley-Clark,South Africa) to remove any debris or potential contaminant of the ejaculate.The sigmoid flexure was straightened manually, extending the glans of the penis out of the prepuce, fixating it with a sterile gauze swab.A lubricated rectal probe (with K-Y*lubricating jelly)was inserted into the rectum.An electro-stimulation was applied for 4-6 s intervals between stimuli for 4 to 5 times.The electrical current was gradually increased until a maximum of 5 voltages was reached[11].
2.4.Evaluation of semen
2.4.1.Subjective evaluation
All semen samples were evaluated macroscopically for ejaculate volume and pH, and microscopically for sperm mass motility,progressive motility, concentration, normal morphology, acrosome integrity, viability and plasma membrane integrity as described previously[16].
2.4.2.Objective evaluation
Sperm VCL (μm/s), VSL (μm/s) and VAP(μm/s) parameters were assessed with a computer assisted semen analyser system (Sperm Class Analyzer, Microptic SL., Barcelona, Spain), using existing species-specific evaluation parameters for bucks.Preset values for the instrument were as follows: for the Basler camera, which can take 60 frames per second, image brightness of 60, contrast of 750, and light of 1 000 were adjusted.The minimum average path at 50 μm/s and >50% progressive motility was accepted.Motility parameters of static, slow (>40 μm/s), medium (>70 μm/s), and rapid(>100 μm/s) were set and kinematic parameters of VCL (>80 μm/s),VSL (>50 μm/s), and VAP (>25 μm/s) were set.The 5 μL of each sample was evaluated in microscopic slides covered with a coverslip.
For each sample, 200 to 300 spermatozoa in three different areas were analyzed to evaluate the motility.Total motility was taken as the sum of progressive and non-progressive motility.The VCL: μm/s,time-averaged velocity of a sperm head along its actual curvilinear path, as perceived in two dimensions with the microscope.The VSL: μm/s, time-averaged velocity of a sperm head along the straight line between its first detected position and its last.The VAP:μm/s, time-averaged velocity of a sperm head along its average path computed by smoothing the curvilinear trajectory[4].Sperm were classified as medium-speed spermatozoa when their velocity was between 45 μm/s and 75 μm/s, and as rapid spermatozoa when their velocity was >75 μm/s.Spermatozoa were considered progressively motile when they travelled straight over at least 80% of their trajectory.
2.4.3.Sperm mitochondrial membrane potential
Post-thaw mitochondrial membrane potential assay of spermatozoa was evaluated with the probe JC-1 (T-3168).About 20 μL JC-1(0.15 mM in dimethylsulfoxide) was added in 100 μL of each sample (sperm concentration; 5×106cells) and was incubated at 37 ℃ for 10 min.Two hundred spermatozoa with orange midpiece (high mitochondrial membrane potential) and with green midpiece (low mitochondrial membrane potential) were evaluated under epifluorescence microscope (magnification ×400; Nikon, Optiphot,Marunouchi, Chiyoda-ku, Tokyo, Japan) with excitation and emission filters around 490 and 550 nm[17].
2.4.4.Sperm DNA integrity
Post-thaw sperm DNA was evaluated according to üstüner et al[18]with a slight modification.Two hundred spermatozoa with intact DNA (green fluorescence) and damaged DNA (yellow-green to red fluorescence) were evaluated under epifluorescence microscope with excitation and emission filters around 490 and 550 nm.
2.5.Cooling procedures
A total of 120 ejaculates were collected from 20 bucks for each semen collection method (artificial vagina and electro-ejaculator).The ejaculates were considered for cooling and freezing using standard criteria established by Bezerra et al[19].Each pooled semen sample was extended using Tris-based extender (pH 6.8) containing Tris 2.422 g, citric acid monohydrate 1.36 g, glucose 1 g, gentamycin 1 000 μg/mL, kanamycin 1 000 μg/mL, egg-yolk (v/v) 20%, glycerol(v/v) 16%, and distilled H2O to final volume (mL).Pooled ejaculates were diluted at a ratio of 1:2 (semen: extender) and mixed gently to ensure homogeneity of the mixture.The diluted semen was transferred into two 15 mL conical tubes for either slow or fast cooling.
2.5.1.Slow cooling rate
For slow cooling, a 15 mL conical tube containing diluted semen was placed inside a 50 mL conical tube and the space between the tubes was filled with warm water at 33 ℃.The combined tubes were transferred into a 500 mL beaker containing water at 33 ℃.The beaker was placed directly in a refrigerator (4 ℃) and kept for 2 h.This allowed a gradual cooling of the semen from 33 ℃ to 4 ℃ in 2 h at 0.22 ℃?min[15].
2.5.2.Fast cooling rate
For fast cooling, a 15 mL conical tube containing extended semen was placed inside a 50 mL conical tube, and the space between the tubes was filled with warm water at 33 ℃.The combined tubes were placed inside a 500 mL beaker containing tap water and ice blocks.The beaker was immediately placed in a refrigerator (4 ℃) and kept for 30 min.This allowed faster cooling rate from 33 ℃ to reaching 4 ℃ after 30 min at 0.55 ℃?min[15].The sperm characteristics were evaluated immediately after cooling period at 4 ℃.
2.6.Freezing procedure
The semen samples from all treatment were diluted by using a 2-step dilution method.In the first step, solution A (without glycerol) was added to semen samples at 33 ℃, to obtain a sperm concentration of 150×109sperm/mL.Diluted semen was cooled for either 30 min (fast cooling) or 2 h (slow cooling) in a refrigerator at 4 ℃.Following this, an equal volume of solution B (containing 16% glycerol) was added at 4 ℃ to obtain a final sperm concentration of 75×109sperm/mL and equilibrated for 2 h.After equilibration time, the semen samples were aspirated into 0.25 mL French straws, sealed with polyvinyl alcohol powder, and suspended in liquid nitrogen vapour inside a styrofoam box container at height 4 cm above liquid nitrogen for 10 min.They were subsequently submerged into liquid nitrogen at -196 ℃, where they were stored at -196 ℃ before analysis[20]; a minimum of 3 straws from each treatment were thawed at 37 ℃ for 30 s in a water bath 24 h after freezing to evaluate post-thaw sperm characteristics.
2.7.Statistical analysis
Statistical analysis was performed by the general linear model procedures using statistical software SPSS (2015) (IBM SPSS Statistics for Windows, Version 23.0.NY, USA).Normality distribution of data was verified prior to its analysis using the Shapiro-Wilk test as a parametric test assumption.All sperm parameters passed the normality test were used in further analysis.For the analysis, a completely randomized block design in a 2×2 factorial arrangement for each collection methods (artificial vagina and electro-ejaculator) and cooling rates (slow and fast) was used.The fixed effects were the semen collection, cooling rate and their interaction.The results were expressed as mean±standard deviation(mean±SD).The analysis of variance using repeated measures was used to test differences between the treatments for each variable.Mean±SD were separated by using Duncan’s multiple range tests.A probability of P<0.05 was considered to be statistically significant.
2.8.Ethics statement
All animal care and procedures used were performed in accordance with Animal Ethics Committee of the University of Pretoria (Project No.EC079-14).
3.Results
3.1.Semen collection methods
Tables 1 and 2 presented the effects of semen collection methods on fresh semen quality of Saneen goat.The mean values for ejaculate volume, semen pH, sperm concentration and sperm viability were significantly (P<0.05) higher in semen collected with artificial vagina than electro-ejaculator (Table 1).The mean values for total motile rapid-speed and progressive motile spermatozoa were significantly (P<0.001) higher in semen collected with artificial vagina than electro-ejaculator.On the other hand, the mean values for medium-speed and non-progressive motility spermatozoa were significantly (P<0.05) higher in semen collected with electroejaculator than artificial vagina.Mean values for VCL and VSL were significantly (P<0.001) higher in semen obtained by artificial vagina than by electro-ejaculator (Table 2).
3.2.Cooling rates
Figures 1 and 2 showed the mean percentages of motility and velocity parameters of slow and fast cooled spermatozoa.Regardless of semen collection methods, slow cooling resulted in higher(P<0.05) percentages of total motile, rapid-speed and progressive motile spermatozoa as compared to fast cooling (Figure 1).The mean values of spermatozoa VCL were significantly (P<0.05) higher in slow cooled semen as compared to fast cooled (Figure 2).
3.3.Interaction between semen collection methods and cooling rates
Tables 3, 4 and 5 showed the interaction between semen collection methods and cooling rates on sperm parameters of goat semen.Mean values for total motile sperm were significantly (P<0.05)higher when slow cooling was used in semen obtained by artificial vagina as compared to electro-ejaculator in post-thaw semen.Mean values for rapid-speed spermatozoa were significantly (P<0.05)higher when slow cooling was used in semen collected by artificial vagina as compared to electro-ejaculator in both cooled and postthaw semen.The mean values of medium-speed spermatozoa were significantly (P<0.05) higher when slow cooling was used in semen collected by electro-ejaculator in cooled semen.In frozenthawed semen, the mean values of sperm progressive motility were significantly (P<0.05) higher when slow cooling was used in semencollected by artificial vagina method.The mean values of nonprogressive motile spermatozoa were significantly (P<0.05) higher when fast cooling was used in semen collected by electro-ejaculator as compared to artificial vagina method in cooled semen (Table 3).
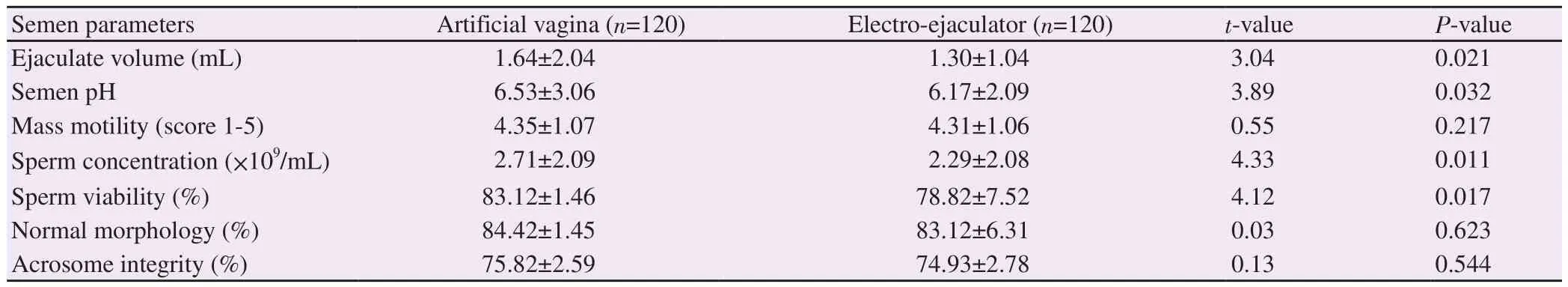
Table 1.Overall fresh semen characteristics obtained by artificial vagina and electro-ejaculator.
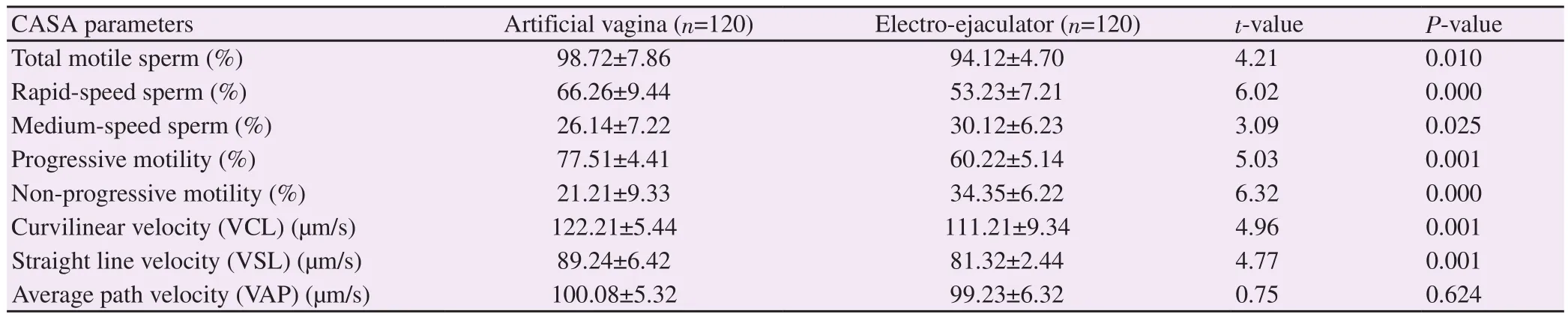
Table 2.Overall sperm motility parameters of sperm in fresh semen collected by using artificial vagina and electro-ejaculator.
Regarding velocity parameters, in cooled semen, the mean values of VCL and VSL were significantly (P<0.001) higher in semen collected by artificial vagina when slow cooling was performed than electro-ejaculator method.In frozen-thawed semen, the mean values of VSL and VAP were significantly (P<0.01) higher when slow cooling was used in semen collected by artificial vagina method(Table 4).
The combination of artificial vagina and slow cooling resulted in significantly (P<0.01) higher percentages of sperm plasma membrane integrity, sperm viability and acrosome integrity in both cooled and post-thaw spermatozoa.Both artificial vagina and electro-ejaculator methods combined with slow cooling rate resulted in significantly (P<0.05) higher post-thaw sperm mitochondrial membrane potential and DNA integrity, as comparedwith fast cooling rate.But, no significant (P>0.05) differences were observed in slow cooling between artificial vagina and electro-ejaculator in post-thawed sperm mitochondrial membrane potential and DNA integrity.The percentages of post-thaw abnormal sperm increased significantly (P<0.05) in fast cooled sperm when electro-ejaculator method was used for semen collection (Table 5).
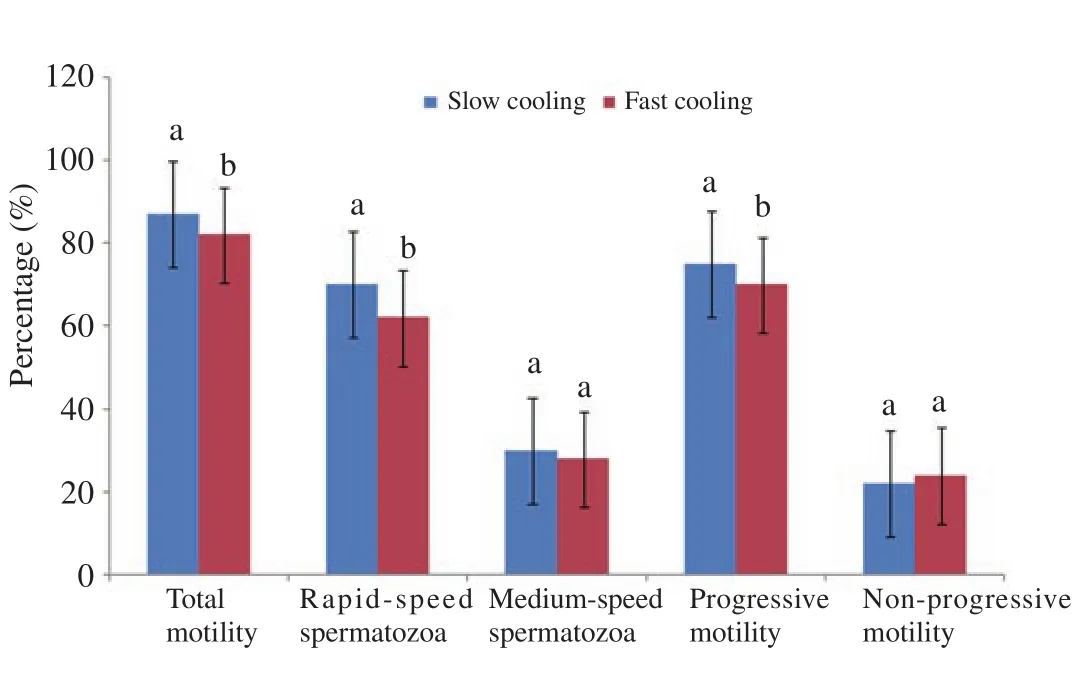
Figure 1.Overall motility parameters of spermatozoa in slow and fast cooled semen.Different superscripts differ significantly at P<0.05.

Figure 2.Overall velocity parameters of spermatozoa in slow and fast cooled semen.Different superscripts differ significantly at P<0.05.
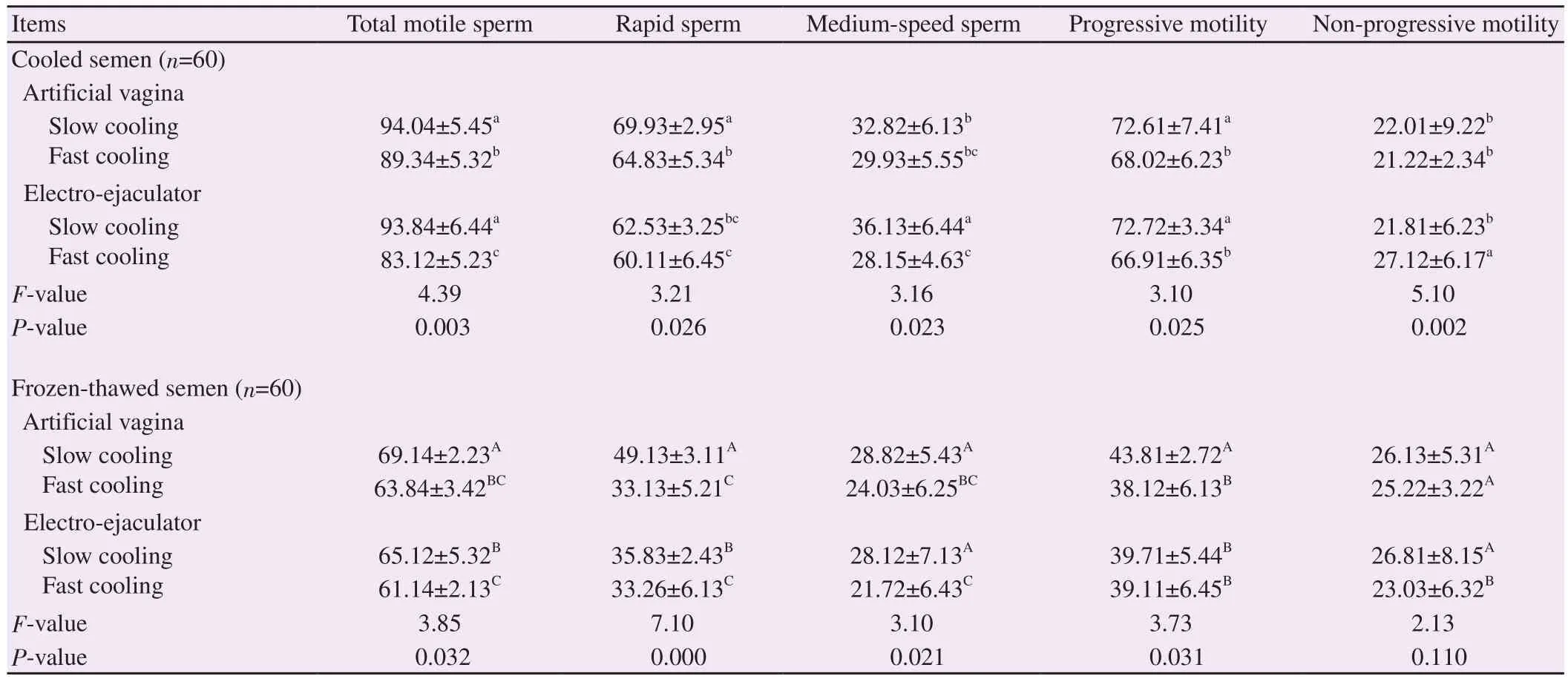
Table 3.Overall sperm motility parameters of cooled and post-thaw Saanen goat semen collected by artificial vagina or electro-ejaculator and cooled using different cooling rates (%).
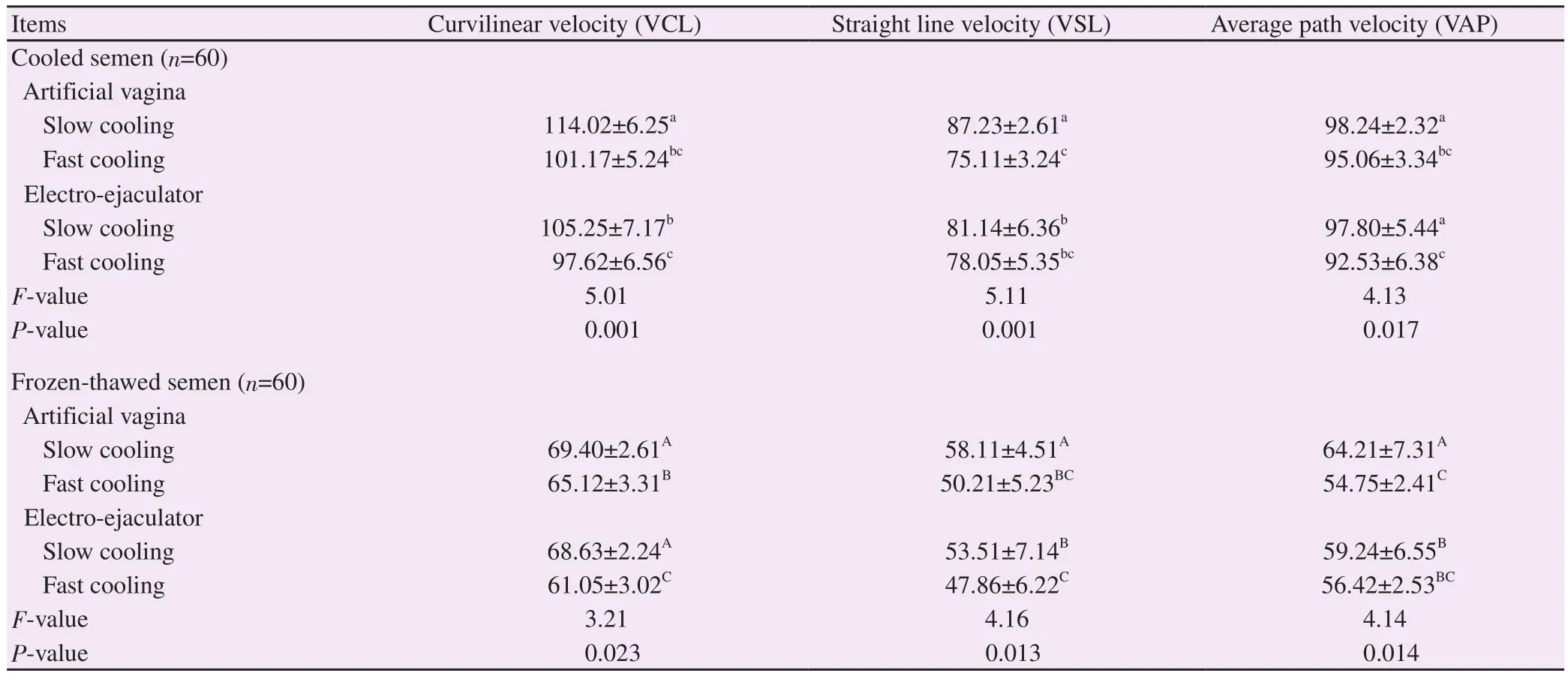
Table 4.Overall sperm velocity parameters of cooled and post-thaw Saanen goat semen collected by artificial vagina or electro-ejaculator and cooled using different cooling rates (μm/s).
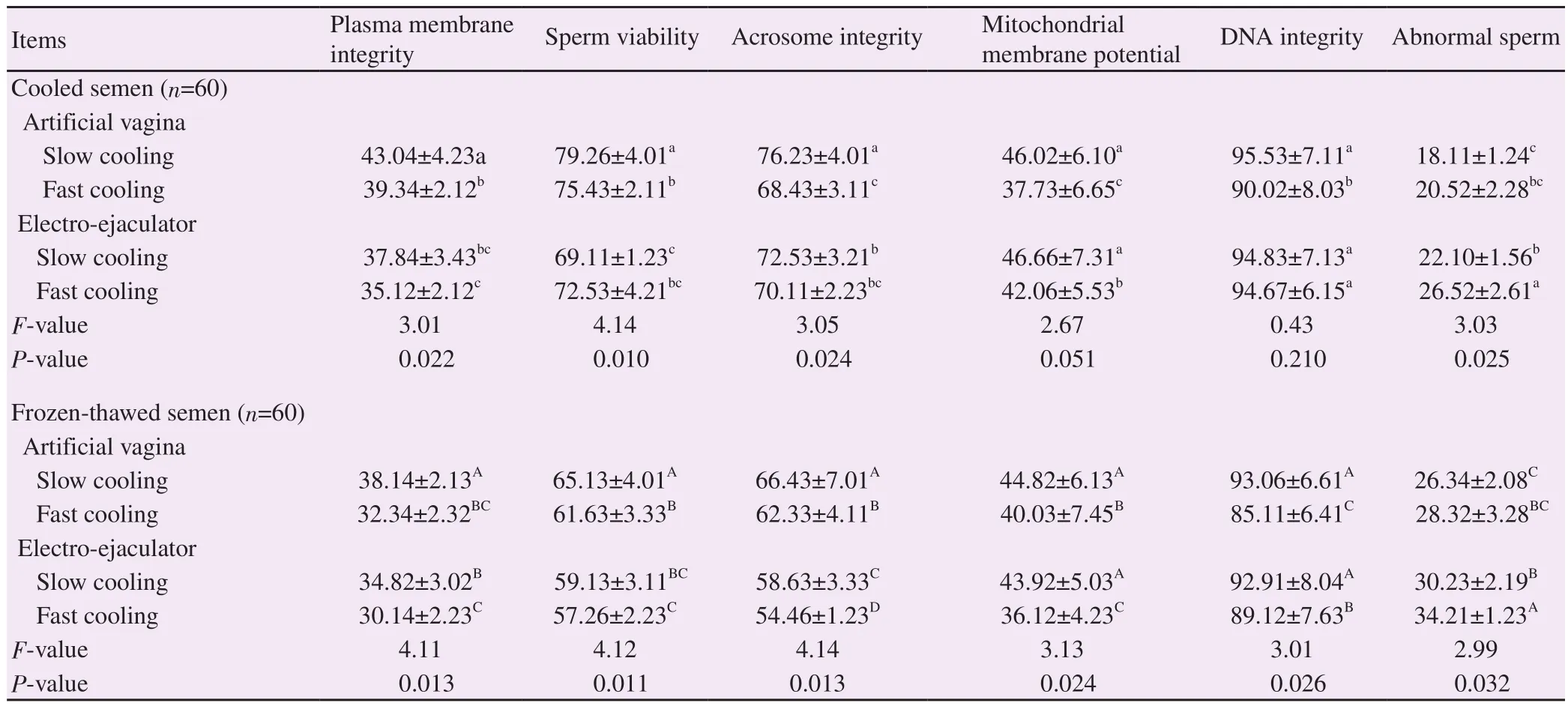
Table 5.Overall of cooled and post-thaw sperm quality parameters of semen collected by artificial vagina or electro-ejaculator and cooled using different cooling rates (%).
4.Discussion
The present study has investigated the effect of combination of semen collection methods with cooling rates on post-thaw sperm attributes.Regardless of the cooling rate, the increased values of ejaculate volume, semen pH, sperm concentration, viability, the total motile, rapid-speed and progressive motile spermatozoa in semen collected with artificial vagina are in agreement with earlier published findings[10,16].The increased percentages of VCL and VSL in semen obtained by using artificial vagina demonstrated that spermatozoa were more resistant to cryo-injuries than those obtained by electro-ejaculator method.Contrary to our results, Jiménez-Rabadán et al[10,11]observed no differences for velocity measures between semen collection methods.These discrepancies could be due to differences between breeds, season, buck or diluents used[11].
The increased mean values of total motility, rapid-speed and progressive motility due to slow cooling have been also reported previously where they found increased percentages of sperm motility when slow cooling was used in goat[15].However, it is obvious that all sperm characteristics are low in fast cooled semen.This may be due to intracellular ice crystal formation and loss of osmotic tolerance[21].In addition, oxidative changes result in disturbance of chemical and physical functions of sperm, leading to imbalanced homeostasis and increase of reactive oxygen species which are detrimental to sperm quality during storage.
The results from the present study demonstrated that sperm motility and velocity of the ejaculate collected with artificial vagina yielded better results than electro-ejaculator method, based on spermatozoa total motility and rapid-speed motility parameters, with the combination artificial vagina and slow cooling yielding superior results.These results are in agreement with the study of Memon et al[15]who reported increased sperm quality with slow cooling when semen was collected using artificial vagina; however, they did not compare different semen collection methods.The collection of semen using artificial vagina method combined with slow cooling rate, as opposed to fast cooling, may have increased the resistance of the outer acrosomal membrane and overlying sperm plasma membrane to cooling and cryo-injury.This suggests that slow cooling was favorable, considering the lowered enzyme leakages as an indirect measure of sperm membrane integrity.Therefore, it can be assumed that to obtain acceptable post-thaw sperm motility and velocity parameters, appropriate semen collection method and optimal cooling rate play important roles in minimizing extra- and intra-cellular stresses.
The increased sperm progressive motility in both artificial vagina and electro-ejaculator when slow cooling was used confirms the importance of this cooling rate on sperm motility.This is supported by the finding that injury of spermatozoa is generally reduced if cooling rate is low[21].The highest values on motility and velocity parameters (VCL, VSL and VAP) obtained with the combination of artificial vagina and slow cooling rate confirmed the report of Memon et al[15]who observed higher sperm survival rate when slow cooling rate was used during cryopreservation.On the other hand, Jiménez-Rabadán et al[10,11]observed higher post-thaw sperm quality in buck when the semen was collected by artificial vagina, as evidenced by increased percentages of motility, intact spermatozoa and viable spermatozoa with active mitochondria.In the present study it clear that the combination of artificial vagina and slow cooling played major roles in protecting sperm motility and velocity during cooled-storage of goat semen.
The increased values of rapid-speed and progressive motility in slow cooling when semen was collected by artificial vagina demonstrated that this rate of cooling was crucial to optimal sperm function post-thaw.This has been also reported by Memon et al[15]who found that a slow cooling rate was optimal and necessary to maintain membrane integrity and motility post-thaw in Boer goats,suggesting that maintaining of sperm velocity post-thaw depends on appropriate interactions between semen collection methods and cooling rates, as evidenced by the increased sperm VSL and VAP observed after thawing in the present study.
The increased values of post-thaw VSL and VAP may be attributed to the increased percentages of sperm plasma membrane integrity,viability and acrosome integrity in both cooled and frozen-thawed semen as observed in the present study under the artificial vagina and slow cooling treatments.Similar to our results, Ahmad et al[12]reported that a slower cooling rate yielded better results than a fast cooling rate, based on several post-thaw sperm characteristics.It is,therefore, suggested that if the cooling rate is appropriate combined with suitable semen collection method would result in less damage of lipid and protein membrane phase caused by cold shock during cryopreservation.
The increase percentages of post-thaw of sperm mitochondrial membrane potential and DNA integrity in both artificial vagina and electro-ejaculator combined with slow cooling agree with the findings of Jiménez-Rabadán et al[11].They reported increased values of mitochondrial membrane potential with artificial vagina method.They also reported increased values of DNA integrity with electro-ejaculator method.This indicates that application of a slow cooling rate in the present study before freezing may have increased the resistance of the mitochondrial membrane and DNA integrity to cooling and cryo-injury in both semen obtained by artificial vagina and electro-ejaculator.
In conclusion, results of the present study indicate that the success of the cooled and frozen-thawed sperm of Saanen goats depends on the semen collection methods and cooling rates used.The artificial vagina method performed better when slow cooling was used in comparison to electro-ejaculator, despite no differences detected in some sperm motility and velocity parameters.These findings support the hypothesis that Saanen goats show a different response to the cryopreservation process depending on the semen collection method used.
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
Kambulu Lukusa contributed substantially in samples collection,data analysis and interpretation as well as drafting of the manuscript.John Kabuba contributed substantially to conception and design,revising it critically for important intellectual content, and final approval of the version to be published.
 Asian Pacific Journal of Reproduction2020年5期
Asian Pacific Journal of Reproduction2020年5期
- Asian Pacific Journal of Reproduction的其它文章
- Socioeconomic, biological and genetic factors influencing preterm birth
- Identification of stable internal control genes for accurate normalization of real-time quantitative PCR data in testicular tissue from two breeds of cattle
- Protective and therapeutic effect of protocatechuic acid in assessment of letrozoleinduced polycystic ovary syndrome in rats
- Predictors of caring behaviors of mothers of premature infants based on the health belief model
- Possible links between COVID-19 and male fertility
